Abstract
Introduction
Retinal thickness measured with optical coherence tomography has been proposed as a noninvasive biomarker for Alzheimer's disease (AD). We therefore measured retinal thickness in well-characterized AD and control participants, considering ophthalmological confounders.
Methods
We included 57 amyloid-proven AD cases and 85 cognitively normal, amyloid-negative controls. All subjects underwent retinal thickness measurements with spectral domain optical coherence tomography and an ophthalmological assessment to exclude ocular disease.
Results
Retinal thickness did not discriminate cases from controls, including stratified analyses for early- versus late-onset AD. We found significant associations between macular thickness and global cortical atrophy [β −0.358; P = .01] and parietal cortical atrophy on magnetic resonance imaging [β −0.371; P < .01] in AD cases.
Discussion
In this study, representing the largest optical coherence tomography cohort with amyloid-proven AD cases, we show that retinal thickness does not discriminate AD from controls, despite evident changes on clinical, neuroimaging, and CSF measures, querying the use of retinal thickness measurements as an AD biomarker.
Keywords: Retinal thickness, Cortical atrophy, Alzheimer's disease, Neurodegeneration, Biomarker
1. Background
The development and use of Alzheimer's disease (AD) pathophysiological biomarkers have revolutionized both AD diagnosis and research [1]. Specific biomarkers for neurodegeneration, such as amyloid(-β) and (phosphorylated) tau, support clinical diagnosis on the one hand and help stratify patients for research purposes on the other [2]. Cross-sectional and longitudinal biomarker studies have increased our insight into AD pathophysiology [3]. These biomarkers are, however, invasive, expensive, or unsuitable for repeated measurements, and noninvasive biomarkers are thus urgently needed to increase understanding of pathophysiology, improve diagnosis, and measure therapy effects.
The retina, an easily accessible part of the central nervous system, might serve as such a noninvasive source of biomarkers because it can be easily visualized with optical instruments on a micrometer scale [4]. Previous research suggests retinal manifestations of AD that might be used as biomarkers [4], [5], [6]. Retinal (layer) thinning measured with optical coherence tomography (OCT) could be a biomarker of neurodegeneration, reflecting cortical atrophy [7], which might be the result of transsynaptic retrograde neurodegeneration from affected cortices or from parallel disease processes in the retina [8].
OCT studies in patients with AD performed to date have presented conflicting findings [9], [10], [11]. Although several studies showed mean peripapillary retinal nerve fiber layer (pRNFL) thinning in AD [9], [10], [11], others showed changes in single pRNFL sectors and others found no changes [12], [13], [14]. In addition, some studies reported absence of RNFL thinning, while thinning of total macular thickness was present [9]. There are several possible reasons why previously observed effects might be an overestimation of true effects. In our recently published meta-analysis, we found an indication of publication bias, with an overrepresentation of positive studies [9]. In addition, it is worth noting that retinal thickness is influenced by age [15] and ophthalmological conditions, such as glaucoma [16], diabetes mellitus (DM) (also without diabetic retinopathy)[17], and age-related macular degeneration (AMD) [18], factors that were not always accounted for. Assessment of these confounders requires ophthalmological screening, as a large proportion of patients with glaucoma, diabetic retinopathy, and AMD are asymptomatic [19], [20], [21]. Because glaucoma, DM, and AMD are, like AD, more prevalent with higher age and because associations between AD, AMD, and glaucoma exist [22], [23], retinal changes could have been falsely attributed to AD in previous studies. OCT studies using well-characterized cases that take important confounders into account are thus needed to assess the role of OCT as a diagnostic biomarker [9], [24], [25]. Furthermore, absence of retinal (layer) thinning in our pilot study with early-onset AD (EOAD) cases [7], confirming an earlier EOAD study [12], suggests that the retina might be differently affected in EOAD compared with late-onset AD (LOAD), indicating age of onset might be important in the interpretation of findings.
The objective of the present study was threefold: (1) to assess retinal thinning in well-phenotyped, amyloid-proven AD cases compared with amyloid-negative controls, taking effects of age and ophthalmological confounders into account, (2) to assess relationships between retinal thickness and established AD biomarkers, and (3) to assess retinal thickness differences between LOAD and EOAD.
2. Methods
2.1. Participants
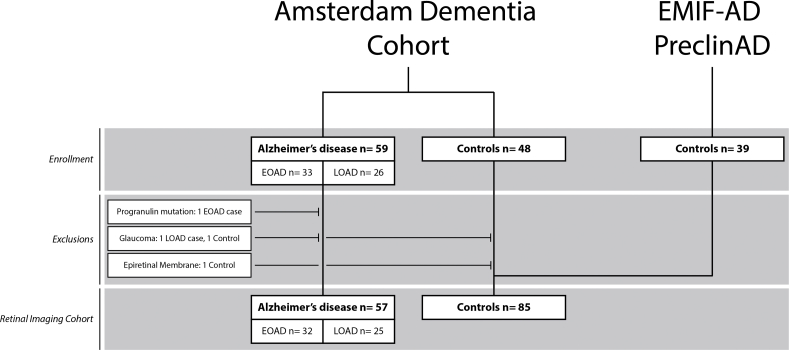
We expanded our pilot cohort, as described earlier in Alzheimer's and Dementia: Diagnosis, Assessment & Disease Monitoring [7]. In total, we enrolled 59 subjects with AD (all Mini–Mental State Examination [MMSE] ≥17, thus capable of giving informed consent) and 48 controls from the screening program of the Alzheimer Center of the Amsterdam UMC (the Amsterdam Dementia Cohort) [26]. Using 65 years of age as cutoff for EOAD versus LOAD, 33 participants were defined as EOAD and 26 as LOAD. Patients and controls underwent a standardized Amsterdam Dementia Cohort screening program including (medical) history, MMSE, neuropsychological evaluation, blood draw for apolipoprotein E (APOE) genotype, magnetic resonance imaging (MRI), or computed tomography scan if contraindications for MRI were present [n = 2], and lumbar puncture. All patients fulfilled NIA-AA criteria of AD and had evidence of amyloid pathology in cerebrospinal fluid (CSF) and/or amyloid positron emission tomography (amyloid-PET) [2]. In all cases with both CSF and PET data available, biomarkers were concordant. Controls were subjects with subjective cognitive decline, defined as subjective cognitive complaints without objective cognitive impairment on neuropsychological evaluation, no signs of neurodegeneration on neuroimaging, and absence of amyloid pathology based on CSF and/or amyloid-PET. In addition, controls were enrolled through the EMIF-AD PreclinAD twin study at our center (n = 39) [27]. One sibling of each monozygotic twin pair was selected to avoid genetic dependency. Controls were eligible if neuropsychological evaluation and MRI were without abnormalities and amyloid-PET showed absence of amyloid pathology.
Exclusion criteria for all participants were (ophthalmological) conditions interfering with OCT quality and/or retinal thickness such as severe cataract, age-related macular degeneration and glaucoma, and neurological or systemic chronic conditions known to interfere with retinal thickness (i.e., multiple sclerosis, Parkinson's disease [PD], and DM). In addition, we excluded subjects with ischemic stroke and/or mild-to-severe white-matter hyperintensities on MRI, operationalized as a Fazekas score of >2 [28]. We excluded one patient with EOAD who was later found to have a progranulin mutation, a mutation known to directly affect retinal integrity [29]. This subject was a significant outlier in our analyses. In addition, we excluded one control and one participant with LOAD because of glaucoma and one control because of bilateral epiretinal membrane (Supplementary Fig. 1).
2.2. Ophthalmological assessment
Subjects underwent the following eye examinations to exclude for ophthalmological pathology: best corrected visual acuity, intraocular pressure using noncontact tonometry (if intraocular pressure >20 mm Hg, contact applanation tonometry was also performed), slit-lamp examination of the anterior and posterior segment, fundus photography (Topcon TRC 50DX type IA), Heidelberg Retina Tomograph optic nerve head analysis, and frequency doubling technology for visual fields. Tropicamide 0.5% was administered for pupil dilation to facilitate optimal ophthalmic examination. We followed the fourth European Glaucoma Guideline criteria: glaucoma was diagnosed when two of the three following measurements were abnormal: ocular pressure (>21 mm Hg), structural glaucomatous changes of the optic nerve head (examined with Heidelberg Retina Tomograph using the Moorfields Regression Analysis), and functional changes in visual fields (examined with frequency doubling technology [C20-1 screening]) [30]. All examinations were interpreted by an experienced ophthalmologist (F.D.V.).
2.3. Spectral domain OCT imaging
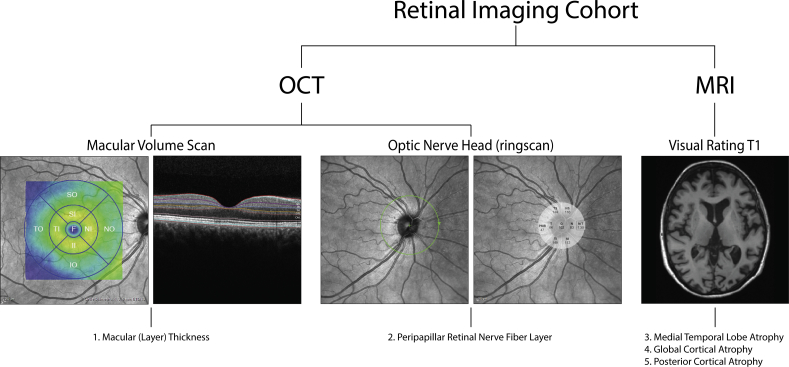
We used Heidelberg Spectralis spectral domain OCT to perform two protocols of each eye for each patient: (1) central retina (macula) dense horizontal scanning; central 20 × 20° area; 49 b-scans (averaging 15 frames per b-scan); 512 a-scans per b-scan and (2) axonal ring scan around the optic nerve head for RNFL (8 frames averaged). Peripapillary RNFL was measured in six sectors provided by the Heidelberg software (temporal superior, nasal superior, nasal, nasal inferior, temporal inferior, and temporal). Macular thickness was measured in the Early Treatment of Diabetic Retinopathy Study map (fovea [Ø 1 mm] and the mean of four quadrants of both the inner ring [Ø 1-3 mm], area 2 to 5, and the outer ring [Ø 3-6 mm], area 6 to 9) (Fig. 1). In the fovea, the inner and the outer ring segmentation analysis was performed with the Heidelberg segmentation software (version 1.9.204.0) to calculate thickness of the following retinal layers: RNFL, ganglion cell layer (GCL), and inner plexiform layer (IPL) (Fig. 1).
Fig. 1.
Overview of retinal imaging cohort. Overview of the retinal imaging cohort that included retinal thickness measurements using optical coherence tomography (OCT) imaging of the optic nerve head and macula and visual rating scores for cortical atrophy on magnetic resonance imaging (MRI).
2.4. Magnetic resonance imaging
MRI visual rating scores were scored by a masked experienced rater (F.B.) before the Amsterdam Dementia Cohort multidisciplinary meeting, where a clinical diagnosis was made by consensus. Medial temporal lobe atrophy (MTA), global cortical atrophy (GCA), parietal cortical atrophy (PCA) [31], [32], [33], and Fazekas score [28] (for white matter hyperintensities) were assessed.
2.5. Cerebrospinal fluid analysis
CSF was analyzed using Innotest ELISA and measured amyloid-β (1–42) (Aβ1–42), tau-181 and pTau. A tau-181/Aβ1–42 ratio of ≥0.52 was considered an AD profile [34].
2.6. Amyloid-PET analysis
A subset of participants (n = 88) was enrolled in research programs that included amyloid-PET scanning with the following tracers: 18F-florbetaben (NeuraCeq, n = 34), 18F-florbetapir (Amyvid, n = 12), 18F-flutemetamol (Vizamyl, n = 39), and 11C-Pittsburgh compound B (11C-PIB, n = 3). Parametric images of amyloid-PET scans were assessed by an experienced rater (B.N.v.B.) and visually interpreted as amyloid positive or amyloid negative by following the guidelines for individual tracers.
2.7. Data extraction
Mean pRNFL as well as in six sectors, total macular thickness, and individual layer thickness in the Early Treatment in Diabetes Retinopathy Study regions were extracted. The mean of both eyes (if both eyes could be examined) was calculated. The means of visual rating scores were calculated for PCA and MTA scores.
2.8. Statistical analysis
2.8.1. Power calculation
Based on our previous meta-analysis, selecting spectral domain OCT scanners in 553 AD cases compared with 486 controls [9], pRNFL thinning of approximately 7 μm can be expected. Assuming a true effect of 7 μm and a standard deviation of 8 μm, 21 subjects in each group are needed to reject the null hypothesis of no difference between the disease and control group with a power of 0.80. In addition, a total macular thickness decrease of approximately 14 μm can be expected. Assuming a true effect of 14 μm and a standard deviation of ±15 μm, 18 subjects in each group are needed to reject the null hypothesis of no difference between the disease and control group with a power of 0.80.
2.8.2. Data analysis
Data were visually tested for a normal distribution using histograms and Q-Q plots. Measures that were normally distributed were tested with an independent t-test, non-normally distributed measures with a Mann-Whitney U test, and binary variables with a χ2 test. Linear regression models were used to assess if changes in retinal (layer) thickness were attributable to diagnosis, corrected for age and sex. All β's reported are standardized β's. Bonferroni correction was used to correct for multiple testing. Data analysis was performed with IBM SPSS Statistics (version 22.0). GraphPad Prism (version 6.0) was used to generate graphs.
3. Results
We included 57 AD cases (32 EOAD, 25 LOAD) and 85 controls (Table 1, Fig. 1) of which 15 EOAD cases and 15 controls were described in our previous pilot study [7]. We found no significant differences in age, sex, and visual acuity. Intraocular pressure showed a statistically significant difference within the normal limits between groups (AD: 16.5 mmHg [±2.3], controls: 15.3 mmHg [±2.7], P < .01). By design, MMSE, atrophy scores on MRI, CSF biomarkers, and amyloid-PET were indicative of an AD diagnosis in AD cases and normal in controls (Table 1). As expected, APOE-ε4 genotype was more prevalent in AD cases (66.6% in AD vs. 24.7% in controls), whereas controls were more often APOE-ε4 negative (33.3% in AD vs. 75.3% in controls, both P < .01) [35].
Table 1.
Cohort characteristics
| Demographics and biomarkers | Alzheimer's disease | Controls | P value |
|---|---|---|---|
| Number | 57 | 85 | |
| Sex (m/f) | 32/25 | 42/43 | .269∗ |
| Age, years | 65.0 (±7.6) | 67.93 (±9.4) | .051† |
| MMSE | 22 (17-28) | 29 (25–30) | .000† |
| APOE-ε4 genotype‡, n (%) | |||
| ε4 homozygous | 12 (22.2) | 1 (1.3) | .000∗ |
| ε4 heterozygous | 24 (44.4) | 18 (23.4) | .009∗ |
| ε4 negative | 18 (33.3) | 58 (75.3) | .000∗ |
| MRI§ | |||
| Global cortical atrophy | 1 (0-2) | 0 (0-2) | .001¶ |
| Medial temporal lobe atrophy | 1.5 (0-2.5) | 0.25 (0-2.5) | .000¶ |
| Parietal cortical atrophy | 1 (0-3) | 1 (0-2) | .000¶ |
| CSF#, (ng/L) | |||
| Aβ1–42 | 551.0 (±107.6) | 1155.9 (±190.6) | .000¶ |
| Tau-181 | 717.8 (±337.4) | 242.2 (±84.7) | .000¶ |
| pTau | 89.0 (±29.6) | 42.5 (±11.6) | .000¶ |
| Tau-181/Aβ1-42 ratio | 1.34 (±.0.8) | 0.21 (±0.1) | .000¶ |
| Aβ-PET∗∗ | |||
| Positive/negative | 23/0 | 0/64 | .000∗ |
| Ophthalmological | |||
| Intra ocular pressure (mmHg) | 16.5 (±2.3) | 15.3 (±2.7) | .005† |
| Visual acuity (LogMAR) | −0.00 (±.07) | −0.02 (±.12) | .191† |
Significant results in bold.
Abbreviations: Aβ, amyloid β; AD, Alzheimer's disease; APOE, apolipoprotein; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; PET, positron emission tomography.
χ2 test.
Independent-samples t-test.
APOE-ε4 genotype was available in 54 AD cases and 77 controls.
MRI was available in 54 AD cases and 80 controls.
Mann-Whitney U test.
CSF was available in 54 AD cases and 36 controls.
Amyloid-PET was available in 23 AD cases and 64 controls.
3.1. Peripapillary RNFL does not discriminate AD from controls
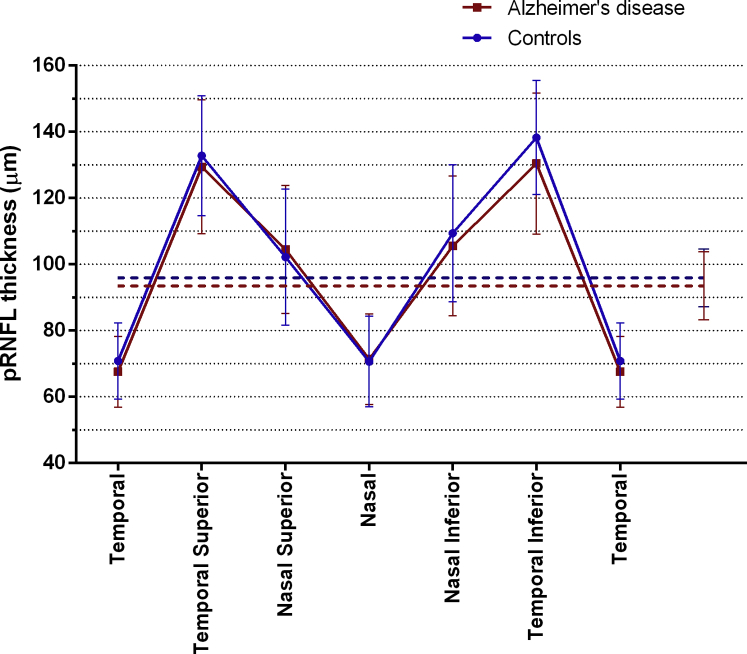
Values of mean pRNFL and in the 6 different sectors (temporal, temporal superior, nasal superior, nasal, nasal inferior, and temporal inferior) showed overlap between AD cases and controls as indicated in a TSNIT plot in Fig. 2. Mean pRNFL was 94.5 μm (±8.7) for AD and 96.0 μm (±10.3) for control participants, with standard deviations exceeding the expected effect size (7 μm). Despite trends of thinning in mean, temporal, temporal superior, and nasal inferior RNFL in AD cases, linear regression models assessing relationships between RNFL and diagnosis, adjusted for age and sex, showed no significant disease effect. Temporal inferior RNFL showed a thinning of 8.7 μm [β −0.222, P = .007], just above the significance level after Bonferroni correction (P = .006). Additional analysis stratified for EOAD and LOAD versus controls showed no significant disease effect (Supplementary Table 1). We found no significant correlation between mean pRNFL thickness and MMSE adjusting for age and sex [β 0.147, P = .078] in the total cohort, nor stratified for diagnosis (AD: [β 0.063, P = .629]; controls: [β 0.017, P = .880]).
Fig. 2.
Peripapillary retinal nerve fiber layer thickness (pRNFL) in patients with Alzheimer's disease (AD) and controls; pRNFL thickness in μm (means, 95% CI) in patients with AD (n = 57, red) and controls (n = 85, blue) in a TSNIT plot, showing pRNFL thickness in different sectors (temporal, temporal superior, nasal superior, nasal, nasal inferior, temporal inferior, and temporal). Mean pRNFL is shown in dashed lines.
3.2. Total and individual macular layer thickness does not discriminate AD from controls
Total retinal thickness and individual layer thickness of RNFL, GCL, and IPL in the inner ring and the outer ring of the Early Treatment in Diabetes Retinopathy Study grid did similarly not discriminate AD cases from controls (Table 2). Despite trends of thinning in AD cases, linear regression models assessing relationships between macular (layer) thickness and diagnosis, adjusted for age and sex, showed no significant disease effect (Table 2). In the same models, total retinal thickness, GCL, and IPL in the inner and outer ring were inversely correlated with age (β's between −0.200 and −0.400, all P < .01). Additional analysis stratified for EOAD and LOAD versus controls yielded similar results (Supplementary Table 1). We found no significant correlation between total retinal thickness (mean inner and outer ring) and MMSE adjusted for age and sex [β 0.078, P = .354], nor for the retinal thickness in the inner ring [β 0.065, P = .440] and the outer ring [β 0.083, P = .321], respectively.
Table 2.
Macular (layer) thickness in ETDRS regions in AD and control participants
| Linear regression | ||||||||
|---|---|---|---|---|---|---|---|---|
| AD | Age | Sex | ||||||
| Retinal thickness measure | Alzheimer's disease (AD) | Controls | β | P value | β | P value | β | P value |
| Total retinal thickness (μm) | ||||||||
| Inner ring | 338.43 (±16.67) | 341.73 (±13.76) | −0.148 | .080 | −0.207 | .015 | 0.082 | .327 |
| Outer ring | 292.38 (±13.12) | 295.17 (±12.30) | −0.143 | .086 | −0.257 | .002 | −0.114 | .166 |
| Retinal nerve fiber layer (μm) | ||||||||
| Inner ring | 21.69 (±1.90) | 21.88 (±1.86) | −0.056 | .515 | 0.008 | .925 | 0.111 | .195 |
| Outer ring | 34.57 (±3.42) | 35.96 (±4.35) | −0.160 | .053 | −0.055 | .505 | −0.263 | .002 |
| Ganglion cell layer (μm) | ||||||||
| Inner ring | 49.71 (±4.66) | 50.15 (±4.58) | −0.110 | .170 | −0.382 | <.001 | 0.005 | .948 |
| Outer ring | 33.96 (±3.17) | 34.17 (±3.13) | −0.098 | .212 | −0.423 | <.001 | −0.053 | .495 |
| Inner plexiform layer (μm) | ||||||||
| Inner ring | 41.22 (±3.11) | 41.33 (±2.98) | −0.089 | .259 | −0.405 | <.001 | 0.073 | .351 |
| Outer ring | 28.35 (±2.53) | 28.33 (±2.12) | −0.051 | .530 | −0.347 | <.001 | −0.020 | .808 |
NOTE. Total retinal thickness, retinal nerve fiber layer thickness, ganglion cell layer thickness, and inner plexiform layer thickness in the fovea, inner ring (Ø 1-3 mm around the fovea) and outer ring (Ø 3–6 mm around the fovea) of the Early Treatment in Diabetes Retinopathy Study (ETDRS) grid in AD (n = 57) and controls (n = 85). Means (±SD), standardized β's, and uncorrected P values of linear regression models with retinal measures as dependent variables and diagnosis, age, and sex (male = 1, female = 0) as independent variables are shown. Significant findings after Bonferroni correction (0.05/8 = 0.006) are shown in bold.
3.3. Macular thickness correlates with visual rating scores for GCA and PCA scores
Next, we assessed relationships between retinal measures and AD biomarkers: visual rating scores on MRI (GCA, PCA, MTA, and Fazekas) and CSF biomarkers (Aβ1-42, Tau-181, pTau, and Tau-181/Aβ1-42 ratio). We found significant inverse associations between perifoveal macular thickness and GCA [β −0.329, P = .002] and PCA [β −0.363 P = .001] scores in the total cohort, after correction for multiple testing using Bonferroni correction. Stratifying for diagnosis, this effect was found to be attributable to AD cases (GCA [β −0.358, P = .010] and PCA [β −0.371, P = .007]). In controls, perifoveal macular thickness did not correlate with GCA (β −0.205, P = .188) or PCA (β −0.088 P = .556). We found no associations between pRNFL thickness and visual rating scores for atrophy on MRI and between pRNFL or macular thickness and any of CSF biomarkers in the total group (Table 3), nor within the total AD patient group (data not shown). In addition, no associations between pRNFL or macular thickness and white matter hyperintensities (Fazekas score) were found.
Table 3.
Relationships between retinal measures and MRI visual rating scores and CSF biomarkers in the total cohort
| A) Magnetic resonance imaging | GCA | PCA | MTA | Fazekas |
|---|---|---|---|---|
| Mean pRNFL | ||||
| β | 0.001 | 0.037 | −0.111 | 0.001 |
| P value | 0.992 | 0.701 | 0.231 | 0.995 |
| Perifoveal macular thickness | ||||
| β | −0.314 | −0.276 | −0.204 | −0.108 |
| P value |
0.001 |
0.003 |
0.028 |
0.298 |
| B) Cerebrospinal fluid analysis |
Aβ1-42 |
Tau-181 |
pTau |
Tau/Aβ |
| Mean pRNFL | ||||
| β | 0.025 | −0.028 | 0.013 | 0.027 |
| P value | 0.811 | 0.790 | 0.903 | 0.795 |
| Perifoveal macular thickness | ||||
| β | 0.142 | 0.047 | 0.112 | 0.033 |
| P value | 0.178 | 0.660 | 0.296 | 0.756 |
NOTE. Linear regression models assessing relationships between mean pRNFL and perifoveal macular thickness (inner + outer ring) with visual rating scores on MRI (GCA, PCA, MTA, and Fazekas) and CSF biomarkers (Aβ1–42, Tau-181, pTau, and Tau/Aβ ratio) adjusted for age and sex (male = 1, female = 0) in the total cohort. Standardized β's and uncorrected P values are shown. Significant findings after Bonferroni correction (0.05/8 = 0.006) are shown in bold. MRI subgroup (n = 134) and CSF subgroup (n = 90).
Abbreviations: GCA, global cortical atrophy; MTA, medial temporal lobe atrophy; PCA, parietal cortical atrophy; pRNFL, peripapillary retinal nerve fiber layer; Aβ, amyloid β; AD, Alzheimer's disease; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; PET, positron emission tomography.
3.4. Retinal measures do not differentiate different APOE genotypes
Finally, we assessed retinal measures stratified for APOE genotype independent of diagnosis; both dichotomous between APOE-ε4 carriers and noncarriers and between APOE-ε4 homozygotes, heterozygotes, and noncarriers. Retinal measures showed no significant differences between different APOE genotypes.
4. Discussion
In this study that represents the largest OCT cohort of well-characterized, amyloid-proven cases of EOAD and LOAD thus far, we show that retinal (layer) thickness does not discriminate AD cases from controls, despite unequivocal changes on clinical, neuroimaging, CSF, and PET measures. Importantly, by using a thorough baseline ophthalmological screening, we were able to exclude confounding from DM, glaucoma, and AMD and measure direct downstream effects of pure AD pathology on retinal structure in AD cases. These results confirm earlier analyses in our pilot cohort [7] and a recent report in a large cohort of AD and mild cognitive impairment participants [14], while contradicting others [9], [11]. Taken together, our results do not support the notion that retinal thickness measurements with OCT can currently serve as a diagnostic biomarker for AD.
Adding to the existing literature, we complemented measurements of pRNFL and total macular thickness with individual layer segmentation of the macula, to assess changes in the RNFL, GCL, and IPL, layers preferentially believed to be involved in AD [36]. Confirming findings from our previous pilot cohort, we did not observe significant differences in inner retinal layer thickness between AD cases and controls, whereas known associations between age and macular GCL and IPL [15] were observed. We did observe trends of retinal (layer) thinning in AD cases that possibly indicate subtle changes in the retina, implying that the effect size of structural changes in the retina might be smaller than in the brain. As we were powered to detect differences of ±6 μm in pRNFL thickness and ±8 μm of total retinal thickness, we doubt that these (or smaller differences) are of clinical relevance given the individual variation of these measures in our cohort and the general population. Given contradictory findings in literature whether retinal (layer) thinning is present and could function as a biomarker in AD, a meta-analysis based on individual data may be a step forward to answer this question. It could also help control for important confounders. In addition, future longitudinal OCT measurements might be more sensitive to subtle changes and could eliminate interindividual differences. A recent study in preclinical AD shows that longitudinal measurements might be sensitive to macular RNFL thinning over time [37]. These studies should ideally also include axial length measurements as this could contribute to interindividual differences and was not accounted for in this study.
By using well-characterized cases and controls, we were able to assess patients confirmed by AD biomarkers in CSF and associate retinal measures with visual rating scores on MRI, CSF biomarkers, and MMSE [2]. We found a significant relationship between total retinal thickness in the macula and measures of neurodegeneration on MRI (GCA and PCA scores) in the total cohort. Interestingly, stratified for diagnosis, these associations were selective for AD, possibly reflecting a selective pathological process in AD cases that involves both the cerebral cortex and the macula. Alternatively, an absence of associations in controls may be the result of a relatively low variance of atrophy scores in controls. In contrast, no relationships between cortical thickness and pRNFL thickness were found. This finding could be a reflection of relationships between cortical thickness and cell bodies of the macula that reflect gray matter, and not with axons in the pRNFL that reflect white matter. Alternatively, it could represent a statistical phenomenon, with less spread and variability in pRNFL thickness. In a previous study, relationships between macular and pRNFL thickness and MTA were reported in a study of cognitively normal controls [38], whereas others described relationships between gray matter volume and retinal layer thickness in controls but not in cognitively impaired cases [39]. Further research is needed to elucidate relationships between brain and retina in AD and controls and could possibly be complemented with cortical thickness and subcortical volume analyses. In line with findings from our pilot cohort, we found no associations between pRNFL and macular thickness and CSF biomarkers or MMSE as measure for disease severity [7].
Our study is the first to test the hypothesis that retinal thinning might be differently involved in EOAD versus LOAD. Previous studies showed differences in cortical atrophy patterns on MRI between EOAD and LOAD, showing a diffuse atrophy pattern with a posterior gradient (posterior cingulate and temporal-parietal cortex and precuneus) or relative absence of atrophy in EOAD and predominant MTA in LOAD [40], [41], [42]. Both adjusted and unadjusted for age, sex, and ophthalmological confounders, we did not observe significant differences in retinal thickness between LOAD and EOAD participants. It's debatable whether 65 or 70 years of age should be used as cutoff for EOAD versus LOAD [43], [44]. We therefore performed additional analyses using 70 years as cutoff (EOAD n = 44, LOAD n = 13, controls n = 85) that yielded similar results.
As retinal (layer) thickness is influenced by multiple variables other than neurodegenerative disease, including AMD, glaucoma, age, DM, and systemic conditions, we foresee that more specific pathological biomarkers in the retina hold more promise as AD biomarkers. Although the presence of retinal tau, Aβ, and neuroinflammation is still controversial, these might be promising molecular biomarkers for diagnosis and possible endpoints in clinical trials [45], [46], [47], [48]. With molecular imaging on the horizon in ophthalmology, optical techniques might enable us to detect such specific retinal changes in the future [49], [50].
5. Conclusion
Retinal thickness does not differ between amyloid-proven AD cases and amyloid-negative controls in a well-characterized sample of patients, taking confounding factors into account. Future studies should focus on longitudinal measurements of retinal layer thickness and specific molecular biomarkers such as amyloid, tau, and neuroinflammation.
Research in context.
-
1.
Systematic review: The authors searched PubMed for all publications assessing retinal thinning in Alzheimer's disease (AD). Well-phenotyped cohorts including amyloid status, visual rating scores on magnetic resonance imaging, and ophthalmological screening have not previously been examined.
-
2.
Interpretation: In this study, representing the largest OCT cohort with amyloid-proven AD cases, we show that retinal thickness does not discriminate patients with AD from controls, despite evident changes on clinical, neuroimaging, and CSF measures, querying the use of retinal thickness measurements as an AD biomarker.
-
3.
Future directions: Future studies, including longitudinal measurements of retinal layer thickness and specific molecular biomarkers such as amyloid, tau, and neuroinflammation, are needed to assess the retina as a potential source of noninvasive AD biomarkers.
Acknowledgments
This study was designed and conducted according to the Declaration of Helsinki, and the study protocol was approved by the Ethical Committee of the Amsterdam Medical Center, location VUmc. All participants gave their written informed consent for participation in this study in the presence of their caregiver. The authors thank all patients, controls, and their families for their participation in our study and Heidelberg for providing the SD OCT for the eye imaging. Research of the Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. The Alzheimer Center Amsterdam is supported by Stichting Alzheimer Nederland and Stichting VUmc fonds. The clinical database structure was developed with funding from Stichting Dioraphte. The EMIF-AD study received support from the EU/EFPIA Innovative Medicines Initiative Joint Undertaking (EMIF grant number 115372) and in kind from GE Healthcare (PET tracer). F.B. is supported by the NIHR biomedical research center at UCLH.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2019.05.002.
Supplementary Data
Supplementary Fig. 1.
Flow diagram showing in-and excluded cases.
References
- 1.Scheltens P., Blennow K., Breteler M.M., de Strooper B., Frisoni G.B., Salloway S. Alzheimer's disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack C.R., Jr., Knopman D.S., Jagust W.J., Petersen R.C., Weiner M.W., Aisen P.S. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.London A., Benhar I., Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53. doi: 10.1038/nrneurol.2012.227. [DOI] [PubMed] [Google Scholar]
- 5.Pelak V.S., Hills W. Vision in Alzheimer's disease: a focus on the anterior afferent pathway. Neurodegener Dis Manag. 2018;8:49–67. doi: 10.2217/nmt-2017-0030. [DOI] [PubMed] [Google Scholar]
- 6.Ong S.S., Proia A.D., Whitson H.E., Farsiu S., Doraiswamy P.M., Lad E.M. Ocular amyloid imaging at the crossroad of Alzheimer's disease and age-related macular degeneration: implications for diagnosis and therapy. J Neurol. 2018 doi: 10.1007/s00415-018-9028-z. [DOI] [PubMed] [Google Scholar]
- 7.den Haan J., Janssen S.F., van de Kreeke J.A., Scheltens P., Verbraak F.D., Bouwman F.H. Retinal thickness correlates with parietal cortical atrophy in early-onset Alzheimer's disease and controls. Alzheimer's Demen (Amsterdam, Netherlands) 2018;10:49–55. doi: 10.1016/j.dadm.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis B.M., Crawley L., Pahlitzsch M., Javaid F., Cordeiro M.F. Glaucoma: the retina and beyond. Acta Neuropathol. 2016;132:807–826. doi: 10.1007/s00401-016-1609-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.den Haan J., Verbraak F.D., Visser P.J., Bouwman F.H. Retinal thickness in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimer's & Dementia: Diagnosis. Assesment Dis Monit. 2017;6:162–170. doi: 10.1016/j.dadm.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson K.L., Yeo J.M., Waddell B., Cameron J.R., Pal S. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimer's Demen (Amsterdam, Netherlands) 2015;1:136–143. doi: 10.1016/j.dadm.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan V.T.T., Sun Z., Tang S., Chen L.J., Wong A., Tham C.C. Spectral-domain OCT measurements in Alzheimer's disease: a systematic review and meta-analysis. Ophthalmology. 2019;126:497–510. doi: 10.1016/j.ophtha.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pillai J.A., Bermel R., Bonner-Jackson A., Rae-Grant A., Fernandez H., Bena J. Retinal nerve fiber layer thinning in Alzheimer's disease: a case-control study in comparison to normal aging, Parkinson's disease, and non-Alzheimer's dementia. Am J Alzheimer's Dis other Demen. 2016;31:430–436. doi: 10.1177/1533317515628053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kromer R., Serbecic N., Hausner L., Froelich L., Aboul-Enein F., Beutelspacher S.C. Detection of retinal nerve fiber layer defects in Alzheimer's disease using SD-OCT. Front Psychiatry. 2014;5:22. doi: 10.3389/fpsyt.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez D., Castilla-Marti M., Rodriguez-Gomez O., Valero S., Piferrer A., Martinez G. Usefulness of peripapillary nerve fiber layer thickness assessed by optical coherence tomography as a biomarker for Alzheimer's disease. Sci Rep. 2018;8:16345. doi: 10.1038/s41598-018-34577-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demirkaya N., van Dijk H.W., van Schuppen S.M., Abramoff M.D., Garvin M.K., Sonka M. Effect of age on individual retinal layer thickness in normal eyes as measured with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:4934–4940. doi: 10.1167/iovs.13-11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S.H., Lee E.J., Kim T.W. Structural characteristics of the acquired optic disc pit and the rate of progressive retinal nerve fiber layer thinning in primary open-angle glaucoma. JAMA Ophthalmol. 2015;133:1151–1158. doi: 10.1001/jamaophthalmol.2015.2453. [DOI] [PubMed] [Google Scholar]
- 17.van Dijk H.W., Verbraak F.D., Kok P.H., Stehouwer M., Garvin M.K., Sonka M. Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2012;53:2715–2719. doi: 10.1167/iovs.11-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nivison-Smith L., Wang H., Assaad N., Kalloniatis M. Retinal thickness changes throughout the natural history of Drusen in age-related macular degeneration. Optom Vis Sci. 2018;95:648–655. doi: 10.1097/OPX.0000000000001256. [DOI] [PubMed] [Google Scholar]
- 19.Quigley H.A. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 20.Cheung N., Mitchell P., Wong T.Y. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 21.Lim L.S., Mitchell P., Seddon J.M., Holz F.G., Wong T.Y. Age-related macular degeneration. Lancet. 2012;379:1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee C.S., Larson E.B., Gibbons L.E., Lee A.Y., McCurry S.M., Bowen J.D. Associations between recent and established ophthalmic conditions and risk of Alzheimer's disease. Alzheimers Dement. 2018;15:34–41. doi: 10.1016/j.jalz.2018.06.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohno-Matsui K. Parallel findings in age-related macular degeneration and Alzheimer's disease. Prog Retin Eye Res. 2011;30:217–238. doi: 10.1016/j.preteyeres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Ong S.S., Doraiswamy P.M., Lad E.M. Controversies and future directions of ocular biomarkers in Alzheimer disease. JAMA Neurol. 2018;75:650–651. doi: 10.1001/jamaneurol.2018.0602. [DOI] [PubMed] [Google Scholar]
- 25.Lee M.J., Abraham A.G., Swenor B.K., Sharrett A.R., Ramulu P.Y. Application of optical coherence tomography in the detection and classification of cognitive decline. J Curr Glaucoma Pract. 2018;12:10–18. doi: 10.5005/jp-journals-10028-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Flier W.M., Scheltens P. Amsterdam dementia cohort: performing research to optimize care. J Alzheimers Dis. 2018;62:1091–1111. doi: 10.3233/JAD-170850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konijnenberg E., Carter S.F., Ten Kate M., den Braber A., Tomassen J., Amadi C. The EMIF-AD PreclinAD study: study design and baseline cohort overview. Alzheimers Res Ther. 2018;10:75. doi: 10.1186/s13195-018-0406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fazekas F., Chawluk J.B., Alavi A., Hurtig H.I., Zimmerman R.A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 29.Kuse Y., Tsuruma K., Mizoguchi T., Shimazawa M., Hara H. Progranulin deficiency causes the retinal ganglion cell loss during development. Sci Rep. 2017;7:1679. doi: 10.1038/s41598-017-01933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terminology and Guidelines for Glaucoma: European Guideline for Glaucoma, 4th ed., 2014. Br J Ophthalmol. 2017;101:130–195. doi: 10.1136/bjophthalmol-2016-EGSguideline.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frisoni G.B., Scheltens P., Galluzzi S., Nobili F.M., Fox N.C., Robert P.H. Neuroimaging tools to rate regional atrophy, subcortical cerebrovascular disease, and regional cerebral blood flow and metabolism: consensus paper of the EADC. J Neurol Neurosurg Psychiatry. 2003;74:1371–1381. doi: 10.1136/jnnp.74.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koedam E.L., Lehmann M., van der Flier W.M., Scheltens P., Pijnenburg Y.A., Fox N. Visual assessment of posterior atrophy development of a MRI rating scale. Eur Radiol. 2011;21:2618–2625. doi: 10.1007/s00330-011-2205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheltens P., Leys D., Barkhof F., Huglo D., Weinstein H.C., Vermersch P. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duits F.H., Teunissen C.E., Bouwman F.H., Visser P.J., Mattsson N., Zetterberg H. The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement. 2014;10:713–723.e2. doi: 10.1016/j.jalz.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Liu C.C., Liu C.C., Kanekiyo T., Xu H., Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hart N.J., Koronyo Y., Black K.L., Koronyo-Hamaoui M. Ocular indicators of Alzheimer's: exploring disease in the retina. Acta Neuropathol. 2016;132:767–787. doi: 10.1007/s00401-016-1613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santos C.Y., Johnson L.N., Sinoff S.E., Festa E.K., Heindel W.C., Snyder P.J. Change in retinal structural anatomy during the preclinical stage of Alzheimer's disease. Alzheimer's Demen (Amsterdam, Netherlands) 2018;10:196–209. doi: 10.1016/j.dadm.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casaletto K.B., Ward M.E., Baker N.S., Bettcher B.M., Gelfand J.M., Li Y. Retinal thinning is uniquely associated with medial temporal lobe atrophy in neurologically normal older adults. Neurobiol Aging. 2016;51:141–147. doi: 10.1016/j.neurobiolaging.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S., Ong Y.T., Hilal S., Loke Y.M., Wong T.Y., Chen C.L. The association between retinal neuronal layer and brain structure is disrupted in patients with cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2016;54:585–595. doi: 10.3233/JAD-160067. [DOI] [PubMed] [Google Scholar]
- 40.van der Flier W.M., Pijnenburg Y.A.L., Fox N.C., Scheltens P. Early-onset versus late-onset Alzheimer's disease: the case of the missing APOE ε4 allele. Lancet Neurol. 2011;10:280–288. doi: 10.1016/S1474-4422(10)70306-9. [DOI] [PubMed] [Google Scholar]
- 41.Karas G., Scheltens P., Rombouts S., van Schijndel R., Klein M., Jones B. Precuneus atrophy in early-onset Alzheimer's disease: a morphometric structural MRI study. Neuroradiology. 2007;49:967–976. doi: 10.1007/s00234-007-0269-2. [DOI] [PubMed] [Google Scholar]
- 42.Frisoni G.B., Testa C., Sabattoli F., Beltramello A., Soininen H., Laakso M.P. Structural correlates of early and late onset Alzheimer's disease: voxel based morphometric study. J Neurol Neurosurg Psychiatry. 2004;76:112–114. doi: 10.1136/jnnp.2003.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koedam E.L., Lauffer V., van der Vlies A.E., van der Flier W.M., Scheltens P., Pijnenburg Y.A. Early-versus late-onset Alzheimer's disease: more than age alone. J Alzheimers Dis. 2010;19:1401–1408. doi: 10.3233/JAD-2010-1337. [DOI] [PubMed] [Google Scholar]
- 44.Palasi A., Gutierrez-Iglesias B., Alegret M., Pujadas F., Olabarrieta M., Liebana D. Differentiated clinical presentation of early and late-onset Alzheimer's disease: is 65 years of age providing a reliable threshold? J Neurol. 2015;262:1238–1246. doi: 10.1007/s00415-015-7698-3. [DOI] [PubMed] [Google Scholar]
- 45.Koronyo Y., Biggs D., Barron E., Boyer D.S., Pearlman J.A., Au W.J. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer's disease. JCI Insight. 2017;2 doi: 10.1172/jci.insight.93621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams E.A., McGuone D., Frosch M.P., Hyman B.T., Laver N., Stemmer-Rachamimov A. Absence of Alzheimer disease neuropathologic changes in eyes of subjects with Alzheimer disease. J Neuropathol Exp Neurol. 2017;76:376–383. doi: 10.1093/jnen/nlx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho C.Y., Troncoso J.C., Knox D., Stark W., Eberhart C.G. Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer's and Parkinson's disease patients. Brain Pathol. 2014;24:25–32. doi: 10.1111/bpa.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schön C., Hoffmann Na, Ochs S.M., Burgold S., Filser S., Steinbach S. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS One. 2012;7:1–9. doi: 10.1371/journal.pone.0053547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cordeiro M.F., Normando E.M., Cardoso M.J., Miodragovic S., Jeylani S., Davis B.M. Real-time imaging of single neuronal cell apoptosis in patients with glaucoma. Brain. 2017;140:1757–1767. doi: 10.1093/brain/awx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie F., Luo W., Zhang Z., Sun D. In vivo molecular imaging in retinal disease. J Ophthalmol. 2012;2012:429387. doi: 10.1155/2012/429387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.