Key Points
Question
Does vitamin D supplementation have any association with cardiovascular disease risk?
Findings
In this meta-analysis of randomized clinical trials that included more than 83 000 participants, vitamin D supplementation was not associated with reduced risks of major adverse cardiovascular events, myocardial infarction, stroke, cardiovascular disease mortality, or all-cause mortality compared with placebo.
Meaning
These results suggest that vitamin D supplementation may not confer cardiovascular protection and may not be indicated for this purpose.
This meta-analysis of 21 randomized clinical trials examines the role of vitamin D supplementation in reducing cardiovascular events and all-cause mortality.
Abstract
Importance
Observational studies have reported an association between low serum vitamin D levels and elevated risk of cardiovascular disease (CVD) events, but such studies cannot prove causation because of possible unmeasured confounding.
Objective
We conducted a meta-analysis of randomized clinical trials that tested the association of vitamin D supplementation with reduced CVD events and all-cause mortality.
Data Sources
Literature search through PubMed, the Cochrane Library, and Embase was completed by 2 reviewers from each database’s inception to December 15, 2018.
Study Selection
Inclusion criteria were randomized clinical trials that reported the effect of long-term (≥1 year) vitamin D supplementation on CVD events and all-cause mortality. Studies that did not include cardiovascular outcomes were excluded.
Data Extraction and Synthesis
Data were abstracted independently by 2 authors. Random-effects models were used to report the risk ratios (RRs) and 95% CIs.
Main Outcomes and Measures
Major adverse cardiovascular events was the primary outcome, and rates of myocardial infarction, stroke or cerebrovascular accident, CVD mortality, and all-cause mortality were the secondary end points.
Results
Twenty-one randomized clinical trials were included (including 83 291 patients, of whom 41 669 received vitamin D and 41 622 received placebos). The mean (SD) age of trial participants was 65.8 (8.4) years; 61 943 (74.4%) were female. Only 4 trials had prespecified CVD as a primary end point. Vitamin D supplementation compared with placebo was not associated with reduced major adverse cardiovascular events (RR, 1.00 [95% CI, 0.95-1.06]; P = .85) nor the secondary end points of myocardial infarction (RR, 1.00 [95% CI, 0.93-1.08]; P = .92), stroke (RR, 1.06 [95% CI, 0.98-1.15]; P = .16), CVD mortality (RR, 0.98 [95% CI, 0.90-1.07]; P = .68), or all-cause mortality (RR, 0.97 [95% CI, 0.93-1.02]; P = .23). Results were generally consistent by sex, baseline 25-hydroxyvitamin D level, vitamin D dosage, formulation (daily vs bolus dosing), and presence or absence of concurrent calcium administration.
Conclusions and Relevance
In this updated meta-analysis, vitamin D supplementation was not associated with reduced major adverse cardiovascular events, individual CVD end points (myocardial infarction, stroke, CVD mortality), or all-cause mortality. The findings suggest that vitamin D supplementation does not confer cardiovascular protection and is not indicated for this purpose.
Introduction
Observational studies have suggested an inverse association between serum 25-hydroxyvitamin D levels and risk of cardiovascular disease (CVD) events.1,2,3 Specifically, low vitamin D levels have been linked to an increased risk of myocardial infarction (MI), stroke, CVD mortality, and heart failure in case-control and other prospective epidemiologic studies.4,5 Additionally, vitamin D receptors are expressed in vascular tissues, including the myocardium and vascular smooth muscle,6 directly influencing calcium influx, muscle relaxation, and diastolic function.7 Vitamin D also has effects on the renin-angiotensin-aldosterone system and parathyroid hormone and may influence endothelial function and arterial thrombogenesis.8,9,10
Vitamin D level supplementation has increased in primary care settings in the United States.11,12 Assessment of vitamin D supplementation for cardiovascular disease prevention has been a subject of growing interest in recent randomized clinical trials (RCTs).13,14,15,16 Owing to insufficient data regarding cardiovascular benefits of screening and treatment of asymptomatic low vitamin D in adults, the US Preventive Services Task Force has not recommended vitamin D supplementation to prevent cardiovascular disease (via I statement, which indicates insufficient evidence).11 Although previous randomized clinical trials assessing vitamin D supplementation and cardiovascular disease have been limited and inconclusive, several recent large-scale trials have added substantial data to the evidence base.13,14,15,16 Therefore, we conducted a meta-analysis of all RCTs to date that evaluate the efficacy of vitamin D supplementation in the prevention of cardiovascular disease.
Methods
Literature Search
For this meta-analysis, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines were followed.17 The meta-analysis was registered with the International Prospective Register of Systematic Reviews (PROSPERO identifier: CRD42019120689). Published trials from Embase, MEDLINE/PubMed, and the Cochrane Library for relevant trials were identified independently by 2 reviewers (A.Y. and S.S.) from inception to December 2018. The search terms vitamin D, cholecalciferol, ergocalciferol, cardiovascular, cardiac, myocardial, and heart were used. Any inconsistency between reviewers was resolved by a third independent reviewer (O.B.). There were no language restrictions. The references of included trials and published meta-analysis were screened for other potential trials.
Eligibility Criteria
In this analysis, only RCTs that evaluated long-term supplementation (≥1-year intervention) with vitamin D, with or without concurrent calcium and with cardiovascular outcomes, were included in this meta-analysis. Any vitamin D or its analogue supplementation was qualified. Studies that did not include cardiovascular outcomes were excluded after reviewing supplementary materials.
Data Extraction
Two reviewers (H.D. and Y.Z.) extracted relevant data independently by using a predetermined data collection table. Any discrepancies between reviewers were resolved by an independent reviewer (B.K.).
Quality Assessment
The Cochrane Collaboration’s tool was used to perform quality assessment and assess the risk of bias in the included RCTs for random sequence generation, allocation concealment, blinding of participants and health care personnel, blinded outcome assessment, completeness of outcome data, evidence of selective reporting, or other biases. Details are in eFigure 1 in the Supplement.
Outcomes of Interest
The primary end point was a composite of major adverse cardiovascular events (MACEs), as defined by each trial (eTable in the Supplement). Secondary end points were MI, stroke/cerebrovascular accident (CVA), CVD mortality, and all-cause mortality. The longest available follow-up time was used for each trial in the analysis.
Statistical Analysis
Results were presented as risk ratios (RRs) and 95% CIs on the basis of the Mantel-Haenszel random-effects model. Heterogeneity was evaluated by using the I2 statistic. Publication bias of the primary end point was assessed by using the funnel plot. We conducted a sensitivity analysis of the primary end point by sequential removal of each trial. Sensitivity analyses of the primary MACE end point were conducted based on age, sex, inclusion of women who were postmenopausal only, use of pretreatment vitamin D level less than 25 ng/mL (to convert to nmol/L, multiply by 2.496), inclusion of patients with chronic kidney disease, exclusion of studies that used vitamin D analogues, and vitamin D dosage and formulation (daily vs bolus dosing). Meta-regression analyses based on study-level covariates (age, sex, follow-up duration, body mass index [BMI; calculated as weight in kilograms divided by height in meters squared], and pretreatment with statin) were conducted to explain any heterogeneity.
To avoid potential spurious inferences from repetitive significance testing and underpowered meta-analysis, we performed trial sequential analysis. By applying trial sequential monitoring boundaries, similar to those of interim analysis in RCTs, we would be able to obtain reliable results.18 We calculated the optimal information (sample) size to maintain a 2-sided type I error at .05 and a type II error at .20 (80% power), with a relative risk reduction of 25% and an incidence of 8.5% MACE in the placebo arm. Sensitivity analysis was performed using a MACE incidence of 7.86% from the included RCTs. We used Review Manager (RevMan) version 5.3 (Cochrane Community), Comprehensive Meta-analysis version 3 (Biostat), and Trial Sequential Analysis version 0.9.5.9 (Copenhagen Trial Unit) software to conduct all analyses. Data analysis was conducted from November 2018 to March 2019.
Results
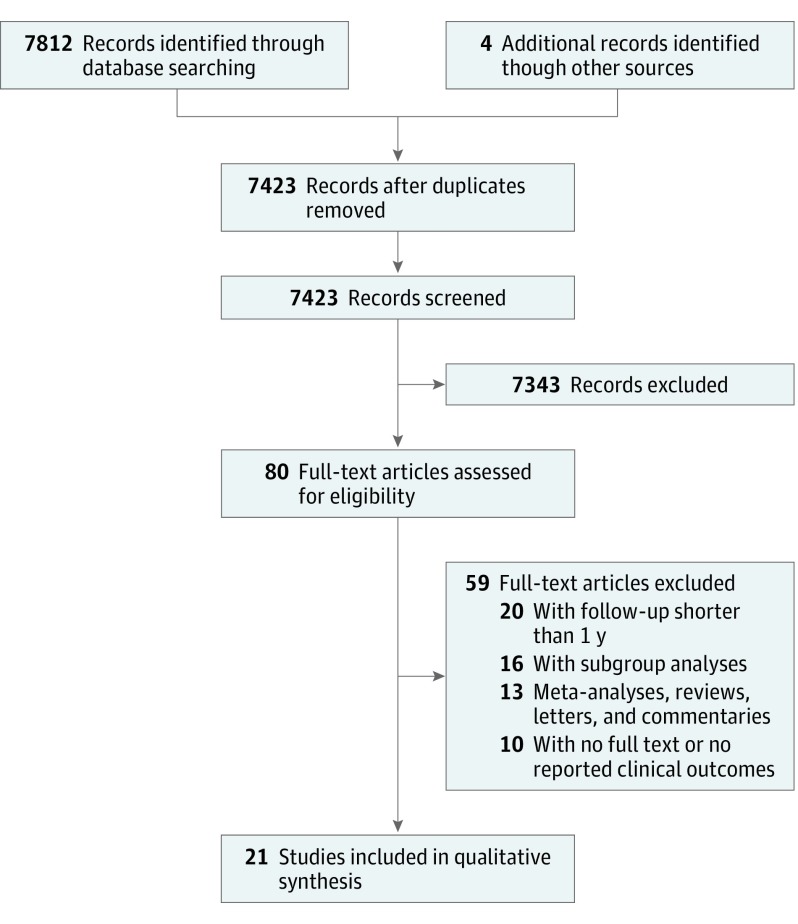
After reviewing 7816 studies from the databases, 7796 studies were excluded. Twenty-one RCTs were included in the final analysis.13,14,15,16,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 Eight trials included postmenopausal women,22,23,24,25,27,29,33,34 9 trials included older patients,20,21,22,24,28,32,33,34,35 2 trials included patients with chronic kidney disease,14,26 2 trials included patients with heart failure,15,30 and 1 trial included patients with chronic obstructive pulmonary disease.31 In total, 83 291 patients were included, 41 669 of whom received vitamin D supplementation and 41 622 of whom received placebo. The mean (SD) age was 65.8 (8.4) years, and 61 943 participants (74.4%) were female. Follow-up durations were variable between the included trials (range, 1-12 years). Fourteen trials used cholecalciferol,13,15,16,19,21,23,24,25,27,30,31,32,33,35 2 trials used ergocalciferol,20,22 and 3 trials used vitamin D analogues (alfacalcidol, paricalcitol, and calcitriol).14,26,34 The search strategy is illustrated in Figure 1. All of the included trials reported the incidence of mortality except the study by Prince et al.20 On the other hand, 3 trials did not report the incidence of MI.15,30,33 For the Women’s Health Initiative trial, we included a follow-up and post hoc analysis that were done on the data.36,37 The characteristics of the included trials with the patients’ demographic features are presented in Table 1 and Table 2.
Figure 1. The Study Selection Strategy.
Table 1. Characteristics of the Involved Trials.
| Source | Year | Patients, No. | Study Period | Vitamin D Type and Dosage | Study Follow-up, y | Country | Major Inclusion Criteria | Primary Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Vitamin D | Placebo | ||||||||
| Aloia et al25 | 1988 | 12 | 15 | NA | Vitamin D3, 400 IU/d | 2 | United States | Women who were postmenopausal and aged 50-80 y, with osteoporosis (diagnosed by the presence of ≥1 nontraumatic vertebral compression fracture) | Bone mineral measurements and fracture incidence |
| Ott et al23 | 1989 | 43 | 43 | NA | Vitamin D3, 1000 mg/d | 2 | United States | Women who were postmenopausal, aged 50-80 y, and ambulatory, with ≥2 compression fractures (>15% reduction in anterior height) and without history of serious trauma or current medications for osteoporosis (except calcium supplements in some cases) | Total body calcium, change in bone mineral density, and fracture rate |
| Komulainen et al29 | 1999 | 112 | 115 | 1989-1991 | Vitamin D3, 300 and 100 IU/d | 5 | Finland | Women in early postmenopause who were nonosteoporotic | Lumber and femoral neck bone mineral density |
| STOP IT/Gallagher et al34 | 2001 | 245 | 244 | NA | Calcitriol, 0.25 μg twice daily | 3 | United States | Women aged 65-77 y with femoral neck density in normal range (SD, ≤2) for their age | Change in bone mineral density of the femoral neck and spine |
| Trivedi et al35 | 2003 | 1345 | 1341 | 1996-1997 | Vitamin D3, 100 000 IU/4 mo | 5 | United Kingdom | Participants aged 65-85 y | Fracture incidence and total mortality by cause |
| RECORD/Grant et al32 | 2005 | 2649 | 2643 | 1999-2002 | Vitamin D3, 800 IU daily | Median (IQR), 3.8 (3.1-4.3) | United Kingdom | Participants aged ≥70 y who had had a low trauma, osteoporotic fracture in the previous 10 y | The incidence of new low-energy fractures |
| Brazier et al33 | 2005 | 95 | 97 | NA | Vitamin D3, 400 IU twice daily | 1 | France | Ambulatory women aged >65 y | Vitamin D treatment–associated adverse events |
| WHI/Jackson et al27 | 2006 | 18 176 | 18 106 | 1995 and 2000 | Vitamin D3, 400 IU/d | 12 | United States | Women aged 50-79 y with no evidence of a medical condition associated with anticipated survival <3 y and no safety, adherence, or retention risks | Total number of fractures |
| Schleithoff et al30 | 2006 | 61 | 62 | 2002-2003 | Vitamin D3, 2000 IU/d | 1.3 | Germany | Participants with New York Heart Association functional class ≥II | Survival rates and biochemical variables, such as natriuretic peptides and cytokines |
| Berggren et al28 | 2007 | 102 | 97 | 2000-2002 | Vitamin D3, 800 IU/d | 1 | Sweden | Participants aged ≥70 y who had femoral neck fractures | Total number of falls |
| Zhu et al22 | 2008 | 39 | 81 | 1998 | Vitamin D3, 1000 IU/d | 5 | Australia | Women aged 70-80 y who were ambulatory | Change in hip bone mineral density, plasma 25-hydroxyvitamin D, biomarkers of bone turnover, parathyroid hormone, and intestinal calcium absorption |
| Prince et al20 | 2008 | 151 | 151 | 2003 - 2004 | Vitamin D3, 1000 IU/d | 1 | Australia | Women aged 70-90 y who were ambulatory | Number of falls |
| Vital D/Sanders et al24 | 2010 | 1131 | 1125 | 2003-2005 | Vitamin D3, 500 000 IU/y | Median (IQR), 2.96 (2.92-3.00) | Australia | Community-dwelling women aged ≥70 y at high risk of fracture (defined by criteria such as maternal hip fracture, past fracture, or self-reported fall) | Number of falls and fractures |
| Lehouck et al31 | 2012 | 91 | 91 | 2008-2009 | Vitamin D, 100 000 IU/mo | 1 | Belgium | Current or former smokers aged ≥50 y who had a chronic obstructive pulmonary disease per diagnosis Global Initiative for Chronic Obstructive Lung Disease definition (postbronchodilator ratio of first second of forced expiration to the forced vital capacity _0.7) and a first second of forced expiration <80% anticipated | Incidence of chronic obstructive pulmonary disease exacerbations |
| VITDISH/Witham et al21 | 2013 | 80 | 79 | 2009-2001 | Vitamin D3, 100 000 IU/3 mo | 1 | United Kingdom | Participants aged ≥70 y with isolated systolic hypertension (supine systolic blood pressure, >140 mm Hg; supine diastolic blood pressure, <90 mm Hg) and baseline 25-hydroxyvitamin D levels <30 ng/mL | Difference in in-office blood pressure, 24-hour blood pressure, arterial stiffness, endothelial function, cholesterol level, insulin resistance, and B-type natriuretic peptide level |
| OPERA/Wang et al26 | 2014 | 30 | 30 | 2008-2010 | Paricalcitol, 1 μg/d | 1 | Hong Kong | Participants with stages 3-5 chronic kidney disease and left ventricle hypertrophy | The change in left ventricle mass index measured by cardiac magnetic resonance imaging |
| Baron et al19 | 2015 | 1130 | 1129 | 2004-2008 | Vitamin D3, 1000 IU\day | 3 | United States | Participants aged 45-75 y who had ≥1 colorectal adenoma removed within 120 d before enrollment and no remaining polyps after a complete colonoscopy | Incidence of colonic adenoma |
| EVITA/Zitterman et al15 | 2017 | 199 | 201 | 2010-2013 | Vitamin D3, 4000 IU/d | 3 | Germany | Participants aged 18-79 y who were classified as having New York Heart Association functional class ≥II | All-cause mortality |
| VIDA/Scragg et al16 | 2017 | 2558 | 2550 | 2011-2015 | Vitamin D3, Initial 200 000 IU, then 100 000 IU/mo | Median (IQR), 3.3 (2.5-4.2) | New Zealand | Participants aged 50-84 y | Incident cardiovascular disease and death |
| J-DAVID/Shoji et al14 | 2018 | 488 | 476 | 2011-2015 | Alfacalcidol, 0.5 μg/d | Median (IQR), Vitamin D: 4.0 (2.6-4.0)a; Placebo: 4.0 (3.5-4.0) | Japan | Participants aged 20-80 y who were receiving maintenance hemodialysis; had calcium ≤10.0 mg/dL, phosphate ≤6.0 mg/dL, and intact parathyroid hormone ≤180 pg/mL, and were not taking any vitamin D receptor activations at randomization | Composite measure of fatal and nonfatal cardiovascular events, including myocardial infarctions, hospitalizations for congestive heart failure, stroke, aortic dissection/rupture, amputation of lower limb owing to ischemia, and cardiac sudden death; coronary revascularization; and leg artery revascularization |
| VITAL/Manson et al13 | 2018 | 12 927 | 12 944 | 2011-2014 | Vitamin D3, 2000 IU/d | Median (IQR), 5.3 (3.8 to 6.1)a | United States | Participants had no history of cancer (except nonmelanoma skin cancer) or cardiovascular disease at trial entry | Incidence of invasive cancer of any type and major cardiovascular events (a composite of myocardial infarction, stroke, or death from cardiovascular causes) |
Abbreviations: EVITA, Effect of Vitamin D on All-Cause Mortality in Heart Failure patients; IQR, interquartile range; J-DAVID, Japan Dialysis Active Vitamin D; OPERA, Oral Paricalcitol in Retarding Cardiac Hypertrophy, Reducing Inflammation and Atherosclerosis in Stage 3-5 Chronic Kidney Disease; RECORD, Randomized Evaluation of Calcium or Vitamin D; STOP IT, Estrogen and Calcitriol in the Prevention of Age-Related Bone Loss; VIDA, Vitamin D Assessment; VITAL, Vitamin D and Omega-3 Trial; VITDISH, Vitamin D in Isolated Systolic Hypertension; WHI, Women’s Health Initiative.
Table 2. Patients’ Demographic and Clinical Characteristics.
| Source | Year | Patients, No. | Subgroup | Age, Mean (SD), y | Male, No. (%) | Current Smoker, No. (%) | BMI, Mean (SD) | Total Cholesterol, Mean (SD), mg/dL | Baseline 25-Hydroxyvitamin D Level, Mean (SD), ng/mL | Statin User, No. (%) | Systolic Blood Pressure, mm Hg, Mean (SD) | Hypertension, No. (%) | Diabetes, No. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aloia et al25 | 1988 | 17 | Vitamin D | 64.1 (1.5) | 0 | NA | NA | NA | 21.9 (7) | NA | NA | NA | NA |
| 17 | Placebo | 64.9 (1.7) | 0 | NA | NA | NA | 26.6 (12) | NA | NA | NA | NA | ||
| Ott et al23 | 1989 | 43 | Vitamin D | 67.9 (1.0) | 0 | NA | NA | NA | 26.7 (1.9) | NA | NA | NA | NA |
| 43 | Placebo | 67.1 (1.2) | 0 | NA | NA | NA | 26.3 (2.4) | NA | NA | NA | NA | ||
| Komulainen et al29 | 1999 | 112 | Vitamin D | 53 (0.3) | 0 | NA | 27.2 (0.3) | NA | NA | NA | NA | NA | 4 (3.6) |
| 115 | Placebo | 53 (0.3) | 0 | NA | 26.6 (0.4) | NA | NA | NA | NA | NA | 3 (3) | ||
| STOP IT/Gallagher et al34 | 2001 | 245 | Vitamin D or vitamin D plus HRT | 71 (3.5) | 0 | NA | 27.3 (5) | NA | 31.2 (11) | NA | NA | NA | NA |
| 244 | Placebo or placebo plus HRT | 71.4 (4) | 0 | NA | 27.4 (6) | NA | 32 (10) | NA | NA | NA | NA | ||
| Trivedi et al35 | 2003 | 1345 | Vitamin D | 74.8 (4.6) | 1019 (75.8) | 59 (4.4) | 24.3 (3.4) | NA | NA | NA | NA | NA | NA |
| 1341 | Placebo | 74.7 (4.6) | 1018 (75.9) | 53 (4.0) | 24.4 (3.0) | NA | NA | NA | NA | NA | NA | ||
| RECORD/Grant et al32 | 2005 | 2649 | Vitamin D or vitamin D plus calcium | 77 (6) | 409 (15.4) | 298 (11.2) | NA | NA | NA | NA | NA | NA | NA |
| 2643 | Placebo and calcium | 77 (6) | 402 (15.2) | 320 (12.1) | NA | NA | NA | NA | NA | NA | NA | ||
| Brazier et al33 | 2005 | 95 | Vitamin D plus calcium | 74.2 (6.4) | 0 | NA | 27.0 (4.4) | NA | 7.3 | NA | 138 (11) | NA | NA |
| 97 | Placebo | 75.0 (7.3) | 0 | NA | 26.4 (4.3) | NA | 7.0 | NA | 138 (14) | NA | NA | ||
| WHI/Jackson et al27 | 2006 | 18 176 | Vitamin D plus calcium | 62.4 (7.0) | 0 | 1405 (7.7) | 29.1 (5.9) | 208.1 | NA | 1178 (6.5) | 127 (17) | 5447 (30.0) | 1055 (5.8) |
| 18 106 | Placebo | 62.4 (6.9) | 0 | 1356 (7.5) | 29.0 (5.9) | 208.1 | NA | 1149 (6.3) | 128 (17) | 5476 (30.2) | 1036 (5.7) | ||
| Schleithoff et al30 | 2006 | 61 | Vitamin D | 57.6 (7.5) | 52 (85.2) | 9 (14.8) | 26.3 (3.8) | NA | Median (IQR), 15.2 (12.0-22.1) | 53 (86.9) | 120 (6) | 38 (62.3) | 20 (32.8) |
| 62 | Placebo | 55.3 (9) | 50 (80.6) | 7 (11.3) | 26 (3.1) | NA | Median (IQR), 15.2 (12.0-22.1) | 42 (67.7) | 125 (8) | 32 (51.6) | 23 (37.1) | ||
| Berggren et al28 | 2007 | 102 | Vitamin D | 82.3 (6.6) | 28 (27.5) | NA | NA | NA | NA | NA | NA | NA | 23 (22.5) |
| 97 | Placebo | 82.0 (5.9) | 23 (23.7) | NA | NA | NA | NA | NA | NA | NA | 17 (17.5) | ||
| Zhu et al22 | 2008 | 39 | Vitamin D plus calcium | 75.4 (2.7) | 0 | NA | 27.6 (4) | NA | 26.8 (10.4) | NA | NA | NA | NA |
| 81 | Placebo or calcium | 74.4 (2.4) | 0 | NA | 28.2 (4.1) | NA | 28 (10.4) | NA | NA | NA | NA | ||
| Prince et al20 | 2008 | 151 | Vitamin D plus calcium | 77.0 (4.2) | 0 | NA | 29.6 (3.5) | NA | 18.1 (5.0) | NA | NA | NA | NA |
| 151 | Placebo plus calcium | 77.4 (5.0) | 0 | NA | 28.2 (3.2) | NA | 17.7 (5.1) | NA | NA | NA | NA | ||
| Vital D/Sanders et al24 | 2010 | 1131 | Vitamin D | 76.4 (5.7) | 0 | NA | NA | NA | 53 (7) | NA | NA | NA | NA |
| 1125 | Placebo | 76.5 (5) | 0 | NA | NA | NA | 47 (5) | NA | NA | NA | NA | ||
| Lehouck et al31 | 2012 | 91 | Vitamin D | 68 (9) | 72 (79.1) | 13 (14.3) | 25 (5) | NA | 20 (12) | NA | NA | NA | NA |
| 91 | Placebo | 68 (8) | 73 (80.2) | 19 (20.9) | 24 (5) | NA | 20 (11) | NA | NA | NA | NA | ||
| VitDISH/Witham et al21 | 2013 | 80 | Vitamin D | 76.9 (4.8) | 40 (50.0) | NA | 28.5 (5.0) | 189 (46) | 18 (6) | 41 (51.3) | 136 (11) | 80 (100.0) | 11 (13.9) |
| 79 | Placebo | 76.7 (4.5) | 42 (53.2) | NA | 27.9 (4.5) | 193 (42) | 18 (6) | 46 (58.2) | 133 (11) | 79 (100.0) | 11 (13.9) | ||
| OPERA/Wang et al26 | 2014 | 30 | Vitamin D | 60.8 (10.2) | 18 (60.0) | 3 (10.0) | 26.6 (4.4) | 167 (39) | NA | 18 (60.0) | 131 (19) | 30 (100.0) | 8 (26.7) |
| 30 | Placebo | 62.2 (10.7) | 14 (46.7) | 3 (10.0) | 26.2 (4.5) | 185 (41) | NA | 20 (66.7) | 135 (15) | 30 (100.0) | 13 (43.3) | ||
| Baron et al19 | 2015 | 1130 | Vitamin D plus calcium | 58.1 (7) | 712 (63.0) | 119 (10.5) | 28.9 (5.0) | NA | 24.7 (8) | NA | NA | NA | NA |
| 1129 | Placebo plus calcium | 58.0 (7) | 711 (63.0) | 96 (8.5) | 29.2 (5.1) | NA | 24.4 (8) | NA | NA | NA | NA | ||
| EVITA/Zitterman et al15 | 2017 | 199 | Vitamin D | 55 (10) | 166 (83.4) | NA | 27.8 (1.7) | NA | 13 (3) | 113 (56.8) | 115 (19) | 57 (28.6) | 51 (26.7) |
| 201 | Placebo | 54 (8) | 166 (82.6) | NA | 27.9 (2.1) | NA | 14 (3) | 105 (52.2) | 117 (18) | 63 (31.3) | 46 (22.9) | ||
| VIDA/Scragg et al16 | 2017 | 2558 | Vitamin D | 65.9 (8.3) | 1512 (59.1) | 164 (6.4) | 27.9 (4.2) | 185 (42) | 25.5 (9.5) | NA | 139 (19) | 955 (37.3) | 265 (10.4) |
| 2550 | Placebo | 65.9 (8.3) | 1457 (57.1) | 156 (6.1) | 27.9 (5.7) | 189 (42) | 25.2 (9.4) | NA | 139 (19) | 930 (36.5) | 239 (9.4) | ||
| J-DAVID/Shoji et al14 | 2018 | 488 | Vitamin D | 65 (10.4) | 301 (61.7) | NA | 21.1 (3) | 153 (32) | NA | 77 (15.8) | 145 (22) | NA | NA |
| 476 | Placebo | 65 (9.67) | 277 (58.2) | NA | 21.1 (3) | 150 (29) | NA | 81 (17.0) | 147 (19) | NA | NA | ||
| VITAL/Manson et al13 | 2018 | 12 927 | Vitamin D | 67.1 (7.0) | 6380 (49.4) | 921 (7.1) | 28.1 (5.7) | NA | 30.9 (10) | 4822 (37.3) | NA | 6352 (49.1) | 1812 (14.0) |
| 12 944 | Placebo | 67.1 (7.1) | 6406 (49.5) | 915 (7.1) | 28.1 (5.8) | NA | 30.8 (10) | 4702 (36.3) | NA | 6439 (49.7) | 1737 (13.4) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); EVITA, Effect of Vitamin D on All-Cause Mortality in Heart Failure Patients; HRT, hormone therapy; IQR, interquartile range; J-DAVID, Japan Dialysis Active Vitamin D; NA, not available; OPERA, Oral Paricalcitol in Retarding Cardiac Hypertrophy, Reducing Inflammation and Atherosclerosis in Stage 3-5 Chronic Kidney Disease; RECORD, Randomized Evaluation of Calcium or Vitamin D; STOP IT, Estrogen and Calcitriol in the Prevention of Age-Related Bone Loss; VIDA, Vitamin D Assessment; VITAL, Vitamin D and Omega-3 Trial; VITDISH, Vitamin D in Isolated Systolic Hypertension; WHI, Women’s Health Initiative.
Primary End Point
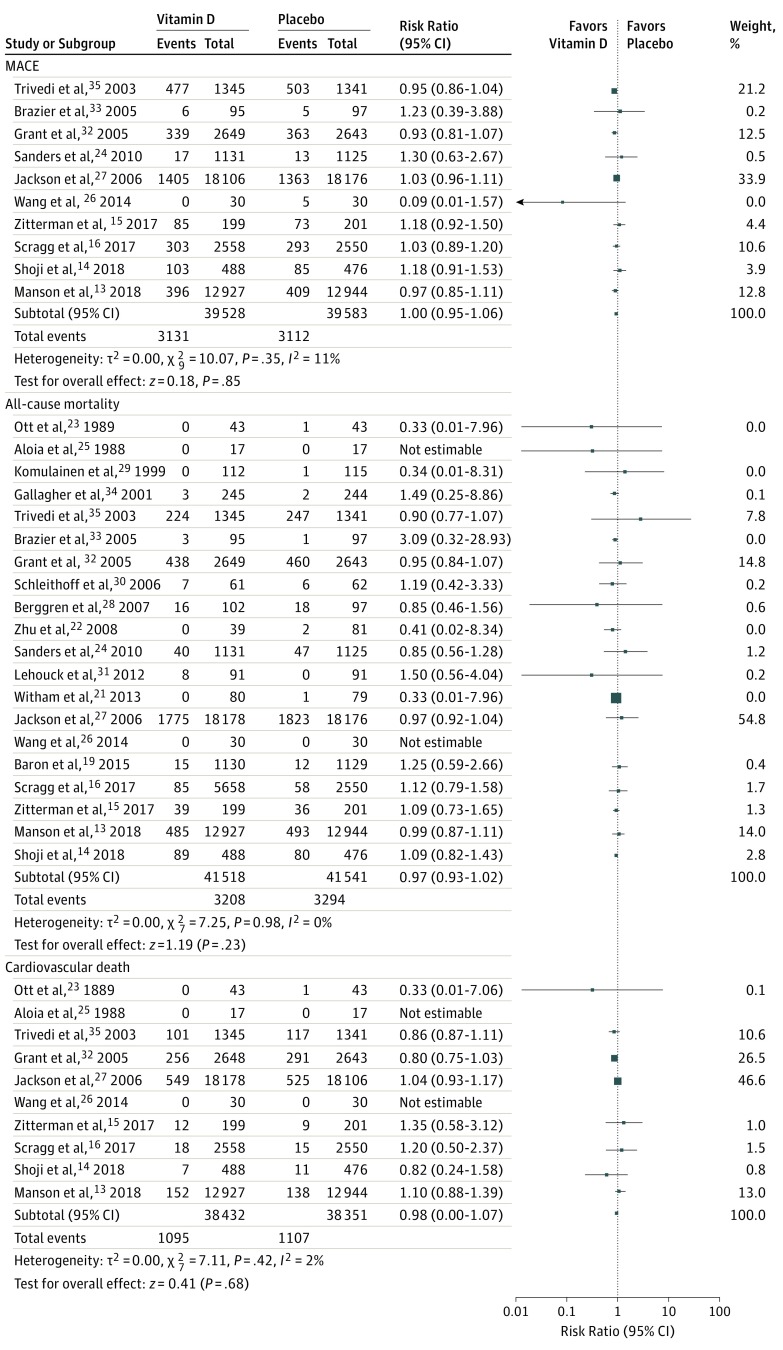
There was no significant difference of vitamin D supplementation between groups with regard to MACE incidence (6243 cases; RR, 1.0 [95% CI, 0.95-1.06]; P = .85; I2 = 11%; Figure 2 and Figure 3). A funnel plot examination for publication bias is provided in eFigure 2 in the Supplement. Sensitivity analyses through removal of each study sequentially and through stratifications by age, sex, inclusion of only postmenopausal women, pretreatment vitamin D levels of less than 25 ng/mL, inclusion of patients with chronic kidney disease, exclusion of studies that used vitamin D analogues, and vitamin D dosage and formulation (daily vs bolus dosing) showed nonsignificant results (eFigures 3-12 in the Supplement). Meta-regression analysis based on sex, BMI, follow-up duration, and age showed a significant association of reduced MACE incidence with advanced age (R2 = 100%; b, −0.01; SE = .004; P = .04), but the P value was not adjusted for multiple comparisons (eFigure 13 in the Supplement).
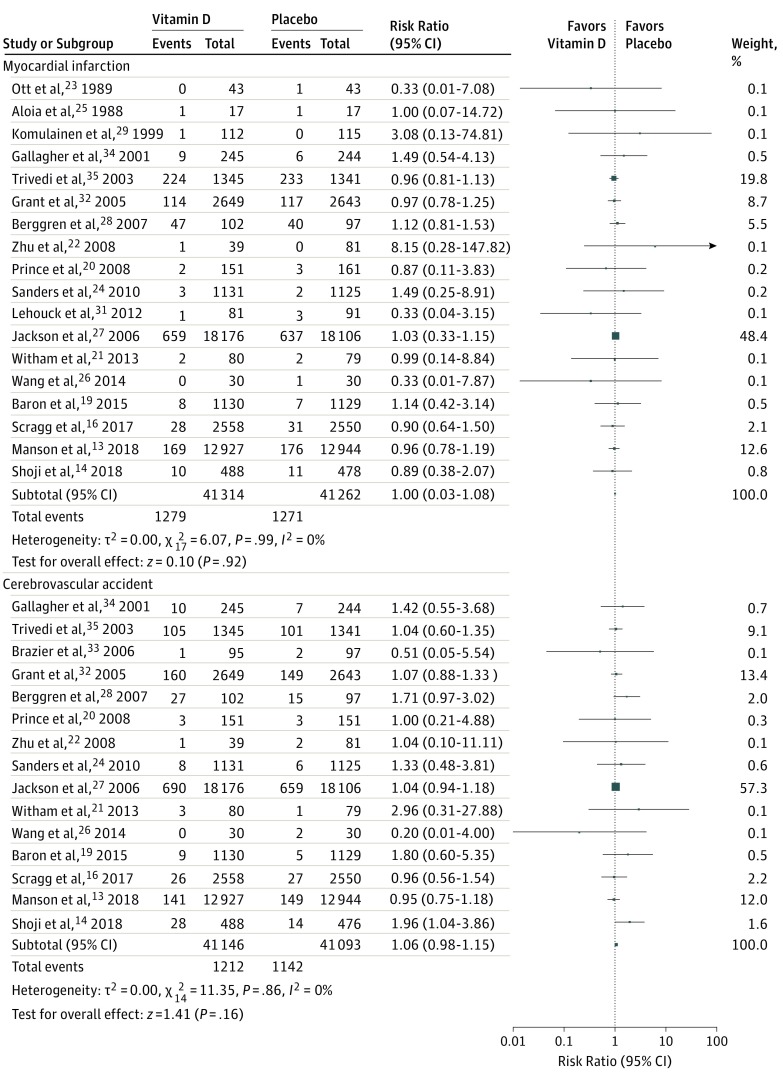
Figure 2. Forest Plot Illustrating the Results of the Primary and Secondary End Points, Part 1.
Figure 3. Forest Plot Illustrating the Results of the Primary and Secondary End Points, Part 2.
In trial sequential analysis, the optimal information size was obtained (diversity adjusted), indicating firm evidence for the lack of an association of MACE reductions with vitamin D supplementation. Details are presented in eFigure 14 in the Supplement.
Secondary End Points
Vitamin D supplementation, compared with placebo, was not associated with a reduced risk of MI (2550 cases; RR, 1.00 [95% CI, 0.93-1.08]; P = .92; I2 = 0%), stroke/CVA (2354 cases; RR, 1.06 [95% CI, 0.98-1.15]; P = .16; I2 = 0%), cardiovascular mortality (2202 cases; RR, 0.98 [95% CI, 0.90-1.07]; P = .68; I2 = 2%), or all-cause mortality (6502 cases; RR, 0.97 [95% CI, 0.93-1.02]; P = .23; I2 = 0%). Figure 2 and Figure 3 present these data.
Discussion
In this comprehensive meta-analysis of randomized clinical trials (n = 83 291 participants) evaluating the cardiovascular effect of vitamin D, we found that vitamin D supplementation was not associated with reduced risk of incident MACE, MI, stroke/CVA, CVD mortality, or all-cause mortality. Observational studies have shown significant associations between low vitamin D level, CVD events, and all-cause mortality.38 However, observational studies are susceptible to uncontrolled confounding by outdoor physical activity, nutritional status, and prevalent chronic disease, which may influence serum 25 hydroxyvitamin D levels.39 Supplementation with vitamin D has not been associated with reduced rates of CVD in previous meta-analyses of RCTs.40,41 In this updated meta-analysis, which extended the earlier findings and included several recent RCTs, we did not find any association between vitamin D supplementation and cardiovascular events. Interestingly, a stratified analysis according to age showed a significantly reduced MACE rate with advanced age, and this was suggested by the Manson et al13 and Trivedi et al35 studies, because vitamin D supplementation in elderly people showed a trend toward lower CVD events. On the other hand, a stratified analysis did not show significant differences by sex, concurrent calcium administration, pretreatment 25-hydroxyvitamin D level (<25 ng/mL vs higher), BMI, vitamin D dosage, formulation (daily vs bolus dosing), or other factors. The regression analysis findings for age should be interpreted cautiously owing to lack of adjustment for multiple comparisons.
In a previous meta-analysis of RCTs,41 vitamin D supplementation showed no benefit in reducing MI, but a potential benefit for heart failure was observed. This meta-analysis confirms an absence of benefit for MI, as well as no reduction in stroke, CVD mortality, or a composite MACE end point. Despite the fact that we aimed in this analysis to study cardiovascular outcomes, most of the trials were not designed to assess CVD events as primary prespecified outcomes; rather, they were designed to assess effects of vitamin D on fracture or osteoporosis and tended to include primarily older patients and women who were postmenopausal.20,21,22,24,28,32,33,34,35 Only 4 trials focused on cardiovascular events as a primary prespecified end point; however, these trials also did not show cardiovascular or mortality benefits.13,14,15,16
Although observational studies have suggested that low serum levels of 25-hydroxyvitamin D are associated with an increased risk of CVD events, the effects of vitamin D supplementation did not appear to vary according to baseline 25-hydroxyvitamin D levels in either the Vitamin D and Omega-3 Trial (VITAL)13or Vitamin D Assessment (VIDA) trials.16 Furthermore, in the sensitivity analysis for trials that had a mean 25-hydroxyvitamin D of 25 ng/mL or less, we did not find an association of vitamin D supplementation with significantly reduced CVD events or all-cause mortality in these participants. Moreover, although several studies focused on patients with chronic kidney disease14,26 because they have low 25-hydroxyvitamin D levels and are at high risk of cardiovascular disease,42 the sensitivity analysis of these trials did not show cardiovascular benefit of vitamin D supplementation in these patients.
Higher prevalence of lower 25-hydroxyvitamin D levels among racial/ethnic groups who are darker skinned than white people are, likely in association with lower vitamin D synthesis in the skin and differences in vitamin D metabolism, has been reported previously.43 However, previous studies have shown no associations of such low levels with CVD events,44 even with vitamin D supplementation.13 Similarly, although low vitamin D levels have been associated with both the risk of CVA and the functional outcome after CVA in observational studies,45 this meta-analysis did not demonstrate a protective effect of vitamin D supplementation with regard to stroke/CVA.40,41 In summary, the included trials, although different in their inclusion criteria, showed consistent findings of no significant benefit of vitamin D supplementation in reducing CVD events and all-cause mortality.
Limitation
This study has limitations that warrant consideration. Most of the included trials had not prespecified CVD as the primary end point and were underpowered for CVD events. Also, the definition of MACE was variable in the included trials, and few trials included data on heart failure. In addition, the results of the subgroup analyses should be interpreted cautiously owing to low data counts, and additional large trials are needed for definitive conclusions. Finally, we lacked patient-level data and could not examine some of the subgroups of interest.
Conclusions
Vitamin D supplementation was not associated with reduced risks of MACE, MI, stroke, cardiovascular mortality, or all-cause mortality. Additional trials of higher-dose vitamin D supplementation, perhaps targeting members of older age groups and with attention to other CVD end points such as heart failure, are of interest.
eTable. Major adverse cardiovascular event definition per each study.
eFigure 1. Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
eFigure 2. Funnel plot of primary endpoints (Major adverse cardiovascular events).
eFigure 3. Forest plot of the sensitivity analysis for elderly patients.
eFigure 4. Forest plot of the sensitivity analysis for female and male patients.
eFigure 5. Forest plot of the sensitivity analysis for postmenopausal women.
eFigure 6. Forest plot of the sensitivity analysis for studies with pretreatment 25-hydroxyvitamin D level less than 25 ng/ml.
eFigure 7. Forest plot of the sensitivity analysis for chronic kidney disease.
eFigure 8. Forest plot of the sensitivity analysis by excluding studies that used vitamin D analogues.
eFigure 9. Forest plot of the sensitivity analysis by including only the studies that used daily vitamin D supplementation.
eFigure 10. Forest plot of the sensitivity analysis by including only the studies that used bolus vitamin D supplementation.
eFigure 11. Forest plot of the sensitivity analysis by including studies that used vitamin D supplementation with calcium.
eFigure 12. Forest plot of the sensitivity analysis by including studies that used vitamin D supplementation without calcium.
eFigure 13. Meta-regression analysis on major adverse cardiovascular events according to age.
eFigure 14. Trial sequential analysis for major adverse cardiovascular events.
References
- 1.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168(11):1174-1180. doi: 10.1001/archinte.168.11.1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pekkanen MP, Ukkola O, Hedberg P, et al. Serum 25-hydroxyvitamin D is associated with major cardiovascular risk factors and cardiac structure and function in patients with coronary artery disease. Nutr Metab Cardiovasc Dis. 2015;25(5):471-478. doi: 10.1016/j.numecd.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 3.Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128(6):1414-1424. doi: 10.1002/ijc.25439 [DOI] [PubMed] [Google Scholar]
- 4.Kheiri B, Abdalla A, Osman M, Ahmed S, Hassan M, Bachuwa G. Vitamin D deficiency and risk of cardiovascular diseases: a narrative review. Clin Hypertens. 2018;24:9. doi: 10.1186/s40885-018-0094-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol. 2008;102(11):1540-1544. doi: 10.1016/j.amjcard.2008.06.067 [DOI] [PubMed] [Google Scholar]
- 6.Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. 2008;88(2):491S-499S. doi: 10.1093/ajcn/88.2.491S [DOI] [PubMed] [Google Scholar]
- 7.Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103(3-5):521-524. doi: 10.1016/j.jsbmb.2006.12.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beveridge LA, Witham MD. Vitamin D and the cardiovascular system. Osteoporos Int. 2013;24(8):2167-2180. doi: 10.1007/s00198-013-2281-1 [DOI] [PubMed] [Google Scholar]
- 9.Al Mheid I, Patel RS, Tangpricha V, Quyyumi AA. Vitamin D and cardiovascular disease: is the evidence solid? Eur Heart J. 2013;34(48):3691-3698. doi: 10.1093/eurheartj/eht166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al Mheid I, Quyyumi AA. Vitamin D and cardiovascular disease: controversy unresolved. J Am Coll Cardiol. 2017;70(1):89-100. doi: 10.1016/j.jacc.2017.05.031 [DOI] [PubMed] [Google Scholar]
- 11.LeFevre ML; U.S. Preventive Services Task Force . Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162(2):133-140. doi: 10.7326/M14-2450 [DOI] [PubMed] [Google Scholar]
- 12.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999-2012. JAMA. 2016;316(14):1464-1474. doi: 10.1001/jama.2016.14403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manson JE, Cook NR, Lee I-M, et al. ; VITAL Research Group . Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33-44. doi: 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoji T, Inaba M, Fukagawa M, et al. ; J-DAVID Investigators . Effect of oral alfacalcidol on clinical outcomes in patients without secondary hyperparathyroidism receiving maintenance hemodialysis: the J-DAVID randomized clinical trial. JAMA. 2018;320(22):2325-2334. doi: 10.1001/jama.2018.17749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zittermann A, Ernst JB, Prokop S, et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J. 2017;38(29):2279-2286. doi: 10.1093/eurheartj/ehx235 [DOI] [PubMed] [Google Scholar]
- 16.Scragg R, Stewart AW, Waayer D, et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study : a randomized clinical trial. JAMA Cardiol. 2017;2(6):608-616. doi: 10.1001/jamacardio.2017.0175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1-e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 18.Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. 2009;38(1):276-286. doi: 10.1093/ije/dyn179 [DOI] [PubMed] [Google Scholar]
- 19.Baron JA, Barry EL, Mott LA, et al. A trial of calcium and vitamin D for the prevention of colorectal adenomas. N Engl J Med. 2015;373(16):1519-1530. doi: 10.1056/NEJMoa1500409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prince RL, Austin N, Devine A, Dick IM, Bruce D, Zhu K. Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Arch Intern Med. 2008;168(1):103-108. doi: 10.1001/archinternmed.2007.31 [DOI] [PubMed] [Google Scholar]
- 21.Witham MD, Price RJG, Struthers AD, et al. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Intern Med. 2013;173(18):1672-1679. doi: 10.1001/jamainternmed.2013.9043 [DOI] [PubMed] [Google Scholar]
- 22.Zhu K, Devine A, Dick IM, Wilson SG, Prince RL. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a five-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93(3):743-749. doi: 10.1210/jc.2007-1466 [DOI] [PubMed] [Google Scholar]
- 23.Ott SM, Chesnut CH III. Calcitriol treatment is not effective in postmenopausal osteoporosis. Ann Intern Med. 1989;110(4):267-274. doi: 10.7326/0003-4819-110-4-267 [DOI] [PubMed] [Google Scholar]
- 24.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815-1822. doi: 10.1001/jama.2010.594 [DOI] [PubMed] [Google Scholar]
- 25.Aloia JF, Vaswani A, Yeh JK, Ellis K, Yasumura S, Cohn SH. Calcitriol in the treatment of postmenopausal osteoporosis. Am J Med. 1988;84(3 pt 1):401-408. doi: 10.1016/0002-9343(88)90259-8 [DOI] [PubMed] [Google Scholar]
- 26.Wang AY-M, Fang F, Chan J, et al. Effect of paricalcitol on left ventricular mass and function in CKD—the OPERA trial. J Am Soc Nephrol. 2014;25(1):175-186. doi: 10.1681/ASN.2013010103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson RD, LaCroix AZ, Gass M, et al. ; Women’s Health Initiative Investigators . Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669-683. doi: 10.1056/NEJMoa055218 [DOI] [PubMed] [Google Scholar]
- 28.Berggren M, Stenvall M, Olofsson B, Gustafson Y. Evaluation of a fall-prevention program in older people after femoral neck fracture: a one-year follow-up. Osteoporos Int. 2008;19(6):801-809. doi: 10.1007/s00198-007-0507-9 [DOI] [PubMed] [Google Scholar]
- 29.Komulainen M, Kröger H, Tuppurainen MT, et al. Prevention of femoral and lumbar bone loss with hormone replacement therapy and vitamin D3 in early postmenopausal women: a population-based 5-year randomized trial. J Clin Endocrinol Metab. 1999;84(2):546-552. doi: 10.1210/jcem.84.2.5496 [DOI] [PubMed] [Google Scholar]
- 30.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754-759. doi: 10.1093/ajcn/83.4.754 [DOI] [PubMed] [Google Scholar]
- 31.Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105-114. doi: 10.7326/0003-4819-156-2-201201170-00004 [DOI] [PubMed] [Google Scholar]
- 32.Grant AM, Avenell A, Campbell MK, et al. ; RECORD Trial Group . Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium or Vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621-1628. doi: 10.1016/S0140-6736(05)63013-9 [DOI] [PubMed] [Google Scholar]
- 33.Brazier M, Grados F, Kamel S, et al. Clinical and laboratory safety of one year’s use of a combination calcium + vitamin D tablet in ambulatory elderly women with vitamin D insufficiency: results of a multicenter, randomized, double-blind, placebo-controlled study. Clin Ther. 2005;27(12):1885-1893. doi: 10.1016/j.clinthera.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 34.Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J Clin Endocrinol Metab. 2001;86(8):3618-3628. doi: 10.1210/jcem.86.8.7703 [DOI] [PubMed] [Google Scholar]
- 35.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387):469. doi: 10.1136/bmj.326.7387.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22(11):915-929. doi: 10.1089/jwh.2013.4270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsia J, Heiss G, Ren H, et al. ; Women’s Health Initiative Investigators . Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115(7):846-854. doi: 10.1161/CIRCULATIONAHA.106.673491 [DOI] [PubMed] [Google Scholar]
- 38.Grandi NC, Breitling LP, Brenner H. Vitamin D and cardiovascular disease: systematic review and meta-analysis of prospective studies. Prev Med. 2010;51(3-4):228-233. doi: 10.1016/j.ypmed.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 39.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press;2011. doi: 10.17226/13050. [DOI] [PubMed] [Google Scholar]
- 40.Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(7):1931-1942. doi: 10.1210/jc.2011-0398 [DOI] [PubMed] [Google Scholar]
- 41.Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M; RECORD Trial Group . Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100(3):746-755. doi: 10.3945/ajcn.113.082602 [DOI] [PubMed] [Google Scholar]
- 42.Sarnak MJ, Levey AS, Schoolwerth AC, et al. ; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention . Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154-2169. doi: 10.1161/01.CIR.0000095676.90936.80 [DOI] [PubMed] [Google Scholar]
- 43.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136(4):1126-1129. doi: 10.1093/jn/136.4.1126 [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Tu W, Manson JE, et al. Racial/ethnic differences in 25-hydroxy Vitamin D and parathyroid hormone levels and cardiovascular disease risk among postmenopausal women. J Am Heart Assoc. 2019;8(4):e011021. doi: 10.1161/JAHA.118.011021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Ji H, Tong Y, Zhang ZB. Prognostic value of serum 25-hydroxyvitamin D in patients with stroke. Neurochem Res. 2014;39(7):1332-1337. doi: 10.1007/s11064-014-1316-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Major adverse cardiovascular event definition per each study.
eFigure 1. Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
eFigure 2. Funnel plot of primary endpoints (Major adverse cardiovascular events).
eFigure 3. Forest plot of the sensitivity analysis for elderly patients.
eFigure 4. Forest plot of the sensitivity analysis for female and male patients.
eFigure 5. Forest plot of the sensitivity analysis for postmenopausal women.
eFigure 6. Forest plot of the sensitivity analysis for studies with pretreatment 25-hydroxyvitamin D level less than 25 ng/ml.
eFigure 7. Forest plot of the sensitivity analysis for chronic kidney disease.
eFigure 8. Forest plot of the sensitivity analysis by excluding studies that used vitamin D analogues.
eFigure 9. Forest plot of the sensitivity analysis by including only the studies that used daily vitamin D supplementation.
eFigure 10. Forest plot of the sensitivity analysis by including only the studies that used bolus vitamin D supplementation.
eFigure 11. Forest plot of the sensitivity analysis by including studies that used vitamin D supplementation with calcium.
eFigure 12. Forest plot of the sensitivity analysis by including studies that used vitamin D supplementation without calcium.
eFigure 13. Meta-regression analysis on major adverse cardiovascular events according to age.
eFigure 14. Trial sequential analysis for major adverse cardiovascular events.