Abstract
Introduction
This study examined the usefulness of basic fibroblast growth factor impregnated collagen-gelatin sponge (bFGF-CGS) in reconstructive surgery for various acute skin defects including deep dermal burns, facial full-thickness skin defects, and finger amputations as the first clinical application.
Methods
Reconstructive surgery was performed in two stages with bFGF-CGS in 8 male subjects, ranging in age from 6 to 84 years, with acute full-thickness skin defects. Following the adequate debridement of the defect, surgeons prepared a bFGF-CGS with bFGF solution at a dose of 7–14 mg/cm2 approximately 10 min just before application and then secured the bFGF-CGS in place with non-absorbable sutures. Second-stage wound closure was performed with autologous skin grafting following adequate dermis-like tissue regeneration at the site postoperatively. Follow-up was continued for 6 months.
Results
Of the 8 subjects, the mean duration from the adequate vascularization of the dermis-like tissue until the second-stage autologous skin graft was 22 ± 4 days. Wound closure was achieved in all cases; the mean duration until wound closure was 32 ± 8 days. During the 6-month follow-up period, no wound infection, recurrent skin ulceration, and no exposure of tendon, bone, and cartilage were observed, and there were no cases of indirectly restricted range of motion from postoperative scar contracture and none with disfiguring scars.
Conclusion
The authors achieved favorable outcomes following reconstructive surgery with a hybrid artificial dermis impregnated with bFGF for treating acute full-thickness skin defects. bFGF-CGS serves as a convenient regenerative device requiring no specialized medical facilities.
Keywords: Drug delivery system, Basic fibroblast growth factor, Artificial dermis
Abbreviations: bFGF, basic fibroblast growth factor; bFGF-CGS, basic fibroblast growth factor-impregnated collagen gelatin sponge
Highlights
-
•
This study examined the usefulness of bFGF-impregnated collagen gelatin sponge for skin defects.
-
•
bFGF-CGS achieved wound closure with acute full-thickness skin defects in a short period of time.
-
•
bFGF-CGS is a convenient device without requiring specialized medical facilities.
1. Introduction
Various drugs and wound healing devices are used in the field of wound care, particularly for full-thickness skin defects, and the use of artificial dermis as a scaffolding material for skin regeneration is recognized as the standard treatment. Artificial dermis is a biomaterial being useful as a reconstructive material for skin defects after extensive burns [1], acute traumatic skin defects [2], and in reconstructive surgery [3]. Artificial dermis has been also the subject of numerous previous reports. However, for achieving perfect wound closure and epithelialization with the materials, surgery must be performed in two stages. In the first stage, artificial dermis is transplanted, and then an autologous skin graft is placed on the top of dermis-like tissue, which appears 2–3 weeks after the initial transplant before the artificial dermis is adequately vascularized [4]. This 2- to 3-week waiting period causes a number of issues such as prolonged burden on the patient, increased incidence of wound infection, and a lower-partial-skin-graft success rates of 50%–80% [5]. Accordingly, reducing the length of this waiting period would increase the usefulness of artificial dermis in the clinical setting. In view of these challenges, in 2013 Morimoto et al. develop a new hybrid artificial dermis having a drug delivery system for basic fibroblast growth factor (bFGF) and conduct a clinical trial on the treatment of chronic ulcer [6]. This basic fibroblast growth factor-impregnated collagen-gelatin sponge (bFGF-CGS) consists of normal artificial dermis to which 10% alkali-treated gelatin is added. This alkali-treated gelatin attaches to positively charged bFGF, allowing bFGF to be released gradually into the wound surface for more than 3 weeks until the gelatin is absorbed by the body [7]. bFGF formulations are already in clinical use in Japan as a highly effective form of treatment for skin ulcers, and in recent years, indications for its use have gradually expanded to various conditions such as skin avulsion injuries to the extremities [8] and second-degree burns [9]. However, the use of bFGF to promote the generation of dermis-like tissue from artificial dermis in vivo raises the issue of exogenous bFGF having a short half-life in vivo. If bFGF solution is disseminated in vivo as-is, the level of bFGF activity decreases down to less than 10% at 24 h after administration [10]. In other words, spraying a solution containing a bFGF formulation only once during the initial surgery for facilitating the regeneration of dermis-like tissue, which takes a few weeks, is unable to be expected to be effective. Accordingly, compared with the use of artificial dermis alone, the use of bFGF-CGS having a capability to play a bFGF drug delivery system would promote angiogenesis and shorten the regeneration period of dermis-like tissue. The authors believe that bFGF-CGS would in turn reduce the risk of infection and contribute to reducing the duration of patient burden [11]. In this article, the authors documented the usefulness of bFGF-CGS in reconstructive surgery for various acute skin defects such as deep dermal burns, facial full-thickness skin defects, and finger amputations as the first clinical application.
2. Methods
This study was approved by the Ethics Committee of Tokyo Women's Medical University. All patients provided written informed consent to participate in the study.
2.1. Preparation of the bFGF-CGS
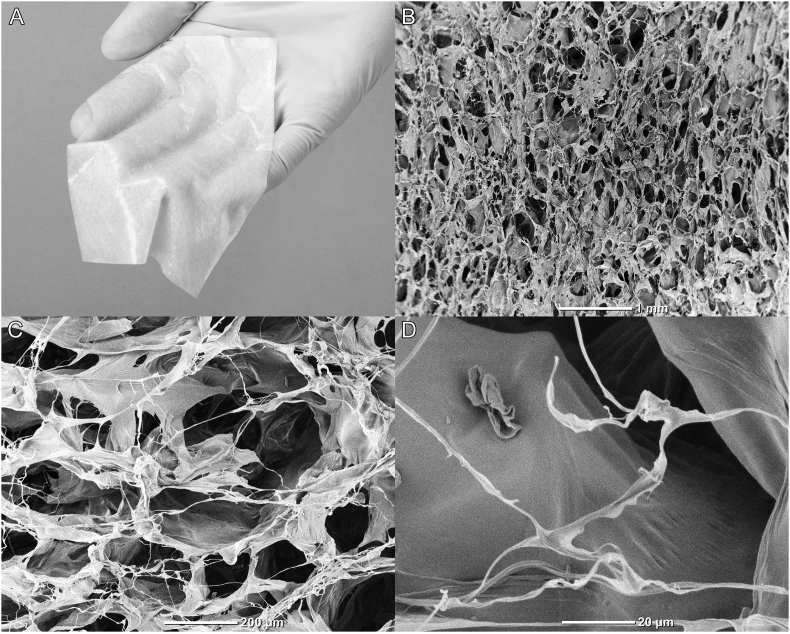
A bFGF-CGS (PELNAC Gplus®, Gunze, Kyoto, Japan) used in this study was a modified version of conventional bilayered artificial dermis and consists of an upper silicone sheet with a thickness of 0.12 mm and a lower collagen sponge with alkali-treated gelatin with a thickness of 3 mm (Fig. 1A). Upon the preparation of bFGF-CGS, the sponge portion of bFGF-CGS is made from atelocollagen and gelatin. Atelocollagen is produced by treating porcine collagen with pepsin under an acidic condition for removing the telopeptide component. Telopeptide removal reduces antigenicity, resulting in imparting a superior biological safety and compatibility. Gelatin is derived from porcine skin. First the epidermis and hairs are removed, and then the skin is subjected to alkali treatment by immersion in lime, followed by heating in warm water and extracting gelatin. Alkali-treated gelatin is acidic and negatively charged, and typically forms electrostatic bonds with growth factors, which are positively charged. Type I collagen derived from porcine tendon is mixed with the gelatin derived from porcine skin at a gelatin ratio of 10 wt%, and the mixture is then dissolved in distilled water to form an aqueous solution. This aqueous solution is cast into a mold, frozen for 1 h at −40 °C, and subsequently lyophilized to obtain a primary lyophilisate. Thermal cross-linking of the primary lyophilisate is performed by vacuum processing at 110 °C. Chemical cross-linking is then performed by immersing the primary lyophilisate in an aqueous acetic-acid solution containing glutaraldehyde. Excess glutaraldehyde is removed by flushing with water, and the primary lyophilisate is again lyophilized to create the sponge portion (Fig. 1B–D). The sponge is then coated with a silicone film to prevent contamination and drying, then cut to size, and sterilized with ethylene oxide gas.
Fig. 1.
Macro and scanning electron microscope images of basic fibroblast growth factor-impregnated collagen gelatin sponge (bFGF-CGS). (A) Photograph shows the appearance of bFGF-CGS. (B–D) Scanning electron microscopy images show the internal structure of bFGF-CGS at various magnifications. The scale bars in image B, C, and D indicate 1 mm, 200 μm, 20 μm, respectively.
2.2. Surgical procedure
This study performed reconstructive surgery with bFGF-CGS in 8 cases with acute full-thickness skin defects. The patients were all male, and their ages were 6–84 years. They were required surgical treatment at the Department of Plastic and Reconstructive Surgery, Yachiyo Medical Center, Tokyo Women's Medical University from 2018 to 2019. Surgeons prepared bFGF-CGS with bFGF solution at a dose of 7–14 mg/cm2 in an operating room approximately 10 min just before the application, and in this study, human recombinant bFGF (Fiblast® spray; Kaken Pharmaceutical, Tokyo, Japan) was used. The dose of bFGF used was the same as that used with CGS for chronic wound care during the clinical trial and is previously confirmed to be safe and effective [6]. In addition, a special caution was taken for ensuring that the total daily dose would be unable to exceed 1000 μg, which is written in the guidelines for using bFGF. The surgeon first performed the adequate debridement of necrotic tissue from the full-thickness skin defect, washed the area with physiological saline, and after achieving hemostasis, secured the bFGF-CGS in the place with a non-absorbable suture. The wound was either covered with ointment-impregnated gauze dressing or negative pressure wound therapy, which was performed with a negative pressure wound therapy system (RENASYS™, Smith & Nephew Wound Management, London, UK). No additional bFGF formulation was used from the postoperative period until complete wound closure. At 14 days after application, the sutures and the silicone sheet of bFGF-CGS were removed. Adequate regeneration of dermis-like tissue at the graft site was confirmed by 2 wound management specialists, and continuously, the second-stage wound closure was performed with an autologous skin graft. No autologous skin grafts were used for patients who were observed to have progressive epithelialization surrounding the dermis-like tissue after the silicone sheet was removed. Instead, these patients continued to be managed conservatively with ointment until epithelialization was complete. Postoperative follow-up was continued for 6 months.
3. Results
Of 8 subjects, 3 had deep dermal burns, 3 had complete digital amputation, 1 had open fracture, and 1 had congenital preauricular sinus. The site of reconstructive surgery was the upper limb in 4 subjects, the lower limb in 3 subjects, and the face in 1 subject (Table 1). Reconstruction with bFGF-CGS was performed in cases with the exposure of bone, tendon, or cartilage at the ulcer surface, cases that had undergone autologous skin grafting during the first stage of treatment, or those whose wound closure by secondary healing was predicted to be difficult if ointment-based dressing was used. Complete wound closure was achieved in 3 cases after the regenerated dermis-like tissue with bFGF-CGS had epithelialized over the entire extent of the wound. Also, wound closure occurred after full-thickness skin grafting was performed during the second-stage surgery in 2 cases and after split-thickness skin grafting was performed during the same stage in 3 cases. The mean duration from adequate vascularization of the dermis-like tissue until the second-stage autologous skin graft was 22 ± 4 days. Wound closure was achieved in all cases, and the mean duration until wound closure was 32 ± 8 days. The mean duration until complete wound closure when the dermis-like tissue epithelialized the entire extent of the wound was 26 ± 8 days. During the 6-month follow-up period, no wound infection, recurrent skin ulceration, and exposure of tendon, bone, and cartilage were observed. Similarly, no cases with indirectly restricted range of motion from postoperative scar contracture and disfiguring scars were found in this study.
Table 1.
Clinical cases.
| Case no. | Sex | Age (years) | Disease | Location | Exposed bone and tendon | Skin defect Size (mm) | bFGF-CGS securing method | Secondary operation procedure (period from first operative days) | Period of complete healing (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 72 | Deep dermal burn | Left ankle joint | Achilles tendon | 130 × 30 | NPWT | FTSG (17) | 46 |
| 2 | Male | 25 | Congenital auricular sinus | Left preauricular region | Auricular cartilage | 50 × 25 | Tie over dressing | STSG (21) | 28 |
| 3 | Male | 6 | Complete digital amputation | Right fifth digit | Phalangeal bone | 10 × 10 | Bandage | N/A | 22 |
| 4 | Male | 72 | Deep dermal burn | Left first toe | Phalanx bone | 60 × 30 | NPWT | STSG (25) | 39 |
| 5 | Male | 49 | Open fracture | right ankle | Tibia | 55 × 25 | NPWT | FTSG (26) | 33 |
| 6 | Male | 84 | Deep dermal burn | Right hand | Extensor digitorum | 40 × 30, 80 × 35 | Tie over dressing | STSG (21) | 31 |
| 7 | Male | 7 | Complete digital amputation | Left index finger | Phalangeal bone | 10 × 10 | Bandage | N/A | 36 |
| 8 | Male | 31 | Complete digital amputation | Right fifth digit | Phalangeal bone | 20 × 10 | Bandage | N/A | 21 |
NPWT indicated negative pressure wound therapy system.
bFGF-CGS indicate basic fibroblast growth factor impregnated collagen-gelatin sponge.
STSG and FTSG indicate split- and full-thickness skin grafts, respectively.
4. Clinical cases
4.1. Case 1
Patient was a 72-year-old man. While riding on his motorbike, the back of his left heal was allowed to be touched with and ground by the high-speed rotating rear tire, and he suffered a deep dermal burn. A burn wound with adherent white necrotic tissue with a size of 130 × 30 mm on the left Achilles tendon was found and third-degree burn with a total-body surface area of 1% was diagnosed (Fig. 4A). On day 9 after the injury, debridement was performed under general anesthesia with a hydrogel jet system (VERSAJET™) (Smith & Nephew Wound Management) to remove the necrotic tissue down to layers where favorable petechiae were observed. This however led to the partial exposure of the Achilles tendon and the sural nerve (Fig. 4B). After adequate hemostasis was achieved, the area was washed, and bFGF-CGS was used for covering the area, which was secured with a negative pressure wound therapy system at a suction pressure of −60 mmHg (Fig. 2C). The silicone sheet of bFGF-CGS was removed on postoperative day (POD) 14, and the exposed Achilles tendon and sural nerve were seen to be completely covered by a firm layer of dermis-like tissue. Minimal curettage of the dermis-like tissue was performed with a curette on POD 17 after the initial surgery, following full-thickness skin grafting was performed with donor skin taken from the left inguinal region (Fig. 2D and E). Negative pressure wound therapy (RENASYS) was once again applied to secure the donor skin at a suction pressure of −60 mmHg. The negative pressure wound therapy system was removed on POD 7, and the proper attachment of the donor skin was confirmed. Subsequent treatment was administered conservatively with ointment, and complete wound closure was achieved a total of 46 days after the injury. No motor dysfunction of the ankle joint due to postoperative scar contracture was observed at 6 months postoperatively (Fig. 2F).
Fig. 4.
Findings during the course of treatment in Case 3. (A) The image shows findings during the initial examination of a 6-year-old boy with the complete amputation of the distal phalanx of the right little finger. (B) The area was covered with basic fibroblast growth factor-impregnated collagen gelatin sponge (bFGF-CGS) with a size of 10 × 10-mm, bFGF-CGS was secured to the wound with a 5-0 nylon suture, and then the area was covered with ointment-impregnated gauze on the same day. (C) The image shows the regenerated dermis-like tissue on day 14 after the initial surgery. (D) Findings at 6 months after surgery are shown.
Fig. 2.
Findings during the course of treatment in Case 1. (A) Findings at initial examination of a 72-year-old man with third degree burn on the left Achilles tendon with a size of 130 × 30 mm, which was estimated to be 1% of total body surface area, are shown. (B) On the debridement of necrotic tissue at 9 days after the injury, the Achilles tendon (*) and sural nerve (black arrowhead) were observed to partially appear. (C) The area was covered with basic fibroblast growth factor-impregnated collagen gelatin sponge (bFGF-CGS). (D) Findings on day 17 after the initial surgery are shown. (E) A full-thickness skin graft was performed with donor skin from the left inguinal region. (F) Findings at 6 months after surgery are shown.
4.2. Case 2
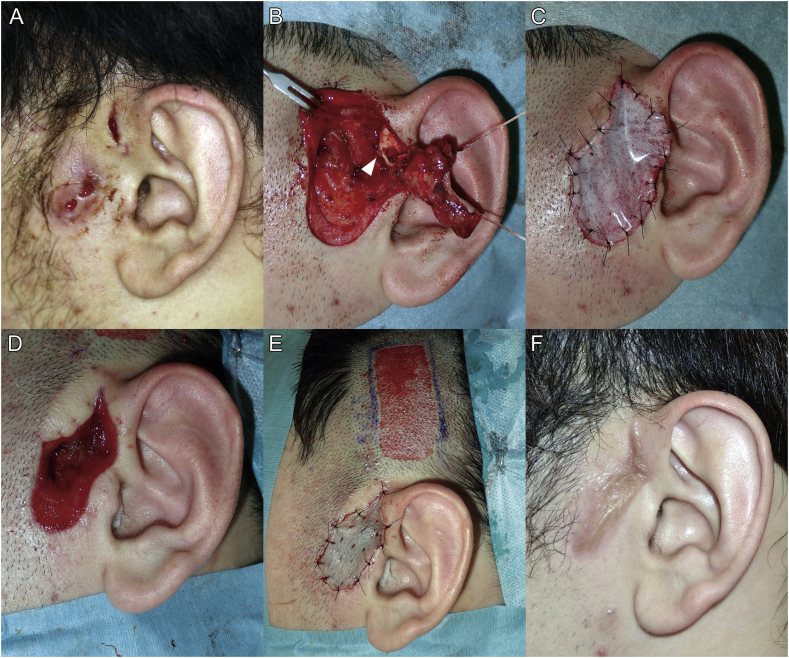
Patient was a 22-year-old man with a congenital left preauricular sinus. He had recurrent inflammation due to localized infection from childhood, which never improved though repeated incision and drainage treatments performed at another hospital. Eventually, he was referred to the authors' department for requesting radical treatment. With daily wound irrigation, the inflammation was reduced to the greatest extent possible, and then resection of the sinus was performed under general anesthesia (Fig. 3A). After resection, the patient was left with a 50 × 25-mm skin defect in the preauricular region with partially exposed auricular cartilage (Fig. 3B). The skin defect was covered with a bFGF-CGS and secured with a tie-over dressing (Fig. 3C). When the silicone sheet on the bFGF-CGS was removed on POD 14, the favorable proliferation of dermis-like tissue was observed, and the auricular cartilage was completely covered. Minimal curettage of the dermis-like tissue was performed with a curette on POD 21 after the initial surgery, and split-thickness skin grafting with a thickness of 10/1000 inches taken with donor skin from the cranial region was performed (Fig. 3D and E). The tie-over dressing was removed on POD 7, and extremely favorable skin graft adherence was observed. No signs of infection, skin ulceration, and the deviation of the pinna due to scar contracture were observed, and the esthetic outcome was favorable (Fig. 3F).
Fig. 3.
Findings during the course of treatment in Case 2. (A) The patient was a 22-year-old man with a congenital left preauricular sinus. (B) Findings during the resection of the preauricular sinus are shown. After the resection of the sinus, the patient was left with a 50 × 25-mm skin defect in the preauricular region with partially exposed auricular cartilage (the white arrowhead). (C) Findings after the area was covered with basic fibroblast growth factor-impregnated collagen gelatin sponge (bFGF-CGS) are shown. (D) Image of the regenerated dermis-like tissue on day 21, and (E) the image of the split-thickness skin graft are shown. (F) Findings at 6 months after surgery are shown.
4.3. Case 3
Patient was a 6-year-old boy who collided with a guardrail while riding his bicycle, resulting in the complete amputation of the distal phalanx of the right little finger, and the amputation was classified as zoneⅠby the Tamai's classification, and subzone II by the Ishikawa's classification. The amputated finger had been severely crushed, and replantation surgery was deemed impossible (Fig. 4A). Extensive irrigation of the amputated surface and exposed phalangeal bone was performed under general anesthesia, then the area was covered with a 10 × 10-mm bFGF-CGS, which secured to the wound with a 5-0 nylon suture and then covered with ointment-impregnated gauze (Fig. 4B). On removing the artificial dermis silicone sheet on POD 14, the presence of favorable proliferation of dermis-like tissue was confirmed, and the distal phalanx was covered in granulation tissue. Since epithelialization progressed around the sutured wound (Fig. 4C), conservative treatment with ointment was initiated, and a complete epithelialization was found on the wound on POD 22. No phalangeal bone exposure, digital motor dysfunction, and digital apex pain were observed at 6 months postoperatively, and the clinical course was favorable (Fig. 4D).
5. Discussion
The present study highlights a number of benefits of the full-thickness skin defect reconstruction method with bFGF-CGS. First, the sponge was useful for treating wounds with the exposure of bone and tendon, allowing the regeneration of firm dermis-like tissue, because bFGF promotes fibroblast proliferation [12] and angiogenesis [13]. Jinno et al. perform a comparative study of the wound-healing effects between bFGF-CGS and conventional artificial dermis in a rat skin full-thickness defect model, and report that significant improvements are observed in the neo-epithelial length, the dermis-like tissue area, and the number of newly formed capillaries in the group of rats that are treated with bFGF-CGS [11]. The authors transplanted bFGF-CGSs onto wound with the exposure of tendon, cartilage, and bone. When normal artificial dermis is used alone, the regeneration of dermis-like tissue requires a longer time. However, in this study, the formation of firm dermis-like tissue took approximately 2 weeks, as described in Case 3 where the patient had the amputation of the digital apex and a small wound surface area, or Case 1 where the patient had the exposure of the Achilles tendon and poor angiogenesis in the transplant bed. This form of treatment may present a new effective option for treating patients with wounds with exposure of tendon or bone in weight-bearing regions, and these patients previously have required highly invasive surgery such as a free flap transplantation [14].
Second, this treatment modality prevented scar contracture at the reconstruction site. In recent years, reports indicate that bFGF not only promotes wound healing but also improves the quality of scars that form after epithelialization, and prevents keloid formation and scar contracture [15]. As the mechanism of these effects, bFGF promotes fibroblast proliferation during the proliferation phase of wound healing and then enhances the apoptosis of fibroblasts and myofibroblasts during the reconstruction phase of wound healing to decrease their numbers, and these subsequently inhibits excessive wound contraction after the reconstruction phase [16]. Akita et al., in their use of bFGF during skin grafting to treat burn wounds, report the reduced hardness of the scars appearing after the skin graft [9]. These same effects were also observed in the present study, for example, in Case 1 where a full-thickness skin defect was found at the joint surface, and the Achilles tendon was exposed. From bFGF-CGS, the controlled release of bFGF, which facilitated the formation of more flexible dermis-like tissue, indicating that this in turn prevented the restricted joint mobility that could appear due to scar contracture. Furthermore, this modality was expected to be useful for preventing dislocation in the some parts of the face due to postoperative scar contracture in cases that require the reconstruction of full-thickness facial skin defects, as seen in Case 3. The authors would like to perform further comparative research for comparing the degree of contracture between bFGF-CGS and conventional artificial dermis without bFGF.
Third, the epithelializing effect of bFGF-CGS alone require no subsequent second-stage skin graft. For example, in the present report, the transplantation of bFGF-CGS was performed in Case 3 where the patient had the complete amputation of the distal phalanx with exposed bone, because replantation was impossible, and wound closure was achieved in the absence of an autologous skin graft after the initial operation. The authors believed this was due to the bFGF-induced proliferation of keratinocytes, which in turn is known to promote epithelialization [17], suggesting that use bFGF-CGS may be possibly applied to small skin defects to avoid harvesting donor tissue to perform autologous skin grafts. The use of bFGF-CGS is expected to give considerable benefit particularly in pediatric and extensive burns patients who have a relatively small skin surface area and difficulty in providing a donor site for skin graft.
Fourth, the graft technique for bFGF-CGS was easy and versatile. The authors previously created a hybrid artificial dermis by inducing adipose tissue-derived stem cells and adipose-derived aldehyde dehydrogenase-expressing cells. During the animal experiments with a murine full-thickness skin defect model, the regeneration of dermis-like tissue is reported to be promoted by pluripotency and paracrine signaling, which are characteristic of stem cells [18]. However, there are numerous drawbacks in using this method in clinical practice, for example, several weeks for cell culture are necessary to obtain the sufficient number of cells required for this method, and flow cytometry is required for cell sorting, resulting in the limited number of hospitals that will be able to perform this treatment. By contrast, bFGF-CGS used in this study requires only bFGF formulations that are already commercially available for clinical use at present, and this biomaterial device requires no cell source, making the device extremely cost-effective form of treatment because of no requirements of special-cell culture facilities and staff.
However, in the present case series, the average period to achieve an adaptive vascularization in the wound with bFGF-CGF was unable to exceed sufficiently the period with conventional artificial dermis, because some cases were speculated to have tendon and bone exposure on the wound surface with a poor angiogenesis ability. Further comparative studies with a greater number of cases are needed for proving the wound-closure-period shortening effect of bFGF-CGS. In addition, the authors also previously report that an artificial nerve conduit containing a bFGF drug delivery system promote nerve regeneration in a rat facial nerve defect model [19] and expect that there may be improved sensory recovery with the use of bFGF-CGS compared with the conventional artificial dermis alone. This study will conduct a comparative study of sensory recovery in the plural number of groups in the future.
6. Conclusions
This study successfully achieved favorable outcomes after performing reconstructive surgery with a hybrid artificial dermis impregnated with bFGF for treating acute full-thickness skin defects of various sizes. bFGF-CGS was used as a simple regenerative device without specialized medical facilities and allowed surgeons to perform minimally invasive treatment procedures and achieve the wound closure of full-thickness skin defects in a short period of time.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Heimbach D., Luterman A., Burke J., Cram A., Herndon D., Hunt J. Artificial dermis for major burns. A multi-center randomized clinical trial. Ann Surg. 1988:208313–208320. doi: 10.1097/00000658-198809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeong E.K., Yu Y.C., Chan Z.H., Roan T.L. Is artificial dermis an effective tool in the treatment of tendon-exposed wounds? J Burn Care Res. 2013;34:161–167. doi: 10.1097/BCR.0b013e3182685f0a. [DOI] [PubMed] [Google Scholar]
- 3.Soejima K., Nozaki M., Sasaki K., Takeuchi M., Negishi N. Reconstruction of burn deformity using artificial dermis combined with thin split-skin grafting. Burns. 1997;23:501–504. doi: 10.1016/s0305-4179(97)88485-9. [DOI] [PubMed] [Google Scholar]
- 4.Moiemen N.S., Vlachou E., Staiano J.J., Thawy Y., Frame J.D. Reconstructive surgery with Integra dermal regeneration template: histologic study, clinical evaluation, and current practice. Plast Reconstr Surg. 2006;117(7 Suppl):160S–174S. doi: 10.1097/01.prs.0000222609.40461.68. [DOI] [PubMed] [Google Scholar]
- 5.Heimbach D.M., Warden G.D., Luterman A., Jordan M.H., Ozobia N., Ryan C.M. Multicenter postapproval clinical trial of Integra® dermal regeneration template for burn treatment. J Burn Care Rehabil. 2003;24:42–48. doi: 10.1097/00004630-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Morimoto N., Yoshimura K., Niimi M., Ito T., Aya R., Fujitaka J. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: clinical trial for chronic skin ulcers. Tissue Eng. 2013;19:1931–1940. doi: 10.1089/ten.tea.2012.0634. [DOI] [PubMed] [Google Scholar]
- 7.Takemoto S., Morimoto N., Kimura Y., Taira T., Kitagawa T., Tomihata K. Preparation of collagen/gelatin sponge scaffold for sustained release of bFGF. Tissue Eng. 2008;14:1629–1638. doi: 10.1089/ten.tea.2007.0215. [DOI] [PubMed] [Google Scholar]
- 8.Matsumine H. Treatment of skin avulsion injuries with basic fibroblast growth factor. Plast Reconstr Surg Glob Open. 2015;3:e371. doi: 10.1097/GOX.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akita S., Akino K., Imaizumi T., Hirano A. A basic fibroblast growth factor improved the quality of skin grafting in burn patients. Burns. 2005;31:855–858. doi: 10.1016/j.burns.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto M., Ikada Y., Tabata Y. Controlled release of growth factors based on biodegradation of gelatin hydrogel. J Biomater Sci Polym Ed. 2001;12:77–88. doi: 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- 11.Jinno C., Morimoto N., Ito R., Sakamoto M., Ogino S., Taira T. A comparison of conventional collagen sponge and collagen-gelatin sponge in wound healing. BioMed Res Int. 2016;2016:4567146. doi: 10.1155/2016/4567146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono I. The effects of basic fibroblast growth factor (bFGF) on the breaking strength of acute incisional wounds. J Dermatol Sci. 2002;29:104–113. doi: 10.1016/s0923-1811(02)00019-1. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka E., Ase K., Okuda T., Okumura M., Nogimori K. Mechanism of acceleration of wound healing by basic fibroblast growth factor in genetically diabetic mice. Biol Pharm Bull. 1996;19:1141–1148. doi: 10.1248/bpb.19.1141. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu M., Matsumine H., Takeuchi M. Reconstruction of Chopart's amputation stump using artificial dermis combined with free anterolateral thigh flap. Plast Reconstr Surg Glob Open. 2015;3:e558. doi: 10.1097/GOX.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu X., Shen Z., Chen Y., Xie J., Guo Z., Zhang M. Recombinant bovine basic fibroblast growth factor accelerates wound healing in patients with burns, donor sites and chronic dermal ulcers. Chin Med J (Engl). 2000;113:367–371. [PubMed] [Google Scholar]
- 16.Muneuchi G., Suzuki S., Moriue T., Igawa H.H. Combined treatment using artificial dermis and basic fibroblast growth factor (bFGF) for intractable fingertip ulcers caused by atypical burn injuries. Burns. 2005;31:514–517. doi: 10.1016/j.burns.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 17.O'Keefe E.J., Chiu M.L., Payne R.E., Jr. Stimulation of growth of keratinocytes by basic fibroblast growth factor. J Investig Dermatol. 1988;90:767–769. doi: 10.1111/1523-1747.ep12560956. [DOI] [PubMed] [Google Scholar]
- 18.Matsumine H., Numakura K., Tsunoda S., Wang H., Matsumine R., Climov M. Adipose-derived aldehyde dehydrogenase–expressing cells promote dermal regenerative potential with collagen-glycosaminoglycan scaffold. Wound Repair Regen. 2017;25:109–119. doi: 10.1111/wrr.12494. [DOI] [PubMed] [Google Scholar]
- 19.Matsumine H., Sasaki R., Tabata Y., Matsui M., Yamato M., Okano T. Facial nerve regeneration using basic fibroblast growth factor-impregnated gelatin microspheres in a rat model. J Tissue Eng Regenerat Med. 2016;10:E559–E567. doi: 10.1002/term.1884. [DOI] [PubMed] [Google Scholar]