Abstract
Background: Peritoneal metastasis, associated with poor prognosis in gastric cancer, is difficult to discriminate from advanced gastric cancer preoperatively. However, operative diagnosis could bring both mental and physical trauma and economic burden for patients. Consequently, a non-invasive biomarker is necessary to reduce the burden of operative diagnosis and improve survival quality of patients. This study aims to elucidate the correlation between Immunoglobulin G (IgG) N-glycome and peritoneal metastasis and find potential biomarkers in preoperative discrimination of peritoneal metastasis from advanced gastric cancer based on the comprehensive sample set.
Methods: A total of 373 gastric cancer patients were enrolled and randomly sorted into training cohort (n=249) and validation cohort (n=124). The IgG N-glycome composition was analyzed by ultra-performance liquid chromatography.
Results: Twenty-four glycan peaks were directly detected and 15 traits based on the same structures were evaluated between peritoneal metastasis group and advanced gastric cancer group. Several differences in IgG glycosylation were found: sialylation and fucosylation were increased in peritoneal metastasis, while neutral glycosylation, monogalacosylation and bisecting GlcNAc were decreased. Based on the significant glycomics profile, a glyco-model composed of five glycan peaks (GP6, GP9, GP11, GP21 and GP23) was established with area under the receiver operating characteristic curve (AUC) value of 0.80 (training cohort) and 0.77 (validation cohort), which showed good potential in discriminating peritoneal metastasis from advanced gastric cancer. The diagnostic performance of this model was further validated in a combined cohort (AUC=0.79). Two patients with gastric cancer were selected to perform and demonstrate the usage of the diagnostic workflow.
Conclusions: Here we firstly present IgG glycome profiles in a large number of preoperative peritoneal metastasis serums. The IgG glycan was highly associated with peritoneal metastasis. These findings enhance the understanding of peritoneal metastasis. Besides, our results suggested that the newly established glyco-model could be a reliable predictor of the presence of peritoneal metastasis in patients with advanced gastric cancer.
Keywords: Biomarkers, Gastric cancer, Glycosylation, Immunoglobulin G, Peritoneal metastasis
Introduction
Gastric cancer is one of the most aggressive gastrointestinal malignancy and third leading cause of cancer related deaths worldwide 1. Nearly 50% of deaths of gastric cancer were caused by peritoneal metastasis which is frequently present in patients with advanced gastric cancer (AGC), while is very rare in early gastric cancer 2, 3. In most cases, gastric cancer with peritoneal metastasis (PMGC) is asymptomatic during a long period and therefore often initially diagnosed intraoperatively which is not benefit for surgeons to determine the most appropriate therapeutic approach 4. Thus, effective method to discriminate PMGC from AGC timely is essential to improve the patients' quality of life.
Operative diagnostic methods including staging laparoscopy, laparotomy and abdominal cytology are currently used in discrimination of peritoneal metastasis 5, 6. However, these methods bring patients high cost, time-consumption and complications from invasiveness such as intra-abdominal organ iatrogenic damages, hemorrhage and infective processes 7. Consequently, non-invasive biomarkers are urgently needed to reduce not only mental and physical trauma but also economic burden of peritoneal metastasis patients. The existing non-invasive diagnostic methods are imaging examinations, such as Computed Tomography (CT), Positron Emission Tomography-Computed Tomography (PET-CT), Magnetic Resonance Imaging (MRI), which have some limitations inaccuracy for preoperatively differentiating PMGC patients from AGC patients2, 8, 9. Recent researches had preliminarily demonstrated the predictive effect of serum tumor markers and inflammation associated factors and indexes in differentiating PMGC patients from AGC patients. However, for serum tumor markers including CA125, CA72-4, CA19-9 and CEA, the sensitivity of each individual tumor marker for PMGC prediction was below 50% 10, 11. For inflammation associated factors and indexes, although platelet to lymphocyte ratio (PLR) and neutrophil to lymphocyte ratio (NLR) were evaluated in PMGC patients, the area under the receiver operating characteristic curve (AUC) values were only 0.60 and 0.68 respectively for the PMGC diagnosis, respectively 2, 10, 12. Besides, the number of patients with peritoneal metastasis in most studies of biomarkers is less than 100, which is insufficient to validate the predictive performance 11-16. Consequently, these markers are deficient for precise diagnosis of peritoneal metastasis because of the absence of accuracy and experimental validation. It is desirable to find new non-invasive predictive factors to differentiate PMGC from AGC in a larger and comprehensive sample set.
Considering the relationship between N-glycans and tumor metastasis, serum N-glycome was analyzed using MALDI-MS in our previous study to discovery the biomarker of PMGC 17. Two of multi-branched N-glycosylation with N-acetylneruaminic acid were significantly decreased in PMGC. Although serum N-glycomics analysis could give a comprehensive overlook of N-glycans, protein-specific glycosylation information is lost. The glycomics analysis of specific glycoprotein would provide more insight into glycosylation actual changes. Immunoglobulin G (IgG), the most abundant glycoprotein in serum, contains a conserved N-glycosylation site at asparagine 297 of its Fc fragment 18. Alternative glycosylation of IgG has significant consequences on cancer immunosurveillance and could be used as predictive markers for gastric cancer. Through LC-ESI-MS, Kristel Kodar et al. profiled the Fc N-glycans of serum IgG samples in 80 patients with gastric cancer and 51 healthy controls. They found the agalactosylated N-glycan was increased in patients with gastric cancer, while galactosylated and monosialylated N-glycans were decreased 19. Besides, IgG N-glycan profile was determined in 35 patients with benign gastric disease and 31 patients with gastric cancer using nano LC-MS. Comparing with benign gastric disease patients, bisected N-glycan and sialylated N-glycans were decreased in gastric cancer patients 20. The analysis of serum IgG N-glycans by FACE was developed in 6 patients with AGC and 6 patients with metastatic gastric cancer, and agalactosylated fucosylated N-glycan was increased in patients with metastatic gastric cancer 21. Nonetheless, the relationship between agalactosylated fucosylated N-glycan and peritoneal metastasis was not evaluated, and the sample size in this study was insufficient to provide a comprehensive analysis of IgG glycome in gastric cancer with metastasis. Until now, no studies have reported IgG N-glycan profiles in PMGC and evaluated their clinical values in distinguishing PMGC from AGC patients.
In the present study, 373 patients with gastric cancer (AGC: n=246, PMGC: n=127) were enrolled to profile specific IgG N-glycans of PMGC patients for the first time. The aim of present study was to (i) identify whether significant differences exist in IgG N-glycans between PMGC patients and AGC patients, and (ii) investigate the predictive values of the alternated IgG N-glycans for patients with PMGC, and (iii) construct an available preoperative prediction glyco-model to differentiate PMGC patients from AGC patients.
Materials and Methods
Study population and sample collection
All serum samples were collected from Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai, China, from March 2015 to February 2018. Exclusion criteria: (1) Patients who ever received chemotherapy or radiation therapy; (2) Patients with distant metastasis; (3) Patients with gastric stump carcinoma. Inclusion criteria: (1) Patients over 18 years old; (2) Patients with histologically proven adenocarcinoma of the stomach or esophagogastric junction (Siewert type 2 or 3); (3) Patients who received CT or PET-CT scan before surgery. Every further postoperative pathological analysis was done for surgery patients, and peritoneal metastasis was confirmed by multidisciplinary team at Zhongshan Hospital.
The cohort composing of 373 serum samples from patients with gastric cancer were randomly assigned into training cohort (161 AGC patients and 88 PMGC patients) and validation cohort (85 AGC patients and 39 PMGC patients) based on a 2:1 ratio. All serum layers were collected and stored at -80°C until analysis. No more than three cycles of freezing/thaw were allowed for any sample. Clinical data of all enrolled patients were retrospectively collected from the database of Zhongshan Hospital and were summarized in Table 1. The cut-off levels recommended by the manufacturer for CEA, CA19-9, CA125 and CA72-4 were 5.0μg/L, 37U/L, 35U/mL and 10U/mL, respectively. This study was carried out in accordance with the recommendations of the ethical standards of the Medical Ethics Committee, Zhongshan Hospital of Fudan University. The protocol was approved by the Zhongshan Hospital of Fudan University. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Table 1.
Clinicopathological characteristics of all patients
| Training cohort N=249 | Validation cohort N=124 | ||||
|---|---|---|---|---|---|
| AGCa | PMGCb | AGC | PMGC | ||
| N | 161 | 88 | 85 | 39 | |
| Age (min-max) | 59.71 (33-83) | 60.51 (27-87) | 59.84 (31-83) | 57.87 (38-81) | |
| Gender (male/female) | 119/42 | 55/33 | 67/18 | 24/15 | |
| Tumor location | Upper 1/3 | 25 | 8 | 10 | 5 |
| Middle 1/3 | 45 | 30 | 28 | 13 | |
| Lower 1/3 | 70 | 33 | 36 | 10 | |
| Mixed | 16 | 13 | 10 | 9 | |
| Data Absent | 5 | 4 | 1 | 2 | |
| Differentiation | High + Moderate | 28 | 28 | 16 | 8 |
| Poor | 125 | 43 | 67 | 23 | |
| Data Absent | 8 | 17 | 2 | 8 | |
| Lauren classification | Intestinal | 37 | 8 | 22 | 2 |
| Diffuse | 44 | 15 | 18 | 4 | |
| Mixed | 50 | 6 | 29 | 9 | |
| Data Absent | 30 | 59 | 16 | 24 | |
| CEA | Mean (Min-Max) | 8.48 (0.4-94.9) | 9.11 (0.5-209.5) | 14.94 (0.4-1.9) | 16.40 (0.5-372.8) |
| <5ng/mL | 88 | 42 | 55 | 18 | |
| ≥5ng/mL | 38 | 21 | 14 | 12 | |
| Data Absent | 35 | 25 | 16 | 9 | |
| CA19-9 | Mean (Min-Max) | 140.95 (1-8982) | 237.01 (1-5682) | 23.38 (1.5-170.9) | 428.02 (4.8-4210) |
| <37U/mL | 94 | 38 | 49 | 12 | |
| ≥37U/mL | 23 | 25 | 11 | 16 | |
| Data Absent | 44 | 25 | 25 | 11 | |
| CA125 | Mean (Min-Max) | 20.95 (5.5-225.3) | 52.89 (4-281.2) | 31.76 (5.6-401.3) | 46.94 (9-246) |
| <35U/mL | 86 | 34 | 46 | 14 | |
| ≥35U/mL | 13 | 22 | 7 | 8 | |
| Data Absent | 62 | 32 | 32 | 17 | |
| CA72-4 | Mean (Min-Max) | 16.73 (0.6-300) | 43.58 (0.8-300) | 18.97 (0.35-257.3) | 30.23 (1-300) |
| <10U/mL | 70 | 24 | 39 | 14 | |
| ≥10U/mL | 19 | 28 | 15 | 6 | |
| Data Absent | 72 | 36 | 31 | 19 | |
aAGC: Advanced gastric cancer; bPMGC: Peritoneal metastasis gastric cancer.
IgG purification from human plasma
Purification of IgG was described in previous study 22. IgGs from serum samples were isolated using Protein A IgG Purification Kit (Thermo Fisher Scientific). The isolation was manipulated according to the manufacturer's instructions. Briefly, 50μL serum was diluted to 100μL using a proprietary Protein A IgG Binding Buffer. Then the mixture was applied to the protein A plate and washed with 500μL of Binding Buffer three times to remove unbound proteins. Last, the bound IgGs were eluted with 200μL of the proprietary IgG Elution Buffer three times in three separate plates. To determine which fractions contained IgG, the absorbance of each fraction was measured at 280nm by a bicinchoninic acid (BCA) test (Thermo Fisher Scientific). And the fractions containing IgG were stored at -20 °C until the N-glycan release.
IgG N-glycans release, enrichment and labeling
As described in previous study, IgG N-glycans were released from IgGs containing elution by incubating with 1μL PNGase F (New England Biolabs) for 12h at 37°C22. Subsequently, the released N-glycans were purified by porous graphic carbon (PGC) solid-phase extraction. Briefly, a PGC-containing 96-well plate was washed with 200μL of 0.1% trifluoroacetic acid (TFA) (v/v) (Merck) in 80% acetonitrile (ACN) (v/v) (Biosolve) and followed by 0.1% TFA (v/v). The solution of released N-glycans was applied to the PGC containing 96-well plate 3 times to allow complete N-glycans adsorption. Then, 100μL H2O was added to remove salts and buffer twice. The N-glycans derived from IgG were eluted with 100μL of 0.05% TFA (v/v) in 25% ACN (v/v). Then the elute was dried in concentrator (Eppendorf) for 3h on 45-degree manual mode followed by labelling with 2-aminobenzamide (2-AB) described by Maja Pucic et al. 23. The labelling mixture was freshly prepared by dissolving 50mg 2-AB (Sigma-Aldrich) and 60mg Sodium cyanoborohydride (Sigma-Aldrich) in 1mL solvent with Dimethyl sulfoxide (DMSO) (Sigma-Aldrich) and glacial acetic acid (AcOH) (Merck) mixture (7:3, v/v). A volume of 3μL of labelling mixture was added to each N-glycan sample. Mixing was achieved by shaking for 2min, followed by 2h incubation at 60°C. The labeling reaction was stopped by adding 50μL H2O per sample.
Hydrophilic Interaction Chromatography (HILIC)-Ultra Performance Liquid Chromatography (UPLC)
The labelled N-glycans were separated by HILIC on a Nexera UHPLC LC-30A (Shimadzu) with fluorescence detector set with excitation and emission wavelengths of 330 and 420nm, respectively. The instrument was under the control of LabSolution software (Shimadzu). Labelled N-glycans were separated on a Waters BEH Amide chromatography column (Waters), 100×2.1mm, 1.7μm BEH particles, with 100mM ammonium formate, pH 4.5, as solvent A and ACN as solvent B. Separation method used linear gradient of 79-56% acetonitrile (v/v) at flow rate of 0.5mL/min in a 26min analytical run. Samples were maintained at 4°C before injection, and the separation temperature of chromatography column was 70°C. Data processing was performed using an automatic processing method with a traditional integration algorithm after which each chromatogram was manually corrected to maintain the same intervals of integration for all the samples. The chromatograms were all separated in the same manner into 24 peaks. In addition to 24 directly measured glycan structures, 15 derived traits were summarized based on the same features from related studies 24, 25.
Data normalization and statistical analysis
In order to normalize the measurement of IgG glycans, each peak area of glycan was divided by total area of corresponding chromatogram. Multiple t-tests with correction for multiple comparisons, the Sidak-Bonferroni method, was chosen for continuous variables. Specifically, for 15 derived traits analysis, P values smaller than 3.33E-03 (0.05/15) were considered statistically significant and for 24 directly measured glycan peaks, P value should smaller than 2.10E-03 (0.05/24). In order to differentiate patients with PMGC from patients with AGC, multivariate logistic regression model was applied with R package “rms”. Only significant differences of 24 directly detect peaks were used as predictors in the model. Receiver operating characteristic (ROC) curves was used to compare the sensitivity and specificity for the predictive performance of glyco-model, and values of area under the curve (AUC) were performed to indicate the accuracy of test. If the AUC value was greater than 0.9 that indicates a “highly accurate” test, while values between 0.8 and 0.9 were considered to be “accurate”. When the AUC value was between 0.7 and 0.8, the test was concluded to be “moderately accurate.” An “uninformative” test resulted in an AUC value that was between 0.5 and 0.7. All the statistical analyses were performed using R 3.4.3 software and IBM SPSS Statistics version 20.0 software.
Results
Serum IgG N-glycan profiles
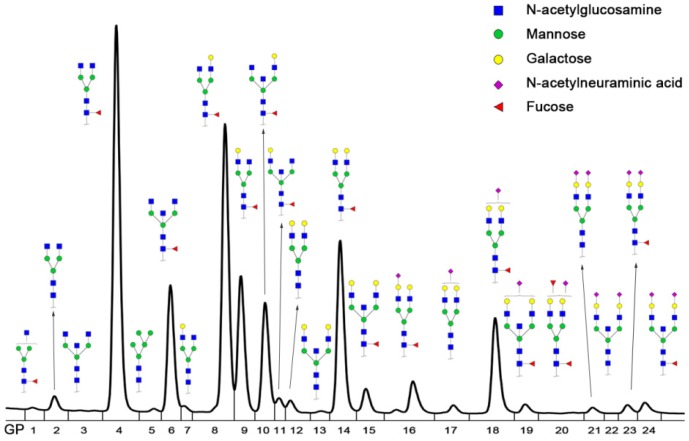
IgG N-glycan profiles were analyzed in 246 patients with AGC and 127 patients with PMGC. These patients were randomly assigned into two groups, with nearly two-thirds in the training group (n=249) and a third in the validation group (n=124). The demographic characteristics of the enrolled patients were presented in Table 1. The age and gender were matched between AGC patients and PMGC patients as far as possible. IgG N-glycomics structures and compositions were determined by UPLC analysis of 2AB-labeled glycans in previous literatures 24, 25. The typical glycomics profile was shown in Figure 1, which is a representative chromatogram for 24 directly measured glycan peaks. And compositions of 24 directly measured glycan peaks by UPLC were shown in Table S1. Besides, fifteen derived traits were calculated based on the same structural features such as fucosylation, galactosylation, sialylation and bisecting type N-glycans (Table S2).
Figure 1.
Representative Ultra Performance Liquid Chromatography (UPLC) chromatogram of serum IgG N-glycan profiles. A total of 24 chromatographic peaks was shown.
Derived traits differences between patients with AGC and PMGC
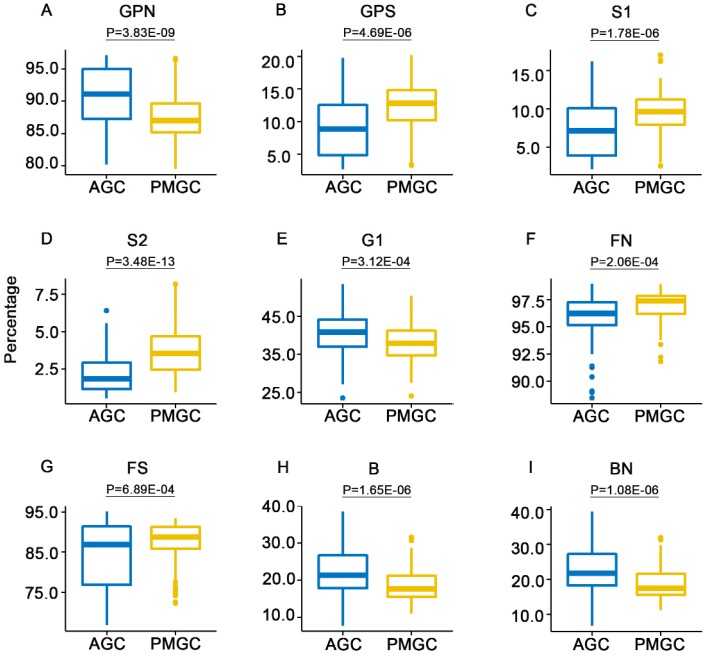
In order to profile the unique IgG N-glycans of patients with PMGC, fifteen derived traits were compared between patients with AGC (n=161) and patients with PMGC (n=88) in the training cohort. Significant differences were observed in several derived traits (Table 2, Figure 2). Neutral N-glycans (GPN, P=3.83E-09, Figure 2A) were decreased in PMGC group. Relatively, total sialylation (GPS, P=4.69E-06, Figure 2B) showed an opposite level in PMGC group compared with AGC group. Monosialylation (S1, P=1.78E-06, Figure 2C) and disialylation (S2, P=3.48E-13, Figure 2D) which contribute to the amounts of total sialylation both showed increased level in PMGC group. Besides, monogalactosylation (G1, P=3.12E-04, Figure 2E) decreased in PMGC group. In this cohort, we also found bisecting N-glycan (B, P=1.65E-06, Figure 2H) and bisecting N-glycan of neutral IgG glycans (BN, P=1.08E-06, Figure 2I) decreased, while fucosylation of neutral glycans (FN, P=2.06E-04, Figure 2F) and fucosylation of sialylated glycans (FS, P=6.89E-04, Figure 2G) increased in PMGC group compared with AGC group. Furthermore, we evaluate the potential diagnostic performance of these traits using ROC curves (Table 2). Notably, the AUC values of GPN, GPS and S2 were 0.72 (95%CI: 0.65 to 0.78), 0.71(95%CI: 0.65 to 0.78) and 0.76 (95%CI: 0.69 to 0.82) respectively, which showed moderately accurate differential performance of patients with PMGC.
Table 2.
Serum IgG N-glycans derived traits in advanced gastric cancer with or without peritoneal metastasis
| Glycan traits | Significant | Tendency in PMGC | P value | Mean of AGC | Mean of PMGC | AUC | 95% CI |
|---|---|---|---|---|---|---|---|
| GPN | * | ↓ | 3.83E-09 | 90.87 | 87.51 | 0.72 | 0.65 to 0.78 |
| GPS | * | ↑ | 4.69E-06 | 9.04 | 12.41 | 0.71 | 0.65 to 0.78 |
| S1 | * | ↑ | 1.78E-06 | 7.30 | 9.47 | 0.67 | 0.60 to 0.74 |
| S2 | * | ↑ | 3.48E-13 | 1.74 | 2.94 | 0.76 | 0.69 to 0.82 |
| G0 | 0.13 | 37.86 | 36.12 | / | / | ||
| G1 | * | ↓ | 3.12E-04 | 37.70 | 36.47 | 0.64 | 0.57 to 0.71 |
| G2 | 0.73 | 14.93 | 14.73 | / | / | ||
| F | 0.52 | 93.60 | 93.75 | / | / | ||
| FN | * | ↑ | 2.06E-04 | 95.95 | 96.86 | 0.66 | 0.59 to 0.73 |
| FS | * | ↑ | 6.89E-04 | 84.07 | 87.38 | 0.58 | 0.51 to 0.65 |
| B | * | ↓ | 1.65E-06 | 22.23 | 18.61 | 0.69 | 0.62 to 0.75 |
| BN | * | ↓ | 1.08E-06 | 22.61 | 18.75 | 0.69 | 0.62 to 0.76 |
| BS | 0.38 | 16.75 | 17.25 | / | / | ||
| FG1 | 0.04 | 28.29 | 29.09 | / | / | ||
| Gal-ratio | 0.60 | 0.56 | 0.55 | / | / |
Figure 2.
The abundance of the nine representative derived traits in patients with PMGC and patients with AGC in the training cohort. The N-glycans were grouped according to their structural features: neutral N-glycans (GPN) (A); total sialylation (GPS) (B); monosialylation (S1) (C); disialylation (S2) (D); monogalactosylation (G1) (E); fucosylation of neutral glycans (FN) (F); fucosylation of sialylated glycans (FS) (G); bisecting N-glycan (B) (H); bisecting N-glycan of neutral glycans (BN) (I).
Predictive model establishment based on the training cohort
Although GPN, GPS and S2 derived from directly measured glycan peaks had a moderately accurate differential performance of patients with PMGC, a predictive glyco-model constructed by directly measured glycan peaks might have a better predictive performance. As displayed in Table 3, six directly detected glycan peaks were significantly increased in patients with PMGC than in patients with AGC in the training cohort. By contrast, five glycan peaks showed a decreased level in patients with PMGC.
Table 3.
List of the 11 serum IgG N-glycans that were evaluated to be significantly different between PMGC and AGC
| Glycan peak | Significant | Tendency in PMGC | P value | Mean of PMGC | Mean of AGC |
|---|---|---|---|---|---|
| GP6 | * | ↑ | 2.53E-05 | 7.60 | 9.52 |
| GP7 | * | ↑ | 2.67E-04 | 0.39 | 0.51 |
| GP9 | * | ↓ | 1.73E-03 | 9.70 | 8.95 |
| GP10 | * | ↑ | 5.84E-08 | 6.20 | 7.85 |
| GP11 | * | ↑ | 3.97E-08 | 0.79 | 1.04 |
| GP15 | * | ↑ | 6.90E-05 | 1.60 | 1.96 |
| GP16 | * | ↓ | 1.82E-08 | 2.17 | 1.64 |
| GP18 | * | ↓ | 1.92E-05 | 5.77 | 4.33 |
| GP21 | * | ↓ | 7.29E-05 | 0.75 | 0.57 |
| GP23 | * | ↓ | 2.09E-13 | 0.95 | 0.44 |
| GP24 | * | ↑ | 1.46E-09 | 1.21 | 0.71 |
Considering that these glycan peaks showed strong association with PMGC, we attempted to build a predictive glyco-model using multivariate logistic regression in the training cohort (Table 3). Logistic regression coefficients were utilized to estimate the odds ratios for each of the independent variables. Score estimation of glyco-model, composed by GP6 (H3N5F1), GP9 (H4N4F1(3)), GP11 (H4N5F1(3)), GP21 (H5N4S2) and GP23 (H5N4F1S2), was established on the mathematic formula:
| Score=4.49 * GP6 + 5.42 * GP9 - 50 * GP11 + 30.19 * GP21+ 26.53* GP23+88.33 |
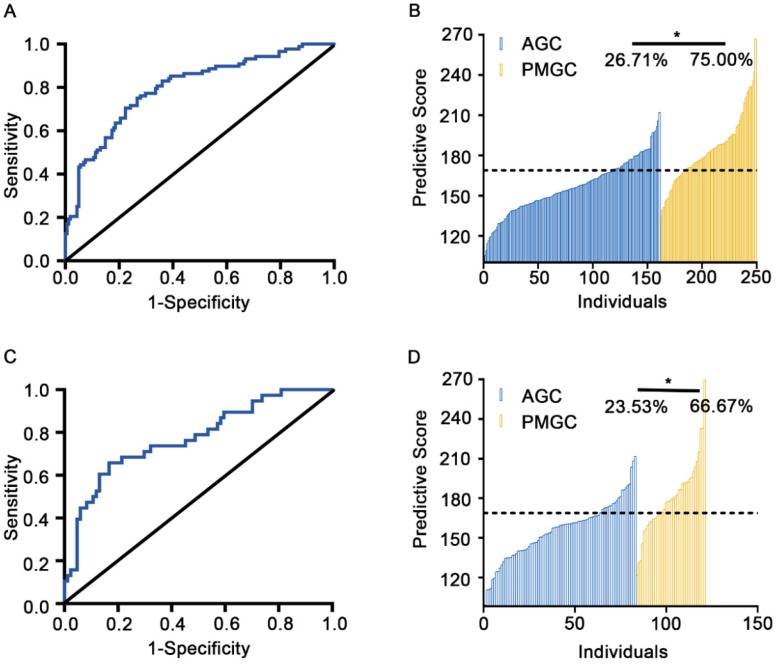
Based on the ROC analysis (AUC=0.80, 95%CI: 0.74 to 0.86) in training cohort, we set up the optimal cut-off value of predictive score as 168.60. Patients with predictive score greater than 168.60 will be classified as PMGC cases, values equal to or less than 168.60 will be classified as AGC cases. Under the cut-off value, the discrimination model was able to distinguish the PMGC patients from AGC ones with a sensitivity of 75.00% and specificity of 73.29% (Figures 3A and B, Table S3).
Figure 3.
Efficacy prediction of discriminate glyco-model of the training cohort and the validation cohort. A and C, Plots of ROC results for distinguishing PMGC samples from the AGC samples. Glyco-model shows good diagnostic efficacy in predicting PMGC in training cohort (AUC=0.80, 95%CI: 0.74 to 0.86) (A) and validation cohort (AUC=0.77, 95%CI: 0.68 to 0.86) (C). B and D, the logistic regression predictive score for each patient of the training (B) and validation set (D). Logistic regression predictive score was calculated with the formula, Score=4.49 * GP6 + 5.42 * GP9-50 * GP11 + 30.19 * GP21+ 26.53* GP23+88.33.
Validation of the predictive model
To validate the accuracy of the predictive model, serum IgG N-glycomics analysis was performed in the aforementioned validation sample set composed of 124 serum specimens (AGC: n=85, PMGC: n=39). This model demonstrated an AUC value of 0.77 (95%CI: 0.68 to 0.86) with a sensitivity of 66.67% and a specificity of 76.47%, suggesting a moderately accurate diagnosis (Figures 3C and D, Table S3). Furthermore, we combined the training cohort and the validation cohort as a combined cohort (AGC: n=246, PMGC: n=127) to further validate the performance of the predictable glyco-model. In this cohort, the AUC value was 0.79 (95%CI: 0.74 to 0.84) with a sensitivity of 72.44% and a specificity of 74.39%, making this glyco-model predictable for differentiation of patients with PMGC (Figure S1, Table S3). The diagnostic performance of the glyco-model was much better than those serum tumor markers including CEA, CA19-9, CA125 and CA72-4 (Table S4).
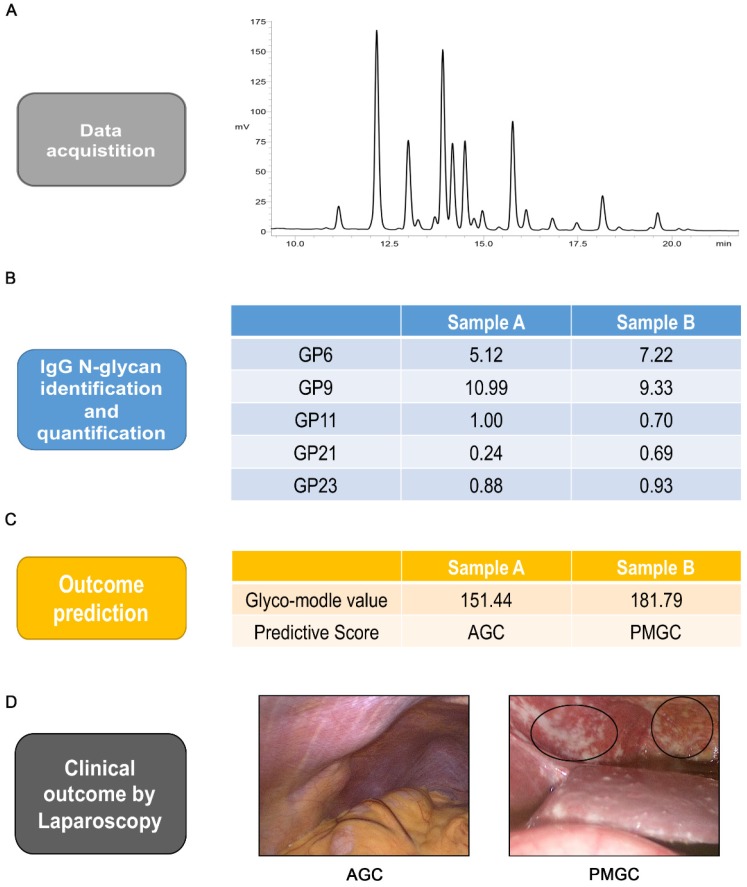
Finally, to illustrate the predictive workflow of the discriminated glyco-model, we selected two preoperative serum samples with or without peritoneal metastasis. Serum IgG N-glycomics analysis of samples and application of the model was conducted (Figures 4A and B). The predictive score of these two samples were calculated as 151.44 and 181.79, respectively (Figure 4C). Under the cut-off value of predictive score of 168.60, we predicted that sample A was from AGC cases and that sample B was from PMGC case. These predictions from the model were in line with laparoscopic results (Figure 4D).
Figure 4.
Analysis workflow of prediction. Typical base peak of the serum specimen in positive ion mode(A). Identification and quantification of the five IgG glycan of GP6, GP9, GP11, GP21 and GP23 (B). Logistic regression predictive score and outcome prediction of the two samples(C). Typical images of abdominal cavity by staging laparoscopy (D). The circled parts are typical peritoneal metastasis.
Discussion
This study represents the first comprehensive analysis of IgG N-glycans in gastric cancer with peritoneal metastasis. By analyzing 127 patients with PMGC and 246 matching controls with advanced gastric cancer, we firstly find that peritoneal metastasis is associated with alterations in the IgG N-glycome composition: (i) increase in IgG sialylation, fucosylation of both neutral and sialylated IgG glycosylation, (ii) decrease in neutral IgG glycosylation, IgG monogalactosylation and bisecting IgG glycosylation. These data may be important to help understand the role of IgG glycosylation in immunosurveillance and mechanism of PMGC.
Peritoneal metastasis, mainly induced by the dissemination of free tumor cells from the primary gastric cancer, is usually associated with a poor prognosis in patients with AGC 26. Median survival of patients without metastasis was 14 months, but only 4 months for patients with peritoneal metastasis 27. As we know, treatment option, decision-making and prognosis of gastric cancer are strongly dependent on the extent of tumor 28. Compared to curable gastrectomy for AGC, an untreatable condition existed in PMGC 29. From this point of view, PMGC needs to be precisely differentiated from AGC before surgery for surgeons to determine the most appropriate therapeutic approach, and avoid unnecessary extensive surgery.
Unfortunately, in clinical practice, it is often difficult to make diagnosis of peritoneal metastasis preoperatively by conventional imaging modalities due to the low sensitivity and cost. A systematic review of imaging in PMGC indicated that although the specificity of imaging methods was more than 0.90, the poor sensitivity for US, EUS, CT and 18F-FDG PET in detecting peritoneal metastasis were 0.09 (95% CI: 0.03 to 0.21), 0.34 (95% CI: 0.10 to 0.69), 0.33 (95% CI: 0.16 to 0.56) and 0.28 (95% CI: 0.17 to 0.44) respectively 8. Therefore, a sensitive examination was urgently needed to be developed to improve the diagnosis of peritoneal metastasis preoperatively.
Due to the non-invasiveness and convenience, serum biomarkers were investigated to predict peritoneal metastasis. Many papers have been published on the relationship between peritoneal metastasis and different tumor markers such as CEA, CA19-9, CA125 and CA72-4. A retrospective study containing 88 patients with PMGC evaluated the diagnostic performance of serum biomarkers. It showed that the sensitivity of CEA, CA19-9 and CA125 were 31.8%, 37.5% and 38.6%, respectively 30. In another study, CEA and CA125 showed a better predictive performance of peritoneal metastasis with a sensitivity of 46.9% and 46.1% 10. Of the combinations of the two markers, CA125 and CA72-4 together showed a higher sensitivity of 68% 10. When combined all four tumor markers and lysyl oxidase (LOX), the diagnostic sensitivity increased to 91.3% 11. In the present study, we also evaluate the performance of these tumor biomarkers. Compared with AGC group, all these four markers were increased in PMGC group. The AUC values of CEA, CA19-9, CA125 and CA72-4 were 0.54, 0.63, 0.63 and 0.62, respectively (Table S4). Consistent with the literatures, our results showed these serum tumor markers cannot predict PMGC effectively. Recently, some serum markers reflecting the systemic inflammatory response and immune responses of gastric cancer were indicated to be associated with peritoneal metastasis. Platelet to lymphocyte ratio (PLR) was an independent indicator to predict peritoneal metastasis, the AUC value was 0.60 with a sensitivity of 69.3% and a specificity of 51% 2. The neutrophil to lymphocyte ratio (NLR) was also evaluated in PMGC group, and the AUC value was 0.68 with a sensitivity of 63.8% and a specificity of 67.4% 12. Besides, serum C-reactive protein (CRP) and serum albumin (Alb) were significantly related to the presence of peritoneal metastasis 12. Therefore, the relationship between tumor and the body inflammatory status has received increasing attention. However, none of these serum markers showed satisfied accuracy to predict PMGC.
As one of the key factors in humoral immunity, IgG is involved in antibody-dependent cell-mediated cytotoxicity (ADCC), opsonization, and complement-dependent cytotoxicity (CDC) 31. The structure and biological activity of IgG is influenced by its Fc-glycosylation. Decreased IgG galactosylation and sialylation lead to pro-inflammatory antibody response. Furthermore, elevated occurrence of bisecting GlcNAc and lack of core fucose results in an increased ADCC activity 25. Altered IgG glycosylation profiles have been described in many kinds of cancers including gastric cancer 31-33. The galactosylated and sialylated IgG N-glycans were decreased and core fucosylated N-glycan was increased in gastric cancer 19, 20. However, there is no study identify whether significant difference in IgG N-glycans existed between patients with PMGC and patients with AGC. In addition, we previously conducted serum N-glycomics analysis in gastric cancer including 46 PMGC patients and 46 AGC patients to find potential biomarkers, and found H6N5F2L2E1 and H7N6F1L2E1 decreased in PMGC group(H: hexose, N: N-acetylhexosamine; F: fucose, L: α-2,3 N-acetylneruaminic acid; E: α-2,6 N-acetylneruaminic acid) 17. Since the relative abundance of these two glycans is low and N-glycomics analysis requires more professional operations, we want to find a more effective predictive biomarker for PMGC.
Based on the aforementioned information, we focused on the significance of IgG N-glycans in differential diagnosis of peritoneal metastasis by using UPLC analysis. We analyzed IgG N-glycan profiles in 373 patients including 127 PMGC patients. Since the incidence of peritoneal metastasis accounted for 14% in gastric cancer, it is difficult to collect PMGC patients 27. This is the first time IgG N-glycans profiled in such a large group with peritoneal metastasis. Based on the large sample size, significant changes in peritoneal metastasis were founded to build and further validate the predictive model. Through comparing 15 derived traits in training cohort, we found that neutral N-glycans (GPN) were decreased in PMGC group. Relatively, sialylated N-glycans (GPS) including monosialylation (S1) and disialylation (S2) were increased in PMGC group. And monogalactosylation showed a decreased level in PMGC group. Interestingly, the results showed an opposite trend to the previous study 19, 20. Therefore, we compared these derived traits between healthy controls (n= 94) with both AGC group (n=246) and PMGC group (n=127), and we found the trend of galactosylation and sialylation in gastric cancer was consistent with the previous literature (data not shown). These results showed that IgG glycosylation changes were dynamic in gastric cancer and could reflect immune response in patients with PMGC. Increased sialylation indicated anti-inflammatory antibody response in peritoneal metastasis. We also found there was an increased level in fucosylation of both neutral N-glycans (FN) and sialylated N-glycans (FS) in PMGC group. Differently, total bisecting GlcNAc (B) and bisecting GlcNAc of neutral N-glycans (BN) showed a decrease level in patients with PMGC. These results suggested that ADCC activity might be decreased in peritoneal metastasis.
Several derived traits have potential to be differential biomarkers of peritoneal metastasis. The AUC values of GPN, GPS and S2 were more than 0.70, which means moderately accurate diagnostic performance. In order to find a more accurate diagnostic model, we analyzed 24 directly measured glycan peaks in the training cohort. In order construction the predicted logistic regression model, five differentially expressed IgG glycans were selected including GP6, GP9, GP11, GP21, GP23, each were weighted with a corresponding coefficient. The final score =4.49 * GP6 + 5.42 * GP9 - 50 * GP11 + 30.19 * GP21+ 26.53* GP23+88.33. The glyco-model showed a more accurate differential performance for peritoneal metastasis with a sensitivity of 75.00% and a specificity of 73.29%. And the performance of this glyco-model were validated in both the validation cohort (n=124, AUC=0.77) and the combined cohort (n=373, AUC=0.79). To our knowledge, this is the first study to identify specific IgG N-glycan features of PMGC, providing potential glyco-model to distinguish the PMGC from AGC. Accurate diagnosis of peritoneal metastasis before the operation is beneficial for PMGC patients to avoid the mental and physical trauma from unnecessary surgery.
In conclusion, we profiled IgG N-glycans in advanced gastric cancer patients with or without peritoneal metastasis and identified several derived traits either up-regulated or down-regulated in patients with peritoneal metastasis for the first time. Based on the largest sample of PMGC as we know, we established and validated a glyco-model to predict peritoneal metastasis. Further studies are still needed to validate the differential potential of this glyco-model in a larger study and identify the role of protein glycosylation in pathology of peritoneal metastasis.
Supplementary Material
Supplementary figure and tables.
Acknowledgments
We deeply thank Doctor Zhaoqing Tang for providing images by staging laparoscopy. This study was funded by grants from National Key Research and Development Program of China (2016YFC1303100, 2016YFA0501303), National Natural Science Foundation of China (31630088, 31770858).
Abbreviations
- IgG
Immunoglobulin G
- GlcNAc
N-acetylglucosamine
- GP
glycan peaks
- ROC
Receiver operating characteristic
- AUC
area under the ROC curve
- AGC
advanced gastric cancer
- PMGC
Gastric cancer with peritoneal metastasis
- CT
Computed Tomography
- PET-CT
Positron Emission Tomography-Computed Tomography
- MRI
Magnetic Resonance Imaging
- PLR
lymphocyte ratio
- NLR
neutrophil to lymphocyte ratio
- MALDI-MS
Matrix-Assisted Laser Desorption/ Ionization t Mass Spectrometry
- LC-ESI-MS
Liquid chromatography electrospray ionisation tandem mass spectrometry
- FACE
Fluorophore-Assisted Carbohydrate Electrophoresis
- UPLC
Ultra Performance Liquid Chromatography
- CA
carbohydrate antigen
- BCA
bicinchoninic acid
- PGC
porous graphic carbon
- TFA
trifluoroacetic acid
- ACN
acetonitrile
- 2-AB
2-aminobenzamide
- DMSO
Dimethyl sulfoxide
- AcOH
glacial acetic acid
- HILIC
Hydrophilic Interaction Chromatography
- GPN
Neutral N-glycans
- GPS
total sialylation
- S1
Monosialylation
- S2
disialylation
- G1
monogalactosylation
- B
bisecting N-glycan
- BN
bisecting N-glycan of neutral glycans
- FN
fucosylation of neutral glycans
- FS
fucosylation of sialylated glycans
- CRP
C-reactive protein
- Alb
albumin
- ADCC
antibody-dependent cell-mediated cytotoxicity
- CDC
complement-dependent cytotoxicity
- H
hexose
- N
N-acetylhexosamine
- F
fucose
- L
α-2,3 N-acetylneruaminic acid
- E
α-2,6 N-acetylneruaminic acid
- S
N-acetylneruaminic acid
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Chen XD, Mao CC, Wu RS, Zhang WT, Lin J, Sun XW. et al. Use of the combination of the preoperative platelet-to-lymphocyte ratio and tumor characteristics to predict peritoneal metastasis in patients with gastric cancer. PLoS One. 2017;12:e0175074. doi: 10.1371/journal.pone.0175074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87:353–7. doi: 10.1046/j.1365-2168.2000.01358.x. [DOI] [PubMed] [Google Scholar]
- 4.Glockzin G, Piso P. Current status and future directions in gastric cancer with peritoneal dissemination. Surg Oncol Clin N Am. 2012;21:625–33. doi: 10.1016/j.soc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Yoon H, Lee DH. New approaches to gastric cancer staging: beyond endoscopic ultrasound, computed tomography and positron emission tomography. World J Gastroenterol. 2014;20:13783–90. doi: 10.3748/wjg.v20.i38.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yonemura Y, Elnemr A, Endou Y, Hirano M, Mizumoto A, Takao N. et al. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J Gastrointest Oncol. 2010;2:85–97. doi: 10.4251/wjgo.v2.i2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rausei S, Ruspi L, Mangano A, Lianos GD, Galli F, Boni L. et al. Advantages of staging laparoscopy in gastric cancer: they are so obvious that they are not evident. Future Oncol. 2015;11:369–72. doi: 10.2217/fon.14.283. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Chen JQ. Imaging in assessing hepatic and peritoneal metastases of gastric cancer: a systematic review. BMC Gastroenterol. 2011;11:19. doi: 10.1186/1471-230X-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikuchi H, Kamiya K, Hiramatsu Y, Miyazaki S, Yamamoto M, Ohta M. et al. Laparoscopic narrow-band imaging for the diagnosis of peritoneal metastasis in gastric cancer. Ann Surg Oncol. 2014;21:3954–62. doi: 10.1245/s10434-014-3781-8. [DOI] [PubMed] [Google Scholar]
- 10.Emoto S, Ishigami H, Yamashita H, Yamaguchi H, Kaisaki S, Kitayama J. Clinical significance of CA125 and CA72-4 in gastric cancer with peritoneal dissemination. Gastric Cancer. 2012;15:154–61. doi: 10.1007/s10120-011-0091-8. [DOI] [PubMed] [Google Scholar]
- 11.Lai H, Jin Q, Lin Y, Mo X, Li B, He K. et al. Combined use of lysyl oxidase, carcino-embryonic antigen, and carbohydrate antigens improves the sensitivity of biomarkers in predicting lymph node metastasis and peritoneal metastasis in gastric cancer. Tumour Biol. 2014;35:10547–54. doi: 10.1007/s13277-014-2355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama Y, Gotohda N, Shibasaki H, Nomura S, Kinoshita T, Hayashi R. Usefulness of the neutrophil/lymphocyte ratio measured preoperatively as a predictor of peritoneal metastasis in patients with advanced gastric cancer. Surg Today. 2014;44:2146–52. doi: 10.1007/s00595-014-0917-1. [DOI] [PubMed] [Google Scholar]
- 13.Sakakura C, Takemura M, Hagiwara A, Shimomura K, Miyagawa K, Nakashima S. et al. Overexpression of dopa decarboxylase in peritoneal dissemination of gastric cancer and its potential as a novel marker for the detection of peritoneal micrometastases with real-time RT-PCR. Br J Cancer. 2004;90:665–71. doi: 10.1038/sj.bjc.6601544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimomura K, Sakakura C, Takemura M, Takagi T, Fukuda K, Kin S. et al. Combination of L-3-phosphoserine phosphatase and CEA using real-time RT-PCR improves accuracy in detection of peritoneal micrometastasis of gastric cancer. Anticancer Res. 2004;24:1113–20. [PubMed] [Google Scholar]
- 15.Huang CJ, Jiang JK, Chang SC, Lin JK, Yang SH. Serum CA125 concentration as a predictor of peritoneal dissemination of colorectal cancer in men and women. Medicine (Baltimore) 2016;95:e5177. doi: 10.1097/MD.0000000000005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T, Wei Y, Tian L, Song H, Ma Y, Yao Q. et al. C-C motif chemokine ligand 5 (CCL5) levels in gastric cancer patient sera predict occult peritoneal metastasis and a poorer prognosis. Int J Surg. 2016;32:136–42. doi: 10.1016/j.ijsu.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Qin R, Zhao J, Qin W, Zhang Z, Zhao R, Han J. et al. Discovery of Non-invasive Glycan Biomarkers for Detection and Surveillance of Gastric Cancer. J Cancer. 2017;8:1908–16. doi: 10.7150/jca.17900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gornik O, Pavic T, Lauc G. Alternative glycosylation modulates function of IgG and other proteins - implications on evolution and disease. Biochim Biophys Acta. 2012;1820:1318–26. doi: 10.1016/j.bbagen.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Kodar K, Stadlmann J, Klaamas K, Sergeyev B, Kurtenkov O. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconj J. 2012;29:57–66. doi: 10.1007/s10719-011-9364-z. [DOI] [PubMed] [Google Scholar]
- 20.Ruhaak LR, Barkauskas DA, Torres J, Cooke CL, Wu LD, Stroble C. et al. The Serum Immunoglobulin G Glycosylation Signature of Gastric Cancer. EuPA Open Proteom. 2015;6:1–9. doi: 10.1016/j.euprot.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanoh Y, Mashiko T, Danbara M, Takayama Y, Ohtani S, Imasaki T. et al. Analysis of the oligosaccharide chain of human serum immunoglobulin g in patients with localized or metastatic cancer. Oncology. 2004;66:365–70. doi: 10.1159/000079484. [DOI] [PubMed] [Google Scholar]
- 22.Qin W, Pei H, Qin R, Zhao R, Han J, Zhang Z. et al. Alteration of Serum IgG Galactosylation as a Potential Biomarker for Diagnosis of Neuroblastoma. J Cancer. 2018;9:906–13. doi: 10.7150/jca.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pucic M, Knezevic A, Vidic J, Adamczyk B, Novokmet M, Polasek O. et al. High throughput isolation and glycosylation analysis of IgG-variability and heritability of the IgG glycome in three isolated human populations. Mol Cell Proteomics. 2011;10:M111. doi: 10.1074/mcp.M111.010090. 010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vuckovic F, Theodoratou E, Thaci K, Timofeeva M, Vojta A, Stambuk J. et al. IgG Glycome in Colorectal Cancer. Clin Cancer Res. 2016;22:3078–86. doi: 10.1158/1078-0432.CCR-15-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theodoratou E, Thaci K, Agakov F, Timofeeva MN, Stambuk J, Pucic-Bakovic M. et al. Glycosylation of plasma IgG in colorectal cancer prognosis. Sci Rep. 2016;6:28098. doi: 10.1038/srep28098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed A, Ukwenya AY, Makama JG, Mohammad I. Management and outcome of gastric carcinoma in Zaria, Nigeria. Afr Health Sci. 2011;11:353–61. [PMC free article] [PubMed] [Google Scholar]
- 27.Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW. et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622–8. doi: 10.1002/ijc.28373. [DOI] [PubMed] [Google Scholar]
- 28.Ikeguchi M, Oka A, Tsujitani S, Maeta M, Kaibara N. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res. 1994;14:2131–4. [PubMed] [Google Scholar]
- 29.Gill RS, Al-Adra DP, Nagendran J, Campbell S, Shi X, Haase E. et al. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: a systematic review of survival, mortality, and morbidity. J Surg Oncol. 2011;104:692–8. doi: 10.1002/jso.22017. [DOI] [PubMed] [Google Scholar]
- 30.Hwang GI, Yoo CH, Sohn BH, Shin JH, Park YL, Kim HD. et al. Predictive value of preoperative serum CEA, CA19-9 and CA125 levels for peritoneal metastasis in patients with gastric carcinoma. Cancer Res Treat. 2004;36:178–81. doi: 10.4143/crt.2004.36.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawaguchi-Sakita N, Kaneshiro-Nakagawa K, Kawashima M, Sugimoto M, Tokiwa M, Suzuki E. et al. Serum immunoglobulin G Fc region N-glycosylation profiling by matrix-assisted laser desorption/ionization mass spectrometry can distinguish breast cancer patients from cancer-free controls. Biochem Biophys Res Commun. 2016;469:1140–5. doi: 10.1016/j.bbrc.2015.12.114. [DOI] [PubMed] [Google Scholar]
- 32.Qian Y, Wang Y, Zhang X, Zhou L, Zhang Z, Xu J. et al. Quantitative analysis of serum IgG galactosylation assists differential diagnosis of ovarian cancer. J Proteome Res. 2013;12:4046–55. doi: 10.1021/pr4003992. [DOI] [PubMed] [Google Scholar]
- 33.Yi CH, Weng HL, Zhou FG, Fang M, Ji J, Cheng C. et al. Elevated core-fucosylated IgG is a new marker for hepatitis B virus-related hepatocellular carcinoma. Oncoimmunology. 2015;4:e1011503. doi: 10.1080/2162402X.2015.1011503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure and tables.