Abstract
Background
Prior to treatment, breast cancer patients are less physically fit compared to peers; during cancer treatment, their fitness typically declines. Depressive symptoms are associated with reduced activity up to 5 years post-treatment, but research has not identified mechanisms linking depression and lower activity. The current study assessed relationships among breast cancer patients’ depression and perceived exertion during exercise as well as heart rate, an objective indicator of exertion.
Methods
Participants were 106 breast cancer patients, stages I–IIIA, who completed surgery but had not started adjuvant treatment. Heart rate and self-rated exertion, measured using the Borg Scale of Perceived Exertion, were assessed every 2 min during a graded exercise test. Depression was assessed using the CES-D and a structured clinical interview.
Results
Compared to women below the CES-D clinical cutoff, women with significant depressive symptoms reported steeper increases in exertion during the exercise test (p = .010) but had similar heart rates (p = .224) compared to women below the cutoff. Major depression history was unrelated to perceived exertion (ps > .224) and heart rate (ps > .200) during exercise.
Conclusions
Women with currently elevated depressive symptoms experienced exercise as more difficult compared to women below the CES-D cutoff, but these self-perceptions did not reflect actual heart rate differences. Depression may make exercise feel more demanding, which could ultimately decrease patients’ likelihood of engaging in regular exercise. Results support the use of depression screening tools following breast cancer surgery to identify and intervene on individuals at risk for decreased physical activity during survivorship.
Keywords: Breast cancer, Physical activity, Exercise, Perceived exertion, Depression, Depressive symptoms
Introduction
Physical activity benefits breast cancer patients during treatment and throughout survivorship. Accordingly, the American Cancer Society recommends that breast cancer patients engage in regular physical activity, defined as 150 min of moderate-to-vigorous activity per week, even during adjuvant cancer treatment like chemotherapy or radiation [1]. Meta-analytic evidence suggests that regular physical activity after breast cancer diagnosis reduces rates of early all-cause mortality, breast cancer mortality, and cancer recurrence [2]. In addition, regular moderate-to-vigorous physical activity has been linked to lower fatigue, less cancer-related worry, and better physical functioning [3]. During adjuvant treatment, physical activity interventions have also reduced fatigue [4]. However, despite the wealth of evidence supporting the benefits of physical activity during all stages of cancer survivorship, most breast cancer patients do not meet national exercise guidelines [5]. Additionally, women with breast cancer have poorer cardiovascular fitness compared to their age-matched peers before, during, and after adjuvant treatment, and their fitness further deteriorates over the course of cancer treatment [6, 7].
Several factors may contribute to declining physical activity and fitness throughout cancer survivorship. In particular, depressive symptoms are more common among breast cancer patients compared to the general population; studies estimate that 13–18% of patients experience clinically significant depressive symptoms following surgery [8, 9]. Additionally, depressive symptoms are associated with reduced physical activity after cancer treatment. One study found that women reporting more depressive symptoms prior to adjuvant treatment were less active over the next 5 years compared to those with fewer symptoms [10]. Another study of 2819 early-stage breast cancer survivors found a similar pattern of results; approximately 2 years after diagnosis, women reporting heightened depressive symptoms also reported less physical activity [11].
Little is known about why depressed breast cancer patients become increasingly sedentary and less physically fit with time. One possibility is that the negative cognitive biases that are characteristic of depressive disorders may distort perceptions of exertion during exercise. The cognitive model of depression suggests that negative thinking patterns, or negatively biased information processing, characterizes depression [12]. Brain imaging studies support this theory. For example, increased amygdala activation among depressed individuals appears to heighten sensitivity to negative information [13]. Additionally, depressed individuals show decreased activation of the prefrontal cortex in response to negative information, which appears to make it more difficult for them to disengage from negative affect and physical discomfort [13, 14]. Indeed, depressed individuals show greater pain sensitivity [15–18] and have more difficulties interpreting internal bodily signals such as heart rate, i.e., interoceptive stimuli [19]. Taken together, depressed individuals may have distorted perceptions of body sensations, including those produced during exercise. Thus, depressed individuals might perceive exercise as more demanding compared to their non-depressed peers.
Depression may also influence objective measures of exercise exertion, such as heart rate. Depression has well-documented effects on cardiovascular reactivity during psychological stress tasks [20–23]; however, the direction of the effect differs between studies, and very little research has investigated whether cardiovascular reactivity during exercise, a physiological stressor, differs between depressed and non-depressed individuals. The scant available evidence suggests that depressed individuals have smaller increases in heart rate during exercise tests, but no data are available on perceptions of effort [24, 25].
The current study investigated the effect of clinically elevated depressive symptoms on perceived exertion and objective exertion (i.e., heart rate) during an exercise test among breast cancer patients. Given prior literature, we hypothesized that individuals with elevated depressive symptoms would report greater increases in exercise-related exertion, but would show smaller objective increases in heart rate compared to their counterparts without elevated depressive symptoms. Prior data is not available regarding objective and perceived exercise exertion among individuals with a lifetime history of major depression. Therefore, major depression history was explored to determine whether lifetime depression history was related to exertion.
Methods
Participants
The sample included 106 breast cancer patients (stages I–IIIA) who were recruited for a longitudinal parent study examining the effects of cancer treatment on metabolic and immune functioning. Institutional review board approval was obtained for the study protocol, and data collected as part of the parent trial have not been previously published. The current study utilized data from participants’ baseline visit, which occurred after their initial breast cancer surgery but prior to beginning chemotherapy or radiation.
Researchers screened participants for study eligibility using electronic medical records in collaboration with medical and surgical oncologists at a large, Midwestern hospital. After an initial screen of patients’ electronic medical records, 443 individuals were approached either in person or by phone to assess their interest and complete a more in-depth eligibility screening. Of those 443 individuals, more than half declined to participate when approached (n = 271) while others were initially interested but were later deemed ineligible or canceled their visits before participating (n = 68). This left a total of 106 total participants with available data for analysis.
Exclusion criteria included prior history of cancer other than current breast cancer (except basal or squamous cell carcinoma), prior cancer treatment, and serious medical comorbidities, such as diabetes, heart disease, autoimmune disease, and drug/alcohol abuse. Women taking medications known to influence lipid metabolism and immunity (e.g., fibrates and steroids) were also excluded. Finally, women were excluded if they were deemed unfit to exercise based on cardiovascular risk factors.
Procedure
Informed consent was obtained from all individual participants included in the study. As part ofthe parent study, women completed two post-surgery visits at a clinical research hospital. At one of these visits, participants completed a graded cycle ergometer test to measure maximal aerobic capacity (VO2max). During the test, ratings of perceived exertion and heart rate were collected every 2 min. At their second post-surgical visit, women spent 9.5 h at the clinical research hospital where self-report data regarding psychosocial functioning was collected, including a questionnaire measuring depressive symptoms and a diagnostic interview to capture lifetime major depression history. As part of the parent study, women also provided blood samples and metabolic data approximately every hour. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Cycle Ergometer Test
The graded cycle ergometer test was conducted using an Excalibur cycle ergometer (Lode B.V., Groningen, The Netherlands). Exercise testing was overseen by a trained exercise physiologist, and all participants received clearance from their oncologist before completing the exercise test. The graded cycle ergometer test is appropriate for use among participants of varying ages and exercise experience [26]. Participants were asked to cycle at a constant speed (between 50 and 60 rpm) while resistance was increased every 2 min. Each 2-min interval represents an exercise stage. Participants were asked to continue exercising until they felt they could no longer continue or reached an exercise time of 15 min. In the current sample, participant test duration ranged from 4.4 to 15.0 min. The exercise physiologist also rated participant effort during the test as either poor, fair, good, or excellent, based on their peak respiratory exchange ratio (RER), a ratio of carbon dioxide output to oxygen consumption. Peak RER is a validated measure of subject effort during cardiovascular exercise testing [27]. 94.3% of the sample received effort ratings of “good” or “excellent” from the exercise physiology team (i.e., RER> 1.11); none received a “poor” rating.
Physical Fitness
Cardiovascular fitness was measured using participants’ peak oxygen consumption (VO2max). Participants wore a mask during testing, which allowed for continuous, direct measurement of oxygen consumption (VO2) and carbon dioxide production (VCO2). This measure is appropriate for use among breast cancer patients and predicted metastatic disease survival in one study [6].
Heart Rate
Heart rate measurements were collected using a CASE P2 Series electrocardiogram machine (GE Healthcare, Chicago, IL, USA). Measurements were recorded by the exercise physiology team every 2 min throughout the cycle ergometer test, i.e., at the beginning of each new exercise stage.
Perceived Exertion
Participants reported their level of perceived exertion at the beginning of each exercise stage using the Borg Scale of Perceived Exertion [28]. The Borg assesses subjective, physical exertion by asking participants to choose a number on a scale from 6 to 20 indicating how hard they feel they are working. The scale was designed to approximate a participant’s heart rate divided by 10 (e.g., if the participant reports a Borg rating of 11, their heart rate should be approximately 110). Although previous studies have found high correlations between heart rate and perceived exertion, it should be noted that heart rate is not the only factor that drives perceived exertion [29]. Factors such as respiration rate, body temperature, sweat production, and general fatigue are likely considered when participants provide ratings of perceived exertion. The Borg scale demonstrates adequate criterion validity when compared to heart rate and muscle lactate concentration [30].
Depression
Self-Reported Symptoms
The Center for Epidemiologic Studies Depression Scale (CES-D) measured participants’ depressive symptoms [31]. The CES-D is a 20-item scale that asks participants to indicate how frequently they have experienced depressive symptoms in the past week. Scores on the CES-D range from 0 to 60. This scale is appropriate for use among cancer patients [32]. The scale was used as a continuous measure as well as a dichotomized measure, using the clinical cutoff score of 16 [33] to indicate whether the participant was experiencing clinically elevated depressive symptoms. In this sample, the internal reliability estimate for this scale was α = .77.
Diagnostic Interview
The mood disorder modules of the Structured Clinical Interview-Nonpatient Version for DSM-IV, (SCID-NP) were used to assess current or past history of major depressive disorder [34]. Trained clinical psychology graduate students and staff administered diagnostic interviews, which were recorded and reviewed in consensus meetings to obtain diagnoses. Present and past diagnosis of major depressive disorder (MDD) were collapsed to index whether or not the participant had any lifetime history of MDD; only four women were experiencing a current depressive episode in our sample (n = 4). Therefore, we were unable to investigate the effect of a current depressive episode on perceived exertion.
Demographic Information and Control Variables
Nursing staff collected height and weight from all participants to calculate BMI. Women self-reported their age, race, education, and physical activity. Beta blocker use, comorbidities, cancer stage, and cancer treatment were assessed using electronic medical records.
Comorbidities
Medical comorbidities were quantified using the Charlson Comorbidity Index (CCI) [35, 36]. A study of the CCI’s predictive validity among 26,377 breast cancer patients found that the CCI was significantly associated with 2-year non-cancer mortality [37].
Physical Activity
The Godin-Shephard Leisure-Time Physical Activity Questionnaire was used to collect data on participants’ current physical activity [38]. Using the Godin, participants indicated how many times they engage in strenuous, moderate, or light exercise for longer than 15 min during a typical week. Participants’ physical activity level was indexed using the Godin Activity Score, which is calculated using the equation: Activity Score = (9 × Strenuous activity frequency) + (5 × Moderate) + (3 × Light). Evidence suggests that the Godin is acceptable for use in oncology populations [39].
Data Analysis
Primary hypotheses were tested using linear mixed models in SAS (SAS 9.4, Cary, NC). Subject-specific random effects were used to account for the correlation within-subjects. Heart rate and perceived exertion ratings were measured every 2 min during the exercise test (i.e., at the beginning of each exercise stage). Ratings measured after 12 min of the cycle ergometer test were removed from analyses, because < 3% of the sample provided data beyond 10 min. Including data beyond 12 min of the exercise test did not significantly alter results.
All models included the following fixed effect covariates: age, cancer stage, education level, race, BMI, physical activity, and comorbidities. Cancer stage (stages I, II, and III), education level (some high school, high school, some college, college, and Masters or professional degree), and race (White or non-White) were treated as class variables. Models predicting heart rate also included beta blocker use as a fixed effect. Continuous variables were mean-centered to aid in interpretation of results.
Separate models were used for each of the three independent variables: (1) lifetime history of MDD (2) current clinically elevated depressive symptoms, and (3) current depressive symptom severity. Lifetime history of MDD was measured using the SCID interview. Participants were dichotomized as either having clinically elevated depressive symptoms or not, based on whether they met the validated cutoff score of 16 on the CES-D. The CES-D was also tested as a continuous measure of depressive symptoms, to determine whether there is a linear association between depressive symptom severity and perceived exertion. The main effect of each depression measure on perceived exertion was tested; in a second step, we tested the interaction between depression and time. This procedure was repeated to test for effects of depression on heart rate during the cycle ergometer test. Measurement stage was treated as a continuous measure of time in all models. Using the same covariates, secondary analyses tested the main effects of depression history and depressive symptoms on cardiovascular fitness using multiple regression (i.e., VO2max).
Results
On average, participants were middle-aged (M = 52.1, SD = 10.2, range = 26.1–75.4), Caucasian (87.7%), and had at least some college education (86.8%). The sample’s CES-D depression scores ranged 0–29 (M = 9.35, SD = 5.95), and 13.2% (n = 14) scored above 16, indicating that they had clinically elevated depressive symptoms. Thirty-one percent of women had a lifetime history of major depressive disorder, based on their diagnostic interview. Additional clinical and demographic characteristics are available in Table 1.
Table 1.
Sample demographic and clinical information (N =106)
| Measure | N (%) | M (SD) | Range | |
|---|---|---|---|---|
| Age | 52.1(10.2) | 26.1–75.4 | ||
| BMI (kg/m2) | 28.4(5.7) | 16.8–49.6 | ||
| Race | White | 93(87.7%) | ||
| Non-White | 13(12.3%) | |||
| Education | Some high school | 3(2.8%) | ||
| High school | 11(10.4%) | |||
| Some college | 24(22.6%) | |||
| College | 34(32.1%) | |||
| Master’s/professional | 34(32.1%) | |||
| Cancer stage | I | 53(50.0%) | ||
| II | 49(46.2%) | |||
| III | 4(3.8%) | |||
| Time since diagnosis (days) | 94.5(32.0) | 28.0–204.0 | ||
| Beta blocker usage (% yes) | 12(11.3%) | |||
| Physical activity level* | 23.9(18.3) | 0–85 | ||
Measured using the Godin Leisure-Time Activity Score
According to bivariate correlations, individuals who had higher average perceived exertion ratings during the exercise test reported exercising less frequently (r = – 0.26, p = .008) and had lower cardiorespiratory fitness (r = – 0.42, p <.001) compared to those who reported lower exertion. Correlations did not reveal a significant association between depression variables and self-reported physical activity or cardiovascular fitness in this sample (all ps > .103). See Table 2 for bivariate correlations among all study variables.
Table 2.
Bivariate correlations between study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Depressive symptoms (continuous) |
– | .75** | .28** | – .11 | .01 | − .11 | .11 | .01 | − .09 | –.10 | –.11 | .08 | .01 | − .04 | .07 |
| 2. Depressive symptoms (dichotomous) |
– | .16 | .03 | .02 | − .06 | .06 | .16† | − .04 | –.12 | − .05 | .02 | − .10 | −.16 | .05 | |
| 3. MDD lifetime history | – | – .07 | .01 | − .12 | < .01 | .05 | –.10 | .09 | − .05 | .12 | .05 | − .05 | − .06 | ||
| 4. Age | – | − .01 | − .08 | .06 | − .01 | − .07 | .13 | .22* | .14 | − .48** | − .36** | − .48** | |||
| 5. Cancer stage | – | − .09 | < .01 | .06 | .01 | − .12 | − .18† | − .01 | .11 | −.15 | − .12 | ||||
| 6. Education | – | − .09 | −.15 | .25* | −.11 | −.10 | − .22* | .09 | .26** | .34** | |||||
| 7. Race (White or non-White) | – | − .08 | .02 | .12 | −.14 | .02 | − .05 | <.01 | .10 | ||||||
| 8. BMI (kg/m2) | – | − .26** | − .05 | .23* | .14 | − .02 | − .65** | − .20* | |||||||
| 9. Physical activity | – | −.12 | − .23* | − .26** | − .03 | .40** | .39** | ||||||||
| 10. Comorbidities | – | .15 | .08 | − .16† | − .01 | − .04 | |||||||||
| 11. Beta blocker use | – | .03 | − .40** | −.31** | − .28** | ||||||||||
| 12. Average Borg rating | – | .02 | − .42** | − .57** | |||||||||||
| 13. Average heart rate | – | .14 | .05 | ||||||||||||
| 14. Cardiorespiratory fitness (VO2 max) |
– | .67** | |||||||||||||
| 15. Exercise test duration (minutes) |
– | ||||||||||||||
p < .01;
p < .05;
p <.10
Depression and Perceived Exertion
Self-rated exertion did not differ between women who met the CES-D depression cutoff and those who did not (b = – 0.28, SE = 0.43, p = .517; see Table 3). However, a significant interaction between clinically elevated symptoms and time emerged (b =0.29, SE =0.11, p = .010; see Fig. 1 panel a), such that individuals with elevated depressive symptoms reported significantly larger increases in exertion (b = 2.30, SE = 0.10, p < .0001) compared to those who did not meet the clinical cutoff (b = 2.01, SE = 0.04, p < .0001). Women with a MDD history did not report significant differences in perceived exertion throughout the exercise task (b = 0.41, SE = 0.31, p = .192), and there was no significant interaction between exercise time and MDD history (b = – 0.03, SE = 0.08,p = .737). Continuously measured depressive symptoms were not significantly associated with perceived exertion ratings and did not interact with time to predict perceived exertion (all ps > .140).
Table 3.
The effect of elevated depressive symptoms on perceived exertion and heart rate
| Perceived exertion (main effect model) |
Perceived exertion (interaction model) |
Heart rate (main effect model) |
Heart rate (interaction model) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | SE | p | b | SE | P | b | SE | p | b | SE | P | |
| Intercept | 9.04 | 0.79 | <.001 | 9.13 | 0.79 | < .001 | 106.89 | 6.86 | <.001 | 106.91 | 6.86 | <.001 |
| Age | 0.04 | 0.01 | .004 | 0.04 | 0.01 | .003 | − 0.39 | 0.13 | .004 | −0.39 | 0.13 | .004 |
| Cancer stage | ||||||||||||
| I | 0.17 | 0.78 | .830 | 0.15 | 0.78 | .853 | − 6.98 | 6.77 | .305 | −6.98 | 6.77 | .305 |
| II | 0.42 | 0.79 | .591 | 0.39 | 0.78 | .616 | −5.85 | 6.80 | .392 | −5.85 | 6.80 | .392 |
| III | 0 | – | – | 0 | – | – | 0 | – | – | 0 | – | – |
| Education level | ||||||||||||
| Some high school | 2.63 | 1.14 | .023 | 2.60 | 1.14 | .024 | 8.17 | 8.38 | .332 | 8.17 | 8.38 | .332 |
| High school | 0.62 | 0.54 | .255 | 0.64 | 0.54 | .234 | − 1.07 | 4.69 | .821 | −1.07 | 4.69 | .821 |
| Some college | 0.54 | 0.41 | .188 | 0.54 | 0.41 | .189 | .37 | 3.52 | .918 | 0.36 | 3.52 | .918 |
| College | − 0.06 | 0.37 | .868 | −0.06 | 0.37 | .882 | 0.37 | 3.32 | .912 | 0.37 | 3.32 | .911 |
| Master’s/professional | 0 | – | – | 0 | – | – | 0 | – | – | 0 | – | – |
| Race | ||||||||||||
| Non-White | − 0.18 | 0.48 | .710 | − 0.18 | 0.48 | .712 | 5.08 | 4.00 | .207 | 5.08 | 4.00 | .207 |
| White | 0 | – | – | 0 | – | – | 0 | – | – | 0 | – | – |
| BMI | 0.03 | 0.03 | .212 | 0.03 | 0.03 | .210 | 0.08 | 0.24 | .739 | 0.08 | 0.24 | .739 |
| Comorbidities | − 0.004 | 0.30 | .988 | − 0.01 | 0.30 | .987 | −1.52 | 2.63 | .565 | −1.52 | 2.63 | .565 |
| Physical activity | − 0.03 | 0.01 | .004 | − 0.03 | 0.01 | .004 | −0.15 | 0.08 | .055 | −0.15 | 0.08 | .002 |
| Beta blocker use | − 14.73 | 4.58 | .002 | − 14.59 | 2.30 | <.001 | ||||||
| Time | 2.05 | 0.04 | <.001 | 2.01 | 0.04 | <.001 | 15.37 | 0.21 | <.001 | 15.37 | 0.22 | <.001 |
| Elevated depressive symptoms | − 0.28 | 0.43 | .517 | − 0.84 | 0.48 | .082 | −4.62 | 3.78 | .224 | −4.73 | 3.95 | .234 |
| Time × depressive symptoms | 0.29 | 0.11 | .010 | 0.06 | .591 | .925 | ||||||
Fig. 1.
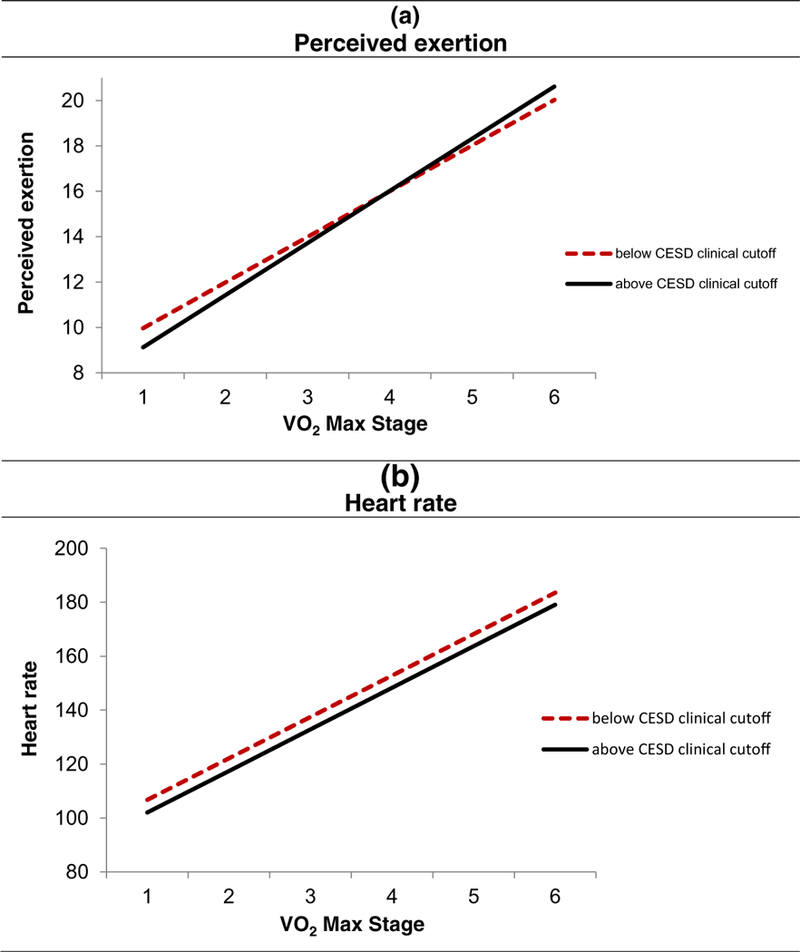
Effect of elevated depressive symptoms on perceived exertion and heart rate. Each stage represents a 2-min interval during the exercise test. Women above the CES-D clinical cutoff reported greater increases in perceived exertion compared to women below the cutoff (b = 0.29, SE = 0.11, p = .010, panel a); of note, they did not differ significantly at baseline (b = −0.54, SE = 0.43, p = .221). In contrast, elevated depressive symptoms were unrelated to exercise heart rate (all ps > .224, panel b)
Covariates were associated with perceived exertion in expected ways. For example, older women reported higher levels of perceived exertion compared to younger women (b = 0.04, SE = 0.01, p = .003). Those who had higher levels of physical activity reported lower exertion (b = – 0.03, SE = 0.01, p = .004).
Depression and Heart Rate
Heart rate and heart rate changes during the exercise test did not differ between women with elevated depressive symptoms compared to those who scored below the CES-D clinical cutoff (all ps>.224; see Fig. 1 panel b). Continuously measured depressive symptoms were also unrelated to exercise heart rate (all ps>.123). Similarly, women with an MDD history did not demonstrate differences in heart rate and heart rate changes during the exercise test compared to those without a major depression history (all ps > .200). In terms of important covariates, older age, beta blocker use, and higher levels of physical activity were significantly associated with lower heart rate (see Table 3).
Secondary Analyses
Secondary analyses tested the effect of depression and depressive symptoms on cardiovascular fitness (i.e., VO2max). None of the depression variables were significantly associated with cardiovascular fitness (allps > .486).
Discussion
Breast cancer patients with clinically elevated depressive symptoms experienced steeper increases in exercise difficulty compared to those without current depression risk. However, they did not show differences in heart rate compared to those with minimal depressive symptoms. In contrast, women with an MDD history did not report differences in perceived exertion compared to women without a depression history. Depression was unrelated to cardiovascular fitness performance on the test. These results indicate that current, but not formerly, depressed patients experienced the exercise test as more difficult compared to those without elevated depressive symptoms, even though this was not reflected in their actual, objective exertion. These effects held after accounting for important covariates such as age, routine physical activity, and medication use.
Findings from this study are consistent with prior literature indicating that individuals with depression are more likely than their non-depressed peers to judge neutral information negatively and have trouble disengaging from negative information [40, 41]. Although theories of negative information processing have not been extended to exercise, it is possible that our breast cancer patients with elevated depressive symptoms perceived the sensations associated with exercise as more negative and had a harder time disengaging from these sensations. Additionally, depressed individuals show impairments in interoceptive accuracy, or the ability to detect internal bodily sensations [19]. For example, depressed individuals (or those with elevated depressive symptoms) perform more poorly on tasks measuring a person’s ability to perceive and detect their own heartbeat (e.g., [41–43]). Importantly, interoception deficits appear to normalize following depression remission [44], which may explain why women with a lifetime MDD history in the current study did not show differences in perceived exertion during exercise compared to women without such a history.
Despite the finding that women with current depression risk experienced exercise as more demanding, the breast cancer patients with elevated depressive symptoms had similar heart rates to those reporting minimal depressive symptoms. This finding is contrary to prior research indicating that individuals with depressive disorders tend to have smaller increases in heart rate during exercise [24, 25] but is consistent with a contradictory finding that depressed individuals show similar rates of heart rate change compared to non-depressed peers [25].
Prior studies have not investigated the effect of depression on ratings of exertion during exercise, but a few have reported effects of chronic stress and mood on perceived exertion, with mixed results. For example, one study found that individuals primed with happy faces prior to an exercise test reported lower levels of exertion [45], while another study found no relationship between current mood and perceived exertion during exercise [46]. One study that investigated perceived exertion and chronic stress found that individuals who reported more stressful life events over 6 weeks showed smaller increases in perceived exertion ratings during workout sessions [47]. The authors suggested that chronic stress may desensitize individuals to noxious stimuli, resulting in blunted affective and physiological responses to unpleasant sensations, such as those that may occur during exercise. Although their chronic stress finding differs from our results with depression, deficits in negative information processing biases and interoception that are depression-specific likely account for the discrepancy.
Results of the current study suggest that depressed breast cancer patients perceive exercise as more physically demanding compared to their non-depressed peers, a novel factor which may contribute to low physical activity levels in this population. The finding that individuals with elevated depressive symptoms experience exercise as more effortful is important, because these perceptions could influence their exercise-related attitudes, self-efficacy, and perceived behavioral control (i.e., the extent to which they believe they are able to exercise). Indeed, individuals with elevated depressive symptoms report less perceived behavioral control over physical activity [48] and poorer conscious and non-conscious self-efficacy [49, 50], all of which predict exercise behavior [51, 52]. Individuals with more severe depressive symptoms also report more negative experiences with exercise, which could contribute to more strongly negative implicit attitudes towards exercise [53, 54]. Additionally, depressed individuals appear to have deficits in self-regulation [55–57]. A combination of negative attitudes and poor self-regulatory skills, in particular, may leave a depressed individual especially vulnerable to decreased physical activity, as previous findings suggest that individuals with poor self-regulatory capacity and negative implicit attitudes have shorter average workout durations [58].
Limitations
It should be noted that only 13% of our sample (n =14) scored above the clinical cutoff on the CES-D. Although this figure is representative of prior studies estimating that elevated depressive symptoms occur in 13–18% of post-surgery breast cancer patients [8, 9], it is a relatively small number of participants given our sample size. Additionally, most women in the study were well-educated, Caucasian and younger than the average breast cancer patient, potentially limiting generalizability. The current study’s cross-sectional design limits our ability to test a mediation pathway linking depression, perceived exertion, and physical activity. However, our finding of significant bivariate associations among average perceived exertion, self-reported physical activity, and objectively measured cardiorespiratory fitness support provide preliminary support for the notion that depression may result in reduced activity via heightened perceptions of exercise difficulty.
Implications and Future Directions
Depression may cause exercise to feel more effortful which could ultimately decrease patients’ likelihood of engaging in regular exercise, resulting in lower cardiovascular fitness over the course of survivorship. Study results underscore the importance of screening post-surgery breast cancer patients for depressive symptoms, as recommended by The National Comprehensive Cancer Network Distress Management Clinical Practice Guidelines in Oncology [59]. Exercise interventions may be especially important for depressed patients, as such interventions improve both fitness and depressive symptoms [60,61]; however, depressive symptoms have been shown to attenuate the effects of such interventions on physical activity itself [62]. Given this, it may be important to develop novel physical activity interventions that are tailored to address specific deficits depressed breast cancer patients likely face, such as negative perceptions of exercise sensations and reduced interoceptive awareness.
These results are especially important given that breast cancer patients were studied at a crucial, but understudied treatment timepoint: after surgery but prior to initiating chemotherapy or radiation. Breast cancer patients who have recovered from surgery can and should be exercising during the postoperative period, in order to stave off the negative short and long-term effects of physical fitness declines [63–65]. The current study indicates that breast cancer patients who are at risk for depression following breast cancer surgery perceive exercise as more unpleasant compared to their peers, which may ultimately result in lower physical activity and fitness among these women.
Acknowledgments
Funding This work was supported in part by the National Institutes of Health grant nos. R01 CA186720, UL1TR001070, K05 CA172296, T32 DE014320, and a Pelotonia Postdoctoral Fellowship from Ohio State University’s Comprehensive Cancer Center. The first author also receives support from the National Institutes of Health grant no. R01 CA186720– 02S2, and the third author receives support from the National Institutes of Health grant no. R01 CA186720–02S1.
Footnotes
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest The authors declare that they have no conflict of interest.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictionalclaims in published maps and institutional affiliations.
References
- 1.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Coumeya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:242–74. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28:753–65. [DOI] [PubMed] [Google Scholar]
- 3.Phillips SM, Awick EA, Conroy DE, Pellegrini CA, Mailey EL, McAuley E. Objectively measured physical activity and sedentary behavior and quality of life indicators in survivors of breast cancer: activity sedentary time quality of life. Cancer. 2015;121:4044–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Vulpen JK, Peeters PHM, Velthuis MJ, van der Wall E, May AM. Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: a meta-analysis. Maturitas. 2016;85:104–11. [DOI] [PubMed] [Google Scholar]
- 5.Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36:1484–91. [PMC free article] [PubMed] [Google Scholar]
- 6.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30: 2530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones LW, Habel LA, Weltzien E, Castillo A, Gupta D, Kroenke CH, et al. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol. 2016;34:2743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen S, Zachariae R, Jensen AB, Vaeth M, Moller S, Ravnsbaek J, et al. Prevalence and risk of depressive symptoms 3–4 months post-surgery in a nationwide cohort study of Danish women treated for early stage breast-cancer. Breast Cancer Res Treat. 2009;113:339–55. [DOI] [PubMed] [Google Scholar]
- 9.Golden-Kreutz DM, Andersen BL. Depressive symptoms after breast cancer surgery: relationships with global, cancer-related, and life event stress. Psycho-Oncology. 2004;13:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emery CF, Yang H-C, Frierson GM, Peterson LJ, Suh S. Determinants of physical activity among women treated for breast cancer in a 5-year longitudinal follow-up investigation. Psycho-Oncology. 2009;18:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong S, Bardwell WA, Natarajan L, Flatt SW, Rock CL, Newman VA, et al. Correlates of physical activity level in breast cancer survivors participating in the Women’s Healthy Eating and Living (WHEL) Study. Breast Cancer Res Treat. 2007;101:225–32. [DOI] [PubMed] [Google Scholar]
- 12.Beck AT. Depression: clinical, experimental, and theoretical aspects. New York: Harper & Row; 1967. [Google Scholar]
- 13.Beck AT. The evolution ofthe cognitive model of depression and its neurobiological correlates. Am J Psychiatry. 2008;165:969–77. [DOI] [PubMed] [Google Scholar]
- 14.Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. NeuroImage. 2007;47: 987–94. [DOI] [PubMed] [Google Scholar]
- 15.Adler G, Gattaz WF. Pain perception threshold in major depression. Biol Psychiatry. 1993;34:687–9. [DOI] [PubMed] [Google Scholar]
- 16.Dickens C, McGowan L, Dale S. Impact of depression on experimental pain perception: a systematic review of the literature with meta-analysis. Psychosom Med. 2003;65:369–75. [DOI] [PubMed] [Google Scholar]
- 17.Euteneuer F, Schwarz MJ, Hennings A, Riemer S, Stapf T, Selberdinger V, et al. Depression, cytokines and experimental pain: evidence for sex-related association patterns. J Affect Disord. 2011;131:143–9. [DOI] [PubMed] [Google Scholar]
- 18.Hermesdorf M, Berger K, Baune BT, Wellmann J, Ruscheweyh R, Wersching H. Pain sensitivity in patients with major depression: differential effect of pain sensitivity measures, somatic cofactors, and disease characteristics. J Pain. 2016;17:606–16. [DOI] [PubMed] [Google Scholar]
- 19.Harshaw C Interoceptive dysfunction: toward an integrated framework for understanding somatic and affective disturbance in depression. Psychol Bull. 2015;141:311–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kibler JL, Ma M. Depressive symptoms and cardiovascular reactivity to laboratory behavioral stress. Int J Behav Med. 2004;11:81–7. [DOI] [PubMed] [Google Scholar]
- 21.Carroll D, Phillips AC, Hunt K, Der G. Symptoms of depression and cardiovascular reactions to acute psychological stress: evidence from a population study Biol Psychol. 2007;75:68–74. [DOI] [PubMed] [Google Scholar]
- 22.Phillips AC, Hunt K, Der G, Carroll D . Blunted cardiac reactions to acute psychological stress predict symptoms of depression five years later: evidence from a large community study. Psychophysiology. 2011;48:142–8. [DOI] [PubMed] [Google Scholar]
- 23.York KM, Hassan M, Li Q, Li H, Fillingim RB, Sheps DS. Coronary artery disease and depression: patients with more depressive symptoms have lower cardiovascular reactivity during laboratory-induced mental stress. Psychosom Med. 2007;69:521–8. [DOI] [PubMed] [Google Scholar]
- 24.Lavoie KL, Fleet RP, Lespérance F, Arsenault A, Laurin C, Frasure-Smith N, et al. Are exercise stress tests appropriate for assessing myocardial ischemia in patients with major depressive disorder? Am Heart J. 2004;148:621–7. [DOI] [PubMed] [Google Scholar]
- 25.Pelletier R, Lavoie KL, Gordon J, Arsenault A, Campbell TS, Bacon SL. The role of mood disorders in exercise-induced cardiovascular reactivity. Psychosom Med. 2009;71:301–7. [DOI] [PubMed] [Google Scholar]
- 26.Eriksen L, Grønbaek M, Helge JW, Tolstrnp JS. Cardiorespiratory fitness in 16,025 adults aged 18–91 years and associations with physical activity and sitting time. Scand J Med Sci Sports. 2015. [DOI] [PubMed] [Google Scholar]
- 27.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 28.Borg G Pyschophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–81. [PubMed] [Google Scholar]
- 29.Borg G Borg’s perceived exertion and pain scales. Champaign: Human Kinetics; 1998. [Google Scholar]
- 30.Chen MJ, Fan XT, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873–99. [DOI] [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D scale. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 32.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: evaluation of the Center for Epidemiological Studies Depression Scale (CES-D). J Psychosom Res. 1999;46:437–43. [DOI] [PubMed] [Google Scholar]
- 33.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12:277–87. [DOI] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, non-patient edition (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 35.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 36.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- 37.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–90. [DOI] [PubMed] [Google Scholar]
- 38.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985;10:141–6. [PubMed] [Google Scholar]
- 39.Amireault S, Godin G, Lacombe J, Sabiston CM. Validation of the Godin-Shephard Leisure-Time Physical Activity Questionnaire classification coding system using accelerometer assessment among breast cancer survivors. J Cancer Surviv. 2015;9:532–40. [DOI] [PubMed] [Google Scholar]
- 40.Everaert J, Koster EHW, Derakshan N. The combined cognitive bias hypothesis in depression. Clin Psychol Rev. 2012;32:413–24. [DOI] [PubMed] [Google Scholar]
- 41.Joormann J, D’Avanzato C. Emotion regulation in depression: examining the role of cognitive processes. CognEmot. 2010;24:913–39. [Google Scholar]
- 42.Terhaar J, Viola FC, Bär K-J, Debener S. Heartbeat evoked potentials mirror altered body perception in depressed patients. Clin Neurophysiol. 2012;123:1950–7. [DOI] [PubMed] [Google Scholar]
- 43.Furman DJ, Waugh CE, Bhattacharjee K, Thompson RJ, Gotlib IH. Interoceptive awareness, positive affect, and decision making in major depressive disorder. J Affect Disord. 2013;151:780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiebking C, de Greck M, Duncan NW, Tempelmann C, Bajbouj M, Northoff G. Interoception in insula subregions as a possible state marker for depression—an exploratory fMRI study investigating healthy, depressed and remitted participants. Front Behav Neurosci. 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanchfield A, Hardy J, Marcora S. Non-conscious visual cues related to affect and action alter perception of effort and endurance performance. Front Hum Neurosci. 2014;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Viana BF, Pires FO, Inoue A, Micklewright D, Santos TM. Correlates of mood and RPE during multi-lap off-road cycling. Appl Psychophysiol Biofeedback. 2016;41:1–7. [DOI] [PubMed] [Google Scholar]
- 47.Stults-Kolehmainen MA, Lu T, Ciccolo JT, Bartholomew JB, Brotnow L, Sinha R. Higher chronic psychological stress is associated with blunted affective responses to strenuous resistance exercise: RPE, pleasure, pain. Psychol Sport Exerc. 2016;22:27–36. [Google Scholar]
- 48.Hemmis L, de Vries H, Vandelanotte C, Short CE, Duncan MJ, Burton NW, et al. Depressive symptoms associated with psychological correlates of physical activity and perceived helpfulness of intervention features. Ment Health and Phys Act. 2015;9:16–23. [Google Scholar]
- 49.Maeda U, Shen B-J, Schwarz ER, Farrell KA, Mallon S. Self-efficacy mediates the associations of social support and depression with treatment adherence in heart failure patients. Int J Behav Med. 2013;20:88–96. [DOI] [PubMed] [Google Scholar]
- 50.Risch AK, Buba A, Birk U, Morina N, Steffens MC, Stangier U. Implicit self-esteem in recurrently depressed patients. J Behav Ther Exp Psychiatry. 2010;41:199–206. [DOI] [PubMed] [Google Scholar]
- 51.Neupert SD, Lachman ME, Whitbourne SB. Exercise self-efficacy and control beliefs predict exercise behavior after an exercise intervention for older adults. J Aging Phys Act. 2009;17:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godin G, Valois P, Lepage L. The pattern of influence of perceived behavioral control upon exercising behavior: an application of Ajzen’s theory of planned behavior. JBehavMed. 1993;16:81–102. [DOI] [PubMed] [Google Scholar]
- 53.Pomp S, Fleig L, Schwarzer R, Lippke S. Depressive symptoms interfere with post-rehabilitation exercise: outcome expectancies and experience as mediators. Psychol Health Med. 2012;17:698–708. [DOI] [PubMed] [Google Scholar]
- 54.Greenwald AG, Banaji MR. Implicit social cognition: attitudes, self-esteem, and stereotypes. Psychol Rev. 1995;102:4–27. [DOI] [PubMed] [Google Scholar]
- 55.Lonigan CJ, Vasey MW. Negative affectivity, effortful control, and attention to threat-relevant stimuli. J Abnorm Child Psychol. 2009;37:387–99. [DOI] [PubMed] [Google Scholar]
- 56.Strauman TJ. Self-regulation and depression. Self Identity. 2002;1: 151–7. [Google Scholar]
- 57.Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychol Bull. 2008;134:912–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Padin AC, Emery CF, Vasey M, Kiecolt-Glaser JK. Self-regulation and implicit attitudes toward physical activity influence exercise behavior. J Sport Exerc Psychol. 2017;39:237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holland JC, Andersen B, Breitbart WS, Buchmann LO, Compas B, Deshields TL, et al. Distress management: clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2013;11:190–209. [DOI] [PubMed] [Google Scholar]
- 60.Chase J-AD, Conn VS. Meta-analysis of fitness outcomes from motivational physical activity interventions. Nurs Res. 2013;62: 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Josefsson T, Lindwall M, Archer T. Physical exercise intervention in depressive disorders: meta-analysis and systematic review. Scand J Med Sci Sports. 2014;24:259–72. [DOI] [PubMed] [Google Scholar]
- 62.Schoeny ME, Fogg L, Buchholz SW, Miller A, Wilbur J. Barriers to physical activity as moderators of intervention effects. Prev Med Rep. 2017;5:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson RT, Kimmick GG, McCoy TP, Hopkins J, Levine E, Miller G, et al. A randomized trial of exercise on well-being and function following breast cancer surgery: the RESTORE trial. J Cancer Surviv. 2012;6:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McNeely ML, Campbell K, Ospina M, Rowe BH, Dabbs K, Klassen TP, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010: CD005211. [DOI] [PubMed] [Google Scholar]
- 65.Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev. 2016;9:CD005001. [DOI] [PMC free article] [PubMed] [Google Scholar]


