Abstract
Introduction
Reward processing is a key aspect of cognitive control processes, putatively instantiated by mesolimbic and mesocortical brain circuits. Deficient signaling within these circuits has been associated with psychopathology. We applied a network discovery approach to assess specific functional networks associated with reward processing in participants with attention‐deficit/hyperactivity disorder (ADHD).
Methods
To describe task‐related processes in terms of integrated functional networks, we applied independent component analysis (ICA) to task response maps of 60 healthy participants who performed a monetary incentive delay (MID) task. The resulting components were interpreted on the basis of their similarity with group‐level task responses as well as their similarity with brain networks derived from resting state fMRI analyses. ADHD‐related effects on network characteristics including functional connectivity and communication between networks were examined in an independent sample comprising 150 participants with ADHD and 48 healthy controls.
Results
We identified 23 components to be associated with 4 large‐scale functional networks: the default‐mode, visual, executive control, and salience networks. The salience network showed a specific association with reward processing as well as the highest degree of within‐network integration. ADHD was associated with decreased functional connectivity between the salience and executive control networks as well as with peripheral brain regions.
Conclusions
Reward processing as measured with the MID task involves one reward‐specific and three general functional networks. Participants with ADHD exhibited alterations in connectivity of both the salience and executive control networks and associated brain regions during task performance. Hum Brain Mapp 38:2359–2369, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: imaging, brain networks, ADHD, reward processing
INTRODUCTION
Reward is essential to human behavior. For example, reward elicits approach behaviors and learning as we try to maximize rewards. Moreover, it impacts affective experiences associated with behavior, for example, by inducing pleasure when receiving a reward [Berridge and Robinson, 2003]. It is clear that such reward processes affect how and what kind of decisions we make, what kind of preferences we have, and, as they are directly related to cognitive control, how many cognitive resources we allocate for performance in a given situation [Aarts et al., 2011].
Given their central role in behavior, researchers have started investigating reward processes in clinical populations. For instance in attention‐deficit/hyperactivity disorder (ADHD), several studies have been conducted that employed reward‐related manipulations. Based on reports of altered performance observed in participants with ADHD, impairment in reward‐related processes was hypothesized as potential mechanism underlying ADHD and incorporated in theoretical models of psychopathology that explain ADHD in terms of neurochemical [Tripp and Wickens, 2009], brain pathway [Sonuga‐Barke, 2002], or neurocomputational [Frank, 2005] deficits [for review see Luman et al., 2010]. However, inconsistent replication of these findings in subsequent studies highlights that reward‐related alterations in ADHD are far from understood and an active topic of debate in the community [e.g., Demurie et al., 2016].
On a neural level ADHD is associated with aberrant signaling in structures of the brain that are thought to govern reward processing. Structures involved in reward processing include mesolimbic and mesocortical brain circuits consisting of midbrain, ventral striatum, anterior cingulate cortex (ACC), and orbitofrontal cortex (OFC) [Frank and Fossella, 2011]. Functional Magnetic Resonance imaging (fMRI) studies investigating reward‐related brain processes in participants with ADHD mostly apply a monetary incentive delay (MID) task. These studies most consistently report attenuated responses of the ventral striatum during reward anticipation [for review see Plichta and Scheres, 2014]. In addition, other studies reported reward‐related alterations in the orbitofrontal cortex. The direction of these alterations, however, is less consistent and depends largely on the used paradigm (increases reported by Ströhle [Ströhle et al., 2008] and Von Rhein [Von Rhein et al., 2015] using a MID task (during reward receipt); decreases reported by Rubia using a temporal discounting [Rubia et al, 2009a] and rewarded continuous performance task [Rubia et al., 2009b]). Together, these findings provide support for the role of dysfunctional mesolimbic and mesocortical brain networks in ADHD.
The functional units of the brain are networks of specialized, neural structures that communicate with each other [Mesulam, 1998; Poldrack, 2012]. However, the ability to investigate network characteristics using conventional task‐based analytical approaches is limited. Such approaches commonly use univariate models that rely on calculating averaged responses of the brain to manipulations, either for a region of interest (ROI) or at the whole‐brain level (voxel‐wise). Although such approaches are powerful in localizing cognitive functions based on the blood oxygen‐level dependent (BOLD) response amplitude, they are blind to relational aspects among neural structures. These aspects are, however, crucial for a comprehensive and integrated description of the functional properties of the neural circuits underlying cognitive functions. Accordingly, a model that takes the brain's highly connected neural structure into account is likely to provide a biologically more valid description of neural processes compared to a model that assumes functional independence [Sporns et al., 2004].
Here we provide a network discovery approach to define brain areas implicated in reward processing and to assess whether reward‐related network characteristics differ between participants with ADHD and controls. Specifically, we performed an independent component analysis (ICA) on participant‐level reward‐related activation maps to define those regions that co‐activate across participants. ICA is a data‐driven approach that separates neural data into a set of spatially independent components (ICs). By identifying components that reflect neuroanatomical systems we were able to describe task responses in terms of associated larger functional networks. Because these components are determined based on the consistency of brain response patterns, this method is also sensitive to responses with low amplitude if they are consistent across participants. Finally, component selection allows focused investigations on specific components of interest, thus avoiding interpretation of components that relate to noise or components that are invariant to task demands. Investigation of network characteristics in the context of ADHD is of particular interest as studies applying network analyses using resting state‐fMRI report ADHD effects on a network level (for review see [Oldehinkel, et al., 2013]). ADHD has been associated with decreased connectivity between ventral striatum (VS) and orbitofrontal cortex (OFC) [Posner et al., 2013]. Moreover, several studies report aberrant connectivity in the default‐mode network (DMN) in ADHD (for review see [Konrad and Eickhoff, 2010; Posner et al., 2014]). By applying network analysis to reward‐related task responses we were able to specifically assess the association between ADHD and network characteristics of reward‐related functional networks. Based on the prominent role of mesolimbic and mesocortical brain circuits in reward processing and reported alterations within these circuits in participants with ADHD, we expected to find diagnostic changes on network characteristics particularly for components that include frontal striatal structures such as OFC and VS.
METHODS
To identify reward‐related functional networks based on spatially coherent response patterns we performed ICA on task‐based response maps derived from 60 healthy control participants. We refer to these participants as the “discovery sample.” In addition, we used an independent “test sample” including 48 normal controls and 150 participants with ADHD to investigate ADHD‐control differences in the spatial and temporal characteristics of the derived functional networks. An overview of the analytical steps is presented in the Supporting Information (Fig. S1), and each step is described in detail below.
Participants
Both the participants for the discovery sample as for the test sample were selected from the NeuroIMAGE study. Detailed description of the recruitment and selection procedure for the entire cohort can be found in the supplemental material and in Von Rhein et al. [2014]. Here, we included all participants from ADHD and control families who underwent a MRI scan session that included administration of a monetary incentive delay (MID) task (N = 370). Participants with ADHD were required to have a current ADHD diagnosis according to the DSM‐5 definition, healthy controls were not allowed to display more than 2 ADHD‐related symptoms (see [Von Rhein et al., 2014] for detailed diagnostic procedure). Exclusion criteria were use of medication (other than ADHD medication) or illicit drugs (n = 7), acute psychiatric conditions such as psychosis (n = 3), and qualitatively insufficient or incomplete data (n = 102; see Supporting Information for details). Applying these selection criteria resulted in the inclusion of 258 participants in the current analyses (150 ADHD, 108 healthy controls). These participants were the same as described in a univariate task‐based case‐control comparison presented elsewhere [Von Rhein et al., 2015]. As the study was conducted in families, participants included siblings (n = 45).
As indicated above, the 258 participants were divided into a discovery and test sample. The discovery sample was entirely composed of control participants to ensure an unbiased definition of reward‐related networks. Sex was unequally distributed between controls and participants with ADHD, with more girls in the control group and more boys in the ADHD group (for sensitivity analyses on the effects of this imbalance we refer to the Supporting Information). To ensure that at least 25% of participants in each sample were male we randomly assigned 25% (n = 15) of the control boys to the discovery sample. All remaining control boys (n = 33) were assigned to the control test sample. Subsequently, we added female control participants to the control test sample until the ADHD and the healthy control test sample had a comparable percentage of male participants (∼70%). This was achieved by adding 15 randomly selected females to the control test sample. All other control females were added to the discovery sample. Demographics for all samples are displayed in Table 1.
Table 1.
Group characteristics
| Discovery sample | Test sample | Statistics | ||||
|---|---|---|---|---|---|---|
| Controls | Controls | ADHD | S | |||
| N | 60 | 48 | 150 | |||
| Comorbid (ODD/CD) | 0 | 0 | 34/8 | |||
| Number of males (%) | 15 (25) | 33 (69) | 105 (70) | |||
| M (SD) | M (SD) | M (SD) | ||||
| Age | 17.3 (2.8) | 16.9 (3.2) | 17.7 (3) | F(2,255) = 1.39 | ||
| IQ | 108 (15) | 107 (13) | 98 (15) | F(2,253) = 13.5* | (Con = Con)>ADHD | |
| Inattentive symptoms | 0.3 (1.1) | 0.8 (1.5) | 7.2 (1.8) | F(2,255) = 551* | (Con = Con)<ADHD | |
| Hyperactive symptoms | 0.1 (0.4) | 0.6 (1) | 6 (2.4) | F(2,255) = 283* | (Con = Con)<ADHD | |
*P < 0.05.
Paradigm
Participants performed a monetary incentive delay (MID) task, in which they needed to respond to the occurrence of a target stimulus by pressing a button. The core manipulation of this task relates to the target‐preceding cue. The color of this cue informs the participant whether a button press is potentially rewarded or not. Difficulty of the task was adapted to the performance of each participant by adjusting the time window in which participants were allowed to respond (20 ms shorter after hits and 10 ms longer after misses), separately for rewarded and neutral cues. This resulted in an expected hit rate of 33% for each trial type. Common measures of the MID task are reaction times and reaction time variability as well as neural responses in reward processing brain structures including VS and OFC during reward anticipation and receipt [Fairchild, 2011; Knutson et al., 2001]. A detailed description of the task is available in the Supporting Information and in Von Rhein et al. [2015].
Brain Network Analysis of Task‐Related Responses
Network identification and decomposition
To recover networks implicated in reward processing we focused on task‐based activation maps of the participants in the discovery sample. First, we identified all brain regions dedicated to reward processing by performing a typical task‐based fMRI analysis (first‐level) on preprocessed fMRI data with onset times of rewarded and neutral cues (regressor 1 and 2), hits (regressor 3 and 4), and misses (regressor 5 and 6) as regressors of interest (see Von Rhein et al. [2015] and Supporting Information for details of data acquisition/preprocessing and first‐level statistics). This analysis resulted in participant‐level spatial maps (zstat) for these six regressors. To investigate the relationship between these simple activation maps and higher‐order contrasts hypothesized to capture key reward processes, we included the within‐subject contrasts reward cue versus neutral cue (reward anticipation; spatial map 7) and rewarded versus neutral accuracy (hit vs. miss; reward receipt, spatial map 8). All eight maps were transformed to a common space (MNI152) for group analysis (see Supporting Information for details).
To decompose the reward network into independent sources, we concatenated unthresholded participant‐level zstat maps into one time series. On these data, we applied ICA as implemented in FSL MELODIC [Jenkinson et al., 2012]. To maximize component reliability, we ran 50 ICA decompositions, each including data from 40 randomly selected participants from the discovery sample. We requested extraction of 15 independent components (ICs) for each ICA decomposition. We chose this number to allow sufficient differentiation between noise and potential non‐noise components, while avoiding unreasonably scattered functional networks. The spatial maps of all ICs gained from these multiple ICA decompositions were thresholded by means of mixture modeling at P < 0.5 [Woolrich et al, 2005] and entered into a meta‐ICA decomposition with a dimensionality of 30 components [Biswal et al. 2010]. The 30 resulting components were again thresholded using mixture modeling at P < 0.5 [Woolrich et al., 2005] to reveal the final spatial maps. DvR and MM visually inspected the spatial maps in order to check the validity of the ensuing component solution and to identify components that clearly represented noise.
Network interpretation
To facilitate interpretation of the obtained meta‐ICA components in terms of their relation with task aspects, we investigated spatial similarity between each component and the group‐level task response maps. The group‐level task response maps were derived from group‐level statistical analysis on the participant‐level zstat maps with age, gender, and scan site as covariates. Group‐level statistical maps were thresholded using Gaussian Random field (GRF) theory‐based cluster statistics (P < 0.05) after initial thresholding (Z > 2.3). We obtained group‐level maps for the following eight contrasts: rewarded cue, neutral cue, rewarded hit, neutral hit, rewarded miss, neutral miss, reward cue versus neutral cue, and rewarded (hit vs. miss) versus neutral (hit vs. miss) receipt.
We conducted a spatial regression for each of the obtained group response maps against all 30 ICs from the meta‐ICA. This provided a unique loading (beta) for each IC on the task response maps, effectively indexing the spatial similarity of each component with each of the eight task response maps. Combining the eight beta weights into one vector per IC yielded for each IC a profile that resembled similarity between the IC and the response maps. Noise components were excluded from further analyses (see Supporting Information Fig. S4 for reference).
Since many obtained profiles showed similar patterns, we reduced the number of profiles using a k‐means clustering algorithm. This algorithm allocates individual data points to clusters by means of maximizing between‐cluster differences and minimizing within‐cluster differences. The number of clusters (k) was determined iteratively (n = 1,000) and by comparing the explained variance of all possible k's for our data to explained variance obtained for randomly generated data (see Supporting Information for details). We performed clustering with k ranging from two to the amount of non‐noise components (i.e., 2–23). This step yielded a limited number of distinct profiles represented by cluster‐averaged beta weights.
To identify the larger functional networks represented by the distinct profiles resulting from the clustering procedure, we calculated averaged spatial maps of the corresponding network components and mapped these visually onto functional networks gained from previous resting‐state functional connectivity analyses [Seeley et al., 2007; Smith et al., 2009].
Network Characteristics in Healthy Controls and Participants with ADHD
The second aim of our study was to assess the effects of ADHD on reward‐related brain networks. To this end, we used the networks identified using the discovery sample to investigate network‐related metrics in an independent sample (the test sample) of healthy control participants and adolescents with ADHD.
Network integrity
To investigate integrity of the identified network components, we applied a dual regression analysis using all ICs from the meta‐ICA [Filippini et al., 2009]. This analysis consisted of two stages. In the first stage, we used every participant's full task‐related time series to derive the time series for each of the ICs, by entering all ICs as spatial regressors in a multivariate GLM. In a second step, the obtained time courses were used as temporal regressors in a multivariate GLM to calculate spatial maps of each component for each individual. For further group comparisons, these spatial maps were transformed into MNI152 space using a custom study template (see Supporting Information).
To investigate diagnostic effects we applied a group‐level GLM to the subject‐specific spatial maps with diagnosis, age, gender, scan site, comorbidity with ODD/CD, and head motion summary scores as covariates. Significance for the effect of group was assessed using permutation testing (FSL randomize) with 10,000 permutations. Clusters were considered significant if they comprised at least 20 voxels and reached an FDR‐corrected P‐value < 0.05 [Smith et al., 2004]. Finally, to assess the relation between neural measures being sensitive to diagnostic effects and behavior, we correlated mean z‐stat values extracted from significant clusters of our case‐control comparison with reaction times and reaction time variability (for detailed description of these behavioral parameters see [Von Rhein et al., 2015]).
Communication between network components
Finally, we investigated the synchronicity of brain responses within and between different networks. For this analysis, we used the FSLNets toolbox (fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets). This Matlab‐based toolbox uses the time course of each IC (result of the first step of dual regression described above) to calculate normalized regression coefficients for each IC‐IC pairing (ridge regression). To assess the degree of network integration of the larger brain networks, we created a correlation matrix of all regression coefficients, arranged by the result of the clustering algorithm. For each cell indicating within‐ or between‐cluster functional connections we calculated the proportion of positive coefficients relative to all coefficients. These observed proportions were subsequently used to test against the expected population proportion (50% positive coefficients per cell) by means of z‐statistics. Using a 5% Type I error criterion, absolute z‐values greater than 1.96 were considered as statistically significant.
To test regression coefficients at the group‐level, we modeled group as between‐subject factor and age, gender, scan site, comorbidity with ODD/CD, and head motion summary scores as covariates. Statistical inference for the group contrast (ADHD vs. CON) was done using permutation testing with 10,000 permutations. Coefficients of IC‐IC pairings were considered significant if they exhibited a P‐value < 0.05 (FDR‐corrected).
RESULTS
Brain Network Analyses
Network identification and decomposition
The meta‐ICA applied to the ICA components resulting from iterative decomposition on the participant‐level task‐related maps yielded 7 components that were considered noise components based on visual inspection (see Fig. S4 in the Supporting Information), leaving 23 components for further investigation. Figure 1 illustrates the spatial maps for each non‐noise component.
Figure 1.
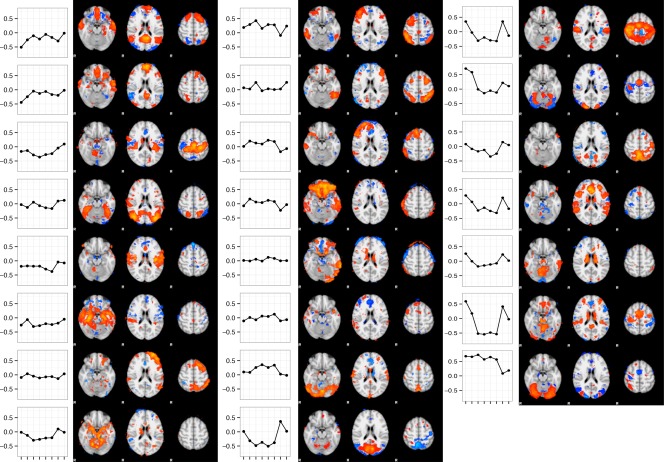
Profiles and spatial maps of all non‐noise ICs gained from meta‐ICA. Profiles indicate relation between task response maps and ICs expressed as beta estimates (y‐axis) of the multiple linear regression with task response map (x‐axis) as dependent measure and ICs from the meta‐ICA as independent measure. Task response maps are (from left to right): rewarded cue, neutral cue, rewarded hits, rewarded misses, neutral hits, neutral misses, reward anticipation, and reward receipt. [Color figure can be viewed at http://wileyonlinelibrary.com]
Network interpretation
To interpret the components resulting from the meta‐ICA, we compared the component spatial maps to the group‐level task activation maps obtained from the MID task. Group‐level activation maps are shown in Supporting Information Figure S5. We determined the correspondence between the component spatial map and each of the task contrast maps using spatial multiple regression. The resulting beta‐loadings for each component on each of the task contrasts are depicted in Figure 1.
Applying k‐means clustering to all 23 series of beta‐loadings yielded an optimal number of four specific clusters (see Supporting Information for detailed clustering results). For each cluster, the average beta‐series as well as the aggregated brain map is displayed in Figure 2. The beta‐series of the first three clusters respectively included eight (cluster 1), seven (cluster 2), and one (cluster 3) components and loaded on almost all task aspects. This was not the case for the fourth beta‐series, which loaded specifically on the reward manipulation. The first general cluster included regions in posterior cingulate gyrus (PCC), bilateral lateral parietal cortex, and ventromedial prefrontal cortex. Compared with functional networks revealed by resting‐state functional connectivity (RSFC) studies, these structures primarily resembled the default‐mode network (DMN) [Buckner et al., 2008; Smith et al., 2009]. Yet, few other regions were also associated with this cluster, including cortical structures such as bilateral temporal cortex and motor cortex, and subcortical regions including putamen, hippocampus, and amygdala.
Figure 2.
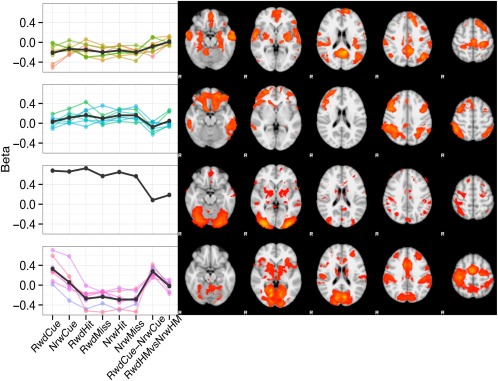
K‐means clustered profiles and spatial maps of non‐noise components. Black lines in profile plots indicate mean for each cluster. Spatial maps of independent components are averaged across cluster and thresholded (z > 2.3). Major networks that correspond with the different clusters are: (1) Default‐Mode, (2) Executive control, (3) Lateral Visual, and (4) Salience networks. [Color figure can be viewed at http://wileyonlinelibrary.com]
The second cluster was associated with dorso‐ and ventrolateral PFC and lateral parietal cortex. These regions fit best with the executive control network (ECN) [Seeley et al., 2007]. Other regions showing significant association with this cluster were ventromedial PFC, inferior temporal gyrus, and cerebellum.
The third cluster was formed by one single component. It strongly loaded on all task‐event response maps and was strongly associated with the lateral visual cortex. Including bilateral thalamus, this cluster fits best with a lateral visual network [Seeley et al., 2007]. Other regions associated with this cluster were bilateral putamen, inferior and superior frontal gyrus, and ventromedial PFC.
Finally, a fourth cluster was formed by seven components and demonstrated reward‐specific loadings. This cluster had strong loading on response maps for rewarded cues and the reward anticipation contrast and moderate loading on all receipt events. Associated regions comprised anterior cingulate cortex (ACC), supplementary motor cortex, bilateral fronto‐insular cortex, nucleus accumbens, putamen, brain stem, and thalamus. Compared with functional networks at rest, this cluster showed high spatial similarity with the salience network [Seeley et al., 2007]. Additional involved regions were motor cortex, visual cortex, dorso‐lateral PFC, and cerebellum.
ADHD‐Related Effects on Network Characteristics
Network integrity
Group maps of the functional connectivity analysis in the test sample are displayed in Supporting Information Figure S6. These maps replicated network components identified with the meta‐ICA in the discovery sample (Figure 2). Compared with the ICs from the meta‐ICA three of these networks exhibited extensions into adjacent regions. For the executive control network, adjacent regions included bilateral caudate nucleus and medial visual cortex. The visual network included precentral gyrus, dorsomedial PFC, and cerebellum; the salience network further included posterior cingulate gyrus, and caudate nucleus.
Case‐control differences were found for four networks. As indicated in Table 2, two of these components were associated with the salience clustered network and two with the executive control clustered network. We observed significantly stronger connectivity for control participants relative to participants with ADHD for both components constituting the executive control network: one component had stronger functional connections with the inferior frontal gyrus (IFG), and the other with the cerebellum. The salience network had one component with stronger connectivity for participants with ADHD relative to controls, the other component showed an effect in the opposite direction. The region with stronger connectivity for participants with ADHD was located in the cerebellum, the region with stronger connectivity for control participants in the inferior temporal gyrus. An overview of all significant clusters is given in Table 2 and in Supporting Information Figure S7. Correlations with behavioral measures were not significant (all P > 0.05).
Table 2.
Significant clusters from whole‐brain connectivity analysis
| Comparison | ICa | Sizeb | Z‐score | Xc | Yc | Zc | Region | Clustered network |
|---|---|---|---|---|---|---|---|---|
| CON vs. ADHD | 0 | 27 | 3.82 | 62 | −48 | −12 | Inferior temporal gyrus | Salience |
| 25 | 88 | 3.61 | 56 | 16 | 4 | Inferior frontal gyrus | Executive control | |
| 28 | 31 | 4.13 | −18 | −62 | −52 | Cerebellum | Executive control | |
| ADHD vs. CON | 5 | 20 | 4.41 | 6 | −50 | −16 | Cerebellum | Salience |
Independent component.
In voxel.
Coordinates in MNI.
Network communication
A matrix showing the regression coefficients of all IC‐IC pairings is displayed in Figure 3. The regression coefficients illustrate connectivity between the ICs included in each cluster, illustrating both within‐ as well as between‐cluster communication. The salience network exhibited significant within‐cluster integration between its composing ICs, as the majority of within‐cluster functional connections was positive (76%, P < 0.05). In contrast, the salience cluster and ICs from the DMN cluster and executive control cluster exhibited greater amount of negative functional connections (EC‐S: 28%, DMN‐S: 36%). Statistical tests of the proportion positive versus negative functional connections are summarized in Table 3. Statistical comparison of the IC‐IC matrices obtained for both groups did not reveal significant diagnosis‐related differences.
Figure 3.
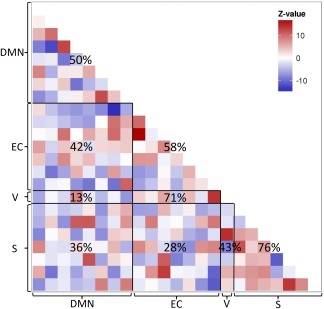
Matrix of the discovery sample regression coefficients of all IC‐IC pairings. Letters at x‐ and y‐axis indicate cluster to which IC belongs (DMN: default‐mode network, EC: executive control network; V: lateral visual network; S: Salience network). Digits in matrix indicate percentage of positive functional connections within or between clusters. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Statistical tests of proportion (positive vs. negative) within‐ and between‐network correlations
| Connection | Proportion | z‐score | P < 0.05 |
|---|---|---|---|
| DMN‐DMN | 50 | 0 | |
| DMN‐EC | 42 | −1.13 | |
| DMN‐V | 12 | −2.12 | * |
| DMN‐S | 36 | −2.06 | * |
| EC‐EC | 58 | 0.69 | |
| EC‐V | 71 | 1.13 | |
| EC‐S | 28 | −2.95 | * |
| V‐V | n.a.a | ||
| V‐S | 43 | −0.38 | |
| S‐S | 76 | 2.4 | * |
Networks are default‐mode network (DMN), executive control (EC), visual (V) and salience (S) network
Not applicable.
DISCUSSION
In this study we set out to identify functional networks on the basis of reward‐related task responses to describe reward processing in terms of a limited set of brain networks. We recovered four major brain networks. Three of them resembled general, reward‐independent task‐responses, while one was specifically reward processing oriented. Comparison of these networks with existing resting‐state functional connectivity studies revealed that the obtained networks reflected the executive control, default‐mode, lateral visual network, and salience networks.
The network that was preferentially associated with rewarded task responses exhibited high spatial correspondence with the well‐known salience network [Seeley et al., 2007]. The main functions of the salience network are to integrate information from different modalities such as sensory information and bodily states in order to establish goal‐directed behavior, and to process emotion‐ and reward‐related information such as reward predictions [Seeley et al., 2007]. The association of this network with rewarded cues might therefore reflect brain processes signaling the salience of the cue, but also the need to perform well, elaborating optimal response strategies, or increasing the readiness to respond.
The other three networks derived from our analyses were not specifically related to reward processing but seemed associated with overall task‐performance independent of reward manipulations. These networks were the DMN, the executive control network, and a lateral visual network. The executive control network exhibited a positive association with all task responses. For cue events, involvement of the executive control network might be related to attentional control processes necessary for an adequate behavioral response such as inhibition of a button press before the target occurs. Further, activation of this network during receipt events suggests that operability of this system is maintained during the whole trial.
The positive association between the executive control network and all task responses was paralleled by a negative association between the DMN and all task responses. The DMN is thought to function in the organization of the brain and undirected thoughts; it has also been found to be dynamically linked with the executive control network [Fox et al., 2005; Kelly et al., 2008] suggesting that the brain deactivates the DMN to allocate attentional resources.
Finally, the lateral visual network was represented across task manipulations. This could be explained by the modality of our task, which necessitates processing color information, detecting changes (occurrence of target), and reading feedback information during reward receipt.
Further investigating the connectivity between components constituting the four networks provided additional information about within‐ and between‐network characteristics. The salience network exhibited the most consistent within‐network integration, observed as positive connectivity among all components constituting this network. When investigating between‐network communication, we observed three consistent negative relations, namely between the salience and the DMN, the salience and the EC network, and between the DMN and the visual network. This result suggests that the salience network is highly segregated from other non‐visual networks. The visual network, on the other hand strongly segregated from the DMN.
Further research could include adequate modeling of these relationships within for instance a Dynamic Causal Modeling context [Friston et al., 2003]. The individual components as recovered through our approach would provide excellent regions of interest to define such causal models.
Although analyses were done on a single dataset investigating reward anticipation and receipt, the approach is easily extendable to other datasets. One interesting next step would be to acquire imaging data from different reward‐related functional tasks and recover reward‐related networks from those. Data sharing initiatives exist that might provide repositories for such efforts (e.g., Neurovault.org; OpenfMRI.org) and comparable attempts have proven fruitful. For instance, Smith et al. matched with the same data‐driven approach network components from task response maps of a large database (>7,000 maps) with functional networks obtained from a resting‐state network analysis [Smith et al., 2009], thereby demonstrating a close link between functional networks underlying diverse cognitive functions and the functional architecture of the brain. Our approach is similar in the sense that we also used task‐based activation maps as our starting point. However, our input maps were unthresholded z‐statistic maps related to a specific cognitive paradigm, compared with Gaussian spheres modeled at peak locations recovered from a database of studies as in Smith et al. As such, we believe that our approach allowed a more comprehensive and specific assessment of reward‐related networks.
We used a network discovery approach to investigate the effects of ADHD on larger brain networks. We observed that participants with ADHD relative to healthy controls exhibited altered functional connectivity of the executive control and the salience network. In both networks, functional connectivity was decreased, namely in the interior frontal gyrus (IFG) and cerebellum (both executive control) and inferior temporal gyrus (salience). The salience network had also increased functional connectivity in the cerebellum. In contrast, we observed no group‐related differences in the between‐ and within‐network integrity measures.
Altered functional connectivity of the executive control and salience network in ADHD may provide new biomarkers for ADHD. We note that the location as well as spatial extent of our results is in contrast to earlier resting‐state fMRI studies evidencing large changes in core structures of reward processing brain networks in ADHD [Costa Dias et al., 2013; Posner et al., 2013]. However, our current results are in line with the ADHD‐related effects observed in a typical task‐based analysis on the same data [Von Rhein et al., 2015]. Using the same sample as used here, this study reported moderately increased reward‐related activity in VS and OFC, core reward processing structures. It should be noted, however, that reward processing is a heterogeneous concept including different processes (see [Berridge and Kringelbach, 2008]), which are associated with signaling in different neural structures. Accordingly, we might have pooled distinct functional networks into one (i.e., salience) network. The use of such a unified network might not be specific enough to capture subtle differences between participants with ADHD and healthy controls in specific aspects of reward processing. When integrating across both our studies, our results suggest that ADHD might be related to altered usage of an otherwise intact reward‐processing network. These results are further confirmed in a second study in the current sample [Oldehinkel et al., 2016], where we observed no ADHD‐related effects in resting‐state functional connectivity between the functional building blocks of the salience network. In contrast, inattention modulated resting‐state functional connectivity within the default mode and fronto‐parietal networks underlying more general task processing. As such, our results support the idea that reward‐related deficits as behaviorally evident in ADHD are not supported by underlying reward‐specific neurobiological deficits. Rather they might result from more generally altered brain function [e.g., Sergeant, 2000], or from another specific deficit such as inhibition as already proposed by Barkley in 1997 [Barkley, 1997].
With respect to the technicalities of our implementation, an important point to discuss is the heterogeneity of the network components that we recovered from the meta‐ICA analysis. To simplify our reported network decomposition, we summarized the large number of components by clustering them together. We interpreted these clusters of components as unified networks assuming shared/common functionality of components within each cluster. However, in light of the assumed functional unity of components, it remains that components differ in their spatial extent as well as their time courses. For instance, some components within a network cluster were negatively correlated, which we currently interpret as a lack of integration of those components within the network. An alternative explanation would be that the clustered networks include structures that do actually not belong to the functional network.
A second discussion point relates to the inclusion of the higher‐order contrasts (rewarded cue vs. neutral cue and rewarded versus neutral accuracy [hit vs. miss]) in the component identification procedure. Components constituting the three general networks did not show high loadings for these higher‐order contrasts questioning their additional value. For the salience network, however, the loading of the reward anticipation contrast mirrored the positive loading observed for rewarded cues. The inclusion of higher‐order contrasts thus enabled us to investigate which task aspect was captured by a contrast, thereby facilitating its interpretation. For instance for the contrast rewarded versus neutral cue, finding high spatial similarity with rewarded cues supports the interpretation that this contrasts captures reward processes.
A third point of discussion relates to our functional interpretation of the brain networks. We estimated the relation between brain networks and different task aspects by calculating the unique association of the brain response to each task aspect and each brain network. These coefficients were used to form profiles per brain network, which were subsequently used to interpret the functional aspect of brain network. This interpretation was done qualitatively as assessing the differential pattern of the profiles’ coefficients (opposed to one‐by‐one) provides reasonable explanation of possible functional implications. Accordingly, these interpretations were not based on statistical differences between the obtained profiles.
To conclude, we discovered brain networks on the basis of reward‐related task responses. Using a data‐driven approach, we were able to recover four major brain networks involved in reward processing: the salience network, the executive control network, the lateral visual network, and the default mode network. This finding provides a comprehensive picture of involved brain networks and their specific task‐related role. Only the salience network was selectively associated with rewarded task aspects, whereas the other three networks seemed to be related to more general cognitive processes not specifically related to processing of reward. Between‐component connectivity analysis revealed a high degree of network integrity in the salience network, which was less evident in the other networks. Comparison of healthy participants and participants with ADHD revealed altered functional connectivity within the salience and executive control networks, while functional connectivity within the lateral visual network and the DMN did not differ between the two groups.
Supporting information
Supporting Information
ACKNOWLEDGMENT
Jan K Buitelaar has in the past 3 years been a consultant to/member of advisory board of/and/or speaker for Janssen Cilag BV, Eli Lilly, Lundbeck, Shire, Roche, Medice, Novartis, and Servier. He has received research support from Roche and Vifor. He is not an employee of any of these companies, and not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, royalties. Dr. Hoekstra has received research support from Shire and has been member of an advisory board of Shire. In the past year, Dr. Faraone received income, potential income, travel expenses and/or research support from Lundbeck, Rhodes, Arbor, Pfizer, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA Pharma, NeuroLifeSciences and NACE. With his institution, he has US patent US20130217707 A1 for the use of sodium‐hydrogen exchange inhibitors in the treatment of ADHD. In the past 3 years, Dr. Oosterlaan has received an investigator‐initiated grant from Shire Pharmaceuticals. All other authors (Dr Von Rhein, Luman, Cools, Hartman and Heslenfeld) have no conflicting interests to declare. The authors thank the Department of Pediatrics of the VU University Medical Center for the opportunity to use the mock scanner for preparation of the study participants as well as Paul Gaalman, technical engineer at the Donders Centre for Cognitive Neuroimaging, for his technical support at the site Nijmegen.
REFERENCES
- Aarts E, van Holstein M, Cools R (2011): Striatal dopamine and the interface between motivation and cognition. Front Psychol 35:1943–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA (1997): Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull 121:65–94. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE (2003): Parsing reward. Trends Neurosci 26:507–513. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML (2008): Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology 199:457–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, et al. (2010): Toward discovery science of human brain function. Proc Natl Acad Sci 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Costa Dias TG, Wilson VB, Bathula DR, et al. (2013): Reward circuit connectivity relates to delay discounting in children with attention‐deficit/hyperactivity disorder. Eur Neuropsychopharmacol 23:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demurie E, Roeyers H, Wiersema JR, Sonuga‐Barke E (2016): No evidence for inhibitory deficits or altered reward processing in adhd: Data from a new integrated monetary incentive delay go/no‐go task. J Attention Disord 20:353–367. [DOI] [PubMed] [Google Scholar]
- Fairchild G (2011): The developmental psychopathology of motivation in adolescence. Accid Anal Prev 1:414–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. (2009): Distinct patterns of brain activity in young carriers of the APOE‐ε4 allele. Proc Natl Acad Sci U S A 106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ (2005): Dynamic dopamine modulation in the basal ganglia: A neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci 17:51–72. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Fossella JA (2011): Neurogenetics and pharmacology of learning, motivation, and cognition. Neuropsychopharmacology 36:133–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W (2003): Dynamic causal modelling. NeuroImage 19:1273–1302. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM (2012): FSL. NeuroImage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP (2008): Competition between functional brain networks mediates behavioral variability. NeuroImage 39:527–537. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D (2001): Dissociation of reward anticipation and outcome with event‐related fMRI. Neuroreport 12:3683–3687. [DOI] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB (2010): Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum Brain Mapp 31:904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A (2010): Identifying the neurobiology of altered reinforcement sensitivity in ADHD: A review and research agenda. Neurosci Biobehav Rev 34:744–754. [DOI] [PubMed] [Google Scholar]
- Mesulam M (1998): From sensation to cognition. Brain 121:1013–1052. [DOI] [PubMed] [Google Scholar]
- Oldehinkel M, Francx W, Beckmann CF, Buitelaar JK, Mennes M (2013): Resting state FMRI research in child psychiatric disorders. Eur Child Adolesc Psychiatry 22:757–770. [DOI] [PubMed] [Google Scholar]
- Oldehinkel M, Beckmann CF, Franke B, Hartman CA, Hoekstra PJ, Oosterlaan J, Heslenfeld D, Buitelaar JK, Mennes M (2016): Functional connectivity in cortico‐subcortical brain networks underlying reward processing in attention‐deficit/hyperactivity disorder. NeuroImage Clin 12:796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Scheres A (2014): Ventral‐striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: A meta‐analytic review of the fMRI literature. Neurosci Biobehav Rev 38:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA (2012): The future of fMRI in cognitive neuroscience. NeuroImage 62:1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Rauh V, Gruber A, Gat I, Wang Z, Peterson BS (2013): Dissociable attentional and affective circuits in medication‐naïve children with attention‐deficit/hyperactivity disorder. Psychiatry Res 213:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J, Park C, Wang Z (2014): Connecting the dots: A review of resting connectivity MRI studies in attention‐deficit/hyperactivity disorder. Neuropsychol Rev 24:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Christakou A, Taylor E (2009a): Impulsiveness as a timing disturbance: Neurocognitive abnormalities in attention‐deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos Trans R Soc B 364:1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E (2009b): Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication‐naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology 57:640–652. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant JA (2000): The cognitive‐energetic model: An empirical approach to attention‐deficit hyperactivity disorder. Neurosci Biobehav Rev 24:7–12. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 Suppl 1:S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith S, Fox P, Miller K (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga‐Barke EJS (2002): Psychological heterogeneity in AD/HD–a dual pathway model of behaviour and cognition. Behav Brain Res 130:29–36. [DOI] [PubMed] [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, Hilgetag CC (2004): Organization, development and function of complex brain networks. Trends Cogn Sci 8:418–425. [DOI] [PubMed] [Google Scholar]
- Ströhle A, Stoy M, Wrase J, et al. (2008): Reward anticipation and outcomes in adult males with attention‐deficit/hyperactivity disorder. Neuroimage 39:966–972. [DOI] [PubMed] [Google Scholar]
- Tripp G, Wickens JR (2009): Neurobiology of ADHD. Neuropharmacology 57:579–589. [DOI] [PubMed] [Google Scholar]
- Von Rhein D, Mennes M, van Ewijk H, Groenman AP, Zwiers MP, Oosterlaan J, et al. (2014): The NeuroIMAGE study: A prospective phenotypic, cognitive, genetic and MRI study in children with attention‐deficit/hyperactivity disorder. Design and descriptives. Eur Child Adolesc Psychiatry 24:265–268. [DOI] [PubMed] [Google Scholar]
- Von Rhein D, Cools R, Zwiers MP, van der Schaaf, et al. (2015): Increased neural responses to reward in adolescents and young adults with attention‐deficit/hyperactivity disorder and their unaffected siblings. J Am Acad Child Adolesc Psychiatry 54:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Smith SM (2005): Mixture models with adaptive spatial regularization for segmentation with an application to FMRI data. IEEE Trans Med Imaging 24:1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


