Abstract
Background
A complete infectious focus survey relies on a thorough physical examination as well as a pelvic examination. Tubo-ovarian abscess, though less likely to occur in senior women, may become a life-threatening disease requiring emergent surgery. Hence, clinical awareness and aggressive management are warranted to avoid delayed diagnosis and subsequent complications.
Case presentation
We report a post-menopausal woman presented with sepsis of unknown origin, which turned out to be a huge tubo-ovarian abscess. Although tubo-ovarian abscess mostly occurs in women of fertile age, it is likely that the immune status of our post-menopausal patient was compromised because of old age and uremia. Moreover, due to underlying dementia, she could not express her discomfort in the early stage. Her sepsis resolved after a unilateral salpingo-oophorectomy surgery and antibiotic treatment. It is crucial to exclude pelvic inflammatory disease (PID) if no specific source of infection can be identified.
Conclusions
Rupture of the tubo-ovarian abscess is a condition of high mortality rate. Although tubo-ovarian abscess is more likely to develop in patients aged 15–25 years old, the tubo-ovarian abscess should be listed as a differential diagnosis in all post-menopausal women, especially those who are immunocompromised or with a palpable pelvic mass, to enable timely management and better prognosis.
Keywords: Fever of unknown origin, Tubo-ovarian abscess, Post-menopausal, Nonagenarian, Chronic kidney disease, Case report
Background
Tubo-ovarian abscess, one entity of pelvic inflammatory diseases (PID), mostly occurs in women of fertile age and may become a life-threatening condition requiring emergent surgery. Therefore, in order to ensure early recognition, it is essential to exclude PID if no specific source of infection can be identified, even in elderly post-menopausal women. In this report, we highlight the pivotal role of pelvic examination in a thorough infectious focus work-up.
Case presentation
A 91-year-old post-menopausal woman without diabetes mellitus or hypertension presented with shortness of breath, fever up to 38.5 degrees, anuria, and conscious disturbance for two days. Tracing back her history, she has dementia for 20 years with chronic kidney disease in stage 5, and she has not received any bowel or adnexal surgery. Last year, a transvaginal ultrasound had been performed by the gynecologist for a palpable pelvic mass, but only endometrial hyperplasia was impressed. Upon this admission, physical examination revealed a palpable mass as well, but there was no evident tenderness initially. Her body mass index was 23 Kg/m2. Laboratory test showed leukocytosis, azotemia with blood urea nitrogen 117 mg/dL, creatinine 12.9 mg/dL, C-reactive protein 26.2 mg/dL, procalcitonin 2.5 ng/mL, and pyuria. We initiated hemodialysis therapy for her uremia. Stool routine and culture showed negative results, indicating that colitis or gastrointestinal bleeding is less likely.
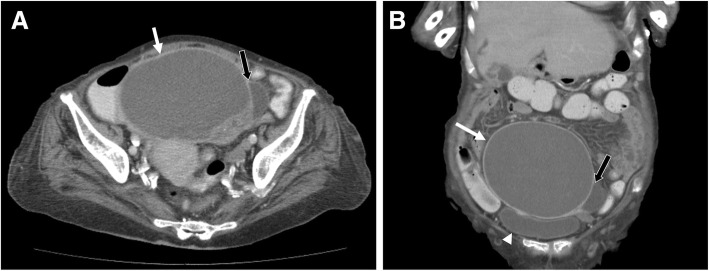
After two weeks of antibiotic treatment, leukocytosis, pyuria, and sepsis resolved, but intermittent fever lasted along with pelvic tenderness. We thus consulted the gynecologist again, who then arranged an urgent abdominal computed tomography (CT) because of the highly possible surgical requirement upon consultation. The CT scan disclosed the presence of a huge cystic mass 13.5 × 11.8 cm with internal septation and mural solid component without any obvious fat stranding at lower abdomen nor any evidence of acute colitis. The urinary bladder was compressed by it (Fig. 1). No significant enlarged lymph nodes were found. Mucinous cystadenoma with ovarian torsion was suspected, and thus surgical intervention was arranged. During the surgery, a 12 × 10 × 10 cm right tubo-ovarian abscess with 800 mL of pus-like content was drained. Right salpingo-oophorectomy and pus culture were performed. The pathological examination showed ovarian tissue with acute and chronic inflammation, inflammatory exudate, and granulation tissue formation, which were compatible with that of a tubo-ovarian abscess, and its pus culture yielded Escherichia coli. Antibiotics were administered based on the culture sensitivity test, and her infection ultimately resolved thereafter.
Fig. 1.
An abdominal computed tomography demonstrated the presence of a huge cystic mass 13.5 × 11.8 cm (white arrows) with internal septation (panel a, black arrows) and solid mural component. The urinary bladder (panel b, arrowhead) was compressed by it. No significant enlarged lymph nodes were found
Discussion and conclusions
In a case series which enrolled 80 tubo-ovarian abscess patients, their age ranged from 15 to 69 years old with an average of 42 years old [1]. In another retrospective study enrolled 63 patients with a surgically confirmed tubo-ovarian abscess, only nine patients were post-menopausal [2], contrasting the rarity of our case who is a nonagenarian. Similarly, literature regarding tubo-ovarian abscess showed that the average age ranges 52–58-year-old in the post-menopausal group, as shown in Table 1 [1–10]. The risk factors for tubo-ovarian abscess include age between 15 to 25 years old, a prior history of pelvic inflammatory disease, and multiple sexual partners.
Table 1.
Literature review of tubo-ovarian abscess in post-menopausal women
| No. | Patient number (Post-menopausal/Total) | Age (year) | Microorganism | Treatment | Treatment outcome | Conclusion | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 1/1 | 91 | Escherichia coli | Exploratory laparotomy with antibiotics | Successful and patient survived | TOA could occur in nonagenarian women, especially those who are immunocompromised, which requires timely management for a better prognosis | Our case |
| 2 | 1/1 | 55 | Clostridium perfringens | Exploratory laparotomy with hysterectomy | Successful and patient survived | Clostridium perfringens can cause adnexal infection in the absence of trauma | 8 |
| 3 | 1/1 | 71 | N/A | Exploratory laparotomy | Successful and patient survived | Chronic TOA may rupture or fistulize to adjacent organs into the ischiorectal space | 7 |
| 4 | 9/63 |
Pre-menopausal: 26 Post-menopausal: 52 |
N/A | Exploratory laparotomy | Successful and patient survived | An attempt at early recognition and surgical management of TOA is vital in post-menopausal women | 2 |
| 5 | 17/80 | Overall: 42 | Anaerobes; negative results | Exploratory laparotomy | Successful and patient survived | Fewer patients were hospitalized in Oslo for PID during the period of 2000–2002 compared with ten years earlier, but a higher percentage of patients had developed TOA compared with the first period (43% compared with 26%), indicating a changing clinical panorama of PID | 1 |
| 6 | 17/93 |
Pre-menopausal: 34 Post-menopausal: 58 |
N/A | Exploratory laparotomy | Successful and patient survived | For post-menopausal women with TOAs, pelvic malignancy should be excluded. Conservative treatment has no place during the menopause | 9 |
| 7 | 20/20 | N/A | N/A | Total hysterectomy | Successful and patient survived | Early detection and treatment of unruptured TOA had less surgery-related complications and had a shorter mean length of hospitalization | 10 |
| 8 | 25/296 | Overall: 34.5 ± 10.3 | N/A | Exploratory laparotomy; laparoscopic treatment; broad-spectrum antibiotics | Successful and patient survived | Post-menopausal status on admission were associated with a failed response to conservative treatment | 6 |
| 9 | 29/64 |
Early laparoscopic: 39.0 Conventional: 38.9 |
Escherichia coli Peptostreptococci baumanmii |
Early laparoscopic treatment; conventional antibiotics | Successful and patient survived | Early laparoscopic treatment is associated with a shorter time of fever resolution, shorter hospitalization, and less blood loss compared with conventional treatment for TOA or pelvic abscess | 4 |
| 10 | 35/318 |
Medical treatment: 35.6 ± 8.1 Medical + Surgical treatment: 37.3 ± 6.2 |
N/A | Exploratory laparotomy with drainage tube; conventional antibiotics | Successful and patient survived | The TOA size, complex multi-cystic mass image, CRP, and ESR are useful indicators as to whether surgical treatment is required for the management of TOA | 5 |
| 11 | 39/144 |
Pre-menopausal: 38.5 ± 7.7 Post-menopausal: 54.3 ± 8.1 |
Group C Streptococcus | Exploratory laparotomy with antibiotics; drainage for premenopausal women only | One post-menopausal woman of TOA had malignancy, but no other women were diagnosed with cancer during a mean follow-up of 7.6 years | In post-menopausal women with TOA, the prevalence of concurrent pelvic malignancy was 2.6%, which is higher than in the general population, but lower than that reported in the literature; 44% were conservatively managed without any apparent cases of misdiagnoses of cancer | 3 |
*Data were presented as mean or mean ± SD. Abbreviations: N/A not available, TOA tubo-ovarian abscess
Heaton et al. reported 20 post-menopausal women with a tubo-ovarian abscess in a case series; only 20% of patients were febrile, 45% presenting with leukocytosis, and 55% having a palpable pelvic mass [11]. In our patient, fever and leukocytosis were presented initially. However, due to her underlying dementia, the patient could not express her discomfort. Meanwhile, the initial physical examination did not reveal any acute abdominal sign, leading to delayed recognition of tubo-ovarian abscess in our case. Hsiao et al. analyzed 74 patients with surgically proved tubo-ovarian abscess, they found that an accurate preoperative diagnosis of the tubo-ovarian abscess was significantly lower in the post-menopausal group as compared to the pre-menopausal group (22% versus 54%), indicating a highly prevalent silent presentation of tubo-ovarian abscess in the post-menopausal group [12]. Also, another predisposing factor of our patient may be her immunocompromised status because of advanced age and uremia, usually manifesting as reduced antigen-presenting dendritic cells, depletion of naïve and central memory T cells and B cells, and impaired phagocytic function of neutrophils and monocytes [13].
Because rupture of a tubo-ovarian abscess is a life-threatening emergency, aggressive medical or surgical management is required immediately [14]. Therefore, during infection work-up, clinicians should always consider PID to avoid delayed management, even if patients are more than 70 years old, as is our patient. A complete infectious focus survey relies on a thorough physical examination as well as a pelvic examination. Also, the tubo-ovarian abscess should be listed as a differential diagnosis in all post-menopausal women, especially those who are immunocompromised or with a palpable pelvic mass, to enable timely management and better prognosis.
Acknowledgements
Not applicable.
Abbreviation
- PID
Pelvic inflammatory disease
Authors’ contributions
Drafting manuscript: KC and CY. Revising manuscript content: JT and CY. Approving final version of manuscript: KC, JT, and CY. All authors make a significant contribution to the project and give final approval of the submitted manuscript.
Funding
This work was in part supported for research purpose by the “Development and Construction Plan” of the School of Medicine, National Yang-MingUniversity, Taiwan (107F-M01), the Ministry of Science and Technology, Taiwan (MOST 108–2633-B-009-001), and Taipei Veterans General Hospital, Taiwan (V106D25–003-MY3). The funders have no role in study design, data collection, analysis, and interpretation, or in writing of the manuscript.
Availability of data and materials
All data presented in this report are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
The son of the patient who was described in this case report had given written informed consent for the publication of this case report.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kuan-Yi Chen, Email: ioudanny520@hotmail.com.
Jen-Yu Tseng, Email: jytseng@vghtpe.gov.tw.
Chih-Yu Yang, Phone: +886-2-2871-2121, Email: cyyang3@vghtpe.gov.tw.
References
- 1.Sorbye IK, Jerve F, Staff AC. Reduction in hospitalized women with pelvic inflammatory disease in Oslo over the past decade. Acta Obstet Gynecol Scand. 2005;84(3):290–296. doi: 10.1111/j.0001-6349.2005.00509.x. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman M, Molpus K, Roberts WS, Lyman GH, Cavanagh D. Tuboovarian abscess in postmenopausal women. J Reprod Med. 1990;35(5):525–528. [PubMed] [Google Scholar]
- 3.Yagur Y, Weitzner O, Man-El G, Schonman R, Klein Z, Fishman A, et al. Conservative management for postmenopausal women with tubo-ovarian abscess. Menopause. 2019. [DOI] [PubMed]
- 4.Chu L, Ma H, Liang J, Li L, Shen A, Wang J, et al. Effectiveness and adverse events of early laparoscopic therapy versus conservative treatment for Tubo-ovarian or pelvic abscess: a single-center retrospective cohort study. Gynecol Obstet Investig. 2019:1–9. [DOI] [PubMed]
- 5.Inal ZO, Inal HA, Gorkem U. Experience of Tubo-ovarian abscess: a retrospective clinical analysis of 318 patients in a single tertiary Center in Middle Turkey. Surg Infect. 2018;19(1):54–60. doi: 10.1089/sur.2017.215. [DOI] [PubMed] [Google Scholar]
- 6.Gungorduk K, Guzel E, Asicioglu O, Yildirim G, Ataser G, Ark C, et al. Experience of tubo-ovarian abscess in western Turkey. Int J Gynaecol Obstet. 2014;124(1):45–50. doi: 10.1016/j.ijgo.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Belli EV, Landmann RG, Koonce SL, Chen AH, Metzger PP. Persistent ischiorectal fistula with supralevator origin secondary to a chronic tubo-ovarian abscess: report of a case and review of the literature. Female Pelvic Med Reconstr Surg. 2012;18(1):66–67. doi: 10.1097/SPV.0b013e31823bdbe2. [DOI] [PubMed] [Google Scholar]
- 8.Wagner A, Russell C, Ponterio JM, Pessolano JC. Ruptured tuboovarian abscess and septic shock with Clostridium perfringens in a postmenopausal woman: a case report. J Reprod Med. 2009;54(10):652–654. [PubMed] [Google Scholar]
- 9.Protopapas AG, Diakomanolis ES, Milingos SD, Rodolakis AJ, Markaki SN, Vlachos GD, et al. Tubo-ovarian abscesses in postmenopausal women: gynecological malignancy until proven otherwise? Eur J Obstet Gynecol Reprod Biol. 2004;114(2):203–209. doi: 10.1016/j.ejogrb.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 10.Chao AS, Chang SY, Soong YK. Postmenopausal tuboovarian abscess. Changgeng Yi Xue Za Zhi. 1992;15(3):128–133. [PubMed] [Google Scholar]
- 11.Heaton FC, Ledger WJ. Postmenopausal tuboovarian abscess. Obstet Gynecol. 1976;47(1):90–94. [PubMed] [Google Scholar]
- 12.Hsiao SM, Hsieh FJ, Lien YR. Tuboovarian abscesses in postmenopausal women. Taiwanese J Obstet Gynecol. 2006;45(3):234–238. doi: 10.1016/S1028-4559(09)60231-X. [DOI] [PubMed] [Google Scholar]
- 13.Vaziri ND, Pahl MV, Crum A, Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012;22(1):149–156. doi: 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedowitz P, Bloomfield RD. Ruptured adnexal abscess (Tuboovarian) with generalized peritonitis. Am J Obstet Gynecol. 1964;88:721–729. doi: 10.1016/0002-9378(64)90604-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data presented in this report are included in this published article.