Abstract
We examined the spatial distribution of antibiotic-resistant coliform bacteria amongst livestock from three distinct cultural groups, where group-level differences in practices (e.g., antibiotic use) may influence the magnitude of antibiotic resistance, while livestock interactions (e.g., mixing herds, shared markets) between these locations may reduce heterogeneity in the distribution of antibiotic resistant bacteria. Data was collected as part of a larger study of antibiotic-resistance in northern Tanzania. Simple regression and generalized linear regression were used to assess livestock management and care practices in relation to the prevalence of multidrug-resistant (MDR) coliform bacteria. Simple and multivariable logistic regression were then used to identify how different management practices affected the odds of households being found within MDR “hotspots.” Households that had a higher median neighbourhood value within a 3000 m radius showed a significant positive correlation with livestock MDR prevalence (β = 4.33, 95% CI: 2.41–6.32). Households were more likely to be found within hotspots if they had taken measures to avoid disease (Adjusted Odds Ratio (AOR) 1.53, CI: 1.08—2.18), and if they reported traveling less than a day to reach the market (AOR 2.66, CI: 1.18—6.01). Hotspot membership was less likely when a greater number of livestock were kept at home (AOR 0.81, CI: 0.69–0.95), if livestock were vaccinated (AOR 0.32, CI: 0.21—0.51), or if distance to nearest village was greater (AOR 0.46, CI: 0.36–0.59). The probability of MDR increases when herds are mixed, consistent with evidence for passive transmission of resistant bacteria between animals. Reduced MDR with vaccination is consistent with many studies showing reduced antibiotic use with less disease burden. The neighbourhood effect has implications for design of intervention studies.
Keywords: Antimicrobial resistance, Multi-drug resistance, Livestock, Spatial epidemiology, Hotspot analysis
Abbreviation: AIC, Akaike's information criterion; AMR, Antimicrobial resistance; AOR, Adjusted odds ratio; IQR, Interquartile range; LMIC, Low- and middle-income country; MDR, Multi-drug resistant; OR, Odds ratio; WHO, World Health Organization
1. Introduction
Our ability to treat infectious diseases has been seriously compromised by the emergence and dissemination of microorganisms that are resistant to antimicrobial agents [1]. Resistant pathogens, particularly multidrug-resistant (MDR) strains, are found globally, irrespective of geographic, economic, or cultural differences. In a 2014 report by the World Health Organization (WHO), high prevalence rates of antimicrobial resistance have been observed in all WHO-defined regions around the world, including the African Region, the Region of the Americas, the South-East Asia Region, the European Region, the Eastern Mediterranean Region, and the Western Pacific Region [2].
Despite being a global phenomenon, antimicrobial resistance (AMR) is particularly acute in low and middle income countries (LMICs), and can be attributed to several different factors [[3], [4], [5], [6], [7]]. High rates of infectious diseases drive greater demand for antibiotics and in turn, results in more widespread resistance [7]. The unregulated sale of antimicrobials for both people and livestock also contributes heavily to this issue [[5], [6], [7], [8]], and is exacerbated by low rates of compliance with best practices for antimicrobial use— a problem likely attributed to reduced access to professional veterinary and medical care in LMICs [[6], [7], [8], [9]]. Finally, financial constraints in LMICs often prevent the widespread availability of the most effective antimicrobials needed to tackle resistant infections, making management and containment of AMR extremely difficult here [5,7,8].
Another factor that is an important contributor to the rise and dissemination of resistant microorganisms is the close relationship between people and livestock in livestock-dependent communities [[10], [11], [12]]. In sub-Saharan Africa, over 600 million people live in households that rear livestock [13], which in turn can be a source of zoonotic and AMR pathogens. For example, in 2013, investigators in Burkina Faso examined 729 fecal samples of production animals for Salmonella; 14% were resistant to antibiotics [14]. Other investigators in Hungary and China have reported possible links between antimicrobial use in cattle and swine, respectively, and resistant infections in humans [12,15].
While many aspects of AMR have been studied extensively worldwide, it is rare to incorporate information about spatial relationships between prevalence of resistant organisms and household- or community-level factors (but see [[16], [17], [18], [19], [20]]). This is a potentially important omission when neighbourhood effects (e.g., sharing of bacteria between adjacent households) impact the variance in the distribution of resistant bacteria. This can be particularly critical when developing and testing interventions because significant neighbourhood effects have the potential to interfere with treatment effects, increasing the probability of type 1 errors and consequently the rejection of test hypotheses. Adopting a spatial perspective is also critical for identifying areas where there is clustering of high AMR because knowledge of such clusters can play a key role in the development of targeted interventions in these areas.
To better partition the variance in the distribution of resistant bacteria, a spatial technique called “hotspot analysis,” can be implemented to evaluate whether high or low values of a variable cluster together [16,17,21]. A study by Kiffer et al. in 2011 used hotspot analysis to detect cluster emergence of ciprofloxacin-resistant E. coli in São Paulo, Brazil, which was then used to help demonstrate a positive correlation between ciprofloxacin use and resistance from a spatial perspective [17]. This is an interesting example of how spatial analyses can be used to elucidate important risk factors for AMR and highlights the potential importance of neighbourhood effects, such as one's increased risk of resistance due to antimicrobial usage by others in the area.
Using data from a recent study by Caudell et al. [22] that examined antimicrobial use and veterinary care amongst three ethnic groups (Maasai pastoralists, Arusha agropastoralists, and Chagga highland farmers) in Tanzania's northern region, we employed a spatial approach to identify household clusters of increased livestock MDR bacteria, and the risk factors related to livestock management and care practices that are significantly associated with these clusters.
2. Methods
2.1. Survey data
This analysis draws on data from a study of AMR in northern Tanzania [22]. A survey instrument was used to collect data on >200 variables covering topics about livelihood, veterinary care, and practices surrounding the use of antimicrobials, livestock movement networks, and geospatial data. The survey was conducted between March 2012 and July 2015 and was administered to over 400 households across 13 villages in the region. A subset of households (n = 289) had accompanying antibiotic resistance data that was used in the current analysis. Detailed descriptions of the original study can be found here [22].
2.2. Study groups
Surveys included three different ethnic groups in northern Tanzania—Maasai, Chagga, and Arusha— each with varying livestock practices. The Maasai are pastoralists, who primarily tend large free-range cattle and some small-stock herds. While cattle are mostly kept for dairy and as household equity, other small-stock are typically sold for cash or represent a source of meat for regular household consumption. The Chagga are highland farmers, and primarily tend small, zero-grazing herds of cattle and small-stock for subsistence and market sales. The Arusha are agro-pastoralists and have animal husbandry systems that share similarities to both the Maasai and Chagga. They also typically tend small herds of cattle and small-stock. These three groups provided a wide range of livestock practices that could be assessed in terms of their contribution to the risk of carrying MDR bacteria. More detailed descriptions about the three groups can be found here [22].
2.3. Sampling
Focal villages were selected based on ethnic composition, and in consultation with local research assistants. Within these villages, households were randomly sampled using census data supplied by local Village Executive Officers. Household heads provided information, and household latitude/longitude were recorded.
2.4. Sampling and antibiotic susceptibility testing
The same sampling protocol was used for each household. Up to three fresh but distinct fecal samples were collected for each species and combined separately by species into sterile plastic bags (sheep and goats were treated as the same species for this analysis). If a household did not own more than three animals, samples were collected from all animals. Samples were then transported to a lab at the Nelson Mandela African Institute of Science and Technology (Arusha, TZ). Samples were plated on MacConkey agar for isolation of lactose-fermenting Gram-negative bacteria (mostly Escherichia coli, Klebsiella and Enterobacter). Up to 48 isolates were selected per sample based on colony morphology consistent with E coli. Isolates (n > 70,000) were then shipped to Washington State University where they were tested for resistance to nine antibiotics using a “breakpoint” assay. With this assay, if a bacterial isolate grows on a MacConkey agar plate in the presence of a concentration of antibiotic that normally inhibits susceptible bacteria, then the isolate is classified as resistant. In-depth methods for sample processing and antibiotic susceptibility testing were published earlier [23].
2.5. Statistical analysis
Antibiotic-resistance testing data (binary result for each antibiotic) was pooled across cattle, sheep and goats. MDR values at the household level represent the prevalence of isolates that were resistant to three or more antibiotics. This MDR value served as the dependent variable (“livestock MDR”) for the subsequent analyses. Simple regression was used to first examine the household practices relating to livestock management and care, as well as spatial factors that might influence the prevalence of livestock MDR. Twelve independent variables relating to livestock management and care were selected for the regression analyses. Variables were chosen based on behaviours that have been theoretically or empirically linked to MDR [26], and through previous ethnographic work in these populations [22]. These can largely be grouped according to the following categories:
Livestock management practices included whether livestock came into contact with other livestock (0 = no, 1 = yes), and the numbers of livestock a household kept at home and managed (continuous).
Livestock feeding patterns included whether a household had access to common grazing land (“communal graze”) (0 = no, 1 = yes), whether or not grazing patterns changed if livestock were sick (0 = no, 1 = yes), and whether wildlife and livestock shared a water source (0 = no, 1 = yes).
Disease avoidance measures included the number of vaccines administered to livestock (“vaccinations”) (continuous), antibiotic use (“antibiotic use”) (measured on a scale from 0 (low) to 5 (high)), whether households participated in withdrawal of milk or meat for a period after treatment of livestock with antimicrobials (0 = no, 1 = yes), and the number of measures taken to avoid disease (continuous),. This last variable represents a variety of possible measures the household may partake in to avoid disease in their livestock, including but not limited to building a shed or pen to prevent contact of livestock with other domestic or wild animals, grazing sick livestock separately, feed supplementation, treatment with allopathic medicine, treatment with traditional medicine, spraying, and vaccinations.
Sales yards and villages included the length of time it takes for a person in the household to reach a sales yard (market) to purchase livestock (“time to sales yard <1 hour”; “time to sales yard <1 day”; “time to sales yard >1 day”; “time to sales yard >2 days”), and also the distance to the nearest village (continuous). Given that only a small percentage of people use cars, and public transportation is not common in these regions, almost all people walk to the nearest village on small paths. The Euclidian metric was therefore chosen to measure the distance to the nearest village as these paths are understandably the closest possible to such a beeline measurement.
To investigate whether households with high MDR values are spatially clustered, a point distance analysis was performed for two different search radii (3000 m and 4000 m) using ArcGIS version 10.5.1. Search radii were pre-determined using incremental spatial autocorrelation. These radii were used to create “neighbourhood boundaries” to determine the median neighbourhood MDR value for each household. The median neighbourhood MDR value for a household was calculated using only the MDR values of those households within the set radius; the household for which the median neighbourhood value was being determined was not included in the calculation. Simple regression was performed for each to examine whether median neighbourhood MDR values are associated with an increased likelihood of households having a higher prevalence of livestock-associated MDR isolates.
Using these spatial variables in addition to the livestock management and care practices for which the regression was significant at P < 0.2, generalized linear regression analyses were performed, eliminating the variable with the highest P value at each step. All variables were tested for multicollinearity prior to model selection. Final model selection was based upon the step with the lowest Akaike Information Criterion (AIC).
2.6. Spatial analysis
Hotspot analysis was performed using the Getis-OrD hotspot tool with a distance band of 4000 m to ascertain whether clusters of statistically increased or decreased levels of livestock MDR are found in these areas. To examine the characteristics of households residing within these clusters, a second spatial analysis categorized households based on membership within a hotspot versus outside a hotspot. Simple and multivariable logistic regression analyses were conducted using this variable and a second explanatory model was developed as above, using the livestock management and care practices as the independent variables.
3. Results
For the 289 households included in this analysis, the average number of livestock (cattle, sheep and goats) was 123 (typical of Maasai herds), with a mode of just two (typical of Chagga and Arusha herds). The median prevalence of MDR from livestock across groups was 0.27. There was some variability in the number of isolates grown and tested for each sample, however pairwise correlation indicated that only a very weak correlation exists (r = 0.0394, P < 0.05) between the number of isolates recovered and herd size. Head-of households were predominantly male (74%) and were interviewed in the majority of cases (77.5%). Thus, there is a possibility for sex bias with responses. Surveyed households represented a wide diversity of livestock management practices (Table 1).
Table 1.
Livestock management and care variables for 289 households.
| Variables | Total, n (%) (N = 289) |
|---|---|
| Number of livestock at homea | |
| (Median, IQR) | (0, 0–4) |
| Livestock come in contact with livestock from other households | |
| No | 135 (46.9%) |
| Yes | 153 (53.1%) |
| Number of livestock managed | |
| (Median, IQR) | (0, 0–0.5) |
| Livestock and wildlife share a water source | |
| No | 24 (8.3%) |
| Yes | 265 (91.7%) |
| Communal graze | |
| No | 146 (50.7%) |
| Yes | 142 (49.3%) |
| Graze change occurs if livestock are sick | |
| No | 185 (64.5%) |
| Yes | 102 (35.5%) |
| Vaccinationsb | |
| (Median, IQR) | (1, 0–2) |
| Number of measures taken to avoid disease | |
| (Median, IQR) | (2, 1–2) |
| Household withdrawal of milk or meat from livestock treated with antimicrobials | |
| No | 146 (50.7%) |
| Yes | 142 (49.3%) |
| Antibiotic use | |
| (Median, IQR) | (2, 0–3) |
| Time to sales yard < 1 h | |
| No | 241 (83.68%) |
| Yes | 47 (16.32%) |
| Time to sales yard < 1 day | |
| No | 167 (57.99%) |
| Yes | 121 (42.01%) |
| Time to sales yard > 1 day | |
| No | 258 (89.58%) |
| Yes | 30 (10.42%) |
| Time to sales yard > 2 days | |
| No | 270 (93.75%) |
| Yes | 18 (6.25%) |
| Nearest village distance (km) | |
| (median, IQR) | (3.44, 1.96–6.40) |
| IQR = interquartile range |
Number of livestock at home refers to livestock that do not leave the house to graze but are brought fodder and water. These animals are kept at the home all day, and differ from livestock that go in and out of the household for grazing and watering.
Vaccinations include anthrax, East Coast fever, foot-and-mouth disease, contagious bovine pleuropneumonia, and rabies.
Five of the sixteen variables were identified from the simple regression analyses for consideration in the model (P < 0.20; Table 2). The adjusted model indicated that after controlling for the other variable in the model, median neighbourhood MDR value for a 3000 m radius showed a significant positive correlation with livestock MDR (β = 4.41, CI: 2.54–6.35) (Table 2).
Table 2.
Generalized linear regression results for livestock management and spatial variables using “livestock MDR”a as dependent variable.
| Variables | Unadjusted estimate |
Adjusted estimate |
|---|---|---|
| (95% CI) | (95% CI) | |
| Number of livestock at home | −0.07 (−0.16–0.01) | – |
| Livestock come in contact with livestock from other households | 0.09 (−0.17–0.35) | – |
| Number of livestock managed | 0.00(−0.01–0.01) | – |
| Livestock and wildlife share a water source | −0.27 (−0.71–0.20) | – |
| Communal graze | 0.05 (−0.21–0.31) | – |
| Graze change occurs if livestock are sick | −0.03 (−0.31–0.24) | – |
| Vaccinations | −0.12 (−0.33–0.07)⁎ | – |
| Number of measures taken to avoid disease | 0.08 (−0.17–0.34) | – |
| Household withdrawal of milk or meat from livestock treated with antimicrobials | −0.08 (−0.34–0.18) | – |
| Antibiotic use | 0.05 (−0.12–0.21) | – |
| Time to sales yard: | ||
| <1 h | 0.00 (−) | - |
| <1 day | 0.11 (−0.15–0.38) | - |
| >1 day | 0.05 (−0.39–0.45) | – |
| >2 days | −0.09 (−0.69–0.42) | – |
| Nearest village distance (km) | −0.06 (−0.13–0.16) | −0.012 (−0.09-0.06) |
| Median neighbourhood MDR 3000 m, | 4.41 (2.54–6.35)⁎ | 4.33(2.41–6.32)** |
| Median neighbourhood MDR 4000 m, | 4.39 (2.52–6.34)⁎ | – |
P < 0.05
Livestock MDR refers to the prevalence of isolates measured per household that were resistant to three or more antibiotics.
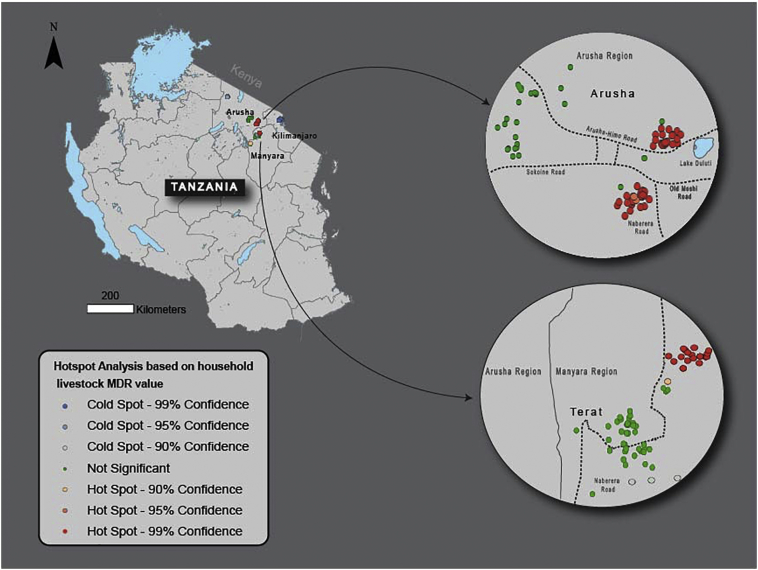
MDR hotspots were found within the Arusha and Manyara regions while coldspots were found within the Kilimanjaro region, an area predominantly inhabited by Chagga people (Fig. 1). When logistic regression was used to analyze household data relative to hotspot membership, 12 of 14 variables were significant with P < 0.20 (Table 3). For the adjusted model, increased measures taken to avoid disease (AOR 1.53, CI: 1.08–2.18) and “time to sales yard < 1 day” (AOR 2.66, CI: 1.18–6.01) were positively correlated with hotspot membership, while keeping livestock at home (AOR 0.81, CI: 0.69–0.95), vaccinating livestock (AOR 0.32, CI: 0.21–0.51), and distance to nearest village (AOR 0.46, CI: 0.36–0.59) were negatively correlated with hotspot membership (Table 3).
Fig. 1.
Map of hotspots based on values of livestock MDR for 289 households.
This figure shows a map of Tanzania with two insets illustrating the two key areas where hotspots were found. These hotspots are areas where households with higher prevalence of MDR cluster together.
Table 3.
Logistic regression results for livestock practices using “within hotspot vs. outside of hotspot” as dependent variable.
| Variables | Odds Ratio (OR) |
|
|---|---|---|
| Unadjusted OR |
Adjusted OR |
|
| (95% CI) | (95% CI) | |
| Number of livestock at home | 0.95 (0.88–1.02) | 0.81 (0.69–0.95)⁎ |
| Livestock come in contact with livestock from other households | 0.52 (0.31–0.87)⁎ | 0.32 (0.10–1.07) |
| Number of livestock managed | 0.84 (0.72–0.99)⁎ | 0.87 (0.72–1.04) |
| Livestock and wildlife share a water source | 0.17 (0.07–0.41)⁎ | 0.32 (0.08–1.37) |
| Communal graze | 0.45 (0.27–0.77)⁎ | – |
| Graze change occurs if livestock are sick | 0.50 (0.28–0.88)⁎ | – |
| Vaccinations | 0.43 (0.31–0.59)⁎ | 0.32 (0.21–0.51)⁎ |
| Number of measures taken to avoid disease | 1.29 (1–1.65)⁎ | 1.53 (1.08–2.18)⁎ |
| Household withdrawal of milk or meat from livestock treated with antimicrobials | 1.94 (1.15–3.26)⁎ | – |
| Antibiotic use | 0.81 (0.68–0.96)⁎ | 0.73 (0.50–1.06) |
| Time to sales yard <1 day | 2.08 (1.24–3.50)⁎ | 2.66 (1.18–6.01)⁎ |
| Time to sales yard >1 day | 0.36 (0.12–1.05) | – |
| Time to sales yard >2 days | 0.00 (0.00–0.00) | – |
| Nearest village distance (km) | 0.53 (0.44–0.65)⁎ | 0.46 (0.36–0.59)⁎ |
P < 0.05
4. Discussion
While many studies have examined risk factors contributing to MDR globally, spatial factors concerning the prevalence of resistant organisms are often overlooked. However, consideration of spatial context and neighbourhood effects are critical to better understand risk factors associated with MDR, and in particular for developing and testing effective interventions, as findings from our research suggest.
The first model examined was the adjusted median neighbourhood analysis model and it identified only one variable as significant— the median neighbourhood livestock MDR value for a 3000 m radius. Households having a higher neighbourhood MDR value for a 3000 m radius showed significant positive correlation with having a higher prevalence of MDR isolates in their livestock population, suggesting a strong spatial component to the spread of MDR beyond the livestock management practices of a particular household. That is, even if a household adheres to recommended best practices for antimicrobial use, having neighbours who do not may be enough to put that household at risk. This finding is important not only for understanding the spread of MDR bacteria but also for understanding interventions to address MDR. Currently, many interventions focus on household-level practices (i.e. using antibiotics more prudently) [9], and not necessarily on practices linked directly to transmission. Nevertheless, if neighbourhood effects do play a role in prevalence of household MDR as these results suggest, then implementing an intervention without considering spatial effects could impact its efficacy. An improved understanding of the spatial component of MDR bacteria is therefore required if we are to develop effective interventions to manage resistance.
Results of the hotspot analysis performed on the study area also indicate statistically significant spatial clusters of households with high (hotspot) or low (coldspot) livestock MDR values, further highlighting the link between proximity and MDR. These hotspots serve as key indicators where multi-drug resistance is most pressing— a critical first step in implementing interventions to reduce the emergence and dissemination of resistance.
The follow-up adjusted hotspot analysis model can be considered the second critical step for interventions, as it offers insight into the potential risk factors giving rise to the distribution of MDR bacteria. Hotspot membership was more likely for households with a shorter time to the sales yard and households that take more measures to avoid disease. Hotspot membership was less likely for households that keep a greater number of livestock at home, households with a greater distance to the nearest village and for households who gave a greater number of vaccinations to livestock.
For households that visited sales yards less than one day away, the increased likelihood result aligns with our expectations that households with shorter travel time might frequent sales yards more often as opposed to those further away, resulting in increased passive transmission [23,27]. For households that take more measures to avoid disease, this result was surprising, however it may reflect greater willingness to use antibiotics in livestock [1]. As this variable represents a variety of different disease avoidance measures, further investigation is needed to better pinpoint which measures specifically used by these households contribute to this result.
Conversely, households that kept a greater number of livestock at home, households with greater distances to the nearest village, and households who demonstrated increased vaccine use all reduced likelihood of hotspot membership. By keeping livestock at home, it is likely that instances of passive transmission that can allow MDR to propagate—including contact with other herds during grazing and watering—were minimized, thereby reducing the transmission of MDR. Several lines of indirect evidence are consistent with the possibility of passive transmission. First, examination of E. coli isolates from waterholes in our research area where people, livestock, and wildlife congregate show resistant profiles similar to livestock [23]. Second, we documented the presence of resistant E. coli in samples from wildlife (consistent with other studies) [27], which could not be the result of direct selection given antibiotics are not used on these populations. The observation regarding households with greater distances to the nearest village is consistent with studies finding that proximity to urban areas increases AMR prevalence [[28], [29], [30]], and reiterates the importance of spatial consideration with regards to resistance. Finally, the observation regarding vaccine use could reflect reduced reliance on antibiotics with livestock even though some of the vaccines were for viral agents, suggesting that increased vaccine use might be an important tool to interrupt the spread of livestock-related AMR.
Potential limitations of the current study include its reliance on some self-reported data that could be skewed by a social-desirability bias [22]. This might be especially true if respondents were aware of behaviours that promote MDR bacteria, like frequent, widespread antimicrobial use. In less literate populations where ethnomedical beliefs often run counter to Western biomedical science [31], this bias may be limited. The primary outcome for these analyses (household prevalence of livestock MDR bacteria) is a quantitative measure that was not self-reported, and consequently not subject to such bias. Furthermore, while fecal samples were easily distinguishable by species, it was not possible to consistently identify the breed and age of the animal producing fecal pats that were sampled. Consequently, these two variables could not be included in the model. Importantly, however, there was no significant species effect on the likelihood of detecting MDR bacteria. This study also only examines factors contributing to bacterial resistance amongst livestock, which is just one facet driving the emergence and transmission of AMR. While some studies have begun to examine the human dimension to this issue [32], more research is required if we are to develop a more substantive and robust explanatory model of AMR.
Acknowledgments
Acknowledgements
We are grateful for the invaluable assistance of many people including Chagga, Arusha, and Maasai study participants; village chairmen of focal villages, Misters Isaya Rumas, Godfrey Naisikye, Lemuta Naisikye, Imma Laiser, Willium Kanunga, Imani Baraka, Paul Sangre, Joseph Tarimo, Joseph Temo, Rigobert Tarimo, Deogratius Mshanga; Profs. Guy Palmer, Terry McElwain, and Jonathan Yoder (Washington State University), Profs. Joann Sharpe (University of Glasgow) and Jonathan Rushton (University of London), and Dr. Julius Keyyu and Dr. Victor Kakengi (Tanzania Wildlife Research Institute).
Funding
This work was supported by the National Science Foundation: Ecology and Evolution of Infectious Diseases [DEB1216040].
Ethics approval
Ethical review for human subjects was approved by the Washington State University (IRB #12533) and by the Tanzania National Institute for Medical Research (Tanzania Commission for Science and Technology permit #2012–151). Research permits were obtained from the Tanzania Commission for Science and Technology, the Tanzania Wildlife Research Institute, and by the regional, district, and ward offices of the Arusha, Manyara, and Kilimanjaro areas where the research was conducted.
Consent for publication
Given high rates of illiteracy amongst the study population, consent for publication was obtained verbally, and the local research assistants signed on their behalf. Approval of this consent procedure was obtained from Washington State University's Institutional Review Board.
Availability of data and material
The data that support the findings of this study are available upon reasonable request from the author [DRC].
Declarations of interest
None.
Funding
National Science Foundation: Ecology and Evolution of Infectious Diseases: DEB1216040.
Authors' contributions
Study concept and design: OA, LR; development of survey protocols and acquisition of data: DRC, MAC; analysis and interpretation of data: OA, LR; drafting of the manuscript: LR; critical revision of the manuscript for important intellectual content and for final approval: OA, DRC, MAC, NS; principal investigator: DRC. All authors have read and approved the final version.
Contributor Information
Leah Rosenkrantz, Email: lrosenkr@sfu.ca.
Ofer Amram, Email: ofer.amram@wsu.edu.
Mark A. Caudell, Email: mcaudell@wsu.edu.
Nadine Schuurman, Email: nadine@sfu.ca.
Douglas R. Call, Email: drcall@wsu.edu.
References
- 1.Ferri M., Ranucci E., Romagnoli P., Giaccone V. Antimicrobial resistance: a global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017;57:2857–2876. doi: 10.1080/10408398.2015.1077192. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . WHO; Geneva: 2014. Antimicrobial resistance: global report on surveillance. [Google Scholar]
- 3.Omulo S., Thumbi S.M., Njenga M.K., Call D.R. A review of 40 years of enteric antimicrobial resistance research in Eastern Africa: what can be done better? Antimicrob Resist Infect Control. 2015;4 doi: 10.1186/s13756-014-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley E.A., Lubell Y., White N.J., Turner P. Antimicrobial susceptibility of bacterial isolates from community acquired infections in sub-Saharan Africa and Asian low and middle income countries. Tropical Med. Int. Health. 2011;16:1167–1179. doi: 10.1111/j.1365-3156.2011.02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Planta M.B. The role of Poverty in antimicrobial resistance. J. Am. Board Fam. Med. 2007;20:533–539. doi: 10.3122/jabfm.2007.06.070019. [DOI] [PubMed] [Google Scholar]
- 6.Ocan M., Obuku E.A., Bwanga F., Akena D., Richard S., Ogwal-Okeng J. Household antimicrobial self-medication: a systematic review and meta-analysis of the burden, risk factors and outcomes in developing countries. BMC Public Health. 2015;15 doi: 10.1186/s12889-015-2109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okeke I.N. Poverty and root causes of resistance in developing countries. In: de Sosa A.J., Byarugaba D.K., Amábile-Cuevas C.F., Hsueh P.-R., Kariuki S., Okeke I.N., editors. Antimicrobial Resistance in Developing Countries. Springer New York; New York, NY: 2010. pp. 27–35. [Google Scholar]
- 8.Okeke I.N., Laxminarayan R., Bhutta Z.A., Duse A.G., Jenkins P., O'Brien T.F. Antimicrobial resistance in developing countries. Part I: recent trends and current status. Lancet Infect. Dis. 2005;5:481–493. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 9.Roulette C.J., Caudell M.A., Roulette J.W., Quinlan R.J., Quinlan M.B., Subbiah M. A two-month follow-up evaluation testing interventions to limit the emergence and spread of antimicrobial resistant bacteria among Maasai of northern Tanzania. BMC Infect. Dis. 2017;17 doi: 10.1186/s12879-017-2857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogaard V.D., E A., London N., Driessen C., Stobberingh E.E. Antibiotic resistance of faecal Escherichia coli in poultry, poultry farmers and poultry slaughterers. J. Antimicrob. Chemother. 2001;47:763–771. doi: 10.1093/jac/47.6.763. [DOI] [PubMed] [Google Scholar]
- 11.Angulo F.J., Nargund V.N., Chiller T.C. Evidence of an association between use of anti-microbial agents in food animals and anti-microbial resistance among Bacteria isolated from humans and the human health consequences of such resistance. J. Veterinary Med. Ser. B. 2004;51:374–379. doi: 10.1111/j.1439-0450.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- 12.Juhász-Kaszanyitzky É., Jánosi S., Somogyi P., Dán Á., vanderGraaf van Bloois L., van Duijkeren E. MRSA transmission between cows and humans. Emerg. Infect. Dis. 2007;13:630–632. doi: 10.3201/eid1304.060833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsh T.L., Yoder J., Deboch T., McElwain T.F., Palmer G.H. Livestock vaccinations translate into increased human capital and school attendance by girls. Sci. Adv. 2016;2:e1601410. doi: 10.1126/sciadv.1601410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kagambèga A., Lienemann T., Aulu L., Traoré A.S., Barro N., Siitonen A. Prevalence and characterization of Salmonella enterica from the feces of cattle, poultry, swine and hedgehogs in Burkina Faso and their comparison to human Salmonellaisolates. BMC Microbiol. 2013;13:253. doi: 10.1186/1471-2180-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu H.-Z., Weng X.-H., Li H., Yin Y.-K., Pang M.-Y., Tang Y.-W. Enterococcus faecium-related outbreak with molecular evidence of transmission from pigs to humans. J. Clin. Microbiol. 2002;40:913–917. doi: 10.1128/JCM.40.3.913-917.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galvin S., Bergin N., Hennessy R., Hanahoe B., Murphy A.W., Cormican M. Exploratory spatial mapping of the occurrence of antimicrobial resistance in E. coli in the community. Antibiotics. 2013;2:328–338. doi: 10.3390/antibiotics2030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiffer C.R., Camargo E.C., Shimakura S.E., Ribeiro P.J., Bailey T.C., Pignatari A.C. A spatial approach for the epidemiology of antibiotic use and resistance in community-based studies: the emergence of urban clusters of Escherichia coli quinolone resistance in Sao Paulo, Brasil. Int. J. Health Geogr. 2011;10:17. doi: 10.1186/1476-072X-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mweu M.M., Nielsen S.S., Halasa T., Toft N. Spatiotemporal patterns, annual baseline and movement-related incidence of Streptococcus agalactiae infection in Danish dairy herds: 2000-2009. Prev. Vet. Med. 2014;113:219–230. doi: 10.1016/j.prevetmed.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Aliyu A.B., Saleha A.A., Jalila A., Zunita Z. Risk factors and spatial distribution of extended spectrum β-lactamase-producing- Escherichia coli at retail poultry meat markets in Malaysia: a cross-sectional study. BMC Public Health. 2016;16 doi: 10.1186/s12889-016-3377-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Jiang S., Liu Y., Wang R., Li X., Yuan Z. Spatial epidemiology and spatial ecology study of worldwide drug-resistant tuberculosis. Int. J. Health Geogr. 2011;10:50. doi: 10.1186/1476-072X-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelner J.L., Murray M.B., Becerra M.C., Galea J., Lecca L., Calderon R. Identifying hotspots of multidrug-resistant tuberculosis transmission using spatial and molecular genetic data. J. Infect. Dis. 2016;213:287–294. doi: 10.1093/infdis/jiv387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caudell M.A., Quinlan M.B., Subbiah M., Call D.R., Roulette C.J., Roulette J.W. Antimicrobial use and veterinary care among agro-pastoralists in Northern Tanzania. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0170328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyimo B., Buza J., Subbiah M., Smith W., Call D.R. Comparison of antibiotic resistant Escherichia coli obtained from drinking water sources in northern Tanzania: a cross-sectional study. BMC Microbiol. 2016;16 doi: 10.1186/s12866-016-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Global Action Plan on Antimicrobial Resistance. World Health Organization; Geneva, Switzerland: 2015. [Google Scholar]
- 27.Katakweba A.A.S., Møller K.S., Muumba J., Muhairwa A.P., Damborg P., Rosenkrantz J.T. Antimicrobial resistance in faecal samples from buffalo, wildebeest and zebra grazing together with and without cattle in Tanzania. J. Appl. Microbiol. 2015;118:966–975. doi: 10.1111/jam.12738. [DOI] [PubMed] [Google Scholar]
- 28.Walson J.L., Marshall B., Pokhrel B.M., Kafle K.K., Levy S.B. 2018. Carriage of Antibiotic-Resistant Fecal Bacteria in Nepal Reflects Proximity to Kathmandu; p. 7. [DOI] [PubMed] [Google Scholar]
- 29.Najjuka C.F., Kateete D.P., Kajumbula H.M., Joloba M.L., Essack S.Y. Antimicrobial susceptibility profiles of Escherichia coli and Klebsiella pneumoniae isolated from outpatients in urban and rural districts of Uganda. BMC Res. Notes. 2016;9 doi: 10.1186/s13104-016-2049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisenberg J.N.S., Goldstick J., Cevallos W., Trueba G., Levy K., Scott J. In-roads to the spread of antibiotic resistance: regional patterns of microbial transmission in northern coastal Ecuador. J. R. Soc. Interface. 2012;9:1029–1039. doi: 10.1098/rsif.2011.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roulette J.W., Roulette C.J., Quinlan R.J., Call D.R., Hewlett B.S., Caudell M.A. Children's ethnobiological notions of contamination and contagions among Maasai agro-pastoralists of northern Tanzania. J. Ethnobiol. 2018;38:261–275. [Google Scholar]
- 32.Mosites E., Mwangi T., Otiang E., Garland-Lewis G., Sammons M., Onyango C. Characterising the taxonomic composition of children and livestock gut microbiomes and of environmental samples and the potential role for household-level microbiome sharing in western Kenya. Lancet Glob. Health. 2016;4:S20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the author [DRC].