Abstract
Large vessel occlusions (LVOs), variably defined as blockages of the proximal intracranial anterior and posterior circulation, account for approximately 24% to 46% of acute ischemic strokes. Commonly refractory to intravenous tissue plasminogen activator (tPA), LVOs place large cerebral territories at ischemic risk and cause high rates of morbidity and mortality without further treatment. Over the past few years, an abundance of high-quality data has demonstrated the efficacy of endovascular thrombectomy for improving clinical outcomes in patients with LVOs, transforming the treatment algorithm for affected patients. In this review, we discuss the epidemiology, pathophysiology, natural history, and clinical presentation of LVOs as a framework for understanding the recent clinical strides of the endovascular era.
Keywords: Large vessel occlusion, Stroke, Endovascular thrombectomy
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- ACA
anterior cerebral arteries
- AHA
American Heart Association
- AIS
acute ischemic stroke
- ASA
American Stroke Association
- BA
basilar artery
- ICA
intracranial internal carotid artery
- LVO
large vessel occlusions
- MCA
middle cerebral arteries
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- PCA
posterior cerebral arteries
- RCT
randomized-controlled trial
- TIA
transient ischemic attack
- tPA
tissue-plasminogen activator
- VA
vertebral artery
Despite some terminological variations, acute blockages of the intracranial internal carotid artery (ICA), proximal posterior, middle, and anterior cerebral arteries (PCA, MCA, and ACA, respectively), intracranial vertebral artery (VA), and/or basilar artery (BA) are commonly referred to as large vessel occlusions (LVOs).1-3 Accounting for up to 46% of acute ischemic strokes (AISs), LVOs possess outsized clinical importance as they more than doubled the risk of death or dependence as compared to non-LVO AISs in the pre-endovascular era.1,4 Over the past several years, multiple randomized-controlled trials (RCTs) have established the efficacy of endovascular thrombectomy for improving outcomes following LVO, initially in selected symptomatic patients presenting within 6 hr of stroke onset,5-11 and more recently in patients up to 24 hr from last known normal with favorable preprocedural imaging.12,13 Knowledge of the epidemiology, pathophysiology, natural history, and clinical presentation of patients with LVOs, as provided in this succinct review, is crucial to understanding the recent paradigm shift in the treatment of this patient population.14
EPIDEMIOLOGY OF STROKE AND LVO
Stroke Epidemiology
Although most epidemiological data combine ischemic and hemorrhagic (including both intraparenchymal hemorrhages and subarachnoid hemorrhages) cerebrovascular disease under the umbrella term stroke, the vast majority of strokes (87%) are ischemic in nature.15 This combined data thus provides a reasonable starting point for understanding the epidemiology of AISs and the included subgroup of LVOs. Reported risk factors for strokes are similar to general cardiovascular disease and include hypertension, diabetes mellitus, atrial fibrillation, elevated cholesterol and lipid levels, smoking/tobacco use, physical inactivity, poor nutrition, kidney disease, and a family history/genetic predisposition.15 Females,racial/ethnic minorities, and those with low educational background are also disproportionately affected by strokes.15,16
Within the US, the prevalence of stroke is approximately 2.7% in people 20 yr of age or older (7.2 million total people), and increases with age to more than 6% and 13% in people over 60 and 80 yr of age, respectively.15 The annual incidence of new or recurrent strokes in the US is nearly 800 000.15 Although stroke mortality rates have declined over the past several decades due to advancements in prevention, diagnosis, and treatment, more than 140 000 people still die annually in the US from strokes, which makes it the fifth leading cause of death.15 Strokes also remain a significant cause of serious long-term disability, with associated annual direct and indirect costs estimated at more than 40 billion dollars in the US alone.15 Globally, the prevalence and annual incidence of stroke are more than 42 million and 16 million people, respectively, and stroke remains the second leading cause of mortality, being responsible for 6.3 million deaths annually.15
LVO Epidemiology
Interpretation of epidemiological data on LVOs is confounded by variations in terminology. While LVOs were largely defined as occlusions of the intracranial ICA or M1 (first segment of the MCA) in the initial wave of RCTs determining the efficacy of thrombectomy,5-9 a subset of these studies also included M2 (second segment of the MCA) and A1 or A2 (first or second segment of the ACA) blockages.5,8 Subsequent studies have also demonstrated the potential utility of thrombectomy for occlusions in the posterior circulation (BA and PCA), and more distal in the anterior circulation tree (M3, third segment of the MCA),10,17 with variations in the terminology used to describe such occlusions.
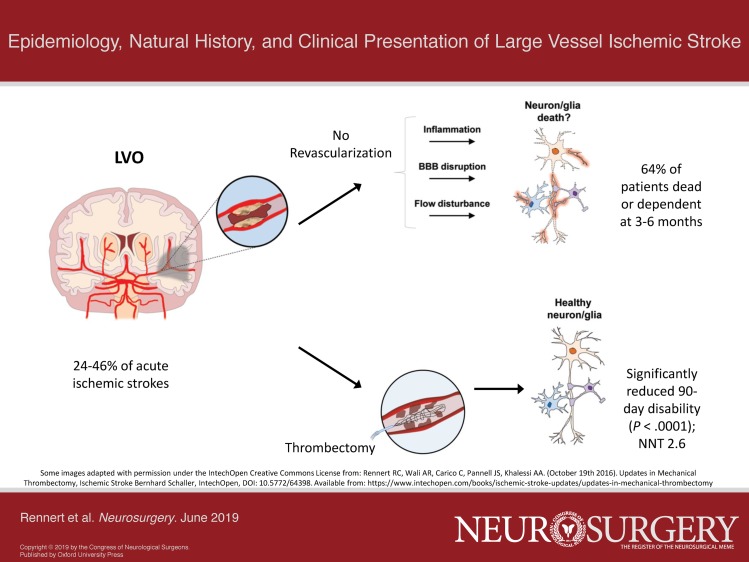
When defined as blockages in the intracranial ICA, M1, M2, A1, intracranial VA, P1, or BA (Table 1), LVOs are estimated to account for 24% to 38% of AISs,1,2 with this number increasing to 46% with the inclusion of the A2 and P2 segments.4 LVOs represent a clinically significant subpopulation of AISs due to their disproportionate morbidity and mortality without treatment and the potential to significantly improve outcomes with endovascular thrombectomy.1,11,14 Even when restrictively defined as occlusions of the M1, ICA terminus, and BA, the estimated annual incidence of LVOs in the US is 24 per 100 000 people per year, totaling close to 80 000 LVOs annually.3 Although only a fraction of these patients would likely be eligible for thrombectomy based on current guidelines,14 the estimated 10 000 annual thrombectomies performed in recent years remains well below LVO incidence3 and suggests a potentially significant future increase in the utilization of endovascular stroke therapies.
TABLE 1.
Inclusive Definition of LVO
| Anterior circulation | |
| Intracranial ICA, M1, M2a, M3a, A1, A2a | |
| Posterior circulation | |
| Intracranial VA, P1, P2a, BA |
A1, first segment of the anterior cerebral artery (ACA); A2, segment of the ACA; BA, basilar artery; ICA, internal carotid artery; M1, first segment of the middle cerebral artery (MCA); M2, second segment of the MCA; M3, third segment of the MCA; P1, first segment of the posterior cerebral artery (PCA); P2, segment of the PCA; VA, vertebral artery.
aVariably referred to as LVOs or distal vessel occlusions.
Based on data from RCTs with less restrictive inclusion criteria, the average age of patients presenting with an anterior circulation LVO is roughly 65 to 70 yr, with no consistent differences observed by gender.5,6,8,12 Similar to patients with ischemic strokes from other etiologies, patients with LVOs in these datasets also had significant rates of atrial fibrillation, hypertension, diabetes mellitus, and tobacco use.5,6,8,12 Patients with LVOs of the posterior circulation have a comparable comorbidity profile, and although of unclear significance, they tend to be slightly younger in age.18,19 Regarding anatomic location, the majority of LVOs (approximately 70%-80%) occur in the anterior circulation and are fairly evenly distributed between the ICA and MCA (with limited ACA LVOs likely due to the takeoff angle of the A1 from the ICA limiting embolic events).4,20 Tandem LVOs, or separate occlusions in both the proximal ICA and MCA, can also occur in less than 10% of cases. LVOs of the posterior circulation are split across the VA, BA, and PCA.4,20
In a multivariate analysis of acute stroke patients (ischemic and hemorrhagic) with and without LVOs (defined as blockages of the intracranial ICA, M1, M2, or BA), age, gender, personal and family history of stroke, history of hypertension, heart failure, diabetes mellitus, hyperlipidemia, kidney disease, and the use of antiplatelet or anticoagulant medications did not significantly differ across groups.21 However, the rate of atrial fibrillation was significantly higher in patients with LVOs as compared to those without (P < .001),21 suggesting this may be a potentially clinically relevant LVO risk factor.
PATHOPHYSIOLOGY OF LVO
LVOs can develop through four mechanisms—occlusion at the primary arterial site secondary to the development of atherosclerosis of an intracranial artery, extracranial artery atherosclerotic embolism or plaque rupture that then results in the occlusion of an intracranial vessel, cardioembolic events related to cardiac disease such as atrial fibrillation resulting in intracranial vessel occlusion, and cryptogenic causes of vessel occlusion.22 LVOs often result in insufficient blood flow to brain parenchyma, causing cellular bioenergetic failure and inflammatory cascades that culminate in the death of neurons, glia, and endothelial cells.23 Although ischemic changes occur within minutes, the ultimate volume of infarcted tissue is determined by the degree and length of hypoperfusion,23 with the level of collateral flow to an ischemic area playing a large role in stroke progression. Accordingly, in one study nearly 15% of patients with transient ischemic attacks (TIAs) were found to have underlying LVOs,4 with the absence of fixed neurologic deficits or strokes in these patients presumably due to adequate collateral perfusion.
The ischemic penumbra, an area of hypoperfused cerebral tissue outside of an irreversibly damaged infarct core, represents clinically important “at risk” tissue that both evolves with time and is potentially salvageable with early revascularization.23 In the initial RCTs establishing the efficacy of thrombectomy for LVOs up to 6 hr, patients with large infarct cores were excluded in order to maximize the amount of salvageable penumbral tissue and increase the chances of demonstrating a procedural benefit.6-9,14 Subsequent RCTs have utilized computed tomography- or magnetic resonance-perfusion imaging to differentiate the ischemic penumbra from the core infarct based on differences in perfusion time (time to maximum of the residue function) and cerebral blood flow (relative cerebral blood flow) (penumbral tissue having delayed perfusion time/near normal flow, ischemic core with delayed perfusion time/significantly decreased flow),12,13 and demonstrated the efficacy of thrombectomy for select LVO patients with favorable penumbral/core ratios up to 24 hr from symptom onset.12-14
NATURAL HISTORY OF LVO
The true natural history of LVOs is difficult to determine due to the administration of tissue-plasminogen activator (tPA) being the standard of care for AIS patients presenting within 3 hr of symptom onset since 1995 (with an extension of this window up to 4.5 hr in 2008),24,25 and cerebrovascular imaging not obtained in these seminal works. LVOs are nonetheless commonly considered refractory to tPA, with resistance to pharmacologic thrombolysis increasing with more proximal occlusions (31%-44% recanalization rate with tPA for M2 occlusions, vs 4%-8% for ICA terminus LVOs).26-28 Observational data from mixed populations of AIS patients (many presenting outside of the tPA window) provide additional insight into the disproportionately high rates of morbidity and mortality associated with LVOs.1 In a recent meta-analysis of AIS data largely preceding the endovascular era, rates of dependence or death at 3 to 6 mo (defined by a modified Rankin Scale [mRS] score of 3-6) were more than double for patients with versus without LVOs (64 vs 24%, P < .0001).1 Six-month mortality rates were also significantly higher in LVO versus non-LVO AIS patients (26.2 vs 1.3%, P < .0001).1 Even amongst patients with TIAs or minor strokes (defined as ≤3 on the 42-point National Institutes of Health Stroke Scale [NIHSS]), the presence of an LVO was found to significantly increase the rates of recurrent stroke (45.8 vs 5.8% P < .001) and functional impairment (37.5 vs 7.7) within 90 d,29 with these secondary insults likely resulting from embolic events distal to the LVO and/or inadequacy of collateral perfusion over time.
The occlusion location and number of occluded vessels in AIS patients were also found to affect stroke severity (as defined by the NIHSS score) and overall clinical outcomes.4 Specifically, LVOs affecting the intracranial ICA and BA were associated with higher NIHSS scores and worse outcomes as compared to occlusions of more distal vessels (M2, A2, and P2).4 Similarly, the mean NIHSS score (a proxy for worse outcomes) was found to increase linearly with the number of vessels occluded on cerebrovascular imaging.4
Neurosurgical Interventions to Alter the Natural History of LVO
The emergence of endovascular therapies has dramatically affected the prognosis for patients with LVOs. In a meta-analysis of the 5 initial RCTs assessing the efficacy of endovascular thrombectomy in proximal anterior circulation occlusions performed up to 6 hr from symptom onset, thrombectomy significantly reduced 90-d disability (P < .0001), with a number needed to treat of 2.6 to reduce the mRS in one patient by at least one point.11 A growing body of randomized retrospective efficacy data also underlies the updated 2018 American Heart Association (AHA)/American Stroke Association (ASA) guidelines stating that endovascular thrombectomy be considered for LVOs of the posterior circulation up to 6 hr from symptom onset, and that intervention is recommended/can be considered up to 16 and 24 hr, respectively, in select patients with anterior circulation LVOs based on perfusion imaging.14
Based on data from multiple RCTs, the performance of a decompressive hemicraniectomy for stroke-related edema with LVOs in patients who are not thrombectomy candidates or have a completed large territory stroke despite intervention should also be considered. Specifically, in a pooled analysis of three RCTs assessing the effect of decompressive hemicraniectomy after a unilateral large territory MCA stroke in patients 18 to 60 yr old, hemicraniectomy reduced overall 12-mo mortality by nearly 50% (78% with medical management vs 29% with surgery, 95% confidence interval [CI] 33-67), and significantly increased the number of patients with moderate or lesser disabilities (mRS ≤ 3) (43% vs 21%, 95% CI 5-41).30 In a cohort more closely age-matched to patients at risk for LVOs, RCT data also support the utility of decompressive hemicraniectomy for significantly reducing mortality in patients >60 yr old with large territory MCA strokes (70% with medical management vs 33% with surgery), although in this patient subset no significant improvement in the number of patients with moderate or lesser disabilities was seen with surgery.31 In a reflection of these data, the 2018 AHA/ASA guidelines state that it is reasonable to perform a decompressive hemicraniectomy in patients with large unilateral MCA strokes ≤60 yr old, and may be considered in patients >60 yr old.14 Based on retrospective data, a decompressive suboccipital craniectomy with dural expansion is also recommended to increase the likelihood of a good outcome in posterior circulation LVO patients with refractory edema, brainstem compression, and neurologic deterioration from acute cerebellar infarctions.14
CLINICAL PRESENTATION OF LVO
Given the ability to alter their natural history with endovascular thrombectomy, early identification of patients with LVOs is critical. Differentiating AIS patients with LVOs from those without based on clinical presentation is nonetheless challenging due to the variable nature of collateral vasculature and the potential for unique “at risk” and symptomatic tissue patterning across patients with the same anatomical site of occlusion. LVOs can also commonly manifest with minimal symptomology.32,33 Accordingly, in one recent retrospective study, there was no significant difference in average NIHSS score between AIS patients with and those without LVOs (23 vs 18.5, P = .55).21 Even when broken down into the 11 NIHSS subcategories (level of consciousness, questions, commands, gaze, visual fields, facial palsy, motor arm and leg, ataxia, sensory, language, dysarthria, and extinction), only an increased severity of facial palsy with LVO (P = .03) was significant on univariate analysis, before losing significance upon multivariate testing (P = .053).21 AIS patients with LVOs were nonetheless significantly more likely to have a lower mean systolic blood pressure upon presentation than those without LVOs (153 vs 179, P < .01 on both univariate and multivariate analysis).21 Conversely, in a separate retrospective analysis of a less symptomatic cohort of patients with AIS, an increased NIHSS was the only clinical predictor of LVO (13 vs 2, P < .01).20
While not pathognomonic, the clinical presentation of patients with LVOs is nonetheless often stereotyped and anatomically matched to the site of occlusion and downstream affected cerebrum (Table 2). Specifically, ICA or proximal MCA occlusions often present with contralateral hemibody and face weakness and/or numbness, contralateral homonymous hemianopsia, and ipsilateral gaze deviation, as well as aphasia for dominant hemispheric lesions and neglect for lesions of the nondominant hemisphere. More nuanced presentations of variable clinical severity and importance are observed with more distal occlusions. One notable distal occlusion site is the M3 branch to the angular gyrus of the dominant hemisphere. Due to the involvement of this vascular territory in speech processing and complex cognition,34 focal occlusions in this location are sometimes more aggressively pursued for thrombectomy in an attempt to preserve speech and cognition as compared to similarly distant blockages in arteries supplying less eloquent cortex.
TABLE 2.
Common Clinical Symptoms of LVO by Occlusion Site
| ICA/Proximal MCA | |
| Contralateral hemibody and face weakness/numbness, contralateral homonymous hemianopsia, ipsilateral gaze deviation, aphasia (dominant hemisphere), neglect (non-dominant hemisphere) | |
| VA/BA | |
| Hemibody weakness or numbness, dizziness, nausea, vomiting, gait and balance issues, or alterations in consciousness | |
| PCA | |
| Contralateral homonymous hemianopsia or quadrantanopia |
BA, basilar artery; ICA, internal carotid artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; VA, vertebral artery.
Posterior circulation LVOs are often more challenging to diagnose than LVOs of the anterior circulation due to a nonspecificity of associated symptoms, which can lead to significant delays in both prehospital and intrahospital management of this patient subset.35 Nonetheless, common symptoms seen with occlusion of the VAs and BA include hemibody weakness or numbness, dizziness, nausea, vomiting, gait and balance issues, or alterations in consciousness.36 Occlusions of the PCA typically result in contralateral vision changes such as a homonymous hemianopsia or quadrantanopia.36
Stemming from the challenges of diagnosing LVOs based on clinical presentation alone, there is an ongoing debate on the efficacy and optimization of prehospital triaging of AIS patients suspected of having an LVO from intravenous-tPA-ready hospitals to facilities with endovascular capacities.37 However, the updated 2018 AHA/ASA guidelines recommend further research on this topic.14
CONCLUSION
Patients with LVOs represent a clinically significant subset of AISs due to disproportionate morbidity and mortality rates and the ability to significantly alter the natural history of the disease with endovascular thrombectomy. Although differentiating LVO from non-LVO AISs based on clinical presentation alone is challenging, awareness of common LVO presentations is critical to expedite workup and initiate timely intervention.
Disclosures
Dr Khalessi has previously received competitive grants from Covidien Ltd and Penumbra Inc, and holds consulting arrangements for physician training with Stryker Neurovascular, Covidien Ltd, and Penumbra Inc. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Video Abstract (Available Online): Summary Video of LVO Epidemiology, Natural History, and Clinical Presentation.5-8,26-28,38-42 (http://dx.doi.org/10.1093/neuros/nyz042)
REFERENCES
- 1. Malhotra K, Gornbein J, Saver JL. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: a review. Front Neurol. 2017;8:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dozois A, Hampton L, Kingston CW et al.. PLUMBER study (prevalence of large vessel occlusion strokes in Mecklenburg county emergency response). Stroke. 2017;48(12):3397-3399. [DOI] [PubMed] [Google Scholar]
- 3. Rai AT, Seldon AE, Boo S et al.. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J NeuroIntervent Surg. 2017;9(8):722-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith WS, Lev MH, English JD et al.. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke. 2009;40(12):3834-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berkhemer OA, Fransen PS, Beumer D et al.. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. PMID: 25517348 [DOI] [PubMed] [Google Scholar]
- 6. Goyal M, Demchuk AM, Menon BK et al.. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. [DOI] [PubMed] [Google Scholar]
- 7. Saver JL, Goyal M, Bonafe A et al.. Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. [DOI] [PubMed] [Google Scholar]
- 8. Campbell BC, Mitchell PJ, Kleinig TJ et al.. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. [DOI] [PubMed] [Google Scholar]
- 9. Jovin TG, Chamorro A, Cobo E et al.. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. [DOI] [PubMed] [Google Scholar]
- 10. Bracard S, Ducrocq X, Mas JL et al.. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15(11):1138-1147. [DOI] [PubMed] [Google Scholar]
- 11. Goyal M, Menon BK, van Zwam WH et al.. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet North Am Ed. 2016;387(10029):1723-1731. [DOI] [PubMed] [Google Scholar]
- 12. Nogueira RG, Jadhav AP, Haussen DC et al.. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. [DOI] [PubMed] [Google Scholar]
- 13. Albers GW, Marks MP, Kemp S et al.. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Powers WJ, Rabinstein AA, Ackerson T et al.. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110. [DOI] [PubMed] [Google Scholar]
- 15. Benjamin EJ, Virani SS, Callaway CW et al.. Heart disease and stroke Statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492. [DOI] [PubMed] [Google Scholar]
- 16. Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol. 2017;81(4):479-484. [DOI] [PubMed] [Google Scholar]
- 17. Grossberg JA, Rebello LC, Haussen DC et al.. Beyond large vessel occlusion strokes. Stroke. 2018;49(7):1662-1668. [DOI] [PubMed] [Google Scholar]
- 18. Raymond S, Rost NS, Schaefer PW et al.. Patient selection for mechanical thrombectomy in posterior circulation emergent large-vessel occlusion. Interv Neuroradiol. 2018;24(3):309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alawieh A, Vargas J, Turner RD et al.. Equivalent favorable outcomes possible after thrombectomy for posterior circulation large vessel occlusion compared with the anterior circulation: the MUSC experience. J NeuroIntervent Surg. 2018;10(8):735-740. [DOI] [PubMed] [Google Scholar]
- 20. Beumer D, Mulder MJHL, Saiedie G et al.. Occurrence of intracranial large vessel occlusion in consecutive, non-referred patients with acute ischemic stroke. Neurovasc Imaging. 2016;2(1):11. [Google Scholar]
- 21. Inoue M, Noda R, Yamaguchi S et al.. Specific factors to predict large-vessel occlusion in acute stroke patients. J Stroke Cerebrovasc Dis. 2018;27(4):886-891. [DOI] [PubMed] [Google Scholar]
- 22. Al Kasab S, Holmstedt CA, Jauch EC, Schrock J. Acute ischemic stroke due to large vessel occlusion. Emerg Med Rep. 2018;39(2):13-22. [Google Scholar]
- 23. Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. 2009;111(6):483-495. [DOI] [PubMed] [Google Scholar]
- 24. Group NIoNDaSr-PSS Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581-1588. [DOI] [PubMed] [Google Scholar]
- 25. Hacke W, Kaste M, Bluhmki E et al.. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. [DOI] [PubMed] [Google Scholar]
- 26. del Zoppo GJ, Poeck K, Pessin MS et al.. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32(1):78-86. [DOI] [PubMed] [Google Scholar]
- 27. Saqqur M UK, Demchuk AM, Molina CA et al.. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38(3):948-954. [DOI] [PubMed] [Google Scholar]
- 28. Bhatia R, Hill MD, Shobha N et al.. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41(10):948-954. [DOI] [PubMed] [Google Scholar]
- 29. Coutts SB, Eliasziw M, Hill MD et al.. An improved scoring system for identifying patients at high early risk of stroke and functional impairment after an acute transient ischemic attack or minor stroke. Int J Stroke. 2008;3(1):3-10. [DOI] [PubMed] [Google Scholar]
- 30. Vahedi K, Hofmeijer J, Juettler E et al.. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6(3):215-222. [DOI] [PubMed] [Google Scholar]
- 31. Jüttler E, Unterberg A, Woitzik J et al.. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014;370(12):1091-1100. [DOI] [PubMed] [Google Scholar]
- 32. Haussen DC, Bouslama M, Grossberg JA et al.. Too good to intervene? Thrombectomy for large vessel occlusion strokes with minimal symptoms: an intention-to-treat analysis. J Neurointerv Surg. 2017;9(10):917-921. [DOI] [PubMed] [Google Scholar]
- 33. Haussen DC, Lima FO, Bouslama M et al.. Thrombectomy versus medical management for large vessel occlusion strokes with minimal symptoms: an analysis from STOPStroke and GESTOR cohorts. J Neurointerv Surg. 2018;10(4):325-329. [DOI] [PubMed] [Google Scholar]
- 34. Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19(1):43-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sommer P, Seyfang L, Posekany A et al.. Prehospital and intra-hospital time delays in posterior circulation stroke: results from the Austrian Stroke Unit Registry. J Neurol. 2017;264(1):131-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nouh A, Remke J, Ruland S. Ischemic posterior circulation stroke: a review of anatomy, clinical presentations, diagnosis, and current management. Front Neurol. 2014;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benoit JL, Khatri P, Adeoye OM et al.. Prehospital triage of acute ischemic stroke patients to an intravenous tPA-Ready versus Endovascular-Ready hospital: a decision analysis. Prehosp Emerg Care. 2018;22(6):722-733. [DOI] [PubMed] [Google Scholar]
- 38. Levy EI, Rahman M, Khalessi AA et al.. Midterm clinical and angiographic follow-up for the first Food and Drug Administration-approved prospective, Single-Arm Trial of Primary Stenting for Stroke: SARIS (Stent-Assisted Recanalization for Acute Ischemic Stroke). Neurosurgery. 2011;69(4):915-920; discussion 920. [DOI] [PubMed] [Google Scholar]
- 39. Nogueira RG, Lutsep HL, Gupta R et al.. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380(9849):1231-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saver JL, Jahan R, Levy EI et al.. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380(9849):1241-1249. [DOI] [PubMed] [Google Scholar]
- 41. Shi ZS, Liebeskind DS, Xiang B et al.. Predictors of functional dependence despite successful revascularization in large-vessel occlusion strokes. Stroke. 2014;45(7):1977-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mechanical thrombectomy trials with the Solitaire™ FR Device: A Global Perspective: Satellite Symposium - Wednesday, May 29, 2013. Interv Neurol. 2013;2(1):52-56. [DOI] [PMC free article] [PubMed] [Google Scholar]