Graphical abstract
Keywords: Heavy metals, Apples, Grapes, Oranges, Estimated daily intake, Tolerable daily intake
Highlights
-
•
Heavy metals Cu, and Ni were present in all fruit samples obtained from Egyptian governorates.
-
•
Washing of grapes and peeling of orange samples greatly reduced the level of Cu and Ni.
-
•
Estimated daily intake of the fruit samples were considered higher than that of the tolerable daily intake.
Abstract
Heavy metals are considered a main public health hazards, they are known to accumulate in fruits, which are heavily consumed by humans because of their unique sweet taste and potential health benefits. Therefore, the aim of this study was to determine the possible contamination levels of cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb) and nickel (Ni) in some selected fruits obtained from four Egyptian governorates and to compare the contamination levels with those of the recommended permissible limits. Results revealed that Pb and Cd were absent in all fruit samples, while Cr was only detected in grapes obtained from Cairo and Fayoum governorates and exceeded the maximum permissible limit (0.10 mg kg−1). Nickel and Cu were detected in all fruit samples. Nickel was reduced more than copper after washing process of grapes especially in samples obtained from Alexandria and Giza governorates. After peeling process, Cu was extremely reduced in orange samples obtained from the following governorates in descending order Cairo, Alexandria, El-Fayoum and Giza. Estimated daily intake of heavy metals in fruit samples were found to be higher than that of the tolerable daily intake, indicating potential risk to human health. Therefore, to decrease the risk to human health, fruits must be washed well before eating to decrease heavy metal concentrations.
1. Introduction
Fruits contamination by heavy metals is one of the major problems that arise from the huge uses of fertilizers, pesticides and other chemicals due to the pursuit for fast economic development by industrial and new agriculture practices [1], and to fulfill the advanced demands of food production for human consumption [2]. Heavy metals are the main contaminants of food supply as they are considered a vital concerns in food safety and quality assurance [[3], [4], [5], [6], [7]], and can be considered as a major problems in the environment (soil, water, air) [[8], [9], [10]]. Heavy metals have the ability to enter the body system through food, air, and water. They have long biological half-time and are not biodegradable [11].
Due to their long half-time, the bio-accumulation of heavy metals could occur over a period of time and hence the undesirable side effects could be due to accumulation in the different body organs [[12], [13], [14]]. Serious threats to human health include chronic toxicity symptoms, kidney injury, renal failure and liver damage [15]. Due to the chronic accumulation of heavy metals in liver and kidney of humans it cause disruption of numerous biochemical processes, leading to cardiovascular, kidney, bone and nervous diseases, which might be due to extensive consumption of Cr through food [16]. Excess consumption of food contaminated with Cr may lead to stomach upset, skin rashes, lung cancer, liver and kidney damage [17]. Excessive content of Pb and Cd in food, are associated with several of diseases [[18], [19], [20]], as well as carcinogenesis, teratogenesis and mutagenesis [21,22]. In Japan, due to Pb and Cd poisoning several lives were lost and many more established bodily abnormalities [[23], [24], [25]]. Also, copper toxicity can lead to iron deficiency, lipid peroxidation and destruction of membranes [8]. Whereas, zinc or iron deficiency, as well as enzymatic malfunctioning, may also be a result of high level of Ni [11].
Fresh fruits are of great significance in the human health due to the presence of vitamins and mineral salts, in addition to water, calcium, potassium, sulfur and iron [26]. Also, fruits are essential and beneficial for the maintenance of health, prevention and treatment of several diseases [27]. These fruits comprise both essential and toxic heavy metals with a wide variety of concentrations [28]. Few papers reported the presence of heavy metals in some types of fruits, such as papayas [29]; avocado [30]; bananas [31]; apples, oranges and bananas [28]; grapes and cherries [32] apples and plums [33]; table grapes and sour cherry [34]; or mangoes [35]. Also, contamination of fruits by heavy metals led to transfer these heavy metals to the common fruits juices [36,37]. Therefore, the main objective of this study was to determine the contamination levels of Cd, Cu, Cr, Pb, and Ni in some selected fruits (apples, grapes, and oranges) obtained from four Egyptian governorates and to compare the contamination levels with those of the recommended permissible limits. Also, to evaluate the potential risk of these heavy metals on public health due to daily consumption of these fruits and suggestion of possible methods for heavy metal removal.
2. Materials and methods
2.1. Sample collection and preparation
A total of 108 samples representing three different fresh fruit samples (apples, grapes and oranges) were purchased from fruit sale points (36 samples for each fruit) in four Egyptian governorates (Cairo, Giza, Alexandria and El-Fayoum). Cairo is located at 30°2′40″North latitude and 31°14′9″East longitude, Giza is located at 30°01′North latitude and 31°13′East longitude Alexandria is located at 31°12′North latitude and 29°55′East longitude and El-Fayoum is located at 29.308374 °North latitude and 30.844105 °East longitude. The sampling of the fruits was carried out at various fruit sale points for each governorate.
The fruit samples included apples (Malus domestica), oranges (Citrus sinensis) and grapes (Vitis vinifera) were stored under refrigeration condition (<10 °C) in polythene bags until analysis. In the laboratory, surface deposits were removed [37], then the fruits were thoroughly homogenized. Five grams of homogenized fruit samples were transferred to crucibles and dried in an oven at 105 °C for 16 h. Dry-ashing process was carried out in an electric muffle furnace at 540 °C with a gradual increase in temperature for 8–12 h until the sample was completely combusted (slightly colored or gray). Ashed samples were cooled to room temperature and 1.0 ml of 36% HCl was added to crucible walls. The samples were then filtered using Whatman filter paper ashless No. 40 and stored under refrigeration condition (<10 °C) until a determination by atomic absorption spectrophotometer [38].
2.2. Heavy metal analysis
Standard solutions of heavy metals: Cd, Cr, Cu, Pb, and Ni were obtained from Merck (Darmstadt, Germany). The standards were prepared from the individual 1000 mg/kg standard in 0.1 N HNO3. Working standards solutions were prepared from the previous stock by dilution using 0.1 N HNO3 till the needed concentrations for atomic absorption spectrophotometer [39,40].
Analysis for investigated heavy metals was performed using atomic absorption spectrophotometer (AAS) (Agilent Technologies 200 Series AA). Maximum absorbance was obtained by adjusting the Cathode lamps at specific slit and wavelengths. Cadmium was measured at (0.5 nm; 228.8 nm), Cr (0.2 nm; 357.9 nm), Cu (0.5 nm; 324.8 nm), Pb (1.0 nm; 283.0 nm), and Ni (0.2 nm; 232.0 nm). Detection limits of heavy metals were 0.1, 0.02, 0.03, 0.1 and 0.06 mg/kg for Pb, Cd, Cu, Ni and Cr, respectively. The readings were taken from the equipment in mg/l and the results were converted to mg/kg which is the actual concentration of heavy metal in sample using the equation of [13].
Whereas K is the concentration of heavy metal in samples (mg kg−1); A is the concentration of the heavy metal in digested solution (mg/ l); V is the final volume after digestion (ml); M is the weight of the sample (g).
2.3. Removal of heavy metals in grapes by washing
Grape samples were washed by tap water, then the samples were kept in a hot air oven at 70–80 °C till complete dryness, then the fruits were thoroughly homogenized and analyzed as mentioned previously.
2.4. Removal of heavy metals in oranges by peeling
Orange samples were peeled and the peeled orange samples were thoroughly homogenized and analyzed as mentioned previously.
2.5. Estimated daily intake (EDI)
The estimated daily intake (EDI) of heavy metals was calculated as follows:
Whereas Ci is the concentration of heavy metals in fruits (mg kg−1); IR is the average daily consumption of fruits (g/person/day), and BW is the body weight (kg). Based on WHO [41], adults in Middle East had an average daily consumption of 7.5, 15.8 and 31.5 g/person/day for apples, grapes and oranges, respectively. The body weight was set to 70 kg.
2.6. Tolerable daily intake (TDI)
Tolerable Daily Intake is an estimate of the amount of a substance in food that can be taken daily over a lifetime without significant health risk.
2.7. Statistical analysis
Statistical analysis of the data was carried out using Microsoft Excel 2010 statistical program. A one-way analysis of variance (ANOVA) was performed. Fisher's Protected Least Significant Difference was also used to determine the difference between different means.
3. Results
3.1. Heavy metal analysis
The concentrations of heavy metals in fruit samples that are commonly consumed in Egypt are presented in Table 1, Table 2, Table 3. Cadmium and Pb were not detected in all collected samples from the different governorates. Chromium was only detected in grapes obtained from Cairo and Fayoum governorates. The levels of Ni and Cu were detected in all fruit samples obtained from the four Egyptian governorates.
Table 1.
Concentration of heavy metals (mg/kg) in apple samples collected from four Egyptian governorates.
Table 2.
Concentration of heavy metals (mg/kg) in grape samples collected from four Egyptian governorates.
| Ggovernorate | Heavy metals (mg/kg) (Mean ± S.E) |
||||
|---|---|---|---|---|---|
| Cd | Cr | Cu | Pb | Ni | |
| Cairo | ND | 1.01*±0.04a | 3.52*±0.74a | ND | 0.30*±0.05c |
| Giza | ND | ND | 0.72*±0.07c | ND | 0.83*±0.31b |
| Alexandria | ND | ND | 2.53*±0.98b | ND | 1.78*±0.77a |
| El-Fayoum | ND | 1.06*±0.06a | 3.21*±0.86a | ND | 0.31*±0.08c |
| MRL | 0.051 | 0.102 | 0.202 | 0.101 | 0.202 |
| LoD | 0.02 | 0.06 | 0.03 | 0. 1 | 0.1 |
Table 3.
Concentration of heavy metals (mg/kg) in orange samples collected from four Egyptian governorates.
Data in Table 1 revealed that Cu was detected in apple samples at a concentration of 1.63, 0.33, and 0.89 mg/kg from Cairo, Giza, and Alexandria governorates respectively, and they exceeded the maximum permissible limit (0.20 mg/kg) [42]. Results also showed that apple samples obtained from El-Fayoum contained Cu at a concentration of 0.14 mg/kg, which is considered below the maximum permissible limit.
Nickel was also detected in apple samples at a concentration of 0.36, 0.19, 0.11 and 0.34 mg/kg, from Cairo, Giza, Alexandria, and El-Fayoum governorates respectively, whereas Ni exceeded the maximum permissible limit (0.20 mg kg−1) in apple samples obtained from Cairo and El-Fayoum governorates. It could be noticed that apples purchased from Cairo contained a high amount of Cu and Ni at a concentration of 1.63 and 0.36 mg/kg respectively, compared to samples obtained from other governorates.
In apple samples (Table 2), Cr was detected in grape samples only and recorded 1.01, and 1.06 mg kg−1for samples obtained Cairo and El-Fayoum governorate respectively, and they exceeded the maximum permissible limit (0.10 mg/kg). On the other hand, Cu was detected in apples, at a high concentration of 3.52, 0.72, 2.53, and 3.21 mg/kg from Cairo, Giza, Alexandria, and El-Fayoum governorates respectively, and exceeded the maximum permissible limit. Nickel was also detected in apple samples at a concentration of 0.30, 0.83, 1.78, and 0.31 mg/kg from Cairo, Giza, Alexandria, and El-Fayoum governorates respectively, and exceeded the maximum permissible limit (0.20 mg/kg) [42].
Data in Table 3 revealed the concentration of heavy metals in orange samples. Orange samples were contaminated with Cu, which recorded 2.20, 0.64, 0.81, and 0.36 mg/kg from Cairo, Giza, Alexandria, and El-Fayoum governorates respectively. On the other hand, Ni was also detected in orange samples at a concentration of 031, and 0.38 mg/kg from Cairo and Giza governorates respectively, whereas Ni exceeded the maximum permissible limit (0.20 mg/kg) [42]. Nickel was also detected in orange below the maximum residue limit (0.20 mg/kg) for samples obtained from Alexandria and El-Fayoum governorates (0.16 and 0.06 mg/kg).
3.2. Removal of heavy metal in grapes and oranges
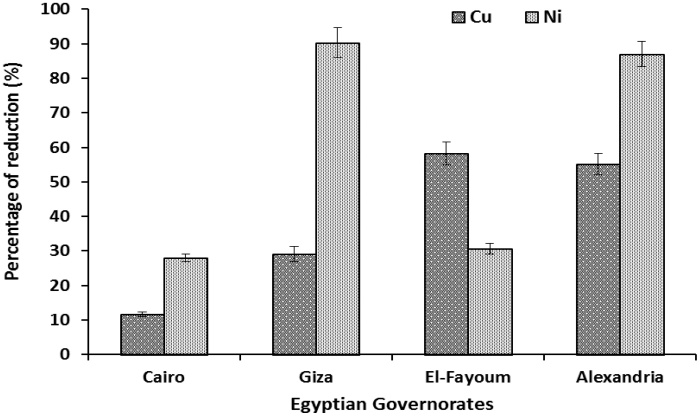
Results indicated that washing of the grape samples obtained from the four governorates reduced the level of Cu and Ni (Fig. 1). Data also revealed that Ni was reduced more than Cu especially in samples obtained from Alexandria (75.27%), Cairo (28.33%), and Giza (96.78%) governorates, whereas in Fayoum governorate Cu (58.02%) was reduced more than Ni (30.64%).
Fig. 1.
Effect of washing grapes on the percentage of heavy metal reduction. Results are mean ± SE (n = 9).
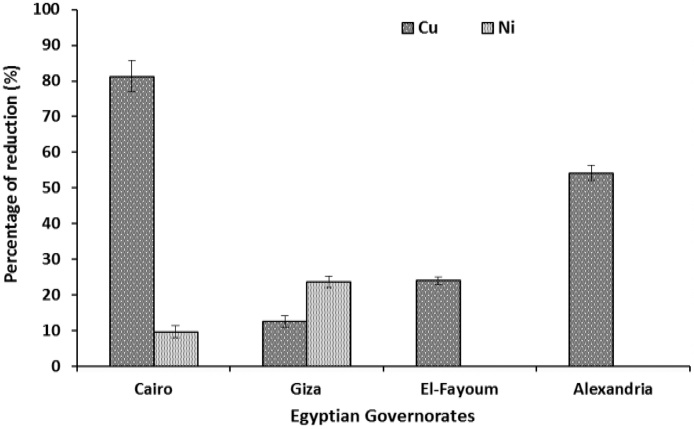
Data in Fig. 2 revealed that peeling of orange samples obtained from the four governorates reduced the level of Cu and Ni. It was also noticed that Cu was extremely reduced by peeling of the orange samples obtained from the following governorates in descending order; Cairo (81.36%), Alexandria (54.32%), Fayoum (24.07%) and Giza (12.56%). Peeling orange samples obtained from Alexandria and Fayoum governorates had no reducing effect on Ni, whereas samples obtained from Cairo and Giza governorates Ni was reduced by 9.67% and 22.61%.
Fig. 2.
Effect of peeling oranges on the percentage of heavy metal reduction. Results are mean ± SE (n = 9).
3.3. Risk assessment of heavy metals
Estimated daily intake (EDI) for adults is shown in Table 4. The results showed that EDI of Cu in apples recorded 0.095, 0.175, 0.015 and 0.059 mg/kg/day for samples obtained from Alexandria, Cairo, Fayoum and Giza governorates respectively. Data also showed that EDI of Ni in grapes recorded 0.402, 0.067, 0.070 and 0.187 mg/kg/day for samples obtained from Alexandria, Cairo, Fayoum and Giza governorates respectively. It was also noticed that TDI recorded 0.003, 0.040, and 0.002 mg/kg/day for Cr, Cu and Ni respectively (Table 4).
Table 4.
Estimated daily intake and tolerable daily intake of heavy metals.
| Fruits | Alexandria |
Cairo |
Fayoum |
Giza |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Cu | Ni | Cr | Cu | Ni | Cr | Cu | Ni | Cr | Cu | Ni | |
| Apples | ND | 0.095* | 0.012 | ND | 0.175* | 0.038* | ND | 0.015 | 0.036* | ND | 0.059* | 0.020 |
| Grapes | ND | 0.724* | 0.402* | 0.228* | 0.794* | 0.067* | 0.239* | 0.724* | 0.070* | ND | 0.162* | 0.187* |
| Oranges | ND | 0.364* | 0.072* | ND | 0.990* | 0.139* | ND | 0.144* | 0.027* | ND | 0.288* | 0.171* |
| TDI | 0.003 | 0.040 | 0.020 | 0.003 | 0.040 | 0.020 | 0.003 | 0.040 | 0.020 | 0.003 | 0.040 | 0.020 |
ND: Not detected, TDI: Tolerable Daily Intake (mg/kg/day) [61], EDI: Estimated Daily intake (mg/kg body weight/day).
EDI above TDI.
4. Discussion
4.1. Heavy metal analysis
Heavy metal analysis showed the absence of Cd and Pb in all fruit samples. These results were considered similar to those of Unaegbu et al. [43] who reported the absence of Cd and Pb in all the fruit samples obtained from Nigeria. Cadmium has been widely dispersed into the environment through the air by usage of phosphate fertilizers, pesticides, pigments, plating, plastics and various industrial uses such as NiCd batteries [44]. Thus, indicating that our fruit samples were grown in farms in rural areas away from industrial areas. High Cr levels found in grapes obtained from Cairo and El-Fayoum only might be an indication that they have originated from different growing regions or through transport chain. The current results were in contrast to those of Ogunkunle et al. [45] who found that fruits from selected markets in Lagos, Nigeria, were free of Cr. It has been reported that sewage, pesticides and fertilizers may release Cr into the environment [17].
The results revealed that grapes from Alexandria, Cairo and El-Fayoum were highly contaminated with Cu. The contamination of grapes with Cu could be due to penetration of heavy metal via the root system into the grapevine plants which accumulates in numerous above ground parts, especially, in the grape rachis grown in a region of industrial activities [46]. Data also revealed that apple and orange samples from Cairo governorate contained high concentrations of Cu. These results are considered similar to those stated by Radwan and Salama [28] who indicated that apples contained 1.47 mg/kg Cu, whereas oranges contained 2.13 mg kg−1 Cu. Meanwhile, these results were considered higher than those observed by Sobukola et al. [47] who found that Cu content in orange samples obtained from Nigeria was 0.002 mg kg-1. Copper are usually ensured through organic or artificial fertilizers as most plants contain insufficient amounts of Cu for normal growth [48]. Also, to maintain a healthy central nervous system for human, Cu is an essential micronutrient to prevent anemia and interrelated with the function of zinc and iron in the body [49].
Nickel was found at high concentration in grapes obtained from Alexandria and Giza governorates. The current results were considered lower than those determined by Ihesinachi and Eresiya [50]. Orisakwe et al. [51] reported the presence of Ni in fruits sold with high concentrations in Owerri, South Eastern Nigeria. Nickel may be mutagenic at levels above the maximum permissible limits [52], whereas mutagens are capable of causing mutations in DNA and leading to cancer. At higher concentration, nickel toxicity is more noticeable [53]. The variation in the different heavy metals concentrations through the fruit samples and the governorates may be due to the nature of the environment in terms of the soil and the ability of the fruits to absorb these heavy metals [54].
4.2. Removal of heavy metal from grapes and oranges
As the increased concentration of heavy metals is associated with the cause of a number of diseases, especially renal, cardiovascular and neurological disorders [55]. Therefore, the maximum permissible levels for toxic metals in human food are set by national and international regulations on food quality; thus, controlling the concentrations of heavy metals in food should be an increasingly important factor of food quality assurance and food safety [28,56].
Washing of grapes lead to the reduction of both Cu and Ni concentration, whereas Ni was reduced more than Cu in Alexandria, Cairo and Giza governorates. Meanwhile, in El-Fayoum governorate, Cu was reduced more than Ni. In the present study, the difference in the heavy metal reduction was due to difference in concentration. These results could also be attributed to the use of different fertilizers, pest management and fruit variety. These results are in agreement with those of Igwegbe et al. [57] who found that washing of crops could lead to the removable of high amounts of the heavy metal that may be present as surface contaminants. Igwegbe et al. [58] indicated that considerable amounts of the Pb and Cd were removed from fruits and vegetable samples by washing proposing that the contamination was a surface type.Peeling of oranges reduced the concentration of Cu and Ni, whereas it was noticed that Cu was extremely reduced more than Ni. Thus indicating that the high percentage of Cu is absorbed from the environment in the orange peel and accordingly can be removed easily by peeling oranges. The difference in the removal percentage of heavy metal could be due to the difference in the concentration of heavy metal in different orange peels. Recently, orange peel was studied for the removal of Cu, Pb and Zn, whereas a removal efficiency was observed [59].
4.3. Risk assessment of heavy metals
Estimated daily intake is presumed to be the daily consumption of a heavy metal residue. On the other hand, tolerable daily intake refers to the daily amount of a heavy metal in food that can be used by human being for a long time with no health effects. It was concluded in our study that some of the values of the EDI were considered higher than those of the TDI. Therefore, there is a considered possible risk to human health due to the consumption of fruits contaminated with high concentration of heavy metals.
5. Conclusion
Heavy metals Cu, and Ni were present in all fruit samples obtained from the four Egyptian governorates. Chromium was present in grape samples only and were above the maximum residue limits. Copper and Ni were also present in all fruit samples above the maximum residue limits. It was also indicated that washing of grapes and peeling of orange samples greatly reduced the level of Cu and Ni. Estimated daily intake of the fruit samples were considered higher than that of the tolerable daily intake. Therefore, it is very important to wash fruits before eating to decrease heavy metal concentrations and increase the safety of human health.
Acknowledgment
This work was funded by the National Research Centre, Cairo, Egypt, under grant No. 11040303.
References
- 1.Otitoloju A.A. University of Lagos. University of Lagos Press; 2016. Today’s Apple: Perspective of an Environmental Toxicologist. 12th Inaugural Lecture; pp. 1–77. [Google Scholar]
- 2.Kooner R., Mahajan B.V., Dhillon W.S. Heavy metal contamination in vegetables, fruits, soil, and water – a Critical Review. Int. J. Agric. Environ. Biotechnol. 2014;7:603–612. [Google Scholar]
- 3.Marshall Y. Crop Post Harvest Program; 2004. Enhancing Food Chain Integrity: Quality Assurance Mechanism for Air Pollution Impacts on Fruits and Vegetables Systems; p. R7530.www.sussex.ac.uk/spru/1-4-7-1-11-1.html Final Technical Report. [Google Scholar]
- 4.Wang X., Sato T., Xing B., Tao S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005;350:28–37. doi: 10.1016/j.scitotenv.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 5.Khan S., Cao Q., Zheng Y.M., Huang Y.Z., Zhu Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing. China. Environ. Pollut. 2008;152:686–692. doi: 10.1016/j.envpol.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 6.Ezemonye L., Adebayo P., Enuneku A., Tongo I., Ogbomida E. Potential health risk consequences of heavy metal concentrations in surface water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from Benin River, Nigeria. Toxicol. Reports. 2019;6:1–9. doi: 10.1016/j.toxrep.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marín S., Pardo O., Sánchez A., Sanchis Y., Vélez D., Devesa V., Font G., Yusà V. Assessment of metal levels in foodstuffs from the Region of Valencia (spain) Toxicol. Reports. 2018;5:654–670. doi: 10.1016/j.toxrep.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidi M.I., Asrar A., Mansoor A., Farooqui M.A. The heavy metal concentrations along roadsides trees of Quetta and its effects on public health. J. Appl. Sci. Faisalabad (Faisalabad) 2005;5:708–711. [Google Scholar]
- 9.Ali S., Gaya M., Abubakar F. Determination of some heavy metals in selected cosmetic products sold in kano metropolis, Nigeria. Toxicol. Reports. 2016;3:866–869. doi: 10.1016/j.toxrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ullah A., Maksud M., Khan S., Lutfa L., Quraishi S. Dietary intake of heavy metals from eight highly consumed species of cultured fish and possible human health risk implications in Bangladesh. Toxicol. Reports. 2017;4:574–579. doi: 10.1016/j.toxrep.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarup L. Hazards of heavy metals contamination. Br. Med. Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 12.Ming-Ho Y. 2nd edition. CRC Press LLC; Boca Raton, USA: 2005. Environmental Toxicology: Biological and Health Effects of Pollutants, Chap. 12. ISBN 1-56670-670-2. [Google Scholar]
- 13.Aderinola O.J., Clarke E.O., Olarinmoye O.M., Kusemiju V., Anatekhai M.A. Heavy metals in surface water, sediments, fish, and periwinkles of Lagos, Lagoon. Am-Eurasian J. Agric. Environ. Sci. 2009;5:609–617. http://www.idosi.org/…/4.pdf. [Google Scholar]
- 14.Taghizadeh S., Davarynejad G., Asili J., Nemati S., Rezaee R., Goumenou M., Tsatsakis A., Karimi G. Health risk assessment of heavy metals via dietary intake of five pistachio (Pistacia vera L.) cultivars collected from different geographical sites of Iran. Food Chem. Toxicol. 2017;107:99–107. doi: 10.1016/j.fct.2017.06.035. [DOI] [PubMed] [Google Scholar]
- 15.Sathawara N.G., Parikish D.J., Agrwal Y.K. Essentials heavy metals in environmental samples from western Indian. Bull. Environ. Cont. Toxicol. 2004;73:756–761. doi: 10.1007/s00128-004-0490-1. [DOI] [PubMed] [Google Scholar]
- 16.Satarug S., Scoh H.G., Mary A.S., Donald A. Cadmium. Environ.exposure health outcomes. 2010;118:182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghani A. Effect of chromium toxicity on growth, chlorophyll and some mineral nutrients of Brassica juncea L. Egyptian Academic J. Biol. Sci. 2011;2:9–15. [Google Scholar]
- 18.World Health Organization . Volume 134. WHO; Geneva: 1992. (Cadmium, Environmental Health Criteria). [Google Scholar]
- 19.World Health Organization . Volume 165. WHO; Geneva: 1995. (Lead. Environmental Health Criteria). [Google Scholar]
- 20.Steenland K., Boffeta P. Lead and cancer in humans: where are we now? Am. J. Ind. Med. 2000;38:295–299. doi: 10.1002/1097-0274(200009)38:3<295::aid-ajim8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.IARC . Beryllium, Cadmium, Mercury, and Exposure in Glass Manufacturing Industry. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. International Agency for Research on Cancer; Lyon: 1993. Cadmium and cadmium compounds; pp. 119–237. 58. [Google Scholar]
- 22.Pitot C.H., Dragan P.Y. Chemical carcinogenesis. In: Casarett D., editor. Toxicology Inter. Edi. 5 th edition. McGraw Hill; New York: 1996. pp. 210–260. [Google Scholar]
- 23.Onianwa P.C., Lawal J.A., Ogunkeye A.A., Orejimi B.M. Cadmium and nickel composition of some nigerian foods. J. Food Anal. 2000;13:961–969. [Google Scholar]
- 24.Karavoltsos S., Sakellari A., Dimopoulos M., Dasenakis M., Scoullos M. Cadmium content in foodstuffs from the Greek market. Food Addit. Contam. 2002;19:954–962. doi: 10.1080/02652030210136973. [DOI] [PubMed] [Google Scholar]
- 25.Z Parveen, Khuhro M.I., Rafiq N. Market basket survey for lead, cadmium, copper, chromium, nickel, and zinc in fruits and vegetables. Bull. Environ. Toxicol. 2003;71:1260–1264. doi: 10.1007/s00128-003-8640-4. [DOI] [PubMed] [Google Scholar]
- 26.Sobukola O.P., Dairo O.U., Sanni L.O., Odunewu A.V., Fafiolu B.O. Thin layer drying process of some leafy vegetables under open sun. Food Sci. Technol. Int. 2007;13:35–40. [Google Scholar]
- 27.D’Mello J.P. CABI Publishing; Wallingford, Oxon, UK, Cambridge, M.A: 2003. Food safety: Contamination and Toxins; p. 480. [Google Scholar]
- 28.Radwan M.A., Salama A.K. Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food Chem. Toxicol. 2006;44:1273–1278. doi: 10.1016/j.fct.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Hardisson A., Rubio C., Báez A., Martin M., Alvarez R. Mineral composition of the papaya (Carica papaya variety sunrise) from Tenerife island. Eur. Food Res. Technol. 2001;212:175–181. [Google Scholar]
- 30.Hardisson A., Rubio C., Báez A., Martin M., Alvarez R. Mineral composition in four varieties of avocado (Persea gratissima, L.) from the island of Tenerife. Eur. Food Res. Technol. 2001;213:225–230. [Google Scholar]
- 31.Hardisson A., Rubio C., Báez A., Martin M., Alvarez R., Diaz E. Mineral composition of the banana (Musa acuminata) from the island of Tenerife. Food Chem. 2001;73:153–161. [Google Scholar]
- 32.Bagdatlioglu N., Nergiz C., Ergonul P.G. Heavy metals levels in leafy vegetables and some selected fruits. J. für Verbraucherschutz Lebensmittelsicherheit. 2010;5:421–428. [Google Scholar]
- 33.Hamurcu M., Özcan M.M., Dursun N., Gezgin S. Mineral and heavy metals levels in some fruits grown at the roadsides. Food Chem. Toxicol. 2010;48:1767–1770. doi: 10.1016/j.fct.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 34.Mitić S.S., Obradović M.V., Mitić M.N., Kostić D.A., Pavlović A.N., Tošić S.B., Stojković S.D. Elemental composition of various sour cherry and table grape cultivars using inductively coupled plasma atomic emission spectrometry method (ICP-OES) Food Anal. Methods. 2012;5:279–286. [Google Scholar]
- 35.Hernández-Sánchez C., Luis G., Moreno I., Cameán A., González A.G., González-Weller D., Castilla A., Gutiérrez A., Rubio C., Hardisson A. Differentiation of mangoes (Magnifera indica L.) conventional and organically cultivated according to their mineral content by using support vector machines. Talanta. 2012;97:325–330. doi: 10.1016/j.talanta.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 36.Anastácio M., dos Santos A.M., Aschner M., Mateus L. Determination of trace metals in fruit juices in the Portuguese market. Toxicol. Reports. 2018;5:434–439. doi: 10.1016/j.toxrep.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdel-Rahman G.N., Ahmed M.B.M., Sabry B.A., Ali S.M. Heavy metals content in some non-alcoholic beverages (carbonated drinks, flavored yogurt drinks, and juice drinks) of the Egyptian markets. Toxicol. Reports. 2019;6:210–214. doi: 10.1016/j.toxrep.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chary N.S., Kamala C.T., Raj D.S.S. Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Safety. 2008;69:513–524. doi: 10.1016/j.ecoenv.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 39.Abdel-Rahman G.N., Ahmed M.B.M., Saleh E.M., Fouzy A.S.M. Estimated heavy metal residues in Egyptian vegetables in comparison with previous studies and recommended tolerable limits. J. Biol. Sci. 2018;18:135–143. [Google Scholar]
- 40.Abdel-Rahman G.N., Ahmed M.B.M., Marrez D.A. Reduction of heavy metals content in contaminated vegetables due to the post-harvest treatments. Egyptian J. Chem. 2018;61:1031–1037. [Google Scholar]
- 41.World Health Organization . WHO; 2003. GEMS/Food Regional Diets: Regional Per Capita Consumption of Raw and Semi-processed Agricultural Commodities / Prepared by the Global Environment Monitoring System/Food Contamination Monitoring and Assessment Programme (GEMS/Food) [Google Scholar]
- 42.World Health Organization / Food and Agriculture Organization (JECFA/76/Sc) 76th Meeting, GenevaWHO/FAO, Summary and Conclusion, Joint FAO/WHO Expert Committee of Food Additives2012. WHO/FAO, Summary and Conclusion, Joint FAO/WHO Expert Committee of Food Additives. 2012 [Google Scholar]
- 43.Unaegbu M., Engwa G.A., Abaa Q.D., Aliozo S.O., Ayuk E.L., Osuji G.A., Onwurah E.I. Heavy metal, nutrient and antioxidant status of selected fruit samples sold in Enugu. Nigeria. Int. J. Food Contam. 2016;3:7. [Google Scholar]
- 44.Agency for Toxic Substances and Disease Registry (ATSDR) US Department of Human and Health Services; 1999. Toxicological Profile for Cadmium. [PubMed] [Google Scholar]
- 45.Ogunkunle A.T.J., Bello O.S., Ojofeitimi O.S. Determination of heavy metal contamination of street-vendor fruits and vegetables in Lagos state, Nigeria. Int. Food Res. J. 2014;21:1725–1730. [Google Scholar]
- 46.Angelova V., Ivanov A.S., Braikov D.M. Heavy metals (Pb, Cu, Zn, and Cd) in the system soil – grapevine – grape. J. Sci. Food Agric. 1999;79:713–721. [Google Scholar]
- 47.Sobukola O.P., Adeniran O.M., Odedairo A.A., Kajihausa O.E. Heavy metal levels of some fruits and leafy vegetables from selected markets in Lagos, Nigeria. Afr. J. Food Sci. 2010;4:389–393. [Google Scholar]
- 48.Itanna F. Metals in leafy vegetables grown in Addis Ababa and toxicology implications. Ethiopian J. Health Develop. 2002;16:295–302. [Google Scholar]
- 49.Akinyele I.O., Osibanjo O. Levels of trace elements in hospital diet. Food Chem. 1982;8:247–251. [Google Scholar]
- 50.Ihesinachi K., Eresiya D. Evaluation of heavy metals in orange, pineapple, avocado pear and pawpaw from a farm in Kaani, Bori, Rivers State Nigeria. Int. Res. J. Public Environ. Health. 2014;1:87–94. http://www.journalissues.org/irjpeh/ [Google Scholar]
- 51.Orisakwe O.E., Kanayochukwu N.J., Nwadiuto A.C., Daniel D., Onyinyechi O. Evaluation of potential dietary toxicity of heavy metals of vegetables. J. Environ. Anal. Toxicol. 2012;3:100–136. www.zju.edu.cn/jzus [Google Scholar]
- 52.Valko M., Morris H., Cronin M.T. Metals, toxicity, and oxidative stress. Curr. Medicinal Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 53.Divrikli U., Horzum N., Soylak M., Elci L. Trace heavy metal contents of some spices and herbal plants from western Anatolia. Turkey. Int. J. Food Sci. Technol. 2006;41:712–716. [Google Scholar]
- 54.Hailemariam T., Aregahegn A., Bekele T., Madhusudhan A. Investigation of the levels of selected metals in edible and medicinal fruits grown in Dilla, Ethiopia. Res. J. Chem. Environ. Sci. 2015;3:44–53. www.aelsindia.com/rjces.htm [Google Scholar]
- 55.Jolaoso A.O., Njoku K.L., Akinola M.O., Adesuyi A.A., Adedokun A.H. Heavy Metal Analysis and Nutritional Composition of Raw and smoked fishes from Ologe and Lagos Lagoon, Lagos, Nigeria. J. Appl. Sci. Environ. Manag. 2016;20:277–285. [Google Scholar]
- 56.Sobukola O.P., Awonorin S.O., Idowu M.A., Bamiro F.O. Chemical and physical hazard profile of ‘robo’ processing – a street vendor melon snack. Int. J. Food Sci. Technol. 2008;43:237–242. [Google Scholar]
- 57.Igwegbe A.O., Belhaj H.M., Hassan T.M., Gibali A.S. Effects of highway’s traffic on the level of lead and cadmium in fruits and vegetables grown along the roadsides. J. Food Safety. 1992;13:7–18. [Google Scholar]
- 58.Igwegbe A.O., Agukwe C.H., Negbenebor C.A. A Survey of heavy metal (lead, cadmium, and copper) contents of selected fruit and vegetable crops from Borno State of Nigeria. Int. J. Engin. Sci. 2013;2:1–5. [Google Scholar]
- 59.Amin M.T., Alazba A.A., Amin M.N. Absorption behaviors of copper, lead, and arsenic in aqueous solution using date palm fibers and orange peel: kinetics and thermodynamics. Pol. J. Environ. Stud. 2017;26:543–557. [Google Scholar]
- 60.European Commission, EC Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. No 629/2008. http://data.europa.eu/eli/reg/2008/629/oj
- 61.USEPA Region III Non-Carcinogen . 2007. Tolerable Daily Intake (TDI) Values From US EPA.http://www.epa.gov/reg3hwmd/risk/human/index.htm [Google Scholar]