Abstract
Background:
A growing body of evidence indicates that gut microbiota characteristics may be closely related to mental dysfunctions. However, no studies have investigated fetal brain development in relation to the maternal gut microbiota, despite the extensive use of antibiotics in obstetric practice.
Objective:
To determine how periconceptional exposure to SuccinylSulfaThiazole (SST), a non-absorbable antibiotic, can affect behavior in rat offspring. This antibiotic drug has previously been shown to substantially perturb the gut microbiota in rats following a 28-day exposure.
Methods:
Female Wistar rats were divided in two groups: control, or exposed during one month before breeding until gestational day 15 to a diet containing 1% SST. We administered behavioral tests to offspring, i.e., open field (post-natal day 20), social interactions (P25), marble burying (P30), elevated plus maze (P35), and prepulse inhibition of the acoustic startle reflex (sensory gating) (P45).
Results:
Both male and female offspring exposed peri-conceptionally to SST showed reduced social interactions, with a decrease of about half in time spent in social interactions compared to controls, reduced exploration of the open arm by 20% in the elevated plus maze test indicating increased anxiety and altered sensorimotor gating, with a 1.5–2-fold decrease in startle inhibition.
Conclusion:
Maternal periconceptional exposure to SST provokes alterations in offspring behavior in the absence of maternal infection. Because we administered SST, a non-absorbable antibiotic, only to the dam, we conclude that these neurobehavioral alterations in the offspring are related to maternal gut microbiota alterations.
Keywords: Antibiotic, Behavior, Rat, Microbiota, SuccinylSulfaThiazole
1. Introduction
A growing body of evidence indicates that the gut microbiota affects mental functions. Several studies in germ-free rats have shown that the absence of gut microbiota leads to stress and anxiety (Crumeyrolle-Arias et al., 2014; O’Mahony et al., 2014). However, experimental studies on the influence of the maternal gut microbiota on fetal brain development are almost non-existent. One experimental study in mice showed that exposure during late pregnancy to a mixture of non-absorbable antibiotics in the drinking water, altered maternal microbiota composition, and provoked decreased locomotor activity in offspring (Tochitani et al., 2016). Moreover, alterations in vaginal microbiota by maternal stress, in a mouse model, have been linked to metabolic reprogramming of the gut and brain in the offspring (Jašarević et al., 2015). The question of whether maternal gut microbiota affect brain development is crucial, because >40% of pregnant women are administered antibiotics (Broe et al., 2014), often to treat urinary tract infections or for prophylaxis for preterm membrane rupture. Exposure to antibiotics has been associated with an increased risk of cerebral palsy in preterm babies (Kenyon et al., 2008). A1.2 to 2-fold increased risk of autism was reported in a Danish cohort following the use of antibiotics during pregnancy, including sulfonamides (Atladóttir et al., 2012). The use of the sulfa antibiotic trimethoprim, which inhibits a key step in the folate pathway, during the 12 weeks before conception was associated with increased risk of congenital malformations including neural tube defects (Andersen et al., 2013). However, epidemiological studies cannot distinguish the effects of the infections from those of antibiotics used to treat them. Therefore, experimental models are required to test the hypothesis that the maternal microbiome affects fetal brain development and to separate the effect on the developing fetus of gut microbiota alterations caused by an antibiotic drug from the effects due to the infection itself. In the present study, we used SuccinylSulfaThiazole (SST), a long-acting non-absorbable sulfonamide antibiotic drug. SST is used in clinical practice mainly to treat intestinal tract infections, since it remains in the gut much longer than absorbable antibiotics and has no systemic toxicity (Patrick, 1995). Once SST, a prodrug, reaches the slightly alkaline large intestine, it is slowly hydrolyzed by bacterial esterases to sulfathiazole, the active form. <4% of SST is absorbed into the bloodstream and >95% is retained in the gut (Patrick, 1995; Welch et al., 1942).
The term “gut-brain axis” refers to the bidirectional relationship between the gut and the brain, as the microbiota activity can directly modify the availability of metabolites and/or precursors used for the synthesis of neurotransmitters (Holzer and Farzi, 2014). In humans, 95% of serotonin, a neuropeptide which influences mood and behavior, is produced in the gut from tryptophan (Camilleri, 2009), and tryptophan availability can be altered by either diet modification (Zhang et al., 2006), or altered microbiota composition as in germ-free mice (Wikoff et al., 2009). Serotonergic neurons are among the earliest neurons to be differentiated and modulate a number of developmental events in the developing brain. Alterations in serotonin homeostasis cause permanent changes to adult behavior and modify the wiring of brain connections (Gaspar et al., 2003).
The objective of this study was to determine if periconceptional perturbation of the maternal gut microbiome by exposure to SST can affect behavior in rat offspring. Given that SST is also used in animal studies in combination with folate deficient diets when modeling folate deficiency, we measured homocysteine in the blood from dams after SST treatment to control for folate status, which is an important determinant of (i) brain development via its role in DNA synthesis and methylation (Bailey, 2009) and (ii) tetrahydrobiopterin (BH4) synthesis, an essential cofactor in the biosynthesis of monoamine neurotransmitters such as serotonin (Miller, 2008). Under conditions of folate/one-carbon deficiency, high homocysteine levels indicate a deficit of one-carbon donors needed for the transformation of homocysteine to methionine.
Our hypothesis is that periconceptional exposure to SST, a nonabsorbable antibiotic, will provoke neurobehavioral alterations in the offspring due to alterations in the gut microbiome in dams. Based on previous findings in germ-free rats (Crumeyrolle-Arias et al., 2014; O’Mahony et al., 2014) and antibiotic exposure in mice (Tochitani et al., 2016), we expect to observe an anxiogenic phenotype and decreased locomotor activity.
2. Methods
2.1. Animals and treatment
We obtained 12 female Wistar rats (250–290 g) and 8 male Wistar rats (310–340 g) from Charles River Laboratories (St. Constant, Québec, Canada). They were housed 6 per for females and 4 per for males in plastic cages with bedding and regulated temperature (21 ± 2 °C) and humidity (50 ± 10%), and a 12 h light/dark cycle (6 h–18 h). Food and water were provided ad libitum. All animals received care in compliance with the Guide to the Care and Use of Experimental Animals from the Canadian Council of Animal Care and the protocol was approved by our institutional animal research ethics committee. Male rats used for breeding were fed with Rodent Chow 5075; Charles River Laboratories. As soon as we received the females, we randomly assigned them to two groups of six dams each: control (Basal Diet 5755, from TestDiet ®), or exposed to the diet with 1% SuccinylSulfaThiazole (SST) (Basal Diet 5755 with SST 1% added, from TestDiet ®). This diet was administered for one month before breeding and was maintained until gestational day 15 (G15), when all dams were fed with standard Rodent Chow 5075, Charles River Laboratories.
After one month on a given diet, a blood sample was taken from the saphenous vein of the female rats which were then placed in a new cage with a male. Blood was immediately centrifuged at 5000 Rotation Per Minute (RPM) for 20 min and the supernatant was aliquoted and stored at −80 °C until analysis. We checked every morning for the presence of a vaginal plug, a sign of breeding during the night. When a plug was found, the female was placed in an individual cage and the day was considered as G1. Two females from the control group and one from the SST group were not pregnant.
Dams were allowed to raise all the litters, and dams and pups were kept in the same cage from birth until weaning at post-natal day 21 (P21). After weaning, males and females were separated. For all behavioral tests, we used 2 males (housed together after weaning) and 2 females (housed together after weaning) from each litter, which results in 16 pups for the control group and 20 pups for the SST-exposed group. Dams and all untested siblings were euthanized at P21. Adult off-spring were euthanized after behavioral testing at P50.
2.2. Analysis of homocysteine and tryptophan levels
For homocysteine dosage, we used the Axis® Homocysteine Enzyme Immunoassay (EIA) kit for quantitative determination of total L-homocysteine in plasma from pre-conception blood in dams. Tryptophan dosages were performed by PhenoSwitch Bioscience Inc. (Sherbrooke, Canada) using liquid chromatography-mass spectrometry (LC-MS). Blood samples from dams were aliquots from pre-conception and euthanasia (P21), and for offspring they were aliquots from euthanasia of the untested siblings (P21). All samples were prepared as follows: for each plasma sample, 20 μl were mixed with acidified methanol, doped with internal standards, to precipitate the proteins. The samples were clarified by centrifugation at 13,000 RPM for 5 min. The supernatant was dried and resuspended in 20 μl of acidified water (0.2% formic acid). A standard curve ranging from 0.09 μM to 500 μM for tryptophan was prepared. Samples are analysed undiluted and 100-fold diluted. Acquisition was performed with a ABSciex TripleTOF 5600 (Sciex, Foster City, CA, USA) equipped with an electrospray interface with a 25 μm iD capillary and coupled to an Eksigent μUHPLC (Eksigent, Redwood City, CA, USA). Analyst TF 1.6 software was used to control the instrument and for data processing and acquisition. The source voltage was set to 5.5 kV and maintained at 400oC, curtain gas was set at 27 psi, gas one at 12 psi and gas two at 15 psi. Acquisition was performed in MRM mode. Separation was performed on a reversed phase Halo PFP column 0.5 mm i.d., 2.7 μm particles, 50 mm long (Advance Materials Technology, Wilmington, DE). Quantification was done using the area under the curve with the MuliQuant software (Sciex).
2.3. Behavioral testing
All behavioral tests were performed between 9 a.m. and 4 p.m. by the same person.
2.3.1. Open field (n = 16 controls and n = 20 SST exposed)
This test measures observable spontaneous motor activities (Bignami, 1996). At P20, the rat was placed in the apparatus, a box with a 40 cm2 base with a 40 lx light intensity and a video camera placed above connected to a computer running ANY-Maze® software (Stoelting CO, USA). Each rat was placed in the same orientation and all trajectories, including time of mobility and distance travelled, were analysed during a 5 min session.
2.3.2. Social interactions (n = 8 pairs of controls and n = 8 pairs of SST exposed, 4 pairs of males and 4 pairs of females in both groups)
At P25, two rats from the same group, of the same sex, and approximately the same weight, but who had never met before, were placed in the same cage (26 cm × 17 cm × 14.5 cm) with a 40 lx light intensity. The day before the test, all animals had a 5 min session alone in the apparatus to become familiarized with the cage. The 10 min session of testing was recorded with a camera placed above the apparatus and was analysed by a person blinded to the exposure group of each pup.A social interaction was scored as an event if it lasted at least one second in one of the following four situations: when the two rats (i) were engaged in playing behavior or (ii) were actively investigating each other (sniffing), or when one rat (iii) was pursuing the other congener, or (iv) when a rat was grooming the other congener. Every time one of these events occurred, the experimenter started a timer. The results of both provided the number of events and the total time spent in interactions).
2.3.3. Marble burying (n = 8 controls and n = 10 SST exposed)
This test is based on the fact that many rodents (including rats and mice) exhibit burying behavior. This test is commonly used to quantify anxiety, but according to Thomas et al. (2009) this test reflects mostly obsessive-compulsive, and repetitive behavior in rodents. At P30, the rat was placed in a cage (26 cm × 17 cm × 14.5 cm) with 5 cm bedding depth and a 40 lx light intensity. 16 marbles were placed in a 4 × 4 grid on the bedding. The 30 min session of testing was recorded with a camera placed above the apparatus and the number of marbles buried was analysed.
2.3.4. Elevated plus-maze (n = 16 controls and n = 20 SST exposed)
This test is commonly used to assess anxiety behavior in rodents. The Elevated Plus-Maze test is based on the conflict induced in subjects by the presence of safe parts of the apparatus that are closed, shadowed and protected, and open ones, bright, aversive and unprotected (Casarrubea et al., 2013). At P35, the rat was placed at the center of the apparatus composed of an elevated cross (50 cm above the floor) with two open arms (16 lx) and two enclosed arms (8 lx). A video camera was placed above the apparatus and was connected to a computer running ANY-Maze® software (Stoelting CO, USA). Each rat was placed in the same orientation and all trajectories, including time, distance, and entries in each compartment, were analysed during a 5 min session. For the analysis, we used the indicators validated as primary anxiety indexes (Pellow et al., 1985): (i) ratio of entries in open vs closed arms calculated as: %open entries = (entries in open arms × 100) / total number of entries; and (ii) ratio of time spent in open vs closed arms calculated as: %open time = (time spent in open arms × 100) / duration of test 500 s. We also used the total number of entries in closed and open arms as validated indicator of general exploratory activity during the test. We also present additional data such as distance travelled.
2.3.5. Prepulse inhibition (PPI) of the acoustic startle reflex (n = 8 controls and n = 10 SST exposed)
The PPI test is used to assess a neurological filtering process present across species, sensorimotor gating, which avoids information overload of the brain. In the case of the PPI test, a strong auditory stimulus will provoke a whole body startle reflex, but when a weaker stimulus precedes this stimulus, the body response will be reduced, with a smaller startle response (Braff and Geyer, 1990; Groves et al., 1974). This test was performed at P45 as previously described (Deslauriers et al., 2013) using a PPI testing apparatus (SR-LAB; San Diego Instruments, CA, USA). The animal was placed in a tubular transparent Plexiglas enclosure which keeps the animal from moving; the enclosure is placed on a platform floor plugged to a piezoelectric transducer in a soundproof box. Before the test began, there was a 5 min acclimatization period with a constant background noise of 71 dB, which continued for the entire test session. The test was divided into 4 main blocks. The first block was composed of 6 pulses of 120 dB (40 ms) each separated by 10 to 20 s. It was directly followed by the second block, composed of 30 trials, separated by 10 to 20 s, and administered in a pseudo-randomized order (the same sequence, created by randomization, was used for all the animals) from these groups: (i) five pulses of 120 dB (40 ms), (ii) five trials with no stimulation, (iii) five trials with a 120 dB pulse (40 ms) preceded (80 ms delay) by a prepulse (20 ms) at + 4 dB above the white noise, (iv) five trials with a 120 dB pulse (40 ms) preceded (80 ms delay) by a prepulse (20 ms) at + 8 dB above the white noise, (v) five trials with a 120 dB pulse (40 ms) preceded (80 ms delay) by a prepulse (20 ms) at + 12 dB above the white noise, (vi) five trials with a 120 dB pulse (40 ms) preceded (80 ms delay) by a prepulse (20 ms) at + 16 dB above the white noise. The data used for analysis is the pressure recorded by the platform floor for each startle of the rat, expressed as a Vmax. The percentage of inhibition of the startle compared to the mean startle reflex was calculated as: The habituation during the test was evaluated as percentage of inhibition of the startle between the first and the last block of 6 pulses of 120 dB as:.
2.4. Statistical analysis
All statistical analyses were performed using SAS/STAT software (SAS Institute, version 9.2), and all data are presented as the mean ± SEM.
For homocysteine analysis, we used a one-way analysis of variance (ANOVA) with the fixed effects of group (control or SST). For tryptophan analysis, we used a two-way analysis of variance (ANOVA) with the fixed effects of group (control or SST), time (conception or weaning), and their interaction.
For behavioral test analysis, we used a mixed-design analysis of variance (ANOVA) with the fixed effects of group (control or SST), sex (males or females) and their interaction. Given that rat pups belong to litters, they are not completely independent samples and the effects associated with being in the same litter must be accounted for. To control for this within-litter effect within groups, a random effect for litter was included in the model. If the effect or interaction was significant (p < 0.05), a Tukey’s post-hoc pairwise comparison test was used to compare means. Individual weights on the day of testing and litter size were introduced in each model as fixed effects, but since these variables were not significantly related, they were removed from the final models.
The statistical significance level was set at p < 0.05 and a nonsignificant effect (0.10 < p < 0.05) was reported in the Results section.
3. Results
3.1. Food consumption, homocysteine and tryptophan levels, and developmental landmarks
We observed no differences in food consumption between groups and no differences in preconceptional blood homocysteine levels (controls: 7.45 ± 0.98 μM vs for SST: 6.37 ± 1.05; F (1,11) = 0.56, p = 0.47). 2-way ANOVA model for plasma tryptophan levels in dams revealed no significant group effect (F (3,15) = 0.45, p = 0.51) or time of dosage effect (F (3,15) = 0.01, p = 0.93), at preconception (controls: 86.1 ± 5.7 μM vs for SST: 83.4 ± 5.7) and weaning (controls: 86.9 ± 6.4 μM vs for SST: 81.7 ± 5.7).We observed no difference in offspring tryptophan levels at weaning (controls: 84.2 ± 2.7 μM vs for SST: 85.5 ± 2.4; F (1,16) = 0.13, p = 0.72).
No change in the timing of developmental landmarks was found in rats exposed to SST vs. controls: fur growth, eye opening, body weight, but we observed a reduced litter size in the group exposed peri-conceptionally to SST (for controls: 17.2 ± 1.9 vs for SST: 12.8 ± 2.4; F (1,7) = 9.18, p = 0.02). Two females from the control group and one female from the SST-exposed group were not bred successfully, which explains the unequal number of litters per group in the results (four control litters and five SST exposed litters).
3.2. Behavioral tests
3.2.1. Open field test
Pups from the control and exposed to SST groups perform identically in all parameters studied: overall distance travelled (F (1,32) = 0.01, p = 0.95), distance travelled in the center (F (1,32) = 0.07, p = 0.80), time of mobility (F (1,32) = 0.40, p = 0.53), or time spent in the center (F (1,32) = 0.11, p = 0.74). No sex effect was significant for either indicators presented.
3.2.2. Social interactions
All social interactions, such as playing, pursuing, sniffing and grooming each other, which happened during the test were grouped and analysed for two indicators: total time spent in social interaction, and total number of social interaction events.
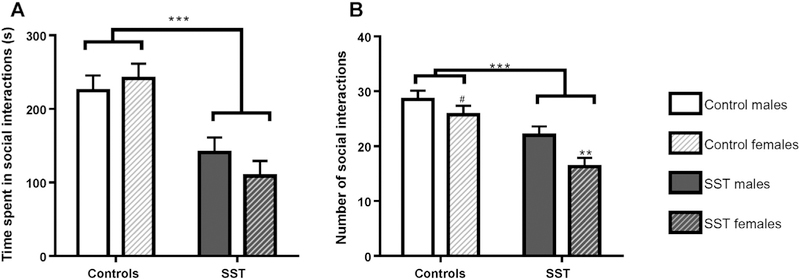
Our statistical model revealed a strong group effect (F (1,12) = 28.59, p < 0.001) for the total time spent in social interactions, but no sex effect (F (1,12) = 0.15, p = 0.70) or sex interaction (F (1,12) = 1.43, p = 0.26). Pups exposed to SST show a ~ 50% decrease in time spent in social interactions compared to controls (See Fig. 1.A). We also observe a strong group effect for the total number of social interaction events (F (1,12) = 25.08, p < 0.001) and a sex effect (F (1,12) = 7.08, p = 0.02) but no statistical interaction for sex (F (1,12) = 0.88, p = 0.37). Pups exposed to SST show a ~ 40% decrease in total number of social interaction events compared to controls, with females having fewer events in both groups (post-hoc Tukey test: p = 0.06 for controls and p = 0.006 for SST females compared to males) (see Fig. 1.B).
Fig. 1.
Social interactions test performed at P25. ***p < 0.001; **p < 0.01; *p < 0.05; #p < 0.1. Control group (in white) n = 8 couples of rats; exposed peri-conceptionally to SST (in black) n = 8 couples of rats. A: Total time spent in social interactions in seconds grouped active playing, pursuing, sniffing and/or grooming each other. B: Number of social interaction event which occurred during the test session.
3.2.3. Marble burying test
No differences were found in the number of marbles at least half-buried between groups (F (1,14) = 1.31, p = 0.12) and there was no sex effect.
3.2.4. Elevated plus maze test
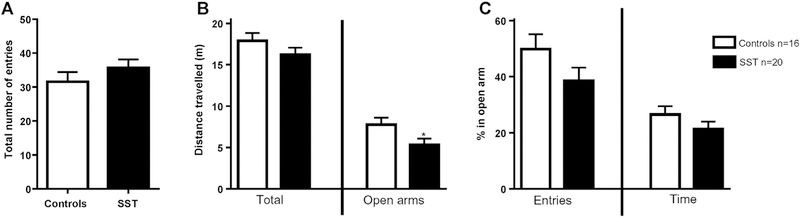
The overall exploratory activity during the test was similar in both groups with no differences in the total number of entries in closed and open arms (F (1,35) = 1.57, p = 0.22) (Fig. 2.A) and no differences in the total distance travelled (F (1,35) = 1.77, p = 0.20) (Fig. 2.B). However there was a non-significant effect (F (1,35) = 3.03, p = 0.09) of ~ 20% decrease in exploratory behavior in open arms given that rats exposed to SST had a smaller percentage of entries in open arms (Fig. 3.C). This non-significant effect was consistent with the ~ 20% decrease in distance travelled in open arms for rats exposed to SST (F (1,35) = 4.93, p = 0.04). Even though it is not statistically significant, we also observed a ~ 20% decrease in the percentage of time spent in the open arm during the 5-min test session (F (1,35) = 1.78, p = 0.19). No sex effect was significant for either of the indicators presented.
Fig. 2.
Elevated Plus Maze test performed at P35. *p < 0.05; control group (in white) n = 16; exposed peri-conceptionally to SST (in black) n = 20. A: Total number of entries in open plus closed arms. B: Distance travelled in total and in the open arms of the apparatus in meters. C: Percentage of entries and time spent in open arm during the test session.
Fig. 3.
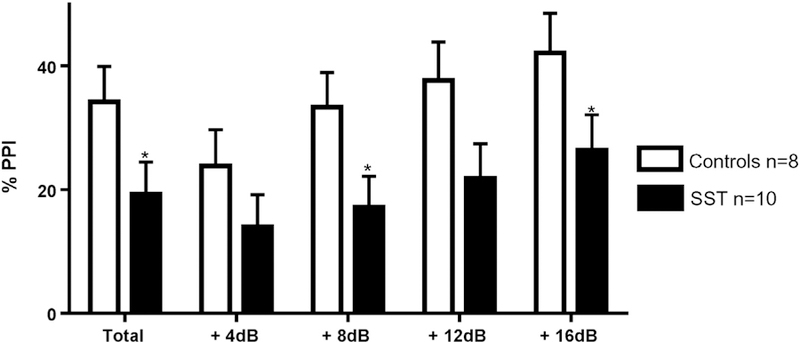
Prepulse inhibition (PPI) of the acoustic startle test performed at P45. ***p b 0.001; **p b 0.01; *p b 0.05; control group (in white) n = 8; exposed peri-conceptionally to SST (in black) n = 10. Percentage of PPI in total and for each prepulse intensities (+4, +8, +12, +16 dB above the white noise of 71 dB).
3.2.5. Prepulse inhibition test
There was a ~ 20% decrease in the total percentage of prepulse inhibition (%PPI) in rats exposed to SST compare to controls (F (1,17) = 7.51, p = 0.03). The %PPI increased proportionally with the intensity of the prepulse in both groups, and the decreased %PPI was consistent in all prepulse intensities: + 4 dB (F (1,17) = 0.88, p = 0.59); + 8 dB (F (1,17) = 9.65, p = 0.02); +12 dB (F (1,17) = 4.29, p = 0.08); +16 dB (F (1,17) = 13.44, p = 0.008) (see Fig. 3). No differences between groups were observed in the percentage of habituation (F (1,17) = 0.80, p = 0.40), and there was no sex effect for any of the indicators.
4. Discussion
Our study is the first to examine behavioral alterations in rodents in which the maternal microbiota was perturbed peri-conceptionally with non-absorbable antibiotics. Our data show that maternal exposure to a sulfonamide antibiotic SST in the absence of infection alters the behavior of the offspring. Offspring exposed to SST showed 20% reduced exploration of the open arms (i.e. increased anxiety) in the elevated plus maze test, and greatly reduced social interactions, at P35 and P25 respectively, which corresponds to the pre-pubertal period in rats (Suckow et al., 2005). Once they reach adult age, they have a reduced capacity to filter out unnecessary information (decreased sensory-motor gating).
In bacteria, antibacterial sulfonamides act as competitive inhibitors of the enzyme dihydropteroate synthase, an enzyme involved in tetrahydrofolate synthesis. Tetrahydrofolate is crucial to the folate cycle as a carrier of one-carbon units, necessary for many biosynthetic pathways. If tetrahydrofolate is no longer synthesized, then many biosynthetic pathways requiring one-carbon donors are disrupted. The biosynthesis of nucleic acids is particularly disrupted and this leads to the cessation of cell growth and division. SST is considered to be bacteriostatic since it does not actively kill bacterial cells (Patrick, 1995; Sepehr et al., 2003). In the rat, a 28-day treatment with SST substantially reduces the number of gut coliforms and lactobacteria (2- and 100-fold, respectively), which is compensated for by increases in enterococci and yeastlike forms (Evenson et al., 1946). The exposure to SST did not alter folate metabolism in the dams, given that homocysteine levels in exposed dams were similar to those in non-exposed dams. Considering that the food we provided contains at least 4.2 mg of folic acid per kg, and that the recommendations from the U.S. National Research Council are 1 mg of folic acid per kg diet for maintenance, reproduction and growth of laboratory rats (National Research Council (US) Subcommittee on Laboratory Animal Nutrition, 1995), behavioral alterations in the offspring cannot be explained by reduced folate availability.
In the Elevated Plus Maze Test, an ethologically and pharmacologically validated tool for anxiety assessment in rodents (Carobrez and Bertoglio, 2005), pups exposed to SST showed anxiety-like behavior, which was characterized by a 20% reduced exploration of the open arms. The elevated plus maze test is a widely used test to assess anxiety in rodents (Wall and Messier, 2001). The ratio of entries and time spent in open arms compared to closed arms used in our analyses has been extensively validated using anxiolytic drugs (Pellow et al., 1985). It has also been shown numerous times that, when a rat is allowed to freely explore the elevated plus maze, it will spend only 20 to 30% of its time in the open arms (Dawson and Tricklebank, 1995). In our experiment, we observed an equal total number of entries in the open plus closed arms for the two groups, which indicates an equivalent exploratory behavior (Weiss et al., 1998), and an equal distance travelled indicating the same level of motor activity. However, the rats in the SST group had 20% less distance travelled, associated with the same nonsignificant effect for less time spent and fewer entries in the open arms (20% decrease), consistent with an anxious state.
Our data show that SST reduced by half the number of social interactions in both males and females. In addition, the exposure to SST reduced the sensorimotor gating, as measured by the PPI test in adult rats. Deficits in PPI are considered to be an endophenotype for schizophrenia and have been characterized in many other mental diseases in humans, such as bipolar disorders, Huntington’s disease, panic disorder, post-traumatic stress disorder, Parkinson’s disease, Tourette’s syndrome, and attention deficit and/or hyperactivity disorder (Kohl et al., 2013).
It has been shown that social interactions in rats correlate with PPI levels. Rats with lower PPI spent less time in social interactions compared to rats with higher PPI. However, in this same study, there was no relation observed between PPI level and anxiety, measured with the elevated plus maze test (Goktalay et al., 2014), suggesting that behavioral alterations observed in social interactions and PPI tests are not solely due to a higher anxiety of the animals exposed periconceptionally to SST.
Given the fact that SST is non absorbable antibiotic, we suggest that all the behavioral alterations we observed are related to its local effects on gut microbiota composition in dams. Although, in our experiment we stopped the administration of SST at gestational day 15, at least 7 days before delivery, we cannot exclude the longer term effect of SST on the dam’s gut microbiota or on early colonisation of the gut in the offspring. Recent studies suggest that microbial metabolites, such as short chain fatty acids, derived from the gut are not only an important energy supply for the developing brain, but are also able to interact with G-protein coupled receptors in the sympathetic nervous system (Samuel et al., 2008; Tolhurst et al., 2012), and participate in several metabolic processes such as tryptophan metabolism (Perry et al., 1967). In addition, they may possess the capacity to influence host gene expression by remodelling host chromatin structure (Selkrig et al., 2014). Gut microorganisms are capable of producing and delivering neuroactive substances such as serotonin (Yano et al., 2015) and gamma-aminobutyric acid (Barrett et al., 2012), which act on the gutbrain axis (Evrensel and Ceylan, 2015). The composition of the human gut microbiota changes during pregnancy and exhibits a distinct metabolic phenotype in the 3rd trimester, reduced in diversity and capable of generating greater energy from the diet (Koren et al., 2012).
Thus, we tested the possibility that SST exposure might induce alterations in tryptophan availability preconceptionally and in dams and pups at weaning. However, we did not observe any changes in plasmatic tryptophan availability across times and we therefore suggest that behavioral alterations observed after periconceptional SST exposure are not due to either folate or tryptophan availability in dams. However, recent studies have shown that prenatal exposure to low-levels of propionic acid, a short chain fatty acid and a metabolic product of antibiotic resistant enteric bacteria, may alter rat offspring’s development and behavior with sexual differences. In addition, the offspring show increased locomotor activity, subtle impairments in social interactions and neonatal olfactory discrimination, increased preference for novel objects, and decreased sensorimotor gating (Foley et al., 2014a,b, 2015).
Although manipulation of gut microbiota in young animals suggests that the hypothalamic-pituitary-adrenal axis is programmed by dietary conditions in early life (Sudo, 2014; Sudo et al., 2004), little is known about the short and longer term effects of manipulating the maternal gut microbiota on brain development in the offspring.
Our conclusions are subject to the following caveats: (i) we assume that SST treatment altered the gut microbiome, based on published results, but we did not confirm this in our study; (ii) we did not measure folate status directly in the rats but relied on the facts that there were high levels of folate in the food and that homocysteine levels did not increase in SST treated rats compared to controls; (iii) in developmental studies, pups from the same litter are not completely independent, and thus the litter should be considered as the subject. We therefore take into account this fact in our statistical analysis by adding a litter random effect.
5. Conclusion
Our findings suggest that the gut microbiota can be one determinant of long term complex behavioral skills such as social interactions, integration of sensorial information, and risk perception (anxiety).
Our results are particularly relevant given the frequent use of antibiotics in pregnancy, not only non-absorbable but also absorbable antibiotics, none of which have been tested for developmental neurotoxicity.
Acknowledgements
The study was supported by internal funding from the Research Center of the CHUS PAFI2014 and also suported by NIH Grant 5R21ES024841. L. Takser and D.J. Hunting are members of the CHUS research Center, which is supported by the FRQ-S. L. Takser was supported by a CIHR (the Canadian Institutes of Health Research) New Investigator Award. S. Degroote was supported by a QTNPR (Québec Training Network in Perinatal Research) studentship. The authors thank Dr. Edward V. Quadros (SUNY-Downstate Health Science Center, Brooklyn, NY, USA) and Dr. René Jacobs (U of Alberta, Canada) for advice on methodology related to folate status.
Abbreviations:
- SST
SuccinylSulfaThiazole
- Gx
Gestational day x
- Py
Post-natal day y
- PPI
Prepulse inhibition
Footnotes
Disclosure
The authors declare no competing financial or personal interests.
References
- Andersen JT, Petersen M, Jimenez-Solem E, Rasmussen JN, Andersen NL, Afzal S, Broedbaek K, Hjelvang BR, Køber L, Torp-Pedersen C, Poulsen HE, 2013. Trimethoprim use prior to pregnancy and the risk of congenital malformation: a register-based nationwide cohort study. Obstet. Gynecol. Int 2013, 364526 10.1155/2013/364526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladóttir HÓ, Henriksen TB, Schendel DE, Parner ET, 2012. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics 130, e1447–e1454. 10.1542/peds.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey LB, 2009. Folate in Health and Disease CRC Press, Second edition. [Google Scholar]
- Barrett E, Ross RP, O’Toole PW, Fitzgerald GF, Stanton C, 2012. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol 113, 411–417. 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- Bignami G, 1996. Economical test methods for developmental neurobehavioral toxicity. Environ. Health Perspect 104 (Suppl. 2), 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, 1990. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch. Gen. Psychiatry 47, 181–188. [DOI] [PubMed] [Google Scholar]
- Broe A, Pottegård A, Lamont RF, Jørgensen JS, Damkier P, 2014. Increasing use of antibiotics in pregnancy during the period 2000–2010: prevalence, timing, category, and demographics. BJOG 121, 988–996. 10.1111/1471-0528.12806. [DOI] [PubMed] [Google Scholar]
- Camilleri M, 2009. Serotonin in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes 16, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ, 2005. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev 29, 1193–1205. 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Casarrubea M, Roy V, Sorbera F, Magnusson MS, Santangelo A, Arabo A, Crescimanno G, 2013. Temporal structure of the rat’s behavior in elevated plus maze test. Behav. Brain Res 237, 290–299. 10.1016/j.bbr.2012.09.049. [DOI] [PubMed] [Google Scholar]
- Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Daugé V, Naudon L, Rabot S, 2014. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 42, 207–217. 10.1016/j.psyneuen.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Tricklebank MD, 1995. Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol. Sci 16, 33–36. [DOI] [PubMed] [Google Scholar]
- Deslauriers J, Larouche A, Sarret P, Grignon S, 2013. Combination of prenatal immune challenge and restraint stress affects prepulse inhibition and dopaminergic/ GABAergic markers. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 45, 156–164. 10.1016/j.pnpbp.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Evenson A, McCoy E, Geyer BR, Elvehjem CA, 1946. Cecal flora of white rats on purified diet and its modification by succinylsulfathiazole. J. Bacteriol 51, 513–521. [PMC free article] [PubMed] [Google Scholar]
- Evrensel A, Ceylan ME, 2015. The gut-brain axis: the missing link in depression. Clin. Psychopharmacol. Neurosci 13, 239–244. 10.9758/cpn.2015.13.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley KA, MacFabe DF, Vaz A, Ossenkopp K-P, Kavaliers M, 2014a. Sexually dimorphic effects of prenatal exposure to propionic acid and lipopolysaccharide on social behavior in neonatal, adolescent, and adult rats: implications for autism spectrum disorders. Int. J. Dev. Neurosci 39, 68–78. 10.1016/j.ijdevneu.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Foley KA, Ossenkopp K-P, Kavaliers M, Macfabe DF, 2014b. Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS One 9, e87072 10.1371/journal.pone.0087072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley KA, MacFabe DF, Kavaliers M, Ossenkopp K-P, 2015. Sexually dimorphic effects of prenatal exposure to lipopolysaccharide, and prenatal and postnatal exposure to propionic acid, on acoustic startle response and prepulse inhibition in adolescent rats: relevance to autism spectrum disorders. Behav. Brain Res 278, 244–256. 10.1016/j.bbr.2014.09.032. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L, 2003. The developmental role of serotonin: news from mouse molecular genetics. Nat. Rev. Neurosci 4, 1002–1012. 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Goktalay G, Kayir H, Ulusoy GK, Uzbay T, 2014. Social interaction of rats is related with baseline prepulse inhibition level. Neurosci. Lett 582, 125–129. 10.1016/j.neulet.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Groves PM, Boyle RD, Welker RL, Miller SW, 1974. On the mechanism of prepulse inhibition. Physiol. Behav 12, 367–375. [DOI] [PubMed] [Google Scholar]
- Holzer P, Farzi A, 2014. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol 817, 195–219. 10.1007/978-1-4939-0897-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Howerton CL, Howard CD, Bale TL, 2015. Alterations in the vaginal microbiome by maternal stress are associated with metabolic reprogramming of the offspring gut and brain. Endocrinology 156, 3265–3276. 10.1210/en.2015-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A, Taylor DJ, 2008. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet 372, 1319–1327. 10.1016/S0140-6736(08)61203-9. [DOI] [PubMed] [Google Scholar]
- Kohl S, Heekeren K, Klosterkötter J, Kuhn J, 2013. Prepulse inhibition in psychiatric disorders–apart from schizophrenia. J. Psychiatr. Res 47, 445–452. 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, Bäckhed F, Isolauri E, Salminen S, Ley RE, 2012. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480. 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, 2008. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern. Med. Rev 13, 216–226. [PubMed] [Google Scholar]
- National Research Council (US) Subcommittee on Laboratory Animal Nutrition, 1995A. Nutrient Requirements of Laboratory Animals Fourth Revised ed. National Academies Press (US), Washington (DC). [PubMed] [Google Scholar]
- O’Mahony SM, Felice VD, Nally K, Savignac HM, Claesson MJ, Scully P, Woznicki J, Hyland NP, Shanahan F, Quigley EM, Marchesi JR, O’Toole PW, Dinan TG, Cryan JF, 2014. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 277, 885–901. 10.1016/j.neuroscience.2014.07.054. [DOI] [PubMed] [Google Scholar]
- Patrick GL, 1995. An Introduction to Medicinal Chemistry Oxford University Press. [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M, 1985. Validation of open: closed arm entries in an elevated plus-maze asa measure of anxiety in the rat. J. Neurosci. Methods 14, 149–167. [DOI] [PubMed] [Google Scholar]
- Perry TL, Hansen S, MacDougall L, Schwarz CJ, 1967. Studies of amines in normal and schizophrenic subjects In: Smythies HE (Ed.), Amines and Schizophrenia; Pergamon, pp. 31–41. [Google Scholar]
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI, 2008. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. U. S. A 105, 16767–16772. 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkrig J, Wong P, Zhang X, Pettersson S, 2014. Metabolic tinkering by the gut microbiome: implications for brain development and function. Gut Microbes 5, 369–380. 10.4161/gmic.28681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehr E, Peace RW, Storey KB, Jee P, Lampi BJ, Brooks SPJ, 2003. Folate derived from cecal bacterial fermentation does not increase liver folate stores in 28-d folate-depleted male Sprague–Dawley rats. J. Nutr 133, 1347–1354. [DOI] [PubMed] [Google Scholar]
- Suckow MA, Weisbroth SH, Franklin CL, 2005. The Laboratory Rat Academic Press. [Google Scholar]
- Sudo N, 2014. Microbiome, HPA axis and production of endocrine hormones in the gut. Adv. Exp. Med. Biol 817, 177–194. 10.1007/978-1-4939-0897-4_8. [DOI] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu X-N, Kubo C, Koga Y, 2004. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. Lond 558, 263–275. 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R, 2009. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 204, 361–373. 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochitani S, Ikeno T, Ito T, Sakurai A, Yamauchi T, Matsuzaki H, 2016. Administration of non-absorbable antibiotics to pregnant mice to perturb the maternal gut microbiota is associated with alterations in offspring behavior. PLoS One 11, e0138293 10.1371/journal.pone.0138293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM, 2012. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371. 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PM, Messier C, 2001. Methodological and conceptual issues in the use of the elevated plus-maze as a psychological measurement instrument of animal anxiety-like behavior. Neurosci. Biobehav. Rev 25, 275–286. [DOI] [PubMed] [Google Scholar]
- Weiss SM, Wadsworth G, Fletcher A, Dourish CT, 1998. Utility of ethological analysis to overcome locomotor confounds in elevated maze models of anxiety. Neurosci. Biobehav. Rev 23, 265–271. [DOI] [PubMed] [Google Scholar]
- Welch AD, Mattis PA, Latven AR, 1942. A toxicological study of succinyl sulfathiazole. J. Pharmacol. Exp. Ther 75, 231–246. [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G, 2009. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U. S. A 106, 3698–3703. 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY, 2015. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161, 264–276. 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Guadarrama L, Corona-Morales AA, Vega-Gonzalez A, Rocha L, Escobar A, 2006. Rats subjected to extended L-tryptophan restriction during early postnatal stage exhibit anxious-depressive features and structural changes. J. Neuropathol. Exp. Neurol 65, 562–570. 10.1097/00005072-200606000-00004. [DOI] [PubMed] [Google Scholar]