Abstract
This study examined concentrations of 15 perfluoroalkyl acids (PFAAs) in tissues from male Mozambique tilapia (Oreochromis mossambicus) collected at Loskop Dam, Mpumalanga, South Africa in 2014 and 2016. Nine of the 15 PFAAs were detected frequently and were included in statistical analysis and included two of the most commonly known PFAAs, perfluorooctanesulfonic acid (PFOS) (median, 41.6 ng/g) and perfluorooctanoic acid (PFOA) (median, 0.0825 ng/g). Of the tissues measured, plasma (2016 and 2014 median, 22.2 ng/g) contained the highest PFAA burden followed by (in descending order): liver (median, 11.6 ng/g), kidney (median, 9.04 ng/g), spleen (median, 5.92 ng/g), adipose (median, 2.54 ng/ g), and muscle (median, 1.11 ng/g). Loskop Dam tilapia have been affected by an inflammatory disease of the adipose tissue known as pansteatitis, so this study also aimed to investigate relationships between PFAA tissue concentrations and incidence of pansteatitis or fish health status. Results revealed that healthy tilapia exhibited an overall higher (p-value < 0.05) PFAA burden than pansteatitis-affected tilapia across all tissues. Further analysis showed that organs previously noted in the literature to contain the highest PFAA concentrations, such as kidney, liver, and plasma, were the organs driving the difference in PFAA burden between the two tilapia groups. Care must be taken in the interpretations we draw from not only the results of our study, but also other PFAA measurements made on populations (human and wildlife alike) under differing health status.
Keywords: Perfluoroalkyl acids, Liquid chromatography mass, spectrometry, Pansteatitis, Perfluorooctanesulfonic acid, Tissue distribution
Introduction
Per- and polyfluoroalkyl substances (PFASs) are a class of man-made chemicals commonly employed in commercial and industrial products such as stain-resistant materials, textiles, and non-stick surfaces. Consequently, PFASs are released into the environment via industrial manufacturing processes and/or indirectly from consumer products. Since their introduction to the market in the 1950s (Renner, 2006), PFASs have made their way into humans (Calafat et al., 2007), domestic pets (Bost et al., 2016), and wildlife (Houde et al., 2011) across the globe. For this reason, PFASs have become a class of chemicals of considerable concern to human and animal health.
Despite a growing knowledge on the pervasiveness of PFASs globally, a limited number of studies on PFASs in humans and wildlife exist within the continent of Africa (Hanssen et al., 2010; Bouwman et al., 2014; Mudumbi et al., 2014; Ololade, 2014; Christie et al., 2016) in comparison to areas in Asia (e.g., China), Europe, and North America (e.g., United States) (Houde et al., 2011). Recent investigations into plasma PFAS levels from the Nile crocodile (Crocodylus niloticus) sampled in South Africa revealed comparable levels of PFASs to those found in the American alligator (Alligator mississippiensis) from the southeastern US (Bangma et al., 2017). However, further analysis needs to be performed to fully understand the burden and distribution of PFASs in Africans and wildlife.
Within PFASs, a family of nonpolymer perfluoroalkyl substances known as perfluoroalkyl acids (PFAAs) is commonly studied. Two subclasses of the PFAA family that will be investigated in this study are perfluoroalkyl carboxylic acids (PFCAs) and perfluoroalkyl sulfonic acids (PFSAs). Structurally, PFCAs and PFSAs have the general chemistry formula CnF2n + 1COOH and CnF2n + 1SO3H, respectively (Buck et al., 2011). It is the numerous carbon–fluorine bonds that provide the chemical and thermal stability of these two subclasses of PFAAs and prevent their breakdown once released into the environment (Moody and Field, 2000). Once internalized by an organism, these PFAAs, which are proteinophilic, are transported within the body and accumulate in protein-heavy matrices like the blood, kidney and liver (Kudo, 2015). Within these matrices, PFAAs have shown an affinity for albumin in the plasma, fatty acid binding protein (FABP) in the liver, and organic anion transporters (OATs) in the kidney in several species (Jones et al., 2003; Hebert and MacManus-Spencer, 2010; Ng and Hungerbühler, 2013). Although species-specific and sex-specific variations in PFAA excretion rates have been observed, the actual mechanism(s) controlling PFAA toxicity is not fully elucidated (Peters and Gonzalez, 2011). Overall, PFAAs are partial to binding to albumin, for most species studied, making these compounds readily measurable in plasma employing current technological methods (Reiner et al., 2011b). This aspect makes non-lethal sampling of potentially exposed organisms beneficial by using blood matrices in field studies.
Despite the obvious advantages for the sampling of blood matrices, tissue distribution studies of PFAAs for individual species and/or organisms are also beneficial for several reasons. Specifically, tissue distribution studies (1) better inform plasma-based studies by serving as a proxy for understanding whole-body PFAA burden and (2) allow the comparison of disparate tissue studies on PFAA burden. Regarding the latter point, contaminant exposure studies often focus on a variety of tissues for multiple purposes and having tissue distribution studies available can allow for comparisons between data sets on the same or similar species across the globe. This comparative ability allows for a more complete understanding of the distribution of PFAAs, which will ultimately allow for an improved examination into patterns, such as chain length patterns, spatial patterns across continents, and potential source patterns of PFAAs. One such example of a spatial pattern is the presence of high levels of perfluorooctanesulfonamide (PFOSA) in wildlife species in the arctic regions (Butt et al., 2010; Reiner et al., 2011a).
To date, tissue distribution studies have investigated PFAAs in multiple animal species including harbor seals (Phoca vitulina) (Van de Vijver et al., 2003), baikal seals (Pusa sibirica) (Ishibashi et al., 2008), arctic foxes (Vulpes lagopus) (Aas et al., 2014), Sprague–Dawley rats (De Silva et al., 2009), rainbow trout (Oncorhynchus mykiss) (Martin et al., 2003), and a variety of bird species (Verreault et al., 2005; Herzke et al., 2009; Rubarth et al., 2011). Shi et al. (2012) investigated PFAAs in liver, brain, muscle and eggs of farmed tilapia from markets in Beijing, China. However, data on PFAA concentrations in wild tilapia are unavailable. We aim to characterize PFAAs in additional tissue matrices, including blood plasma, from a population of wild Mozambique tilapia (Oreochromis mossambicus) at Loskop Dam, Mpumalanga, South Africa. With this additional information, future studies in tilapia species could potentially use non-lethal plasma samples as a proxy to understand the burden of other tissues and also allow a comparison of tilapia plasma PFAA studies to other studies involving disparate tissues.
Loskop Dam is part of the Olifants River/watershed, a water shed that also supplies Kruger National Park, and is located upstream of sites on the Olifants River where elevated concentrations of PFAAs have recently been detected in Nile crocodiles (Christie et al., 2016). Sources of PFAAs in the area are currently unknown; however, we hypothesize that the sources resulting in PFAA exposure in crocodiles in the Olifants River also result in PFAA exposure to wildlife at Loskop Dam.
In recent years, both crocodile and tilapia populations in the Olifants River have been affected by the inflammatory disease pansteatitis. Pansteatitis leads to inflammation and hardening of adipose tissue with severe cases resulting in immobility and death (Lane et al., 2013). Pansteatitis out-breaks have affected wildlife in the Mpumalanga region and nearby Kruger National Park for nearly a decade resulting in a number of fish and crocodile mortality events over the years (Ashton, 2010; Botha et al., 2011). Exposure to PFAAs is unlikely to be solely responsible for these outbreaks, as the levels of PFAAs observed in crocodile plasma in Mpumalanga (Christie et al., 2016) have been observed in crocodilians in other areas of the world where pansteatitis outbreaks have not been recorded (Bangma et al., 2017). However, it is possible that the health status (i.e., pathological condition) of tilapia may be influencing PFAA burden in pansteatitis-affected fish in some manner. Previously, wildlife studies investigating health status and PFAAs have found associations between various pathological conditions and PFAAs in sea otters and other marine mammals (Kannan et al., 2006; Van de Vijver et al., 2003), but no studies to date have investigated similar possibilities in non-mammalian species. The tilapia population at Loskop Dam offers a unique opportunity to not only examine tissue distribution in fish, but also to begin to investigate PFAA burden in relation to health status (pathological condition). Thus, the objectives of this study were to (1) examine PFAA tissue distribution in six tilapia tissues and (2) determine if PFAA level tissues differ between healthy and pansteatitis-affected tilapia.
1. Materials and methods
Methods employed in this study have been previously published and extended descriptions of chemicals and control materials, extraction method, purification, instrumental analysis (Reiner et al., 2011a, 2011b), blood chemistry measures (Bowden et al., 2016), and quality control can be found in the Supplemental information.
1.1. Sample collection
Sexually mature male Mozambique tilapia were collected from Loskop Dam, Mpumalanga, South Africa, in July 2014 and May 2016. Males were selected as a sex-based difference in male and female plasma PFAA levels was observed in a preliminary investigation conducted in 2014 (unpublished data). In addition to significantly higher plasma PFAAs, female tilapia had greater variation in PFAA levels (unpublished data) likely due to reproductive differences among females.
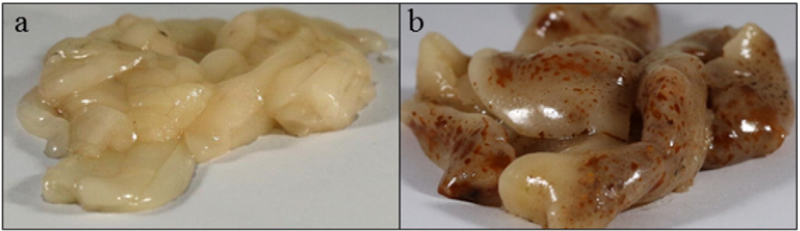
In July 2014, plasma and muscle samples were collected for 10 male tilapia and were separated into two groups, healthy (n = 5) and pansteatitis-affected (n = 5). In 2014, plasma was collected to understand baseline circulation levels of PFAAs in Loskop Dam tilapia, and muscle was collected to understand baseline PFAA tissue levels since locals frequently consume the fish from Loskop Dam. The second collection from Loskop Dam in 2016 was designed to include additional tissues as a follow-up to the preliminary data that showed that there were differences observed between healthy and pansteatitis-affected tilapia in the 2014 specimens. Therefore, in addition to plasma and muscle, the 2016 tilapia were also sampled for spleen, liver, kidney, muscle and adipose. For 2016, 13 tilapia, healthy (n = 6) and pansteatitis-affected (n = 7) individuals, were analyzed. Plasma, kidney, and liver were collected due to the higher levels of PFAAs typically measured in these organs in wildlife and laboratory animals (Houde et al., 2011). Spleen was collected due to additional literature marking it an organ of interest for PFAA burden (Van de Vijver et al., 2005). Finally, adipose was collected since pansteatitis manifests predominately in the adipose tissue (Bowden et al., 2016). Tilapia located at Loskop Dam have large amounts of adipose tissue scattered throughout their body, most notably surrounding the viscera. To standardize adipose collection for this study, approximately 1 g of adipose surrounding the intestines was consistently collected (Fig. 1).
Fig. 1.
(a) Healthy and (b) pansteatitis-affected adipose tissue collected from adipose reserves surrounding the intestines in Mozambique tilapia from Loskop Dam.
For both sampling events, tilapia were captured using composite gill nets. Blood was collected using a syringe and a 23 gauge (G) needle and was drawn immediately from the lateral line upon removal from the net. Blood was kept on ice until centrifugation and then flash frozen in liquid nitrogen for transport and storage at − 20°C. Fish were kept in an oxygenated tank until necropsy. Total length and mass were collected immediately preceding necropsy (Table S1). Approximately 1 g of each dissected tissue was trimmed, wrapped in methanol-rinsed foil and stored at − 20°C until analyzed.
1.2. Disease scoring
While lesions were present throughout various tissues in the pansteatitis-affected fish, fish were assigned healthy or pansteatitis-affected scores based on the condition of the adipose tissue, where the disease was most readily discernable, using a metric that has been established previously (Bowden et al., 2016). In brief, health classification was determined using criteria centered on number, size, and color of the lesions present in the adipose tissue (Bowden et al., 2016). Adipose scores ranged from 0 to 5. Fish with an adipose score <1 were considered healthy, and fish with an adipose score from 1 to 5 were considered pansteatitis-affected. For specimens collected in both 2014 and 2016, the healthiest and most diseased tilapia (highest adipose scores) were selected for PFAA investigations and comparisons. Examples of healthy and pansteatitis-affected adipose can been seen in Fig. 1.
1.3. Statistical methods
Statistical analyses were performed using IBM SPSS statistic software (Version 22.0, IBM Corp., US). Only PFAAs detected in 75% to 100% of the plasma and tissue sampled were statistically analyzed. PFAAs detected in < 50% for a given tissue were excluded from analysis for that tissue type. For those PFAAs included in statistical analyses, compounds less than the reporting limit (RL) were set equal to half the RL prior to running the statistical tests (Keller et al., 2005). t-Tests were used on log normally distributed data and the Mann–Whitney U test was employed for non-normally distributed data.
For comparisons between the healthy and pansteatitis-affected tilapia, 2 sample t-tests were applied to log normally distributed data whereas the Mann–Whitney U test was employed for non-normally distributed data. For correlative measures, Pearson correlations were used for log normally distributed data and Spearman’s correlation for non-parametric data.
2. Results and discussion
2.1. Loskop Dam PFAAs
In the present study, plasma and muscle tissue from 10 adult male tilapia were collected in 2014, and plasma, kidney, liver, spleen and muscle tissue from 13 adult male tilapia we collected in 2016. All samples were analyzed for the presence of 15 PFAAs, of which 10 were included in statistical analysis.
Statistical tests were performed for perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluorododecanoic acid (PFDoA), perfluorotridecanoic acid (PFTriA), perfluorohexanesulfonic acid (PFHxS), and perfluorooctanesulfonic acid (PFOS), which were detected in 75% to 100% of all tissue samples. Statistical tests were also performed for perfluorooctanoic acid (PFOA), PFOSA, perfluoroundecanoic acid (PFUnA), and perfluorotetradecanoic acid (PFTA), when detected in more than 50% of a tissue type (example: plasma) regardless of the detection percentage in all samples. The remaining PFAAs (perfluorobutyric acid (PFBA), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), and perfluorobutanesulfonic acid (PFBS)) were detected in less than 15% of the samples and were excluded from statistical analyses. PFAAs in the muscle and plasma samples from 2014 showed no statistical difference from PFAAs in the muscle and plasma samples collected in 2016, and therefore were combined with 2016 data when applicable.
Plasma and tissue values for individual tilapia collected in 2016 were pooled for each PFAA and the PFOS concentration (median, 41.6 ng/g) was recorded to be the highest concentration, as is commonly observed in both human and wildlife studies (Houde et al., 2011). From highest to lowest, total 2016 tilapia PFAA summed across tissues was as follows: PFOS > PFDA (median, 4.54 ng/g) > PFNA (median, 1.55 ng/g) > PFUnA (median, 1.41 ng/g) = PFDoA (median, 1.41 ng/g) > PFOSA (median, 0.768 ng/g) > PFHxS (median, 0.553 ng/g) > PFTriA (median, 0.250 ng/g) > PFTA (median, 0.210 ng/g) > PFOA (median, 0.0825 ng/g). Muscle and plasma values for individual tilapia collected in 2014 were also pooled for each PFAA and revealed lower medians due to fewer tissues sampled per tilapia, but resulted in the similar trend from highest (PFOS) to lowest (PFOA) PFAA. A number of other PFAA studies involving various fish species across the globe (Ye et al., 2008; Delinsky et al., 2010) have also reported low PFOA levels compared to other PFAAs. This phenomenon may be attributed to the quick clearance of PFOA for some fish species (Mortensen et al., 2011).
Correlations between PFAA plasma levels for all tilapia surveyed in 2014 and 2016 were investigated to examine potential site-specific trends. Significant (p < 0.05) coefficients of correlation (r) displaying high r-values were found between the plasma PFAA tested (Table 1). The highest r value of 0.983 was observed between plasma PFTA and PFTriA, with many other PFAAs had similar strong r with one another. This high co-variation between the various measured PFAAs suggests a high likelihood that PFAA concentrations detected in the tilapia at Loskop Dam originate from a similar or the same source.
Table 1 –
Coefficients of correlation (r) between concentrations of various perfluoroalkyl acids (PFAAs) in the plasma of Mozambique tilapia sampled from Loskop Dam, Mpumalanga, South Africa (2014–2016).
| PFOA | PFNA | PFDA | PFUnA | PFDoA | PFTA | PFTriA | PFHxS | PFOS | |
|---|---|---|---|---|---|---|---|---|---|
| PFOA | − | 0.945** | 0.827** | 0.782** | 0.665* | 0.692* | 0.644* | 0.815** | 0.888** |
| PFNA | − | − | 0.824** | 0.733** | 0.593** | 0.380 | 0.315 | 0.840** | 0.852** |
| PFNA | − | − | − | 0.958** | 0.858** | 0.708** | 0.656** | 0.564** | 0.933** |
| PFUnA | − | − | − | − | 0.934** | 0.801** | 0.776** | 0.454* | 0.886** |
| PFDoA | − | − | − | − | − | 0.909** | 0.911** | 0.256 | 0.813** |
| PFTA | − | − | − | − | − | − | 0.983** | 0.053 | 0.704** |
| PFTriA | − | − | − | − | − | − | − | −0.013 | 0.632** |
| PFHxS | − | − | − | − | − | − | − | − | 0.629** |
| PFOS | − | − | − | − | − | − | − | − | − |
PFOA: perfluorooctanoic acid; PFNA: perfluorononanoic acid; PFDA: perfluorodecanoic acid; PFUnA: perfluoroundecanoic acid; PFDoA: perfluorododecanoic acid; PFTA: perfluorotetradecanoic acid; PFTriA: perfluorotridecanoic acid; PFHxS: perfluorohexanesulfonic acid; PFOS: perfluorooctanesulfonic acid.
Correlations were derived using log-transformed values. All log-transformed PFAA values were normally distributed.
Significant at 0.05 level (2-tailed).
Significant at 0.01 level (2-tailed).
2.2. PFAA profiles across tissues
When each individual tilapia tissue was examined for ΣPFAAs, another trend emerged with plasma (2016 and 2014 median, 22.2 ng/g) containing the highest concentration of measured PFAAs followed by: liver (median, 11.6 ng/g), kidney (median, 9.04 ng/g), spleen (median, 5.92 ng/g), adipose (median, 2.54 ng/g), and muscle (2014 and 2016 median, 1.11 ng/g) (Fig. S1). Information on PFAA levels in each tissue is summarized in Table S2. Our results are similar to trends reported in other fish studies (Labadie and Chevreuil, 2011; Shi et al., 2012), but show some variation from other PFAA tissue studies involving red-throated divers (Rubarth et al., 2011), baikal seals (Ishibashi et al., 2008), and arctic foxes (Aas et al., 2014), where liver tissue contained the highest PFAA concentrations reported followed by plasma or serum and kidney, indicating subtle differences as to how species sequester PFAAs from tissue to tissue. While not the tissue with the highest PFAA burden, our study and others have shown that PFAAs, specifically PFOSA, readily accumulate into the spleen in wildlife as well as laboratory test species (Van de Vijver et al., 2005; Rubarth et al., 2011; Ross et al., 2012). This may be due to the affinity of PFOSA for red blood cells, as PFOSA concentrations have been detected up to 26 times higher in whole blood than in serum and that the spleen’s function is to filter and recycle old blood cells in the body (Kärrman et al., 2006; Ross et al., 2012). High concentrations of PFOSA in the spleen may be an important consideration in future studies investigating immunotoxic effects of PFAAs, given the important immunological function of this organ.
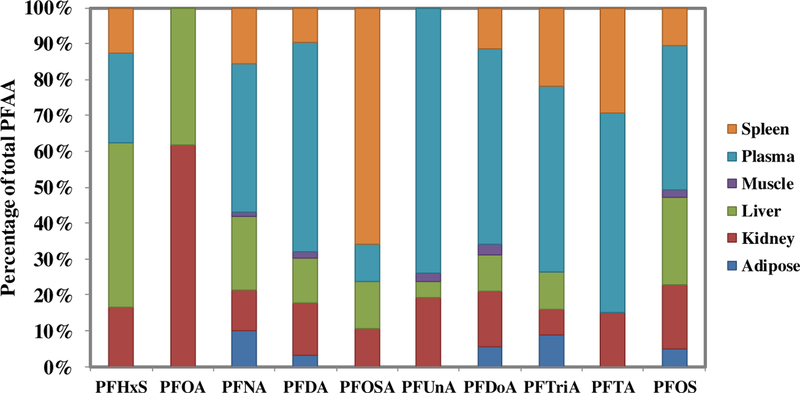
When tissue distribution of each of the nine detected PFAAs was examined separately, variability in PFAA compartmentalization was observed and resulted in a unique fingerprint (Fig. 2). As indicated in the literature, PFOSA, a PFOS precursor (Tomy et al., 2004), revealed a high affinity for the spleen, with the concentrations (median, 0.465 ng/g) higher than all other tissue concentrations combined (sum of medians of remaining tissues, 0.241 ng/g). PFHxS had the highest affinity for the liver compared to other tissues investigated with approximately 46% of PFHxS sequestering in the liver (median, 0.241 ng/g) compared to the remaining tissues (sum of median of remaining tissues, 0.283 ng/g). Over 7% of the PFUnA detected in tilapia tissue was measured in the plasma (median, 1.00 ng/g) compared to considerably less in the remaining tissues (sum of median in remaining tissues, 0.351 ng/g). Even though PFOA was infrequently detected across the majority of tissues investigated, this PFAA revealed a greater affinity for liver and kidney than plasma in this study as it was detected above RL in 62% and 85% of all kidney and liver samples, respectively, compared to only 46% of plasma samples.
Fig. 2.
Tissue compartmentalization of each of the nine measured perfluoroalkyl acids (PFAAs) in Mozambique tilapia from Loskop Dam (2014 and 2016). Values were not included for any PFAA not detected above reporting limit (RL) in more than 50% of a tissue (Example: PFOA in the spleen). PFHxS: perfluorohexanesulfonic acid; PFOA: perfluorooctanoic acid; PFNA: perfluorononanoic acid; PFDA: perfluorodecanoic acid; PFOSA: perfluorooctanesulfonamide; PFUnA: perfluoroundecanoic acid; PFDoA: perfluorododecanoic acid; PFTriA: perfluorotridecanoic acid; PFTA: perfluorotetradecanoic acid; PFOS: perfluorooctanesulfonic acid.
2.3. Health status and PFAAs
To determine if differences existed between healthy (n = 6) and pansteatitis-affected (n = 7) tilapia collected in 2016, overall PFAA burdens (sum of all PFAAs across all organs for each tilapia) were statistically compared between the two groups. Tests revealed a significant difference (p-value < 0.05) with healthy tilapia exhibiting an overall lower PFAA burden than pansteatitis-affected tilapia (Fig. S2). To determine what organs were driving the difference, concentrations of the ΣPFAAs across individual tissues were then statistically compared. Results indicated that the organs previously noted in the literature, that traditionally contribute to the bulk of PFAA burden, such as the kidney, liver, and plasma, were the organs driving the difference in ΣPFAAs between the two tilapia groups (Fig. 3). A further breakdown of individual PFAA contributions to the tissue ΣPFAA differences between healthy and pansteatitis-affected tilapia revealed PFOS and PFHxS as the two PFAAs with the most statistically significant differences between healthy and pansteatitis-affected organs (Table S3).
Fig. 3.
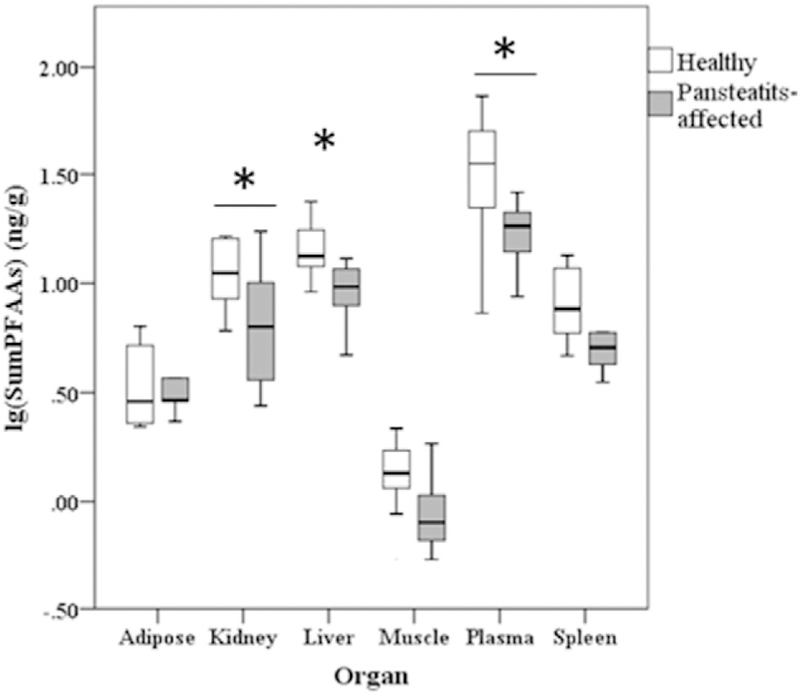
Difference in PFAA levels for 2016 tilapia tissues, between healthy (adipose score < 1, n = 6) and pansteatitis-affected tilapia (adipose score from 1 to 5, n = 7) from Loskop Dam in each organ investigated. Liver (p = 0.030), kidney (p = 0.026), and plasma (p = 0.023) were significantly different. Median values and 95% confidence interval (CI) depicted above. Asterisk (*) denotes significant difference (p ≤ 0.05).
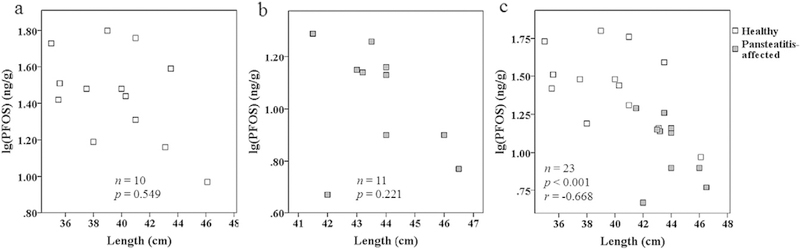
Relationships between fish total length and PFAA concentrations were investigated for all 2014 and 2016 plasma in both healthy and pansteatitis-affected fish. Tilapia total length between the two years was not significantly different and therefore was combined for analysis. Tilapia total length ranged from 35.0 to 46.5 cm (Table S1). A significant negative correlation was observed between total length and plasma PFOS (Fig. 4a) when pooling values recorded and comparing both healthy and pansteatitis-affected tilapia. In contrast, when healthy (Fig. 4b) and pansteatitis-affected (Fig. 4c) tilapia were examined separately, no significant correlations were observed. The negative correlation observed in the combined group could be driven by a number of factors. Research by Bowden et al. (2016) has shown that length is a potential indicator of pansteatitis among Loskop Dam tilapia, with healthy fish being significantly smaller than pansteatitis-affected individuals (Bowden et al., 2016). It is a possibility that length is driving the differences between healthy and pansteatitis-affected PFAAs as has been previously shown in some animal studies (Baduel et al., 2014; Bangma et al., 2017). However, we are not convinced that this is the case in the present study for several reasons. First, no significant correlations were observed when the tilapia were separated into health groups (2014 and 2016 combined). Second, in general, age-related trends in PFAA concentrations are not commonly observed in fish (Reiner and Place, 2015), and while a study by Pan et al. (2014), conducted on tilapia, revealed a positive correlation between length and PFOS (Pan et al., 2014), fish used in Pan et al. (2014) study were smaller in size than those investigated in this study. Third, the negative correlation observed in the combined group in this study could be an artifact of a small sample size. At present, there are no other studies that have investigated PFAA burden in tilapia of similar size and cohort as achieved in this study.
Fig. 4.
Correlations between length and plasma lg(PFOS) for male tilapia collected in 2014 and 2016 from Loskop Dam (a) healthy, (b) pansteatitis-affected, and (c) combination of healthy and pansteatitis-affected tilapia.
We have several hypotheses for the difference observed between pansteatitis-affected tilapia and the higher PFAA-burdened healthy tilapia. Firstly, we hypothesize that a difference in diet may contribute to the differences in PFAA concentrations between the healthy and pansteatitis-affected tilapia. A major source of PFAA bioaccumulation in nature is attributed to food intake (Houde et al., 2011; Mortensen et al., 2011). While the half-life of many PFAAs varies among species and is currently unknown in tilapia, it is possible that pansteatitis-affected tilapia exhibit a reduced appetite and/ or foraging success compared to their healthy counterparts. This could lead to a reduction in food consumption and ultimately the possibility of a decrease in PFAA exposure and accumulation.
Secondly, we hypothesize that changes in physiological conditions such as a reduction in the levels of protein (e.g., albumin) within pansteatitis-affected tilapia can reduce the abundance of proteins available for PFAA to readily bind to and thus PFAAs are more readily excreted from the body compared to healthy tilapia counterparts. While the cause of pansteatitis is still unknown, it has been shown to be an inflammatory disease of the adipose in crocodiles in the same watershed from which tilapia in this study were collected (Lane et al., 2013), and biomarker studies have shown that malnutrition and inflammation can lead to reduced protein levels (Don and Kaysen, 2004). Because inflammatory diseases can present with hypoalbuminemia and PFAAs show an affinity for albumin in the plasma, we investigated the levels of plasma albumin between our two tilapia subpopulations (Methods in Appendix A. Supplementary data). Results revealed that healthy tilapia exhibited significantly higher plasma albumin than pansteatitis-affected tilapia (Fig. S3) (Bowden et al., 2016). It is possible that the plasma of pansteatitis-affected tilapia cannot maintain a similar burden of PFAAs as healthy tilapia, due to the fact that if PFAAs bind readily to plasma albumin and there would be less albumin present in the pansteatitis-affected tilapia. Investigations into polyfluoroalkyl ether sulfonic acids (PFAES) in Crucian Carp (Carassius carassius) have suggested that bioaccumulation potential is concentration dependent with a saturation of serum albumin at higher exposures (Shi et al., 2015). Therefore, if the pansteatitis-affected tilapia maintain less albumin than their healthy counterparts, PFAAs may be saturating the pansteatitis-affected tilapia albumin at lower concentrations, leaving the remaining fraction of unbound PFAAs to be excreted. Future investigations are needed to examine this possibility and to examine potential changes in additional physiological conditions including proteins FABP and OATs in matrices such as the liver and kidney in pansteatitis-affected tilapia.
Numerous studies have investigated health, pathological condition, or body condition in relation to PFAA burden in wildlife and the results are variable. Some studies reported higher levels of PFOS and PFOA in the livers of diseased sea otters compared to their healthy counterparts (Kannan et al., 2006), while other studies observed similar results to our study whereby diseased (bronchopneumonia-affected) harbor seals maintained lower PFOS when compared to their healthy counterparts (Van de Vijver et al., 2003). Another study on harbor seals revealed no relationship between PFAA levels and general health status (Ahrens et al., 2009), while a study on artic foxes showed that differences between lean and fat body condition can correlate with changes in PFAA burden in a variety of tissues (Aas et al., 2014). Interestingly, in a human study, Yueng and colleagues found that patients that underwent liver transplantation for liver disease had lower levels of PFASs in their livers than reported in healthy individuals (Yeung et al., 2013). Similar to our study, Yueng hypothesizes that these differences might be attributed to physiological changes. In their case, the physiological change was fibrous tissue replacing the functional parenchymal in the diseased livers.
We suggest that species, diseases, and/or changes in body condition have the potential to affect PFAA burden differently. Data from our study suggest that PFAA burden in pansteatitis-affected tilapia is affected by disease state, either directly or indirectly via changes in diet or protein content resulting in pansteatitis-affected tilapia with lower levels of PFAAs than their healthy counterparts, but like Van de Vijver and colleagues have suggested, we must take care in the conclusions we draw from our results on the effect PFAAs have on wildlife health (Van de Vijver et al., 2003). The relationship between general health status and PFAA exposure in both wildlife and humans is an area greatly understudied. Like wildlife, humans are impacted by diseases of various types and from the base literature, we know that human PFAA exposure and accumulation is widespread (DeWitt, 2015); however, studies have yet to account for the influence potential changes in health status may have on PFAA burdens in an organism. This is especially significant for human epidemiological studies where chemical contaminant measurements (PFAAs or other xenobiotics of concern) in the blood are made after the onset of disease such as cross-sectional survey designs where an individual is already diagnosed at the time of blood draw. By doing so, key information on PFAA concentrations prior to the onset of disease and the exact role PFAAs play in the disease, if any, may be overlooked.
3. Conclusions
Nine of the 15 PFAAs investigated were detected frequently across collected tissues from all specimens (including perfluorinated carboxylic acids with chain lengths equal to or greater than eight carbons, PFOS, and PFOSA). Overall, plasma from specimens collected in both surveys contained the highest levels of ∑PFAAs (median, 22.2 ng/g) followed by liver (median, 11.6 ng/g), kidney (median, 9.04 ng/g), spleen (median, 5.92 ng/g), adipose (median, 2.54 ng/g), and muscle (median, 1.11 ng/g).
Statistical analyses revealed a significant difference (p-value < 0.05) with lower PFAA levels occurring in tilapia considered healthy compared to those affected by pansteatitis across all tissues tested. Tissues previously noted in the literature to traditionally contribute to the bulk of wildlife PFAA burden (plasma, kidney and liver) were the organs driving the difference in PFAAs between healthy and pansteatitis-affected tilapia.
While our results lead to many questions, the idea that health may alter PFAA tissue burden should not be disregarded. We suggest that researchers in the PFAA community take caution when drawing conclusions from not only our tilapia PFAA levels, but other wildlife PFAA levels, especially if comparing two groups of different health conditions. As shown with the tilapia in this study, health status has the potential to significantly alter the level of PFAAs in several tissues. This finding may also be something to take note of for human studies, especially in human PFAA plasma measurements when comparing health compromised individuals to healthy individuals.
Supplementary Material
Acknowledgments
This project was supported by the Medical University of South Carolina Center for Global Health.
The fieldwork was partly supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa (Grant No 101054). Any opinion, finding and conclusion or recommendation expressed in this material is that of the author(s) and the NRF does not accept any liability in this regard.
We thank the Mpumalanga Tourism and Parks Agency (MTPA) for permission to carry out this research at the Loskop Dam Nature Reserve, and we would like to thank the efforts of Andre Hoffmann for the help of collecting the tilapia specimens used in this study.
Footnotes
Disclaimer
Certain commercial equipment or instruments are identified in the paper and supplemental information to specify adequately the experimental procedures. Such identification does not imply recommendations or endorsement by the National Institutes of Standards and Technology (NIST); nor does it imply that the equipment or instruments are the best available for the purpose.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jes.2017.03.041.
REFERENCES
- Aas CB, Fuglei E, Herzke D, Yoccoz NG, Routti H, 2014. Effect of body condition on tissue distribution of perfluoroalkyl substances (PFASs) in Arctic fox (Vulpes lagopus). Environ. Sci. Technol 48, 11654–11661. [DOI] [PubMed] [Google Scholar]
- Ahrens L, Siebert U, Ebinghaus R, 2009. Temporal trends of polyfluoroalkyl compounds in harbor seals (Phoca vitulina) from the German Bight, 1999–2008. Chemosphere 76, 151–158. [DOI] [PubMed] [Google Scholar]
- Ashton PJ, 2010. The demise of the Nile crocodile (Crocodylus niloticus) as a keystone species for aquatic ecosystem conservation in South Africa: the case of the Olifants River. Aquat. Conserv. Mar. Freshwat. Ecosyst 20, 489–493. [Google Scholar]
- Baduel C, Lai FY, Townsend K, Mueller JF, 2014. Size and age–concentration relationships for perfluoroalkyl substances in stingray livers from eastern Australia. Sci. Total Environ 496, 523–530. [DOI] [PubMed] [Google Scholar]
- Bangma JT, et al. , 2017. Perfluorinated alkyl acids in plasma of American alligators (Alligator mississippiensis) from Florida and South Carolina. Environ. Toxicol. Chem 36 (4), 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost PC, Strynar MJ, Reiner JL, Zweigenbaum JA, Secoura PL, Lindstrom AB, Dye JA, 2016. US domestic cats as sentinels for perfluoroalkyl substances: possible linkages with housing, obesity, and disease. Environ. Res 151, 145–153. [DOI] [PubMed] [Google Scholar]
- Botha H, Van Hoven W, Guillette LJ Jr., 2011. The decline of the Nile crocodile population in Loskop dam, Olifants River, South Africa. Water SA 37, 103–108. [Google Scholar]
- Bouwman H, Booyens P, Govender D, Pienaar D, Polder A, 2014. Chlorinated, brominated, and fluorinated organic pollutants in Nile crocodile eggs from the Kruger National Park, South Africa. Ecotoxicol. Environ. Saf 104, 393–402. [DOI] [PubMed] [Google Scholar]
- Bowden JA, Cantu TM, Chapman RW, Somerville SE, Guillette MP, Botha H, Hoffman A, Luus-Powell WJ, Smit WJ, Lebepe J, 2016. Predictive blood chemistry parameters for pansteatitis-affected Mozambique tilapia (Oreochromis mossambicus). PLoS One 11 (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt p., Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ, 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag 7, 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Berger U, Bossi R, Tomy GT, 2010. Levels and trends of poly-and perfluorinated compounds in the arctic environment. Sci. Total Environ 408, 2936–2965. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL, 2007. Serum concentrations of 11 polyfluoroalkyl compounds in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ. Sci. Technol 41, 2237–2242. [DOI] [PubMed] [Google Scholar]
- Christie I, Reiner JL, Bowden JA, Botha H, Cantu TM, Govender D, Guillette MP, Lowers RH, Luus-Powell WJ, Pienaar D, 2016. Perfluorinated alkyl acids in the plasma of South African crocodiles (Crocodylus niloticus). Chemosphere 154, 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva AO, Benskin JP, Martin LJ, Arsenault G, McCrindle R, Riddell N, Martin JW, Mabury SA, 2009. Disposition of perfluorinated acid isomers in Sprague-Dawley rats; part 2: subchronic dose. Environ. Toxicol. Chem 28, 555–567. [DOI] [PubMed] [Google Scholar]
- Delinsky AD, Strynar MJ, McCann PJ, Varns JL, McMillan L, Nakayama SF, Lindstrom AB, 2010. Geographical distribution of perfluorinated compounds in fish from Minnesota lakes and rivers. Environ. Sci. Technol 44, 2549–2554. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, 2015. Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances Springer. [Google Scholar]
- Don BR, Kaysen G, 2004. Poor nutritional status and inflammation: serum albumin: relationship to inflammation and nutrition. Semin. Dial 17, 432–437. [DOI] [PubMed] [Google Scholar]
- Hanssen L, Röllin H, Odland JØ, Moe MK, Sandanger TM, 2010. Perfluorinated compounds in maternal serum and cord blood from selected areas of South Africa: results of a pilot study. J. Environ. Monit 12, 1355–1361. [DOI] [PubMed] [Google Scholar]
- Hebert PC, MacManus-Spencer LA, 2010. Development of a fluorescence model for the binding of medium-to long-chain perfluoroalkyl acids to human serum albumin through a mechanistic evaluation of spectroscopic evidence. Anal. Chem 82, 6463–6471. [DOI] [PubMed] [Google Scholar]
- Herzke D, Nygård T, Berger U, Huber S, Røv N, 2009. Perfluorinated and other persistent halogenated organic compounds in European shag (Phalacrocorax aristotelis) and common eider (Somateria mollissima) from Norway: a suburban to remote pollutant gradient. Sci. Total Environ 408, 340–348. [DOI] [PubMed] [Google Scholar]
- Houde M, De Silva AO, Muir DCG, Letcher RJ, 2011. Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environ. Sci. Technol 45, 7962–7973. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Iwata H, Kim E-Y, Tao L, Kannan K, Amano M, Miyazaki N, Tanabe S, Batoev VB, Petrov E.a., 2008. Contamination and effects of perfluorochemicals in baikal seal (Pusa sibirica). 1. Residue level, tissue distribution, and temporal trend. Environ. Sci. Technol 42, 2295–2301. [DOI] [PubMed] [Google Scholar]
- Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP, 2003. Binding of perfluorinated fatty acids to serum proteins. Environ. Toxicol. Chem 22, 2639–2649. [DOI] [PubMed] [Google Scholar]
- Kannan K, Perrotta E, Thomas NJ, 2006. Association between perfluorinated compounds and pathological conditions in southern sea otters. Environ. Sci. Technol 40, 4943–4948. [DOI] [PubMed] [Google Scholar]
- Kärrman A, van Bavel B, Järnberg U, Hardell L, Lindström G, 2006. Perfluorinated chemicals in relation to other persistent organic pollutants in human blood. Chemosphere 64, 1582–1591. [DOI] [PubMed] [Google Scholar]
- Keller JM, Kannan K, Taniyasu S, Yamashita N, Day RD, Arendt MD, Segars AL, Kucklick JR, 2005. Perfluorinated compounds in the plasma of loggerhead and Kemp’s ridley sea turtles from the southeastern coast of the United States. Environ. Sci. Technol 39, 9101–9108. [DOI] [PubMed] [Google Scholar]
- Kudo N, 2015. Metabolism and pharmacokinetics. In: DeWitt JC (Ed.), Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances Springer, pp. 151–175. [Google Scholar]
- Labadie P, Chevreuil M, 2011. Partitioning behaviour of perfluorinated alkyl contaminants between water, sediment and fish in the Orge River (nearby Paris, France). Environ. Pollut 159, 391–397. [DOI] [PubMed] [Google Scholar]
- Lane EP, Huchzermeyer FW, Govender D, Bengis RG, Buss PE, Hofmeyr M, Myburgh JG, Steyl JCA, Pienaar DJ, Kotze A, 2013. Pansteatitis of unknown etiology associated with large-scale Nile crocodile (Crocodylus niloticus) mortality in Kruger National Park, South Africa: pathologic findings. J. Zoo. Wildl. Med 44, 899–910. [DOI] [PubMed] [Google Scholar]
- Martin JW, Mabury SA, Solomon KR, Muir DC, 2003. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem 22, 196–204. [PubMed] [Google Scholar]
- Moody CA, Field JA, 2000. Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ. Sci. Technol 34, 3864–3870. [Google Scholar]
- Mortensen AS, Letcher RJ, Cangialosi MV, Chu S, Arukwe A, 2011. Tissue bioaccumulation patterns, xenobiotic biotransformation and steroid hormone levels in Atlantic salmon (Salmo salar) fed a diet containing perfluoroactane sulfonic or perfluorooctane carboxylic acids. Chemosphere 83, 1035–1044. [DOI] [PubMed] [Google Scholar]
- Mudumbi JBN, Ntwampe SKO, Muganza FM, Okonkwo JO, 2014. Perfluorooctanoate and perfluorooctane sulfonate in South African river water. Water Sci. Technol 69, 185–194. [DOI] [PubMed] [Google Scholar]
- Ng CA, Hungerbühler K, 2013. Bioconcentration of perfluorinated alkyl acids: how important is specific binding? Environ. Sci. Technol 47, 7214–7223. [DOI] [PubMed] [Google Scholar]
- Ololade I, 2014. Spatial distribution of perfluorooctane sulfonate (PFOS) in major rivers in southwest Nigeria. Toxicol. Environ. Chem 96, 1356–1365. [Google Scholar]
- Pan C-G, Zhao J-L, Liu Y-S, Zhang Q-Q, Chen Z-F, Lai H-J, Peng F-J, Liu S-S, Ying G-G, 2014. Bioaccumulation and risk assessment of per- and polyfluoroalkyl substances in wild freshwater fish from rivers in the Pearl River Delta region, South China. Ecotoxicol. Environ. Saf 107, 192–199. [DOI] [PubMed] [Google Scholar]
- Peters JM, Gonzalez FJ, 2011. Why toxic equivalency factors are not suitable for perfluoroalkyl chemicals. Chem. Res. Toxicol 24, 1601–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner JL, Place BJ, 2015. Perfluorinated alkyl acids in wildlife. In: DeWitt JC (Ed.), Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances Springer, pp. 127–150. [Google Scholar]
- Reiner JL, O’Connell SG, Moors AJ, Kucklick JR, Becker PR, Keller JM, 2011a. Spatial and temporal trends of perfluorinated compounds in beluga whales (Delphinapterus leucas) from Alaska. Environ. Sci. Technol 45, 8129–8136. [DOI] [PubMed] [Google Scholar]
- Reiner JL, Phinney KW, Keller JM, 2011b. Determination of perfluorinated compounds in human plasma and serum Standard Reference Materials using independent analytical methods. Anal. Bioanal. Chem 401, 2899–2907. [DOI] [PubMed] [Google Scholar]
- Renner R, 2006. The long and the short of perfluorinated replacements. Environ. Sci. Technol 40, 12–13. [DOI] [PubMed] [Google Scholar]
- Ross MS, Wong CS, Martin JW, 2012. Isomer-specific biotransformation of perfluorooctane sulfonamide in Sprague–Dawley rats. Environ. Sci. Technol 46, 3196–3203. [DOI] [PubMed] [Google Scholar]
- Rubarth J, Dreyer A, Guse N, Einax JW, Ebinghaus R, 2011. Perfluorinated compounds in red-throated divers from the German Baltic Sea: new findings from their distribution in 10 different tissues. Environ. Chem 8, 419–428. [Google Scholar]
- Shi Y, Wang J, Pan Y, Cai Y, 2012. Tissue distribution of perfluorinated compounds in farmed freshwater fish and human exposure by consumption. Environ. Toxicol. Chem 31, 717–723. [DOI] [PubMed] [Google Scholar]
- Shi Y, Vestergren R, Zhou Z, Song X, Xu L, Liang Y, Cai Y, 2015. Tissue distribution and whole body burden of the chlorinated polyfluoroalkyl ether sulfonic acid F-53B in crucian carp (Carassius carassius): evidence for a highly bioaccumulative contaminant of emerging concern. Environ. Sci. Technol 49, 14156–14165. [DOI] [PubMed] [Google Scholar]
- Tomy GT, Tittlemier SA, Palace VP, Budakowski WR, Braekevelt E, Brinkworth L, Friesen K, 2004. Biotransformation of N-ethyl perfluorooctanesulfonamide by rainbow trout (Oncorhynchus mykiss) liver microsomes. Environ. Sci. Technol 38, 758–762. [DOI] [PubMed] [Google Scholar]
- Van de Vijver KI, Hoff PT, Das K, Van Dongen W, Esmans EL, Jauniaux T, Bouquegneau J-M, Blust R, De Coen W, 2003. Perfluorinated chemicals infiltrate ocean waters: link between exposure levels and stable isotope ratios in marine mammals. Environ. Sci. Technol 37, 5545–5550. [DOI] [PubMed] [Google Scholar]
- Van de Vijver KI, Hoff P, Das K, Brasseur S, Van Dongen W, Esmans E, Reijnders P, Blust R, De Coen W, 2005. Tissue distribution of perfluorinated chemicals in harbor seals (Phoca vitulina) from the Dutch Wadden Sea. Environ. Sci. Technol 39, 6978–6984. [DOI] [PubMed] [Google Scholar]
- Verreault J, Houde M, Gabrielsen GW, Berger U, Haukås M, Letcher RJ, Muir DC, 2005. Perfluorinated alkyl substances in plasma, liver, brain, and eggs of glaucous gulls (Larus hyperboreus) from the Norwegian Arctic. Environ. Sci. Technol 39, 7439–7445. [DOI] [PubMed] [Google Scholar]
- Ye X, Schoenfuss HL, Jahns ND, Delinsky AD, Strynar MJ, Varns J, Nakayama SF, Helfant L, Lindstrom AB, 2008. Perfluorinated compounds in common carp (Cyprinus carpio) fillets from the Upper Mississippi River. Environ. Int 34, 932–938. [DOI] [PubMed] [Google Scholar]
- Yeung LW, Guruge KS, Taniyasu S, Yamashita N, Angus PW, Herath CB, 2013. Profiles of perfluoroalkyl substances in the liver and serum of patients with liver cancer and cirrhosis in Australia. Ecotoxicol. Environ. Saf 96, 139–146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.