Abstract

The nominal concentration is generally used to express concentration–effect relationships in in vitro toxicity assays. However, the nominal concentration does not necessarily represent the exposure concentration responsible for the observed effect. Surfactants accumulate at interphases and likely sorb to in vitro system components such as serum protein and well plate plastic. The extent of sorption and the consequences of this sorption on in vitro readouts is largely unknown for these chemicals. The aim of this study was to demonstrate the effect of sorption to in vitro components on the observed cytotoxic potency of benzalkonium chlorides (BAC) varying in alkyl chain length (6–18 carbon atoms, C6–18) in a basal cytotoxicity assay with the rainbow trout gill cell line (RTgill-W1). Cells were exposed for 48 h in 96-well plates to increasing concentration of BACs in exposure medium containing 0, 60 μM bovine serum albumin (BSA) or 10% fetal bovine serum (FBS). Before and after exposure, BAC concentrations in exposure medium were analytically determined. Based on freely dissolved concentrations at the end of the exposure, median effect concentrations (EC50) decreased with increasing alkyl chain length up to 14 carbons. For BAC with alkyl chains of 12 or more carbons, EC50’s based on measured concentrations after exposure in supplement-free medium were up to 25-times lower than EC50’s calculated using nominal concentrations. When BSA or FBS was added to the medium, a decrease in cytotoxic potency of up to 22 times was observed for BAC with alkyl chains of eight or more carbons. The results of this study emphasize the importance of expressing the in vitro readouts as a function of a dose metric that is least influenced by assay setup to compare assay sensitivities and chemical potencies.
Introduction
In vitro assays play a central role in toxicity testing in the twenty-first century.1,2 Traditionally, research in in vitro toxicology focused on developing assays for hazard identification. Nowadays, in vitro assays are increasingly used to define toxic doses for hazard characterization.3 In vitro concentration–effect relationships are frequently based on nominal concentrations, i.e., the amount of chemical added to the system divided by the volume of the exposure medium. However, the nominal concentration is not necessarily the concentration reaching cells or target sites where toxic events are initiated. For example, serum in in vitro exposure medium increases the observed effect concentrations of chemicals with high binding affinity to serum constituents.4−7 The increased observed effect concentration has been attributed to a reduction of the free, unbound concentration of the test chemical, which is considered to be available for uptake into cells. The free concentration related more directly to the biologically effective dose (BED, the concentration at the target in cells) than the nominal concentration.8,9 Additionally, evaporation, degradation, metabolism, and sorption to laboratory equipment may further reduce the free and therefore effective concentration in vitro.10−13
In recent years, progress has been made with regard to understanding and characterizing the distribution of test chemicals in in vitro assays.3,13−23 A number of distribution models have been developed relating the octanol–water partition coefficient (log KOW or log D) to the sorption affinity of these chemicals to assay components.6,14,18,19,24−26 However, most of these models have not been validated with analytically measured concentrations of test chemicals in plastic, cells, and exposure medium. Furthermore, chemicals like ionic surfactants fall outside the chemical applicability domain of these models since they do not have a meaningful log KOW.27 The distribution of ionic surfactants is likely to differ significantly from more simple ions because they are amphiphilic; i.e., they have a hydropobic alkyl chain and a hydrophilic headgroup. Knowledge of the in vitro distribution of charged chemicals, particularly ionic surfactants, is limited. This can be considered worrisome as most drugs and many industrial chemicals, including many surfactants, are charged at physiological pH.28,29
Quaternary ammonium surfactants are a group of permanently positively charged surfactants. They are widely used as biocides and antielectrostatic agents in, among others, fabric softeners, personal care products, and antiseptics.30,31 They are expected to accumulate at interfaces in an in vitro system.15 A schematic representation of the distribution of surfactants is depicted in Figure 1. It shows the processes that may reduce the free concentration of surfactants in in vitro assays.
Figure 1.
Schematic representation of the distribution of chemicals in in vitro assays. Chemicals that enter the solution may sorb to serum proteins, well-plate plastic, and cells. In the case of surfactants, they may also associate with the air–medium interface or form micelles at high concentrations. Similar illustrations are found in Groothuis et al.,3 Kramer et al.,6 and Heringa et al.4
The aim of this study was to investigate the effect of assay setup and the dose metric on the observed basal cytotoxic potency of seven benzalkonium chlorides (BACs) with varying alkyl chain length (6–18 carbon atoms, Figure 2) in the rainbow trout gill cell line (RTgill-W1). The RTgill-W1 cell line was chosen in this study as the cells can be exposed to test chemicals in closed chambers, at room temperature, and in serum-free medium. This cell line has been used regularly in the past to study chemical kinetics in vitro, but also as a gill disease model, for the detection of toxicant responses, ranking of chemical potencies and in vitro in vivo extrapolation.20,23,32,33 Cytotoxicity was assessed using alamarBlue and CFDA-AM assays. Median effect concentrations of individual BACs were determined in assays varying in exposure time (24 h versus 48 h), presence of serum proteins, chemical delivery (i.e., direct versus indirect dosing), well plate type (48- versus 96-well plates), and degree of shaking. This study highlights that cytotoxic potencies strongly depend on in vitro assay conditions, especially for cationic surfactants with long alkyl chain lengths. As a result, this dependency can influence the potency ranking of surfactants, which subsequently hampers the quantitative in vitro in vivo extrapolation (QIVIVE).
Figure 2.

Structure of benzalkonium chlorides (R is the alkyl chain containing 6–18 carbons)
Experimental Procedures
The Experimental Procedures section of this study is described in more detail than is customary. This is because the replicability of the study is highly dependent on the labware, pipetting technique, exposure conditions, and extraction methods used.34
Chemicals, Media, and Solvents
Benzalkonium chlorides, essentially fatty-acid and γ-globulin free bovine serum albumin (BSA), Dulbecco’s phosphate buffered saline (PBS), formic acid, and reference chemicals used for the plasma protein binding (PPB) measurements were purchased from Sigma-Aldrich (Zwijndrecht, The Netherlands, Table 1). Benzyldimethylstearylammonium chloride (BAC18) had a purity of >90%; benzyldimethylhexylammonium chloride (BAC6), benzyldimethyloctylammonium chloride (BAC8), benzyldecyldimethylammonium chloride (BAC10), benzyldimethyloctylammonium chloride (BAC12), benzyldimethyltetradecylammonium chloride (BAC14), benzyldimethylhexadecylammonium chloride (BAC16), and 1-dodecylpyridinium chloride monohydrate (C12-Pyr) were at least 96% pure. Solvents (acetonitrile, methanol, isopropanol, and ultrapure water (UPLC/MS grade) were provided by Biosolve (Valkenswaard, The Netherlands). Dulbecco vials and caps were supplied by Grace Discovery Sciences (Breda, The Netherlands): 10 mL precision thread headspace vials with 18 mm Butyl Red/PTFE screw caps and clear 1.5 mL autosampler vials with silicone white/PTFE caps.
Table 1. BSA Binding Constants of Test and Reference Chemicals.
| name | literature KBSA | unit | % bound to 60 μM BSA | log tR | log K | estimated log K with BSA column | estimated % bound to 60 μM BSA | ref |
|---|---|---|---|---|---|---|---|---|
| isoniazid | 0.00 | n/a | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | Goodman and Gilman60 |
| amphetamine | 0.002 | L/μmol | 9.64 | –0.31 | –1.05 | –1.44 | 9.35 | Peltenburg et al.36a |
| amitriptyline | 0.03 | L/μmol | 66.00 | 0.56 | 0.17 | 0.68 | 78.14 | Peltenburg et al.36a |
| diazepam | 0.02 | L/μmol | 59.00 | 0.47 | –0.03 | 0.44 | 66.26 | Peltenburg et al.36a |
| tramadol | 0.003 | L/μmol | 12.72 | –0.16 | –1.24 | –1.08 | 16.55 | Peltenburg et al.36a |
| β-estradiol | 0.09 | L/μmol | 84.23 | 0.56 | 0.63 | 0.68 | 78.09 | Heringa et al.37 |
| testosterone | 0.03 | L/μmol | 64.76 | 0.45 | 0.00 | 0.41 | 54.88 | Moulin38 |
| phenanthrene | 1.10 | L/μmol | 98.51 | 0.77 | 1.36 | 1.19 | 96.34 | Kramer et al.6 |
| bisphenol A | 758.58 | L/kg | 75.21 | 0.57 | 0.40 | 0.70 | 79.26 | Endo and Goss39b |
| pyrene | 8.58 | L/μmol | 99.81 | 0.90 | 1.93 | 1.50 | 98.90 | Kramer et al.40 |
| BAC6 | n/a | n/a | n/a | –0.13 | 0.00 | –1.02 | 17.29 | this studyc |
| BAC8 | n/a | n/a | n/a | 0.30 | 0.00 | 0.05 | 48.77 | this studyc |
| BAC10 | n/a | n/a | n/a | 0.50 | 0.00 | 0.53 | 65.61 | this studyc |
| BAC12 | 0.02 | L/μmol | 59.23 | 0.61 | 0.08 | 0.80 | 80.04 | Chen et al.15 |
| BAC14 | n/a | n/a | n/a | 0.63 | 0.00 | 0.84 | 85.83 | this studyc |
| BAC16 | 0.72 | 0.00 | 1.05 | 91.36 | ||||
| BAC18 | 0.79 | 0.00 | 1.23 | 94.86 |
Values obtained from samples with the lowest tested analyte and highest tested BSA concentration.
KBSA of bisphenol was determined at 37 °C instead of 20 °C.
KBSA calculated using HPAC method as described in ref (44).
The following cell culture media and equipment were supplied by Life Technologies (Breda, The Netherlands): Leibovitz’s L15 medium, versene, trypsin-EDTA, FBS, 10000 U/L penicillin and 10 mg/L streptomycin, culture flasks (75 cm2), Greiner bio-one’s CELLSTAR transparent flat bottom 96 multiwell plates (96WP), 48 multiwell plates (48WP), alamarBlue and CFDA-AM (5-carboxyfluorescein diacetate, acetoxymethyl ester) assays. The RTgill-W1 cell line was purchased from American Type Culture Collection (CCL-163, Manassas, VA) and used from passage 5 to passage 10. Exposure medium (L15/ex) was prepared as described by Schirmer et al.35 using cell culture grade components purchased from Sigma-Aldrich (Zwijndrecht, The Netherlands): 8 g/L sodium chloride, 0.4 g/L potassium chloride, 0.09767 g/L magnesium sulfate, 0.0937 g/L magnesium chloride, 0.19 g/L sodium phosphate dibasic, 0.06 g/L potassium phosphate, 0.14 g/L calcium chloride, 0.55 g/L sodium pyruvate and 0.9 g/L galactose dissolved in ultrapure Millipore water (MP).
Cell Culture and Cytotoxicity Assay
To study the effects of serum constituents on the apparent cytotoxic potency of BACs, L15/ex was used as stand-alone exposure medium or supplemented with either 60 μM (4 g/L) BSA or 10% FBS. The amount of BSA or FBS added to the medium contributed a similar level of proteins (0.8 mg/well), which was confirmed using a fluorescamin assay according to the method described by Kramer et al.41 Assuming BSA is representative of other dissolved proteins in serum with regard to binding affinities, sorption to the protein fractions in medium supplemented with 60 μM BSA or 10% FBS should be comparable. Stock solutions of BACs were prepared in methanol. To obtain the desired test concentrations of BACs in medium (0.01–1000 μM), stock solutions in methanol were diluted 200 times in sterilized glass 10 mL vials with exposure medium.. The methanol concentration in medium was 0.5% (v/v) across all assays. The vials were left overnight on a roller mixer (Stuart SRT9, VWR, Amsterdam, The Netherlands) at 40 rpm, 20 °C in the dark, to ensure proper mixing before adding the spiked medium to the cells. This is referred to as “indirect dosing” since the chemical is first added to the medium and then added to the cells.42
RTgill-W1 cells were grown in culture medium consisting of Leibovitsz’s L15 medium supplemented with 10% (v/v) fetal bovine calf serum (FBS) and 100 μg/mL streptomycin and 100 U/mL penicillin. Cells were left to grow in the dark at 20 °C in closed culture flasks. Unless stated otherwise, cells were seeded in 96WP with a density of 30 × 103 cells/well in 150 μL of culture medium. After 48 h, the culture medium was replaced with 200 μL/well exposure medium containing either BAC, vehicle control (0.5% methanol in medium), or just exposure medium (blanks). There was no difference in viability readouts between blanks and vehicle controls, suggesting the vehicle did not influence viability. Replacement of the culture medium with spiked medium was performed using a multidispenser pipet (Sartorius Biohit, Fischer Scientific, Landsmeer, The Netherlands) with a volume of 1 mL. Pipet tips were flushed once with spiked exposure medium to saturate binding sites on the inside of the tip. Thereafter, 200 μL aliquots were dispensed into triplicate wells and 2 × 200 μL was dispensed into two autosampler vials prefilled with 800 μL of acetonitrile containing 0.1% formic acid (exposure reference standard), using a single pipet tip and single draw of medium into the tip. By dosing the cells in this way, the variability of the concentrations at the start of the exposure to cells (Ct=0h) of the chemicals in medium between replicates was reduced. Another 200 μL aliquot of each concentration was added to a single well without cells in the top and bottom rows (A and H) of the well plate. These wells were used to estimate the sorption to the plastic of the wells of 96WPs, since no further loss because of other processes such as evaporation was expected. All assays were done at least thrice. This includes both three technical replicates and three biological replicates for the cytotoxicity assays described in this study.
After 48 h of exposure, the medium from the wells was transferred to autosampler vials with acidified acetonitrile for LC-MS/MS analysis. Wells were then washed with bare L15/ex and viability of the cells was determined using the alamarBlue and CFDA-AM assays. Assay procedures are described in Kramer et al.41 Briefly, cells were incubated for 45 min in the dark at 20 °C, with 50 μL/well working solutions of 5% v/v alamarBlue and 4 μM CFDA-AM in L15/ex. Fluorescence of alamarBlue and CFDA-AM was determined at 540/590 nm (excitation/emission) and 493/541 nm, respectively, using a Tecan infinite M200 plate reader (Tecan Group, Ltd., Männedorf, Switzerland).
The cell protein concentration in each well was measured to confirm that cell densities between plates were constant. The cell protein content was measured using a fluorescamin assay as described by Kramer et al.41 The alamarBlue and CFDA-AM solutions were discarded and wells were washed with 200 μL of fixative (containing 59 g/L CaCl2 and 0.25% formaldehyde) and 200 μL of PBS. Wells were subsequently filled with 50 μL of Millipore water and frozen at −80 °C for >1 h. Well plates were thawed and 100 μL/well PBS with 50 μL/well acetonitrile containing 48 mg/L fluorescamin was added. No cells were grown in the outer rows of the 96WP, and the initial 50 μL of Millipore water in these wells was discarded and replaced by known BSA (protein) concentrations (0.018–2.25 mg/mL) to calibrate the fluorescamin assay. Well plates were wrapped in aluminum foil and gently shaken (20 rpm, 5°) for 5 min on a plate shaker. Fluorescence was determined on the Tecan infinite M200 spectrophotometer at 360/460 nm. .
Additional assay setup conditions were varied in the RTgill-W1 assay to investigate the impact of assay setup on the apparent cytotoxic potency of benzalkonium chlorides. The impact of assay setup on potency measurements was studied using BAC10, BAC14, and BAC18. Exposure times were reduced to 24 h. Slow shaking of the plates at 10 rpm and 5° height was applied. Seeding densities were reduced to 100 000/mL. Forty-eight well plates (48WP with 1 mL/well exposure medium were used. In addition, cells were “directly dosed” by adding 5 μL of 200× concentrated BAC in methanol to the 200 μL medium/well. The effect of repeated dosing on median effect concentrations was also tested by replacing the exposure medium with freshly spiked exposure medium every 12 h. Finally, DMSO was used as a vehicle instead of methanol and the medium volumes in wells were halved. The experiments were performed using three technical replicates and two biological replicates.
Chemical Analysis
After the exposure period, the complete volume of medium of each well was transferred from the 96WP to glass autosampler vials containing 600 μL of cold (5 °C) acetonitrile with 0.1% formic acid and an internal standard (C12-Pyr). In the case of the 48-well plates (48WP), 200 μL aliquots were sampled from the (1 mL) medium in the wells. Pipet tips used for medium transfer were flushed three times in the autosampler vial with acetonitrile before discarding. This method was used to prevent surfactant loss to pipet tip plastic, allowing accurate measurement of the total amount of dissolved BACs. Samples were kept at 5 °C for at least 30 min before being centrifuged at 4 °C at 1500 rcf (2539 rpm) using a Beckman Coulter Allegra X12-R centrifuge (Beckman Coulter, Woerden, The Netherlands). Clean supernatant (450 μL) was transferred to another autosampler vial and stored at 4 °C until LC-MS/MS analysis. Samples stored for longer than 7 days were kept at −20 °C. Loss of test chemicals to sorption to in vitro system components was calculated by taking the ratio of the measured concentration of the chemical in exposure medium after exposure over the nominal concentration or measured concentration of test chemical in exposure medium at the start of the exposure. The LC-MS/MS consisted of a PerkinElmer (Norwalk, CT) liquid chromatography system equipped with a Kinetex 5 μm XB-C18 column (50 × 2.1 mm; 100 Å) with a C18 guard column, coupled to a triple quadrupole/linear ion trap mass spectrometer (MDS Sciex API 3000 LC-MS/MS System, Applied Biosystems, Foster City, CA). The turbo ion spray source was set in the positive ion mode at 400 °C. The injection volume was 2 μL, and the mobile phase consisted of a gradient flow (0.4 mL/min), starting at 95:5 Millipore:methanol (MP:MeOH) with both containing 0.1% (v/v) formic acid. Between 3.2 and 6.2 min, the gradient was changed nonlinearly (S curve) to 5:95 MP:MeOH. This was further increased to 2:98 MP:MeOH at 7.5 min, after which the mobile phase was reset to 95:5 MP:MeOH. The column was allowed to equilibrate for 1.5 min before the next run. A solvent switch was used to direct the initial eluent (containing salts) to the waste; at 4.6 min the eluent flow was redirected toward the MS/MS. Analyte retention time was typically between 5 and 8 min with LOQ between 1.9 and 6 nM although BAC18 had an LOQ of 17 nM. The m/z of the parent and daughter ions were 220.2/91.0, 248.2/91.0, 276.4/91.0, 304.3/91.0, 332.4/90.4, 360.4/90.9 and 388.1/91.0 for BAC6–BAC18 respectively. The recoveries after 48 h of exposure as percentage of the measured dosed amounts (t = 0 h) were calculated, and lost analyte was assumed to be bound to cells and plastic.
Binding affinities to BSA were measured using a Shimadzu Prominence HPLC system (‘s-Hertogenbosch, The Netherlands), equipped with a LC-20AD pump, SIL-20A autosampler, CTO-20A oven, SPD-20AV UV detector, RF-20A xs fluorescence detector, CBM-20A controller, and Resolvosil BSA-7 column (Machery Nagel). The HPLC and data analysis method was similar to the one developed by Valko et al.43 for a human serum albumin (HSA) column. Details of the method and performance are discussed elsewhere.44 The mobile phase consisted of PBS and isopropanol with a gradient flow (0.7 mL/min) starting with 100% PBS that was increased linearly to 30% isopropanol over 7 min. Between 7 and 25 min, the isopropanol concentration was kept constant, after which the mobile phase was reset to 100% PBS in 1 min. The column was allowed 4 min of equilibration time before the next run.
Data Analysis
Concentration-effect curves were constructed using nonlinear regression: log inhibitor versus response function in Graphpad Prism 7.0 (Graphpad Software Inc., San Diego, CA), requiring log concentrations and the percentage of absorbance compared to the controls (viability). Quantification of the responses was based on the nominal concentration, the measured concentration in medium at the start of exposure (time, t = 0 h) and the measured concentration after exposure (t = 48 h). Median effect concentrations (EC50) were considered as different from one another when the 95% confidence intervals of the EC50 did not overlap.
Sorption of BAC to well plate plastic was calculated by comparing measured medium concentrations before and after exposure for 48 h to wells without cells.. The sorption coefficient to plastic (KPlastic) is expressed as the amount associated with plastic (nmol) per area of plastic (cm2), divided by the concentration in the medium (nM or nmol/cm3) resulting in a single unit (cm or m). The surface area of the exposed plastic was calculated to be 1.56 cm2 for 200 μL volume of medium in a 96WP well. BSA binding constants of the BACs were calculated by comparing the relative retention time (tR) to the relative retention of reference chemicals with known association to BSA (see Table 1). Further details on these calculations can be found in the paper by Valko et al.43 and Groothuis et al.44 BSA binding constants were used to estimate the freely dissolved concentration in medium with 4 g/L BSA. New concentration-effect relationships were constructed using these free concentrations, which were compared to the concentration-effect relationships quantified based on the nominal and measured total concentrations.
Results and Discussion
Unless otherwise stated, results are derived from cytotoxicity assays with RTgill-W1 in 96WPs exposed for 48 h to BACs. The “indirect dosing” procedure was used to spike exposure medium with and without 4 g/L BSA or 10% FBS.
Investigation of Plastic Binding Based on Recoveries
When comparing the analytically determined concentrations (Ct=0h) of BACs in exposure medium containing either BSA or FBS to the nominal concentration (Ct=0h/Cnominal), recovered fractions of BACs were high (82–125%). In exposure medium without serum constituents, the recoveries for BACs with alkyl chains longer than 10 carbons were concentration dependent and ranged from 7% to 117%. The low and variable recoveries indicate losses due to sorption to the glass vials and pipet tips used for transferring medium to the microtiter plates. These results confirm previous findings on adsorptive losses to glass and pipet tips with cationic surfactants.34 To circumvent the issues associated with sorption to pipet tips and glass, replicate aliquots of exposure medium with BACs were added, using a single multidispenser pipet tip, to designated wells in the microtiter plates and autosampler vials containing acetonitrile. BAC concentrations in the autosampler vials with medium and acetonitrile were subsequently analytically determined to discern the concentration of BAC to which cells were exposed at the start of the exposure (Ct=0h). A complete extraction of BAC from exposure medium after exposure to the cells was ensured by transferring all medium per well to autosampler vials with acetonitrile and the pipet tip flushed in the resulting acetonitrile/medium solution.
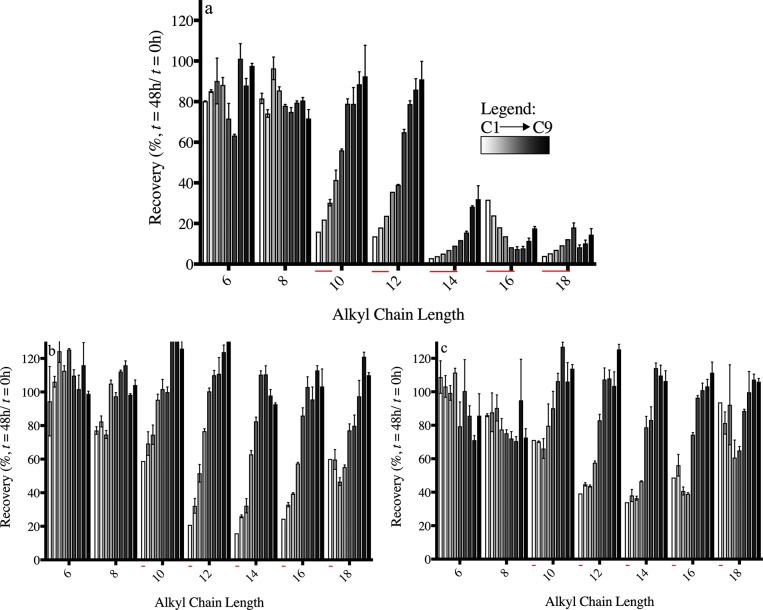
Losses by sorption to plastic microtiter plates are observed when the analytically determined medium concentration at the end of the exposure is compared to the concentration at the start of the exposure (Ct=48h/Ct=0h, Figure 3). Table 2 lists the recovered fraction at a measured dosed concentration between 1 and 2 μM (depicted between brackets). After 48 h of exposure, the analytically determined total concentration in the exposure medium containing serum constituents for chemicals with an alkyl chain length of ≥12 carbons, was reduced to at most 30%, suggesting up to 70% of BACs was associated with the well-plate plastic and cells. In addition, the concentration in medium without medium constituents was reduced further to 3% when comparing Ct=48h to Ct=0h (Figure 3). Recoveries from medium after exposure increase with higher dosing concentrations (Figure 3), indicating that sorption to the microtiter plastic is saturable. These findings indicate that more BAC is retained in solution in the presence of BSA or FBS, likely because of sorption to the proteins in these serum constituents, resulting in a reduction of the fraction lost to plastic or glass. Similarly, greater losses to plastic with decreasing serum concentrations have been reported with polycyclic aromatic hydrocarbons (PAHs) in in vitro bioassays by Schirmer et al.35 and Kramer.6,14
Figure 3.
Percentage (±SD, n = 3) of BAC recovered from exposure medium after 48 h of exposure to RTgill-W1 in 96-well plates. Panels (a)–(c), respectively, depict the percentages recovered from L15/ex, L15/ex with 4 g/L BSA, and L15/ex with 10% FBS. The recovered percentages are sorted by the concentration at the start of the exposure (from low (C1) to high(C9), white to black bars). The concentrations are 0.01–25 μM in L15/ex for BAC10-BAC18, 0.04–50 μM for BAC10-BAC18 in L15/ex with medium constituents, 0.2–250 μM for BAC8, and 0.5–500 μM for BAC6 in L15/ex or 0.5–1000 μM for BAC8 and 1.5–1000 μM for BAC6 in L15/ex with medium constituents. The percentage of chemical not recovered from medium after exposure is assumed to have sorbed to plastic and cells. Note that the red stripes below the bars refer to recoveries calculated using estimated concentrations after exposure. Actual concentrations could not be established because they were below the limit of quantification. Estimations were based on dilution factors.
Table 2. Freundlich Exponents and Concentration Dependent Plastic Association Constants (log Plastic) Obtained from Sorption Isotherms of Benzalkonium Chlorides to Well Plate Plastica.
| chemical name | log KPlastic (cm) at Ct=48h = ± 0.2 μM | n of KPlastic ± SE (Freundlich exponent) | KBSA (μM–1) | % bound in medium with 60 μM BSA | % recovered at 48 h (at Ct=0h in μM) | % recovered at 48 h (at Ct=0h in μM) with 60 μM BSA |
|---|---|---|---|---|---|---|
| BAC6 | –1.30 | 0.90 ± 0.11 | 0.02 | 20.05 | 85.2 (1.3) | 94.5 (1.5) |
| BAC8 | –1.26 | 0.89 ± 0.05 | 0.04 | 53.31 | 96.4 (1.3) | 82.5 (1.8) |
| BAC10 | –0.74 | 0.49 ± 0.03 | 0.06 | 69.62 | 78.9 (1.3) | 101.7 (1.3) |
| BAC12 | –0.21 | 0.43 ± 0.12 | 0.10 | 80.04 | 63.7 (1.0) | 100.4 (1.3) |
| BAC14 | –0.29 | 0.40 ± 0.06 | 0.16 | 87.58 | 28.2 (1.0) | 82.5 (1.4) |
| BAC16 | 0.06 | 0.65 ± 0.08 | 0.26 | 92.37 | 7.8 (1.0) | 85.9 (1.4) |
| BAC18 | 0.18 | 0.85 | 0.43 | 95.41 | 8.3 (1.9) | 77.2 (1.5) |
Log KPlastic is calculated using test concentrations in medium nearest to 0.2 μM. At higher medium concentrations, saturation of the plastic surfaces of wells in well plates was observed.
Isotherms of concentrations of BAC sorbed to plastic versus concentrations in medium after exposure are plotted in Figure 4. Freundlich exponents (n) and concentration dependent plastic association constants (log KPlastic) can be found in Table 2. Values for KPlastic were calculated at a measured concentration after 48 h close to 0.2 μM because saturation of plastic at higher concentrations complicates the comparison of the log KPlastic values between chemicals. The concentration of 0.2 μM was the lowest concentration measured for BAC6 at the end of exposure. BACs with longer alkyl chain lengths have higher plastic binding association constants and smaller Freundlich exponents. When assuming no saturation, log KPlastic may be used to estimate (worst case) the fraction bound to well plate plastic in in vitro assays with varying microtiter plate dimensions and exposure medium volumes.
Figure 4.
Sorption isotherms of benzalkonium chlorides to well plate plastic. Isotherms were fit using the Freundlich equation (R2 of all fits >0.95). In panel (a), estimated concentrations sorbed to the plastic in 96-well plate wells without cells are correlated with concentrations in medium without medium constituents. In panel (b), measured concentrations in medium after exposure are correlated with concentrations in medium without medium constituents before exposure. No sorption to plastic occurs on the 1:1 line.
Another way to characterize partitioning to plastic is to compare concentrations in medium at the end of exposure directly to the concentrations at the start of exposure (Figure 4b). The trend line, especially for BACs with longer alkyl chain lengths, approaches the 1:1 line where no plastic binding occurs. Therefore, the fraction of BAC sorbed to plastic decreases (and the free fraction increases) with increasing medium concentrations of the tested chemicals (saturation), which was also observed from the recoveries (Table 2, Figure 3). This is illustrated further by Table 2, where the listed Freundlich exponents (calculated from Figure 4a) decrease far below 1 for BACs with alkyl chain lengths >10 carbons.
Sorption affinity of BACs to plastic increases with increasing alkyl chain length. Few plastic association constants have been published in literature, making it difficult to place the constants measured in this study into context. Kramer14 reported association constants to well plate plastic (log KPlastic) for polycyclic aromatic hydrocarbons (PAHs) ranging from −2.64 m for fluorene to −0.86 m for benzo(a)pyrene. Timmer and Droge27 proposed to use the distribution coefficient to (artificial) phospholipid membranes (log DMW) as a more suitable parameter compared to the log KOW to predict the affinity to cells and estimate critical (target) membrane burdens of surfactants. The Log DMW of the PAHs tested in Kramer14 ranges from 1.6 to 3.9.14 The log KPlastic at the lowest measured medium concentration ranged from −0.0021 to 0.018 m for BACs with a log DMW range of 4–8.27 The difference in affinity to plastic between PAHs and BACs according to their log DMW may be explained by the lower hydrophobicity of BACs compared to PAHs. Nevertheless, increasing alkyl chain length of the BACs is associated with higher membrane partitioning and stronger lipophilicity,27 which correlates with increasing plastic association constants similar to chemicals within other chemical subgroups such as PAHs, where higher lipophilicity is associated with higher losses to plastic.
Determination of Binding Affinity of Benzalkonium Chlorides to BSA
Binding affinities of BACs to BSA were determined using High Performance Affinity Chromatography (HPAC) in a separate study.44 The binding affinities enable the calculation of free medium concentrations and its relationship with the observed cytotoxic potency. The relative retention times on the HPLC column (logtR) were plotted against the log K (linearized %bound to 60 μM BSA) of reference chemicals with known affinity to BSA (Table 1) and fit using a linear regression model. Log K values for the test chemicals were derived from this regression and used to estimate the percentage bound in medium containing 60 μM (4 g/L) BSA (Table 2). Similar to plastic binding, the percentage BSA bound BAC increases with increasing alkyl chain length. BAC18 is calculated to be 95% bound to protein in medium with 60 μM BSA, while BAC6 is 20% bound. This suggests the bioavailable concentration of BACs with long alkyl chain lengths is expected to be significantly lowered in a cytotoxicity assay with BSA or FBS in the medium compared to bare exposure medium.
Cytotoxic Potency, Effect Concentrations Calculated with Different Dose Metrics
To study the effect of sorption to serum constituents and well plate plastic on the apparent cytotoxic potency of BACs, RTgill-W1 were exposed for 48 h to the BACs in protein free L15/ex medium or L15/ex containing either BSA or FBS. Concentration-effect relationships (Figures 5, S1, and S2) were constructed with different dose metrics (nominal concentrations and analytically determined medium concentrations at Ct=0h and Ct=48h). EC50 values from the CFDA-AM assay for all tested BACs and exposure conditions are summarized in Table 3. EC50 values and concentration-effect relationships from the alamarBlue assay can be found in Table S1 and Figure S3 in the Supporting Information. CFDA-AM assay-based EC50 values are on average 2.8 (1.7–4.0 depending on the dose metric and serum protein levels) times higher than alamarBlue assay-based EC50 values.45,46 AlamarBlue is therefore a more sensitive biomarker of cytotoxicity of BACs than CFDA-AM is. However, both markers show similar trends in potency between chemicals and exposure conditions.
Figure 5.
Concentration–effect relationships of benzalkonium chlorides constructed using different dose metrics. RTGill-W1 were exposed to BAC6–18 for 48 h in supplement-free exposure medium, and the CFDA-AM assay was used to assess cell viability. The different dose metrics used to calculate the concentration–effect relationships were (a) the nominal concentration, (b) the analytically determined dosed concentrations (Ct=0h), and (c) the analytically determined concentration at the end of exposure (Ct=48h). Corresponding EC50 values are listed in Table 3.
Table 3. Median Cytotoxic Concentrations (EC50) of Benzalkonium Chlorides (BAC) Differing in Alkyl Chain Lengtha.
| medium without supplements |
medium with 60 μM BSA | medium with 10% serum | |||
|---|---|---|---|---|---|
| BAC (alkyl chain length) | EC50 ± SE (μM, nominal) | EC50 ± SE (μM, measured t = 0 h) | EC50 ± SE (μM, measured t = 48 h) | EC50 ± SE (μM, measured t = 48 h) | EC50 ± SE (μM, measured t = 48 h) |
| 6 | 247.90 ± 2.94 | 199.10 ± 4.36 | 195.10 ± 4.36 | 305.30 ± 2.32 | 452.00 ± 5.39 |
| 8 | 30.86 ± 0.73 | 32.86 ± 0.70 | 25.01 ± 0.63 | 35.59 ± 0.78 | 61.83 ± 1.24 |
| 10 | 1.27 ± 0.74 | 1.18 ± 0.76 | 0.89 ± 1.02 | 3.03 ± 0.29 | 8.09 ± 0.21 |
| 12 | 0.46 ± 0.04 | 0.27 ± 0.02 | 0.11 ± 0.01 | 1.47 ± 0.56 | 2.28 ± 0.20 |
| 14 | 1.30 ± 0.28 | 0.45 ± 0.04 | 0.05 ± 0.00 | 0.79 ± 0.33 | 1.05 ± 1.39 |
| 16 | 0.70 ± 0.40 | 0.47 ± 0.17 | 0.04 ± 0.00 | 0.82 ± 0.37 | 0.75 ± 0.26 |
| 18 | 1.73 ± 0.32 | 0.48 ± 0.12 | 0.07 ± 0.01 | 1.08 ± 1.61 | 1.62 ± 0.57 |
EC50’s were calculated using three different dose metrics. Cytotoxicity to RTgill-W1 for each BAC was determined using three replicate CFDA-AM assays. BACs were exposed to cells in medium containing no supplements (EC50 in μM measured at t = 48 h), containing 60 μM (4 g/L) BSA (EC50 in μM measured in medium with 60 μM BSA).
Free median effect concentrations were predicted (Figure 6, solid black line) based on a critical cell burden (CCB) for baseline toxicity of 50 mmol/kg lipid in fish47 and membrane association constants as determined by Droge et al.48 and Timmer and Droge.27 Note that the membrane affinity of BACs with a long alkyl chain (BAC16 and BAC18) were extrapolated from the affinity of the BACs with smaller alkyl chain lengths as they could not be measured directly by Droge et al.48 Predicted baseline median effect concentrations were compared to measured median effect concentrations (EC50). The correlation between predicted and measured EC50 was dependent on the dose metric. Cytotoxic potency increases significantly with alkyl chain lengths up to BAC12 (Figure 5, Table 3). When effect concentrations are calculated using Cnominal, the toxic potency decreases for BACs with longer alkyl chain lengths. The toxic potency of BACs with long alkyl chain lengths therefore do not overlap with the predicted free median effect concentrations. However, when quantified using analytically determined (free) concentrations after 48 h of exposure, the toxic potency continues to increase to BAC14 and thus better correlates with predicted free median effect concentrations (Figure 4c, Table 3). The EC50 values of BAC14-BAC18 do not differ from one another as the confidence intervals overlap. In addition, for BAC14–18, up to 25-fold smaller EC50 values are found when based on Ct=48h compared to the EC50 values based on Cnominal. The EC50 values calculated with Ct=0h instead of Cnominal, are significantly higher (11-fold) in comparison to the EC50 values calculated with Ct=48h. Therefore, sorption to plastic of BACs with alkyl chain lengths above 12 carbons significantly impacts the observed cytotoxic potency in these assays.
Figure 6.
Plot showing the relationship between cytotoxic potency (depicted as log EC50 in a CFDA-AM assay with RTgill-W1) and alkyl chain length of benzalkonium chlorides (BACs). EC50 is calculated using different dose metrics: EC50’s depicted as a black solid triangle are calculated by multiplying the lethal membrane concentration in fish (narcosis mode of action) by the affinity for the membrane, as reported by Droge et al.48 The EC50 values were quantified using the nominal concentration (red solid triangle), measured concentration at the start of exposure (orange solid square), measured concentration in the wells after exposure (yellow solid circle), measured total concentration in the presence of BSA after exposure (green plus sign), measured concentration in the presence of FBS after exposure (blue X), and estimated free concentration in the presence of BSA after exposure (purple open triangle).
Although it is difficult to compare different types of surfactants, the toxic potency of the quaternary ammonium surfactants with 12–16 carbons in the alkyl chain used by Sandbacka et al.49 were lower than our values for BACs in a primary culture of rainbow trout gill cells. This can be explained by the absence of the benzyl group in the chemicals used by Sandbacka et al.49 since the presence of this group is assumed to increase the toxic potency by increasing the membrane affinity compared to quaternary trimethylammonium chlorides.27 Additionally, the results may differ because a primary cell culture was used instead of the cell line in our study and their assay had a shorter exposure time and contained more cells, which likely decreased the observed cytotoxicity.
Similarly to our results, Sandbacka et al.49 observed that the toxic potency of their test chemicals calculated based on nominal concentrations, reached a plateau after a “cut-off point” at longer alkyl chain lengths. This phenomenon has been quite often observed with surfactants in particular with regard to hemolytic effects.50−52 One of the most prominent mechanistic explanations of the cutoff point is the free volume theory.51 According to this theory, amphiphilic chemicals can create free volumes in the bilayer membrane. Smaller amphiphilic chemicals will create larger free volumes, destabilizing the membrane to a greater extent compared to the surfactants with longer alkyl chains. On the other hand, surfactants with shorter alkyl chain lengths will have a lower affinity for the membrane. As a result, there might be a maximum level of toxic potency via narcosis as a result of these counteracting effects.
In an earlier study, Isomaa et al.53 investigated the interaction of amphiphilic agents, including cationic surfactants, with in vitro erythrocyte membranes. They observed a similar cutoff point (at an alkyl chain length of 14 carbons) with alkyltrimethylammonium bromides that they tested to protect erythrocytes against hemolysis. The authors suggested that the apparent cutoff point might be caused by an effect of unknown kinetic interactions of the studied chemicals. Interestingly, the cutoff point found in this study does shift from 12 to 14 carbon atoms in the alkyl chain when Ct=48h is used instead of Cnominal or Ct=0h. In addition, the toxic potency of the BACs with longer alkyl chain lengths levels off, instead of decreasing after the cutoff point, which was observed for Cnominal. One explanation could be that the medium contains a small fraction of dissolved organic carbon (DOC) to which the surfactants with long alkyl chain length bind significantly, making them less bioavailable. Chen et al.31 calculated that a DOC content of 0.1 mg/L can reduce the bioavailability of BAC12 with about 50%, while this is often the limit of water purification systems. Since the log DMW of BAC12 is 6, while those of BAC16 and BAC18 are approximately 7.3 and 8 respectively, only a very small fraction of DOC is likely able to reduce the bioavailability of BAC16 and BAC18 to significant extent. Another consideration is that the measured concentration might not represent an amount of chemical evenly distributed in the wells, especially for surfactants with longer alkyl chain lengths. Surfactants are known to accumulate at the interfaces. Therefore, the measured (average) concentration may not directly relate to the amount affecting cells. It is thus not certain whether any of the dose metrics used are appropriate to accurately quantify the observed cytotoxic potency. However, the phenomenon described above may be less prominent in the presence of proteins (e.g., BSA or FBS). Proteins in the medium might facilitate diffusion and enhance homogenization by providing an additional protein–water interface, which is distributed relatively evenly throughout the medium. Concentration–effect relationships determined in media containing proteins may therefore diminish the effect of a nonhomogenous distribution.
When estimated free concentrations in L15/ex medium with BSA are used to calculate EC50 values, the trend and cutoff point is similar to those based on Ct=48h in protein free medium (Figure 5, purple open up triangles). Interestingly no additional shift in cutoff point is observed even though this could have been expected due to a possible homogenization of surfactant in the presence of BSA. There is a small difference when using alamarBlue data (Figure S3) as the cutoff point is shifted from BAC14 to BAC16. The assumption that BSA homogenizes the surfactant distribution is partly supported by the data based on alamarBlue, but not CFDA-AM. The data gathered in this study is therefore partially able to explain the cutoff point based on BAC kinetics of the chemicals by using different dose metrics to quantify toxic potency. Possibly there is indeed a mechanistic explanation for the cutoff point, in addition to a kinetic one. The predicted EC50 values of BACs with longer alkyl chain lengths (BAC16–BAC18) were extrapolated from the BACs with shorter alkyl chain lengths assuming a linear relationship, therefore the predictions could overestimate their toxicity. In addition, the median effect concentration of BAC16 and BAC18 based on any dose metric are higher compared to the predicted baseline toxicity, which may be a result of better integration in the phospholipid membranes without disturbing the integrity in line with the free volume theory. Considering that fish lipid does not solely consist of membranes and the overall negative charge of cell membranes should attract cationic chemicals more than general lipid tissue, estimated free effect concentrations may be lower (an overestimation) than actual values. The free median effect concentrations of the BACs should then fall below the line in Figure 6, which suggests that BACs may not act solely through a narcotic mode of action. The narcotic mode of action might become more significant for BACs with longer alkyl chain lengths compared to a specific mode of action relative to BACs with shorter alkyl chain lengths. Regardless, these considerations may only be conclusively elucidated by analytically measuring membrane concentrations, which is a challenging task and beyond the scope of the work presented in this manuscript.
In summary, this section illustrates that the toxic potency of surfactants increases with alkyl chain length. This is, however, more conclusively observed using analytically determined concentrations to quantify the toxic potency. Additionally, sorption to plastic increases the apparent EC50 up to 11-fold for BACs with long alkyl chain lengths (≥12 carbons), while medium constituents can increase the apparent EC50 up to 22-times. A more detailed determination of the actual amount of benzalkonium chloride that is active at the target site in cells may only be achievable by measuring membrane or cell-associated concentrations.
Impact of Assay Setup on the Cytotoxic Potency
Depending on the assay conditions used, the observed toxic potency differs up to 3 orders of magnitude for BACs with long alkyl chain lengths (Figure 7). The largest cytotoxic potency differences seem to be dose metric driven, e.g., median effect concentrations based on Ct=48h versus Cnominal, and addition of serum or BSA to the medium. However, other assay setup conditions such as exposure time had an influence on observed cytotoxic potencies as well (Figure 7). EC50 values after 48 h of exposure are lower than after 24 h exposure. These findings agree with those of Gülden et al.12,54 where EC50 values of various chemicals decreased with increasing exposure time in cultures of C6 glioma or Balb/c 3T3 cells until an “incipient” EC50 is reached. The incipient EC50 remains constant with longer exposure times. Understanding the effect of exposure duration is important to achieve accurate and reproducible cytotoxic potency determinations in vitro. Gülden et al.54 noted that using the incipient EC50 or at least 72 h EC50, instead of variable or arbitrary exposure times, will make comparisons to other in vitro assays, as well as extrapolations to in vivo experiments more meaningful. However, depending on the cell type and assay, it may not always be possible to expose the cells for 72 h or longer, which is also the case for the RTgill-W1 studied. Toxicokinetic–toxicodynamic (TK-TD) modeling may aid in determining a time independent measure of toxic potency.20,55−57 This could greatly improve applicability of in vitro toxicity data for extrapolation to in vivo and risk assessment.
Figure 7.
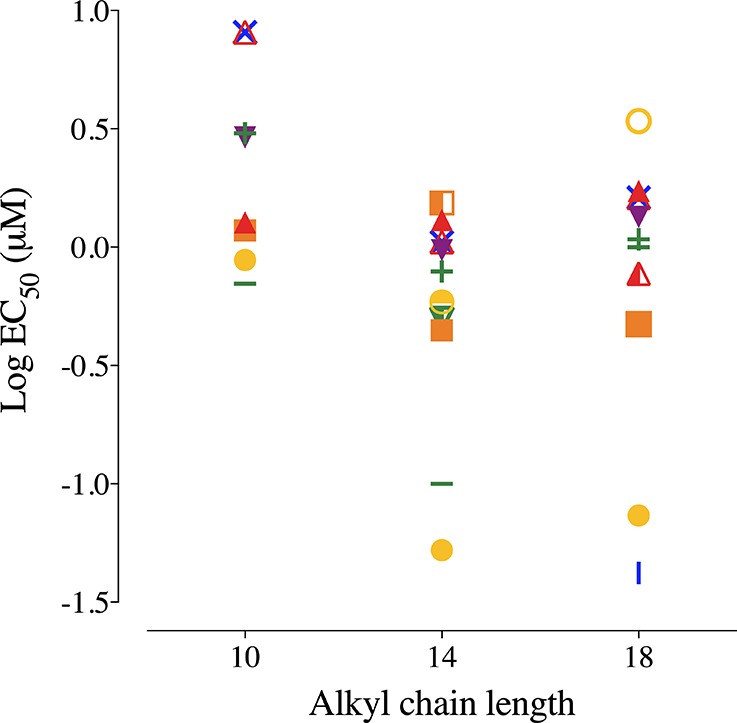
Impact of assay setup on the observed cytotoxic potency of benzalkonium chlorides with 10, 14, or 18 carbons in the alkyl chain. The different experimental conditions result in up to 3 orders of magnitude differences in the observed toxic potency. Unless otherwise stated the setup was with 96-well plates, 48 h exposure, 200 000 cells/mL, indirect and single dosing were used. The shown EC50 values were quantified using the nominal concentration (red solid triangle), measured concentration at the start of exposure (orange solid square), measured concentration in the wells after exposure (yellow solid circle), measured total concentration in the presence of BSA (green +), measured concentration in the presence of FBS (blue X), nominal concentration in the presence of BSA (purple solid triangle), nominal concentration in the presence of FBS (red open triangle), nominal concentration in L15/ex but after 24 h of exposure (orange solid square), nominal concentration but direct dosing (yellow open circle), measured free concentration in L15/ex in 48WP (green dash), analytically measured free concentration, slowly shaken after 24 h exposure (blue dash), repeatedly dosed nominal concentration after 24 h of exposure (red half-solid triangle), nominal concentration in halved volume after 24 h of exposure (orange half-solid square), directly dosed nominal concentration with slow shaking (yellow half-solid circle), and nominal concentration in halved exposure medium and halved cell seeding density.
Repeated dosing, fewer cells, and slow shaking increased the observed toxic potency (Figure 7). Together they increase the apparent toxic potency, while changing one of these factors alone did not necessarily lead to significantly different outcomes. Membrane affinity is higher for BACs with longer alkyl chain lengths.27,48 Therefore, the hydrophobicity is likely higher and diffusion through aqueous solutions and cellular uptake may be slower, compared to surfactants with shorter alkyl chain lengths, as is observed for other chemicals.58 Considering this, the equilibrium time of the chemical distribution in the wells and particularly into the cells, will likely be slower for surfactants with long alkyl chain lengths.54 In this case, 48 h might not be enough for BAC18 to become equilibrated with all assay components and medium constituents. This might explain why the EC50 is higher than what is predicted based on the baseline toxic mechanism of action. It is speculated that increased flux into cells and other compartments caused by slow shaking of the system may increase the observed toxic potency, especially for more hydrophobic chemicals in assay systems where equilibrium is not reached within the exposure time. In addition, BSA and FBS likely facilitate the uptake kinetics into cells and distribution equilibrium as shown for other chemical groups.40,58
Different in vitro setups clearly can result in differing cytotoxic potencies. Chen et al.15 reported effect concentrations for BAC12 that are higher compared to our results (20-fold higher) using the same RTgill-W1 cell line. However, the authors used shorter exposure times (24 h) and 24 microtiter plates as opposed to 96WP in combination with a higher cell density (>60 000/cm2 as opposed to 30 000/cm2 in this study). Shorter exposure times can reduce the observed toxic potency as described earlier, while larger cell density can significantly reduce the observed toxic potency as well.11
Conclusions and Future Perspectives
As observed in this study, the toxic potency of BACs increases with increasing alkyl chain length. By using measured concentrations in medium after exposure rather than nominal concentrations to express cytotoxic potency, the cytotoxic potency of BACs (BAC14 and up) increases with alkyl chain length. The extent of binding to well plate plastic is positively correlated with alkyl chain length. Thus, with increasing chain length, plastic sorption plays an increasingly significant role in reducing the free concentration and the apparent cytotoxicity. The results illustrate the challenge of accurately describing the in vitro cytotoxic potency of BACs and, presumably, surfactants in general. As shown, factors such as sorption to plastic and serum constituents, can influence the apparent toxic potency. Conventional nominal concentrations are suitable to describe the toxic potency of benzalkoniums with short alkyl chain lengths (<10). For these chemicals, medium constituents have limited impact (<4 fold) on the observed toxic potency. However, for benzalkoniums with longer alkyl chain lengths, the fraction available for uptake into cells is reduced (4–30 fold) due to sorption to plastic and, if present, proteins. Other assay setup conditions such as cell density, repeated dosing and exposure time can influence the observed toxic potency as well.
Based on our results, we propose to use the free concentration in exposure medium to compare cytotoxic potencies of BACs between in vitro assays and between in vitro and in vivo bioassays when the BACs have a long alkyl chain length of more than 10 carbons, corresponding to a log DMW of 4 and higher.27 Potencies based on intracellular or membrane concentration can further improve the comparability of toxicity values between assays. In fact, these dose metrics may reduce the impact of other assay setup factors such as cell density.
Although there are a few examples including the studies by Bernhard and Dyer59 and Fischer et al.,58 it is currently difficult to quantify membrane or cell-associated concentrations of surfactants using analytical methods. The determination of free concentrations is often not considered feasible either since it also requires additional expertise, analytical equipment and time. Therefore, algorithms have been developed that model the distribution of chemicals in vitro including free, intracellular and membrane concentrations. Armitage et al.18 and Kramer et al.6 have developed models that estimate the sorption of neutral organic chemicals to in vitro assay compartments once a chemical equilibrium has been established. Recently, Fischer et al.19 extended the model from Armitage et al.18 to include ionized chemicals. The prediction requires input of partition coefficients of the investigated chemicals to serum constituents, microtiter plate plastic. Often these are estimated using the octanol–water partition coefficient, log KOW. Unfortunately, parameters such as log KOW or log D7.4 are not useful descriptors for surfactants because of their amphiphilic properties. The developed models are therefore not suitable to describe the in vitro distribution of surfactants. The models are further limited by the fact that they have not been extensively validated with analytically determined free concentrations but were rather used to investigate the distribution characteristics of chemicals with a wide range of chemical properties. The accuracy of the models to estimate free or cell-associated concentrations is therefore unclear. However, the current study sheds more light on the distribution characteristics of cationic surfactants and can therefore help to improve the modeling of in vitro concentrations in the future.
Using the free concentration for BACs with a log DMW above 4 to calculate effect concentrations will likely improve the reproducibility and comparability of in vitro toxicity test results. This also implies an improved potential to successfully validate an in vitro test for use with these surfactants. Validation is important for regulators to know whether they can rely on the generated data for safety assessment. Finally, since free in vitro concentrations better relate to the target effect levels, potential extrapolations to in vivo may be improved of which the performance will then depend more on the uncertainty of the extrapolation process itself.
Acknowledgments
The authors would like to thank everyone who contributed to and commented on this work and in particular Theo Sinnige who helped with the analytical measurements.
Glossary
Abbreviations
- BAC(s)
benzalkonium chloride(s)
- BSA
bovine serum albumin
- HSA
human serum albumin
- FBS
fetal bovine serum
- QIVIVE
quantitative in vitro in vivo extrapolation
- BAC6
benzyldimethylhexylammonium chloride
- BAC8
benzyldimethyloctylammonium chloride
- BAC10
benzyldecyldimethylammonium chloride
- BAC12
benzyldimethyloctylammonium chloride
- BAC14
benzyldimethyltetradecylammonium chloride
- BAC16
benzyldimethylhexadecylammonium chloride
- BAC18
benzyldimethylstearylammonium chloride
- Pyr-12
1-dodecylpyridinium chloride monohydrate
- CFDA-AM
5-carboxyfluorescein diacetate acetoxymethyl ester
- MP
ultrapure Millipore water
- MeOH
methanol
- LOQ
limit of quantification
- Kplastis
sorption coefficient to plastic
- tR
relative retention time
- Ct=0h
analytically determined dosed concentrations
- Cnominal
the nominal concentration
- Ct=48h
analytically determined concentration at the end of exposure
- PAH
polycyclic aromatic hydrocarbons
- (log)DMW
distribution coefficient to (artificial) phospholipid membranes
- log K
linearized percentage bound to 60 μM BSA
- L15/ex
exposure medium
- DOC
dissolved organic carbon
- EC50
median effect concentration
- TK-TD
toxicokinetic–toxicodynamic
- CCB
critical cell burden
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.chemrestox.8b00412.
Concentration–response relationships of benzalkonium chlorides with CFDA-AM assays using the RTgill-W1 cell line; concentration–response relationships of benzalkonium chlorides with alamarBlue assays using the RTgill-W1 cell line; median effect concentrations (EC50) calculated for benzalkonium chlorides with alamarBlue assays using the RTgill-W1 cell line with exposure medium with or without medium constituents; graphical representation of the median effect concentrations of benzalkonium chlorides in the RTgill-W1 cell line using the alamarBlue assay (PDF)
Author Contributions
F.G. and N.T. performed the cytotoxicity experiments. F.G. drafted the manuscript. N.K. performed the assay variation experiments and lead the research project. B.N. supervised the research and critically reviewed the manuscript. S.D. provided the membrane/water partition coefficient data for BACs and critically reviewed the manuscript. B.B. critically reviewed the manuscript.
For this work, all authors have been funded by Unilever U.K., Safety & Environmental Assurance Centre.
The authors declare no competing financial interest.
Supplementary Material
References
- NRC . (2007) National Research Council, Committee on Toxicity Testing and Assessment of Environmental Agents; Toxicity testing in the 21st century: A vision and a strategy, The National Academies Press. [Google Scholar]
- NAC . (2017) National Academies of Sciences, Engineering, and Medicine; Using 21st Century Science to Improve Risk-Related Evaluations, The National Academies Press. [PubMed] [Google Scholar]
- Groothuis F. A.; Heringa M. B.; Nicol B.; Hermens J. L. M.; Blaauboer B. J.; Kramer N. I. (2015) Dose metric considerations in in vitro assays to improve quantitative in vitro-in vivo dose extrapolations. Toxicology 332, 30–40. 10.1016/j.tox.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Heringa M. B.; Schreurs R. H. M. M.; Busser F.; van der Saag P. T.; van der Burg B.; Hermens J. L. M. (2004) Toward more useful in vitro toxicity data with measured free concentrations. Environ. Sci. Technol. 38, 6263–6270. 10.1021/es049285w. [DOI] [PubMed] [Google Scholar]
- Gülden M.; Seibert H. (1997) Influence of protein binding and lipophilicity on the distribution of chemical compounds in in vitro systems. Toxicol. In Vitro 11, 479–483. 10.1016/S0887-2333(97)00042-8. [DOI] [PubMed] [Google Scholar]
- Kramer N. I.; Krismartina M.; Rico-Rico A.; Blaauboer B. J.; Hermens J. L. M. (2012) Quantifying Processes Determining the Free Concentration of Phenanthrene in Basal Cytotoxicity Assays. Chem. Res. Toxicol. 25, 436–445. 10.1021/tx200479k. [DOI] [PubMed] [Google Scholar]
- Seibert H.; Morchel S.; Gülden M. (2002) Factors influencing nominal effective concentrations of chemical compounds in vitro: medium protein concentration. Toxicol. In Vitro 16, 289–297. 10.1016/S0887-2333(02)00014-0. [DOI] [PubMed] [Google Scholar]
- Escher B. I.; Hermens J. L. M. (2004) Internal exposure: Linking bioavailability to effects. Environ. Sci. Technol. 38, 455A–462A. 10.1021/es0406740. [DOI] [PubMed] [Google Scholar]
- Hermens J. L. M.; Heringa M. B.; ter Laak T. L. (2007) Bioavailability in dose and exposure assessment of organic contaminants in (Eco)toxicology. J. Toxicol. Environ. Health, Part A 70, 727–730. 10.1080/15287390701236157. [DOI] [PubMed] [Google Scholar]
- Riedl J.; Altenburger R. (2007) Physicochemical substance properties as indicators for unreliable exposure in microplate-based bioassays. Chemosphere 67, 2210–2220. 10.1016/j.chemosphere.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Gülden M.; Morchel S.; Seibert H. (2001) Factors influencing nominal effective concentrations of chemical compounds in vitro: cell concentration. Toxicol. In Vitro 15, 233–243. 10.1016/S0887-2333(01)00008-X. [DOI] [PubMed] [Google Scholar]
- Gülden M.; Jess A.; Kammann J.; Maser E.; Seibert H. (2010) Cytotoxic potency of H2O2 in cell cultures: Impact of cell concentration and exposure time. Free Radical Biol. Med. 49, 1298–1305. 10.1016/j.freeradbiomed.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Kramer N. I.; Di Consiglio E.; Blaauboer B. J.; Testai E. (2015) Biokinetics in repeated-dosing in vitro drug toxicity studies. Toxicol. In Vitro 30, 217. 10.1016/j.tiv.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Kramer N. I. (2010) Measuring, Modeling, and Increasing the Free Concentration of Test Chemicals in Cell Assays, In Institute for Risk Assessment Sciences, p 170, PhD Dissertation, Utrecht University, Utrecht. [Google Scholar]
- Chen Y.; Geurts M.; Sjollema S. B.; Kramer N. I.; Hermens J. L. M.; Droge S. T. J. (2014) Acute toxicity of the cationic surfactant C12-benzalkonium in different bioassays: How test design affects bioavailability and effect concentrations. Environ. Toxicol. Chem. 33, 606–615. 10.1002/etc.2465. [DOI] [PubMed] [Google Scholar]
- Gülden M.; Schreiner J.; Seibert H. (2015) In vitro toxicity testing with microplate cell cultures: Impact of cell binding. Toxicology 332, 41–51. 10.1016/j.tox.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Gülden M.; Morchel S.; Tahan S.; Seibert H. (2002) Impact of protein binding on the availability and cytotoxic potency of organochlorine pesticides and chlorophenols in vitro. Toxicology 175, 201–213. 10.1016/S0300-483X(02)00085-9. [DOI] [PubMed] [Google Scholar]
- Armitage J. M.; Wania F.; Arnot J. A. (2014) Application of mass balance models and the chemical activity concept to facilitate the use of in vitro toxicity data for risk assessment. Environ. Sci. Technol. 48, 9770–9779. 10.1021/es501955g. [DOI] [PubMed] [Google Scholar]
- Fischer F. C.; Henneberger L.; König M.; Bittermann K.; Linden L.; Goss K.-U.; Escher B. I. (2017) Modeling Exposure in the Tox21 in Vitro Bioassays. Chem. Res. Toxicol. 30, 1197–1208. 10.1021/acs.chemrestox.7b00023. [DOI] [PubMed] [Google Scholar]
- Stadnicka-Michalak J.; Tanneberger K.; Schirmer K.; Ashauer R. (2014) Measured and modeled toxicokinetics in cultured fish cells and application to in vitro - In vivo toxicity extrapolation. PLoS One 9, e92303. 10.1371/journal.pone.0092303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke H.; Di Consiglio E.; Kreutz R.; Partosch F.; Testai E.; Gundert-Remy U. (2017) The importance of protein binding for the in vitro–in vivo extrapolation (IVIVE)—example of ibuprofen, a highly protein-bound substance. Arch. Toxicol. 91, 1663–1670. 10.1007/s00204-016-1863-z. [DOI] [PubMed] [Google Scholar]
- Pfaller W.; Prieto P.; Dekant W.; Jennings P.; Blaauboer B. J. (2015) The Predict-IV project: Towards predictive toxicology using in vitro techniques. Toxicol. In Vitro 30, 1–3. 10.1016/j.tiv.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Tanneberger K.; Knöbel M.; Busser F. J. M.; Sinnige T. L.; Hermens J. L. M.; Schirmer K. (2013) Predicting fish acute toxicity using a fish gill cell line-based toxicity assay. Environ. Sci. Technol. 47, 1110–1119. 10.1021/es303505z. [DOI] [PubMed] [Google Scholar]
- Stadnicka J.; Schirmer K.; Ashauer R. (2012) Predicting Concentrations of Organic Chemicals in Fish by Using Toxicokinetic Models. Environ. Sci. Technol. 46, 3273–3280. 10.1021/es2043728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G.; Yuan Q.; Yu H.; Lin H.; Chen J.; Hong H. (2017) Development and evaluation of predictive model for bovine serum albumin-water partition coefficients of neutral organic chemicals. Ecotoxicol. Environ. Saf. 138, 92–97. 10.1016/j.ecoenv.2016.12.022. [DOI] [PubMed] [Google Scholar]
- Proenca S., Paini A., Joossens E., Sala Benito J. V.., Berggren E., Worth A., and Prieto P. (2017) Application of the Virtual Cell Based Assay for Simulation of in vitro Chemical fate following Acute Exposure, JRC technical reports, European Union.
- Timmer N.; Droge S. T. J. (2017) Sorption of Cationic Surfactants to Artificial Cell Membranes: Comparing Phospholipid Bilayers with Monolayer Coatings and Molecular Simulations. Environ. Sci. Technol. 51, 2890–2898. 10.1021/acs.est.6b05662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manallack D. T. (2007) The pK(a) Distribution of Drugs: Application to Drug Discovery. Perspect. Med. Chem. 1, 25–38. 10.1177/1177391X0700100003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A.; Ferranti A.; Davidsen C.; Trapp S. (2010) An unexpected challenge: Ionizable compounds in the REACH chemical space. Int. J. Life Cycle Assess. 15, 321–325. 10.1007/s11367-010-0165-6. [DOI] [Google Scholar]
- Ying G. G. (2006) Fate, behavior and effects of surfactants and their degradation products in the environment. Environ. Int. 32, 417–431. 10.1016/j.envint.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Hermens J. L. M.; Droge S. T. J. (2013) Influence of organic matter type and medium composition on the sorption affinity of C12-benzalkonium cation. Environ. Pollut. 179, 153–159. 10.1016/j.envpol.2013.04.017. [DOI] [PubMed] [Google Scholar]
- Lee L. E. J.; Dayeh V. R.; Schirmer K.; Bols N. C. (2009) Applications and potential uses of fish gill cell lines: Examples with RTgill-W1. In Vitro Cell. Dev. Biol.: Anim. 45, 127–134. 10.1007/s11626-008-9173-2. [DOI] [PubMed] [Google Scholar]
- Behrens A.; Schirmer K.; Bols N. C.; Segner H. (2001) Polycyclic aromatic hydrocarbons as inducers of cytochrome P4501A enzyme activity in the rainbow trout liver cell line, RTL-W1 and in primary cultures of rainbow trout hepatocytes. Environ. Toxicol. Chem. 20, 632–643. 10.1002/etc.5620200324. [DOI] [PubMed] [Google Scholar]
- Timmer N.; Scherpenisse P.; Hermens J. L. M.; Droge S. T. J. (2018) Evaluating solid phase (micro-) extraction tools to analyze freely ionizable and permanently charged cationic surfactants. Anal. Chim. Acta 1002, 26–38. 10.1016/j.aca.2017.11.051. [DOI] [PubMed] [Google Scholar]
- Schirmer K.; Chan A. G. J.; Greenberg B. M.; Dixon D. G.; Bols N. C. (1997) Methodology for demonstrating and measuring the photocytotoxicity of fluoranthene to fish cells in culture. Toxicol. In Vitro 11, 107–119. 10.1016/S0887-2333(97)00002-7. [DOI] [PubMed] [Google Scholar]
- Peltenburg H.; Bosman I. J.; Hermens J. L. M. (2015) Sensitive determination of plasma protein binding of cationic drugs using mixed-mode solid-phase microextraction. J. Pharm. Biomed. Anal. 115, 534–542. 10.1016/j.jpba.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Heringa M. B.; Pastor D.; Algra J.; Vaes W. H. J.; Hermens J. L. M. (2002) Negligible depletion solid-phase microextraction with radiolabeled analytes to study free concentrations and protein binding: An example with [3H]estradiol. Anal. Chem. 74, 5993–5997. 10.1021/ac0204552. [DOI] [PubMed] [Google Scholar]
- Moulin J. (2012) Effect of protein binding on the determination of intrinsic clearance of hydrophobic compounds, Master Thesis, Utrecht University. [Google Scholar]
- Endo S.; Goss K. U. (2011) Serum albumin binding of structurally diverse neutral organic compounds: Data and models. Chem. Res. Toxicol. 24, 2293–2301. 10.1021/tx200431b. [DOI] [PubMed] [Google Scholar]
- Kramer N. I.; van Eijkeren J. C. H.; Hermens J. L. M. (2007) Influence of albumin on sorption kinetics in solid-phase microextraction: Consequences for chemical analyses and uptake processes. Anal. Chem. 79, 6941–6948. 10.1021/ac070574n. [DOI] [PubMed] [Google Scholar]
- Kramer N. I.; Busser F. J. M.; Oosterwijk M. T. T.; Schirmer K.; Escher B. I.; Hermens J. L. M. (2010) Development of a partition-controlled dosing system for cell assays. Chem. Res. Toxicol. 23, 1806–1814. 10.1021/tx1002595. [DOI] [PubMed] [Google Scholar]
- Tanneberger K.; Rico-Rico A.; Kramer N. I.; Busser F. J. M.; Hermens J. L. M.; Schirmer K. (2010) Effects of solvents and dosing procedure on chemical toxicity in cell-based in vitro assays. Environ. Sci. Technol. 44, 4775–4781. 10.1021/es100045y. [DOI] [PubMed] [Google Scholar]
- Valko K.; Nunhuck S.; Bevan C.; Abraham M. H.; Reynolds D. P. (2003) Fast Gradient HPLC Method to Determine Compounds Binding to Human Serum Albumin. Relationships with Octanol/Water and Immobilized Artificial Membrane Lipophilicity. J. Pharm. Sci. 92, 2236–2248. 10.1002/jps.10494. [DOI] [PubMed] [Google Scholar]
- Groothuis F. A., Nicol B., Kramer N. I., and Stammler E.. Distribution of Benzalkonium Chlorides in Aqueous Solutions: A Comparison of Methods to Estimate Binding Affinity to Bovine Serum Albumin. Manuscript in preparation. [Google Scholar]
- Schirmer K.; Dixon D. G.; Greenberg B. M.; Bols N. C. (1998) Ability of 16 priority PAHs to be directly cytotoxic to a cell line from the rainbow trout gill. Toxicology 127, 129–141. 10.1016/S0300-483X(98)00030-4. [DOI] [PubMed] [Google Scholar]
- Springer J. E.; Azbill R. D.; Carlson S. L. (1998) A rapid and sensitive assay for measuring mitochondrial metabolic activity in isolated neural tissue. Brain Res. Protoc. 2, 259–263. 10.1016/S1385-299X(97)00045-7. [DOI] [PubMed] [Google Scholar]
- Escher B. I.; Hermens J. L. M. (2002) Modes of action in ecotoxicology: Their role in body burdens, species sensitivity, QSARs, and mixture effects. Environ. Sci. Technol. 36, 4201–4217. 10.1021/es015848h. [DOI] [PubMed] [Google Scholar]
- Droge S. T. J.; Hermens J. L. M.; Rabone J.; Gutsell S.; Hodges G. (2016) Phospholipophilicity of C: XHyN+ amines: Chromatographic descriptors and molecular simulations for understanding partitioning into membranes. Environ. Sci. Process. Impacts 18, 1011–1023. 10.1039/C6EM00118A. [DOI] [PubMed] [Google Scholar]
- Sandbacka M.; Christianson I.; Isomaa B. (2000) The acute toxicity of surfactants on fish cells, Daphnia magna and fish - A comparative study. Toxicol. In Vitro 14, 61–68. 10.1016/S0887-2333(99)00083-1. [DOI] [PubMed] [Google Scholar]
- Manaargadoo-Catin M.; Ali-Cherif A.; Pougnas J. L.; Perrin C. (2016) Hemolysis by surfactants - A review. Adv. Colloid Interface Sci. 228, 1–16. 10.1016/j.cis.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Balgavý P.; Devínsky F. (1996) Cut-off effects in biological activities of surfactants. Adv. Colloid Interface Sci. 66, 23–63. 10.1016/0001-8686(96)00295-3. [DOI] [PubMed] [Google Scholar]
- Przestalski S.; Sarapuk J.; Kleszczyńska H.; Gabrielska J.; Hładyszowski J.; Trela Z.; Kuczera J. (2000) Influence of amphiphilic compounds on membranes. Acta Biochim. Pol. 47, 627–638. [PubMed] [Google Scholar]
- Isomaa B.; Hägerstrand H.; Paatero G.; Engblom A. C. (1986) Permeability alterations and antihaemolysis induced by amphiphiles in human erythrocytes. Biochim. Biophys. Acta, Biomembr. 860, 510–524. 10.1016/0005-2736(86)90548-1. [DOI] [PubMed] [Google Scholar]
- Gülden M.; Kähler D.; Seibert H. (2015) Incipient cytotoxicity: A time-independent measure of cytotoxic potency in vitro. Toxicology 335, 35–45. 10.1016/j.tox.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Stadnicka-Michalak J.; Schirmer K.; Ashauer R. (2015) Toxicology across scales: Cell population growth in vitro predicts reduced fish growth. Sci. Adv. 1, e1500302. 10.1126/sciadv.1500302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeders J. J. W.; Parmentier C.; Truisi G. L.; Jossé R.; Alexandre E.; Savary C. C.; Hewitt P. G.; Mueller S. O.; Guillouzo A.; Richert L.; van Eijkeren J. C. H.; Hermens J. L. M.; Blaauboer B. J. (2015) Biokinetics of chlorpromazine in primary rat and human hepatocytes and human HepaRG cells after repeated exposure. Toxicol. In Vitro 30, 52–61. 10.1016/j.tiv.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Comenges J. M. Z.; Joossens E.; Benito J. V. S.; Worth A.; Paini A. (2017) Theoretical and mathematical foundation of the Virtual Cell Based Assay – A review. Toxicol. In Vitro 45, 209–221. 10.1016/j.tiv.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Fischer F. C.; Abele C.; Droge S. T. J.; Henneberger L.; König M.; Schlichting R.; Scholz S.; Escher B. I. (2018) Cellular Uptake Kinetics of Neutral and Charged Chemicals in in Vitro Assays Measured by Fluorescence Microscopy. Chem. Res. Toxicol. 31, 646–657. 10.1021/acs.chemrestox.8b00019. [DOI] [PubMed] [Google Scholar]
- Bernhard M. J.; Dyer S. D. (2005) Fish critical cellular residues for surfactants and surfactant mixtures. Environ. Toxicol. Chem. 24, 1738–1744. 10.1897/04-234R.1. [DOI] [PubMed] [Google Scholar]
- Gemmill C. L. (1966) The Pharmacological Basis of Therapeutics. J. Med. Chem. 9, 982. 10.1021/jm00324a062. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.