Abstract
Objective:
To evaluate the clinical and cost effectiveness of a group-based memory rehabilitation programme for people with traumatic brain injury.
Design:
Multicentre, pragmatic, observer-blinded, randomized controlled trial in England.
Setting:
Community.
Participants:
People with memory problems following traumatic brain injury, aged 18–69 years, able to travel to group sessions, communicate in English, and give consent.
Interventions:
A total of 10 weekly group sessions of manualized memory rehabilitation plus usual care (intervention) vs. usual care alone (control).
Main measures:
The primary outcome was the patient-reported Everyday Memory Questionnaire (EMQ-p) at six months post randomization. Secondary outcomes were assessed at 6 and 12 months post randomization.
Results:
We randomized 328 participants. There were no clinically important differences in the primary outcome between arms at six-month follow-up (mean EMQ-p score: 38.8 (SD 26.1) in intervention and 44.1 (SD 24.6) in control arms, adjusted difference in means: –2.1, 95% confidence interval (CI): –6.7 to 2.5, p = 0.37) or 12-month follow-up. Objectively assessed memory ability favoured the memory rehabilitation arm at the 6-month, but not at the 12-month outcome. There were no between-arm differences in mood, experience of brain injury, or relative/friend assessment of patient’s everyday memory outcomes, but goal attainment scores favoured the memory rehabilitation arm at both outcome time points. Health economic analyses suggested that the intervention was unlikely to be cost effective. No safety concerns were raised.
Conclusion:
This memory rehabilitation programme did not lead to reduced forgetting in daily life for a heterogeneous sample of people with traumatic brain injury. Further research will need to examine who benefits most from such interventions.
Keywords: Traumatic brain injury, memory rehabilitation, randomized controlled trial
Introduction
Approximately 65% of people with moderate–severe traumatic brain injury report long-term problems with cognitive functioning, with memory being most disrupted.1 Cognitive dysfunctions are persistent and debilitating, reduce quality of life, and are the leading cause of disability in traumatic brain injury.2
Memory rehabilitation aims to retrain memory and help people compensate for deficits. Patients are taught the use of external and internal memory aids, sometimes with computer-assisted delivery, and typically delivered as individual or group interventions in community settings. However, with shrinking healthcare budgets, group formats are increasingly being commissioned in the United Kingdom,3 assuming that these are cost and resource efficient, and clinically beneficial, but research evidence to support such decisions is weak.
National clinical guidelines4,5 for adult traumatic brain injury rehabilitation recommend teaching patients ‘compensatory memory strategies’ to improve everyday functioning.4 However, meta-analyses have suggested that the evidence of effectiveness of such interventions is sparse,6,7 with several small, methodologically weak studies contributing much of the evidence. In fact, a key recommendation from the Lancet Neurology Commission was that ‘Robust evidence is needed to inform guidelines on medical, surgical, and rehabilitation interventions, and hence improve outcomes for patients with TBI [traumatic brain injury]’.8
Our aim was to determine the clinical and cost effectiveness of a group-based memory rehabilitation programme for community-dwelling people with memory problems following traumatic brain injury.
Methods
This was a multicentre, two-arm, parallel group, pragmatic randomized controlled trial of group-based memory rehabilitation plus usual care, compared with usual care alone. Ethical approval was obtained from the National Research Ethics Service (12/EM/0324) and the Ministry of Defence Research Ethics Committee (374/PPE/12). The study was prospectively registered (ISRCTN65792154) and the protocol was published.9 This project was funded by the National Institute for Health Research (NIHR), Health Technology Assessment Programme (project no. 10/57/24) between September 2012 and May 2017. The funder had no role in study design, data collection, analysis, interpretation, or writing of the report.
Participants and procedures
Participants were recruited from nine English National Health Service (NHS) Trusts providing rehabilitation services for people with traumatic brain injury, and from a military rehabilitation centre and an NHS surgical centre treating armed forces personnel. Participants also self-referred following publicity by brain injury charities and patient groups, and by advertising to the general public.
The participants satisfying the following criteria were eligible:
Were admitted to hospital with a traumatic brain injury more than three months prior to recruitment;
Had memory problems, defined as a score ⩾24 on the Everyday Memory Questionnaire – patient version10 or a score <25th percentile on the Rivermead Behavioural Memory Test – version 3;11
Were 18–69 years of age;
Were able to travel to one of the study sites and attend group sessions, and willing to receive treatment in a group if allocated to intervention;
Gave written consent.
We excluded those with the following:
Unable to engage in group treatment if allocated, such as severe hearing or behavioural problems, which was assessed by the clinicians at recruitment sites;
Participating in other psychological intervention studies;
With impairment of language, scoring <17 on the Sheffield Screening Test for Acquired Language Disorders.12
After a screening visit to confirm eligibility and a second visit to collect baseline data and set short- and long-term goals, clusters of 4–6 eligible participants who could attend a group at the same time and place were formed. Participants were randomly allocated, as a cluster, to intervention or usual care on a 1:1 ratio. The allocation sequence was stratified by study site and based on a computer-generated pseudo-random code using random permuted blocks of randomly varying size. Allocation was concealed using a secure web-based system developed and maintained by the Nottingham Clinical Trials Unit. Blinding of participants and Assistant Psychologists delivering the intervention was not possible. Researchers collecting secondary outcome data at 6- and 12-month follow-up visits were blinded to treatment allocation.
All participants received their usual clinical care, which was recorded in the resource use questionnaire. The intervention was memory rehabilitation, as described in our published protocol,9 and delivered following an intervention manual. Details of the intervention following the Template for Intervention Description and Replication (TIDieR) guidelines13 are provided in Supplemental Table 1.
Outcome assessments were completed 6 and 12 months post randomization using self-report questionnaires and face-to-face assessments. Outcome measures reflected the three levels of the International Classification of Function:14 impairment, activity limitations, and participation restrictions, and were selected on the basis of their clinical utility, relevant psychometric properties, and patient feedback. The primary follow-up was 6 months after randomization, with a 12-month assessment to determine longer term effects.
The primary outcome measure was the 28-item patient-reported version of the Everyday Memory Questionnaire – patient version10 that assesses the frequency of memory failures in everyday life over the past month. Each item was rated on a five-point Likert-type scale (‘once or less in the last month/never’ to ‘once or more a day’). We chose a patient-reported outcome because (1) of its ecological validity, (2) our Patient and Public Involvement group felt this was essential, (3) Cochrane Reviews of cognitive rehabilitation have concluded that ‘future research should use outcomes that are deemed important by service users’,15 and (4) subjective cognitive outcomes have been used in several memory rehabilitation trials.16
The secondary outcomes were an objective evaluation of everyday memory (Rivermead Behavioural Memory Test General Memory Index),11 patient-reported outcomes of mood (General Health Questionnaire – 30-item version),17 experience of brain injury (European Brain Injury Questionnaire – patient version modified version),18 and personal short- and long-term goal attainment. Participants also nominated a relative/friend who completed the relative version of the Everyday Memory Questionnaire and the European Brain Injury Questionnaire – relative version.
The cost effectiveness of memory rehabilitation was evaluated from the UK NHS and Personal Social Services perspective. The primary health economic outcomes were a bespoke resource use questionnaire and EuroQol19 Quality of Life – five-dimensional questionnaire, five-level version (EQ-5D-5L).
Intervention sessions were video recorded and a trained independent observer rated these on minute intervals and compared what was covered in the sessions with what was in the manual. Therapist skill at delivering the intervention was also assessed independently and reported elsewhere.20,21
Analyses
To detect a minimum clinically relevant difference in the mean of 12 points on the Everyday Memory Questionnaire – patient version, with 5% two-sided alpha, 90% power, and SD 21.9, requires 71 participants per arm for analysis in an individually randomized trial. Assuming that 10% of variance in outcome would be explained by centre as a fixed effect (based on four centres), allowing for clustering due to the group intervention (cluster size of 6 and intracluster correlation coefficient of 0.1), and accounting for 20% attrition, the target sample size was 312 (calculated using the ‘Optimal Design’ software).22
The main approach to analysis was modified intention-to-treat, that is, analysis according to randomized arm regardless of adherence to allocation and including participants who provided outcome data, within three months of the due date, at follow-up.
We estimated the difference in mean outcome scores between the two arms using a multilevel linear model with site and baseline score as the covariates. Although participants were randomly allocated in clusters, individuals in the usual care arm had no contact with each other and outcomes in this arm were therefore assumed to be independent. However, participants in the intervention arm received memory rehabilitation sessions in groups. We therefore used a fully heteroscedastic model23 recommended for analysis of trials comparing group-based treatments with individual-based treatment, when there is adjustment for individual-level covariates. This model estimated group-level residual variance in the intervention arm and also permitted individual-level residual variance to differ between the intervention and control arms.23 Assumptions for the multilevel linear model were checked using diagnostic plots.
Sensitivity analyses for the primary outcome used multiple imputation for missing outcome data and explored the impact of attendance at group sessions by estimating the complier average causal effect24 at six months using instrumental variable regression. Adherence which was defined as attending at least four memory rehabilitation sessions. Details of the sensitivity analysis are published elsewhere.20
A planned exploratory subgroup analysis on the primary outcome was performed according to memory impairment at baseline using the Rivermead Behavioural Memory Test General Memory Index split into three clinically meaningful groups, based on the test manual25 (significant impairment, borderline/moderate, and average/above-average range), by including an interaction term in the model. Following the planned analyses, trial collaborators suggested that the time since traumatic brain injury could potentially affect intervention effectiveness. We therefore conducted a post hoc subgroup analysis for time since traumatic brain injury.
The EQ-5D-5L scores were used to derive utilities to calculate quality adjusted life year (QALY) scores using the England valuation set.26 Multiple imputation was used to address missing data and baseline differences were controlled for by a multilevel linear model. The multiple-imputation-adjusted costs and outcomes were used for the base case analysis. As the time horizon was 12 months, discounting of costs and effects was not done.
A cost utility analysis was undertaken. An incremental cost effectiveness ratio was used to summarize the incremental cost per additional QALY gained as a result of memory rehabilitation, based on the multiple-imputation-adjusted analysis for the base case. One-way sensitivity analyses were undertaken to evaluate whether changes to costs or effects (based on the lower and upper bound confidence interval (CI) values) influenced the incremental cost effectiveness ratio when other values remained at the base case level. To assess the impact of joint uncertainty, non-parametric bootstrapping was used across 1000 replications with a cost effectiveness acceptability curve produced to ascertain the probability of memory rehabilitation being cost effective across different willingness-to-pay thresholds, with £20,000–£30,000 per QALY gained used to indicate whether memory rehabilitation would be considered cost effective.
All analyses were conducted using Stata/SE 13.1. Details were documented in the statistical analysis plan, which was finalized prior to database lock and release of treatment allocation codes. Trial Steering and Data Monitoring Committees provided independent oversight of the trial.
Results
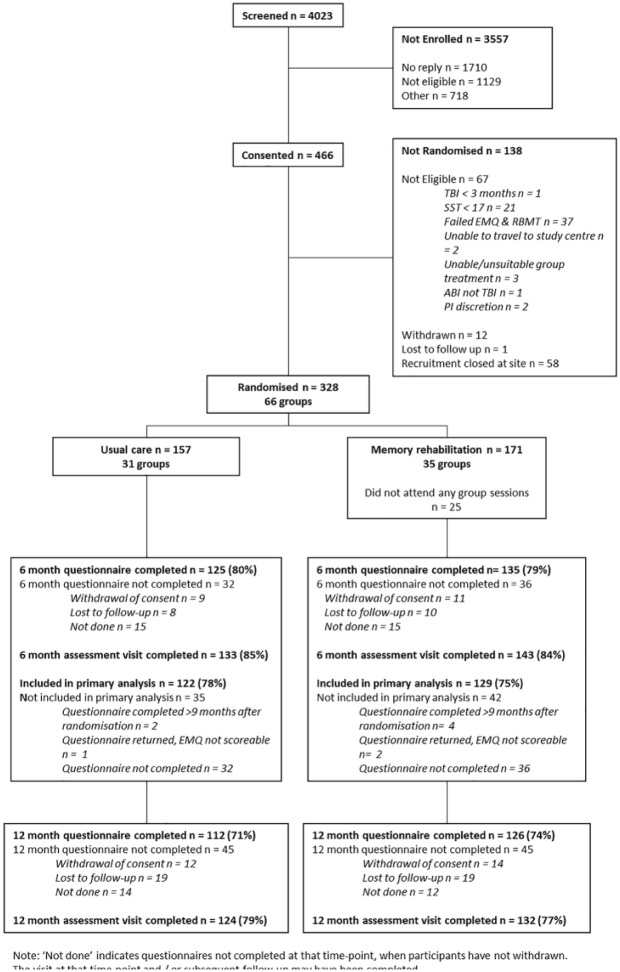
We invited 4023 people with traumatic brain injury to participate between February 2013 and December 2015. Non-eligibility and recruitment being closed at the site were the main reasons participants were not randomized after consent. We exceeded the randomization target because participants were randomized in clusters. Figure 1 outlines the number and flow of participants throughout the trial.
Figure 1.
Flow diagram.
Follow-up was completed between October 2013 and December 2016. Questionnaire return and visit completion were similar in the two arms at both time points. The primary analysis included 129 (75%) participants in the memory rehabilitation arm and 122 (78%) in the usual care arm. The main reason participants were not included was due to not completing questionnaires. We compared the baseline characteristics of participants according to completion of the primary outcome and the allocated group. In both groups, participants with no primary outcome data tended to have a slightly lower level of educational attainment and slightly more memory problems at baseline based on both patient and relative/friend report than participants with primary outcome data.
Participant characteristics were well balanced between arms at baseline (Table 1).
Table 1.
Baseline characteristics.
| Usual care (n = 157) | Memory rehabilitation (n = 171) | |
|---|---|---|
| Age (years) | ||
| Mean [SD] | 45.1 [12.5] | 45.8 [11.5] |
| Gender | ||
| Men | 116 (74%) | 123 (72%) |
| Women | 41 (26%) | 48 (28%) |
| Ethnicity | ||
| White | 147 (94%) | 167 (98%) |
| Black | 6 (3%) | 2 (1%) |
| Mixed race | 3 (2%) | 1 (1%) |
| Other | 1 (1%) | 1 (1%) |
| Residential status | ||
| Living alone | 44 (28%) | 43 (25%) |
| Living with others | 106 (68%) | 120 (70%) |
| Living with informal care | 2 (1%) | 1 (1%) |
| Living with formal care | 2 (1%) | 0 |
| Living in care home | 3 (2%) | 7 (4%) |
| Highest educational attainment | ||
| Below GCSE | 26 (17%) | 29 (17%) |
| GCSE | 54 (34%) | 49 (29%) |
| A-Level | 42 (27%) | 34 (20%) |
| Degree | 24 (15%) | 41 (24%) |
| Higher degree | 10 (6%) | 17 (10%) |
| Not known | 1 (1%) | 1 (1%) |
| Employment status at screening (not mutually exclusive) | ||
| Not employed | 80 (51%) | 85 (50%) |
| Employed full-time | 25 (16%) | 38 (22%) |
| In education full-time | 2 (1%) | 1 (1%) |
| Voluntary full-time | 1 (1%) | – |
| Retired | 17 (11%) | 15 (9%) |
| Employed part-time | 25 (16%) | 19 (11%) |
| Voluntary part-time | 9 (6%) | 17 (10%) |
| Time since TBI (months) | ||
| Median [25th centile, 75th centile] | 46 [23, 116] | 58 [24, 148] |
| EMQ-p (participant-reported frequency of memory problems in everyday life) | ||
| Mean [SD] | 50.1 [24.6] | 47.4 [21.0] |
| RBMT-3 General Memory Index (assessed memory abilities) | ||
| Mean [SD] | 76.3 [14.5] | 77.7 [13.6] |
GCSE: General Certificate of Secondary Education; EMQ-p: Everyday Memory Questionnaire (patient version); TBI: traumatic brain injury; RBMT-3: Rivermead Behavioural Memory Test; GMI: General Memory Index.
EMQ-p scores range from 0 to 112 with higher scores indicating more frequent/important memory problems. RBMT-3 GMI scores range between 52 and 174 and have been standardized to have a mean of 100 and an SD of 15 on a demographically representative sample from the United Kingdom.
Participants in the intervention arm attended a mean of 6.3 memory rehabilitation sessions (SD 3.5); 131 participants (77%) attended four or more sessions. The number of days from the last treatment session to six-month visit and questionnaire were as follows: median: 92 (interquartile range (IQR): 76–105) and median: 80 (IQR: 59–98), respectively.
There was no clinically important difference on the Everyday Memory Questionnaire – patient version at 6 months between the two arms (Table 2) or at 12 months (Table 3). Memory ability on the Rivermead Behavioural Memory Test favoured the memory rehabilitation arm at 6 months; however, there was no evidence of a difference at 12 months. There was no evidence of any difference on the General Health Questionnaire–30 at 6 or 12 months. Goal attainment scores favoured the memory rehabilitation arm at both 6 and 12 months.
Table 2.
Primary and secondary outcomes at six-month follow-up.
| Baseline, mean [SD] | Six-month follow-up, mean [SD] | Adjusted difference in means (95% CI) | |
|---|---|---|---|
| EMQ-p (primary outcome) | |||
| Usual care (n = 122) | 48.9 [23.9] | 44.1 [24.6] | –2.1 (–6.7 to 2.5), p = 0.37 |
| Memory rehabilitation (n = 129) | 45.9 [21.0] | 38.8 [26.1] | |
| General Health Questionnaire 30 | |||
| Usual care (n = 110) | 33.9 [15.7] | 34.1 [16.8] | –1.6 (–5.3 to 2.1) |
| Memory rehabilitation (n = 124) | 36.2 [15.4] | 33.6 [16.3] | |
| RBMT-3 GMI | |||
| Usual care (n = 133) | 77.1 [14.5] | 79.1 [15.0] | 2.5 (0.1 to 4.8) |
| Memory rehabilitation (n = 141) | 78.9 [13.7] | 82.7 [14.0] | |
| Short-term goal attainment average score | |||
| Usual care (n = 131) | – | 1.2 [1.0] | 0.6 (0.3 to 0.9) |
| Memory rehabilitation (n = 141) | – | 1.8 [1.0] | |
| Long-term goal attainment average score | |||
| Usual care (n = 131) | – | 1.0 [0.9] | |
| Memory rehabilitation (n = 141) | – | 1.5 [1.0] | 0.5 (0.2 to 0.7) |
CI: confidence interval; EMQ-p: Everyday Memory Questionnaire (patient version); RBMT-3: Rivermead Behavioural Memory Test; GMI: General Memory Index.
Table 3.
Secondary outcomes at 12-month follow-up.
| Baseline, mean [SD] | 12-month follow-up, mean [SD] | Adjusted difference in means (95% CI) | |
|---|---|---|---|
| EMQ-p | |||
| Usual care (n = 107) | 47.5 [24.6] | 43.0 [26.7] | –4.8 (–9.6 to 0.0) |
| Memory rehabilitation (n = 124) | 46.7 [20.4] | 38.0 [25.0] | |
| General Health Questionnaire 30 | |||
| Usual care (n = 103) | 33.4 [15.8] | 32.5 [18.8] | −0.2 (–4.5 to 4.1) |
| Memory rehabilitation (n = 119) | 35.7 [15.3] | 33.1 [18.5] | |
| RBMT-3 GMI | |||
| Usual care (n = 124) | 76.2 [14.0] | 84.0 [18.4] | 0.5 (–2.6 to 3.6) |
| Memory rehabilitation (n = 131) | 79.5 [12.8] | 87.2 [15.7] | |
| Short term goal attainment average score | |||
| Usual care (n = 123) | – | 1.5 [1.1] | 0.3 (0.0 to 0.5) |
| Memory rehabilitation (n = 131) | – | 1.8 [0.9] | |
| Long term goal attainment average score | |||
| Usual care (n = 123) | – | 1.3 [1.0] | |
| Memory rehabilitation (n = 131) | – | 1.6 [1.0] | 0.4 (0.1 to 0.6) |
CI: confidence interval; EMQ-p: Everyday Memory Questionnaire (patient version); RBMT-3: Rivermead Behavioural Memory Test; GMI: General Memory Index.
Scores from all subscales of the European Brain Injury Questionnaire – patient version were similar in the two arms at 6 and 12 months (Supplemental Table 2). The differences between the two arms at follow-up based on the relative/friend assessments on the Everyday Memory Questionnaire – relative version and European Brain Injury Questionnaire – relative version were consistent with the participant-completed questionnaires (Table 4 and Supple-mental Table 3).
Table 4.
Everyday Memory Questionnaire – relative version (EMQ-r).
| Baseline, mean [SD] | Follow-up, mean [SD] | Adjusted difference in means (95% CI) | |
|---|---|---|---|
| EMQ-r – frequency of problems | |||
| 6-month follow-up | |||
| Usual care (n = 66) | 43.2 [23.1] | 40.9 [25.9] | –4.2 (–10.1 to 1.7) |
| Memory rehabilitation (n = 68) | 39.4 [26.3] | 31.8 [24.5] | |
| 12-month follow-up | |||
| Usual care (n = 57) | 42.9 [23.5] | 37.6 [26.6] | |
| Memory rehabilitation (n = 67) | 40.0 [26.7] | 32.2 [26.2] | −5.3 (–12.0 to 1.4) |
CI: confidence interval.
Sensitivity analyses for the primary outcome found similar results to the primary analysis.20 There was no evidence of difference in the effect of the memory rehabilitation sessions across subgroups based on baseline memory impairment (Rivermead Behavioural Memory Test General Memory Index); the p-value for interaction effect was 0.12. However, the difference in mean Everyday Memory Questionnaire – patient version score in the subgroup of those with borderline/moderate memory impairment favoured the memory rehabilitation arm (adjusted difference in means: –7.1 (95% CI: –13.9 to −0.3, n = 102). There was no evidence of a difference in the intervention effect according to the time since traumatic brain injury (interaction effect p = 0.48; see Supplemental Table 4).
Usual care, based on information from the resource use questionnaire and feedback interview data (published elsewhere),20 suggested that participants largely received no memory rehabilitation, and for the few that received this, it was brief and was terminated shortly after being discharged from hospital.
The cost of the memory rehabilitation programme was estimated at £167 per participant in the memory rehabilitation arm. Memory rehabilitation was £27 (95% CI: –455.13 to 401.34) less expensive compared to usual care; however, this was not statistically significant (p = 0.91; Table 5). Memory rehabilitation generated less QALYs, with 0.011 (95% CI: −0.031 to 0.01) fewer QALYs generated compared to usual care, but this was not statistically significant (p = 0.44). This produced a base case incremental cost effectiveness ratio of £2445 reflecting that memory rehabilitation was less costly but was less effective compared to usual care.
Table 5.
Cost and quality adjusted life year (QALY) outcomes at 12-month follow-up.
| Usual care (n = 157), mean (95% CI) | Memory rehabilitation (n = 171), mean (95% CI) | Incremental (bootstrapped 95% CI) | ICER (£) | ||
|---|---|---|---|---|---|
| Costs | 1423.62 (1031.97, 1815.27) | 1396.72 (1091.91, 1701.54) | −26.89 (–455.13, 401.34) | 2445 | CE plane: southwest quadrant |
| QALYs | 0.004 (−0.017, 0.025) | −0.007 (−0.025, 0.012) | −0.011 (−0.031, 0.011) | Intervention less costly and less effective than usual care | |
CI: confidence interval; ICER: incremental cost effectiveness ratio; CE: cost effectiveness.
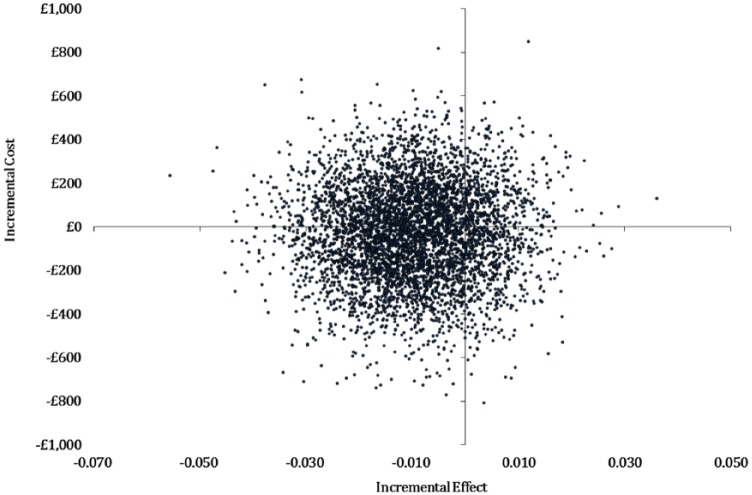
The sensitivity analyses (Supplemental Table 5) demonstrated considerable uncertainty, with results changing based on the imputation method used or when the costs and outcomes were varied by lower and upper CI ranges. Evaluation of joint uncertainty in the cost effectiveness plane (Figure 2) revealed point estimates located across all four quadrants. The probability of memory rehabilitation being cost effective at 12 months at a willingness-to-pay threshold of £20,000 per QALY gain was estimated at 29% and 24% at £30,000 per QALY.
Figure 2.
Cost effectiveness plane for bootstrapped quality adjusted life years (QALYs) at 12 months.
This figure represents the cost effectiveness plane with health outcomes (effects) plotted on the x-axis and costs on the y-axis. The data (represented as individual dots) are derived from 1000 bootstrapped resampling estimations and are plotted on four quadrants. In this graph, the largest proportion of point estimates is located in the bottom-left quadrant consistent with the intervention being less costly and less effective than usual care.
No safety concerns were raised and no deaths reported.
Discussion
Our results suggest that there was no benefit of this group-based memory rehabilitation programme for this heterogeneous sample with traumatic brain injury as a whole, with no clinically important difference on the Everyday Memory Questionnaire – patient version between the two arms at six-month follow-up. We also found consistent results from most of the secondary outcomes. Goal attainment scores, however, indicated that the individual goals were marginally better met in the memory rehabilitation (intervention) arm compared to the usual care (control) arm at both 6- and 12-month follow-ups.
In the following section, we consider why our results are different from much of the extant literature, which has considered memory rehabilitation as largely effective. Indeed, the National Institute for Health and Care Excellence’s (NICE)27 quality statement for community rehabilitation services for adults with traumatic brain injury recommends that patients are offered rehabilitation (‘neuropsychological therapy’) after leaving hospital to help them recover their independence and return to their daily lives, and reviews of effectiveness of cognitive rehabilitation in traumatic brain injury28 have concluded that interventions for memory impairments are beneficial.
A meta-analysis6 of Cicerone et al.’s7,28 reviews identified two crucial issues. First, there were test–retest effects, with a pre–post effect size of 0.21 (standard error = 0.15) in the control group in five studies of memory interventions. This suggests that people in this group were also improving. Second, non-controlled studies appeared to be skewing the evidence of effectiveness, with an effect size of 0.61 (95% CI: 0.37 to 0.85) in 14 single-group pre–post studies of memory training. Based on five studies focusing on memory interventions with a control group, this meta-analysis6 did not find a beneficial effect of memory training on memory measures (effect size: 0.18; 95% CI: −0.16 to 0.53).
Another review of the effectiveness of memory rehabilitation in stroke and traumatic brain injury29 found that dissemination bias was present. This suggests that findings from studies with ‘negative’ or ‘neutral’ results may not have been published. The review authors, therefore, suggest that the overall effect of memory rehabilitation may be overestimated. Furthermore, this review also found ‘spontaneous’ changes not attributable to the memory rehabilitation. Traumatic brain injury–specific data were unavailable, but the authors found a moderate (r = 0.31) and significant (Z = 10.00, p < 0.05) improvement in memory in the control group not attributable to the intervention. They also found a significant (Z = 20.62, p < 0.05) and moderate effect (r = 0.51) of the interventions that could not be attributed to the passage of time or spontaneous recovery. Our data also demonstrate some improvements in memory scores for the control group. This degree of change over time, which for traumatic brain injury can occur over a lifetime,30 warrants further investigation.
Another issue is that some memory rehabilitation interventions that have been evaluated have specifically targeted the constituent components of the wider construct of memory (such as working memory training), which may improve with targeted practice. Therefore, improvements may be seen only when the outcome assessments are similar to the intervention. However, the degree to which such specific interventions are likely to generalize to patients’ daily life is yet to be established.
The lack of ‘positive’ findings could also be attributed to the intervention we delivered (in relation to the content, ‘dose’, therapist skill, etc.), the participants we included, or the outcomes we chose.
The content of our intervention is similar to other published memory rehabilitation studies7,28 and resonates with clinical practice. There is, however, no consensus about what is the appropriate number of intervention sessions for memory rehabilitation. In one meta-analysis,6 the mean treatment duration was 13.3 weeks (SD = 14.2), and ours was 10 sessions over 10 weeks. We assumed, based on our previous studies,31,32 and given the modular structure of the programme, that people would find some benefit from attending at least four sessions. This is also consistent with our clinical practice. Given that our sample had memory problems, attendance at the memory rehabilitation groups was good, with 77% attending four or more sessions. However, it could be argued that attending only four sessions is not enough to effect changes, but research is still needed to determine the dose–effect relationship.
Most memory rehabilitation trials have been small (n ≈ 36).6 The ReMemBrIn trial exceeded the target sample size of 312 randomized participants and provided an estimate of the primary treatment effect that was sufficiently precise to exclude the predefined minimum clinical relevant effect size. The primary analysis included data from 75%–78% of the sample due to non-completion of questionnaires. This level of completion is not uncommon for participants with traumatic brain injury and memory problems.33
One limitation of this study is that we were unable to describe the nature and severity of participants’ traumatic brain injury because of our wide recruitment strategy that included people attending brain injury charity day care centres. We, therefore, did not have access to their medical notes. However, we are unaware of any research that has found differential treatment effects of cognitive rehabilitation based on location or degree of traumatic brain injury, and given the randomization, this variable is likely to be balanced between both intervention and control groups.
The heterogeneity of our sample in terms of demographic characteristics, traumatic brain injury characteristics, and severity of memory problems is both a strength and a limitation. The sample was representative of traumatic brain injury patients seen in many UK community rehabilitation settings. However, the group may have included some who would not typically have received ongoing rehabilitation due to lack of improvement in early rehabilitation. The mean Everyday Memory Questionnaire – patient version scores were consistent with other traumatic brain injury populations,34 but impaired relative to healthy controls.35 However, some with severe memory problems may not have been able to retain enough information from groups to apply it in their daily lives. We also included some people who were not actively seeking help for their problems and were participating for altruistic reasons rather than to help themselves. This may have diluted treatment effects.
We recruited clusters of participants based on who could attend the same day and venue. In clinical practice, however, there are sometimes attempts to group members with similar characteristics together. Our procedure may have led to groups with very mixed memory abilities, attitudes, and lifestyles, which may have made it harder to achieve group cohesion and for participants to learn from each other. However, it would not have been possible to match participants on all such variables when composing a group within the remit of a time-limited trial. Therapists were taught to deal with this heterogeneity when attempting to individually tailor the intervention, particularly for homework, and were able to adjust the intervention delivery based on group composition. Our previous qualitative research showed that participants did not mind the heterogeneity,32 and the high attendance rates in this study may also reflect this. Furthermore, we are unaware of research that has described the ideal composition of memory groups to effect best outcomes, and this is largely based on clinical judgement.
Another strength was that all the trial therapists were trained to deliver the intervention with competence and confidence, and there was little variability in terms of their competence.20 Regular supervision and monitoring (including video recording of sessions) enabled fidelity to the treatment delivery.
The primary outcome was self-reported by participants who were aware of their treatment allocation. We recognize that these subjective accounts could have been influenced by the respondent’s mood,36 and mood improvements have been noted following memory rehabilitation in smaller trials.37–39 However, this was not observed in our study.
It could be argued that because our primary outcome was at six months post randomization, if there were improvements immediately after the intervention, this may have diminished by the time of the assessment. The median number of days between the end of the treatment sessions and the primary outcome assessment was however only 80 days (IQR: 59–98 days). We were mindful of the impact of repeated testing, potential practice effects, and participant burden, so did not consider having more than two outcome time points.
Future research could explore the role of moderator variables in relation to outcomes, and breaking down the constituent parts of everyday memory (based on the factor structure of the Everyday Memory Questionnaire) to determine which aspects are most responsive to memory rehabilitation. We determined the minimum clinically relevant difference in mean Everyday Memory Questionnaire – patient version based on our pilot data and patient interviews; however, further research is required to determine whether this difference should be varied based on time since injury (with the expectation that longer term survivors would show less change than those more recently diagnosed). More work needs to be done to determine who benefits most from memory rehabilitation. Finally, as a complex intervention, outcomes of memory rehabilitation are likely to be determined by not only the content of the intervention, but also by how the intervention is delivered. Therefore, future studies could consider therapist variables and the impact of therapeutic alliance in relation to outcomes.
Clinical messages.
Community-based group memory rehabilitation for a heterogeneous group of long-term survivors of traumatic brain injury does not reduce forgetting in daily life and is unlikely to be cost effective.
Supplemental Material
Supplemental material, Supplemental_Material for Clinical and cost effectiveness of memory rehabilitation following traumatic brain injury: a pragmatic cluster randomized controlled trial by Roshan das Nair, Lucy E Bradshaw, Florence EC Day, Avril Drummond, Shaun RS Harris, Deborah Fitzsimmons, Alan A Montgomery, Gavin Newby, Catherine Sackley and Nadina B Lincoln in Clinical Rehabilitation
Acknowledgments
We would like to acknowledge the support of our collaborators: Anthony Pink, Ceri Phillips, Nicola Brain, Jim Thornton, Ioan Humphreys, and Graham Warren; our trial and data management team: Margo Childs, Kirsty Sprange, Sandip Stapleton, Dawn Coleby, Amy Evans, Jo Hobbs, Pat Morris, Natalie Wakefield, Lucinda Murphy, Brian Barnes, Natasha Bryant, Daniel Simpkins, Essam Eliwa, Keith Whitaker, and Alexandra Erven; our Assistant Psychologists: Joanne Crossley, Emma Marsh, Heather Condon, Emma Sinclair, Kristy-Jane Martin, Lucy Morris, Tanya Burton, Lucy Ratcliffe, Katy Hughes, Alexandra Whitelock, Alexandra Cunliffe, John Wilson, Cormac Duffy, Serena Vanzan, Jessica Budgett, and Natasha Anderson; our outcome assessors: Sara Clarke, Hannah Carpenter, Holly Chappell, Luke Squires, and Rachel Harnell; our feedback interviewers: Kerry Burvil and Hannah Carpenter; our Participant Identification Centre leads: Susan Reed, Nigel Schofield, Graham Lowings, and Janet Walker; our site Principal Investigators: Patrick Vesey, Theresa Powell, Annette Schwartz, Catherine McMahon, Perry Moore, Alistair Atherton, Laura Madeley, Simon Gerhand, Stephen Evans, and Jenna Moffitt; our Trial Steering Committee: Jonathan Evans, Derick Wade, Charlotte Leask, Declan McNicholl, Janice Mackenzie, Dawn Flynn, and Morgan O’Connell; and our Data Monitoring Committee: Andrew Bateman, Diane Playford, and Amanda Farrin. The corresponding author had full access to all study data and had final responsibility for the decision to submit for publication.
Footnotes
Author contributions: R.d.N. (guarantor) and N.B.L. initiated and led the study. F.E.C.D. managed the trial. S.R.S.H. and D.F. led the health economic analyses. L.E.B. and A.A.M. developed the statistical analysis plan and completed the analyses. A.D., G.N., and C.S. offered clinical input. All authors contributed to the design of the study, interpretation of the results, and writing and editing of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the National Institute for Health Research (NIHR) and Health Technology Assessment Programme (project no. 10/57/24) and will be published in full in Health Technology Assessment. Further information available at: https://www.journalslibrary.nihr.ac.uk/programmes/hta/105724/#/. The funder had no role in study design, data collection, analysis, interpretation, or writing of the report. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Roshan das Nair  https://orcid.org/0000-0001-8143-7893
https://orcid.org/0000-0001-8143-7893
References
- 1. Arciniegas DB, Held K, Wagner P. Cognitive impairment following traumatic brain injury. Curr Treat Options Neurol 2002; 4(1): 43–57. [DOI] [PubMed] [Google Scholar]
- 2. Rabinowitz AR, Levin HS. Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am 2014; 37(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thames Valley Strategic Clinical Network. Transforming community neurology: what commissioners need to know, http://tvscn.nhs.uk/wp-content/uploads/2016/06/Transforming-Community-Neurology-Part-A-Transformation-Guide-version-1.pdf (2016, accessed January 2018).
- 4. Scottish Intercollegiate Guidelines Network (SIGN). Brain injury rehabilitation in adults, http://www.sign.ac.uk (2013, accessed September 2017).
- 5. Velikonja D, Tate R, Ponsford J, et al. INCOG recommendations for management of cognition following traumatic brain injury, part V: memory. J Head Trauma Rehabil 2014; 29(4): 369–386. [DOI] [PubMed] [Google Scholar]
- 6. Rohling ML, Faust ME, Beverly B, et al. Effectiveness of cognitive rehabilitation following acquired brain injury: a meta-analytic re-examination of Cicerone et al.’s (2000, 2005) systematic reviews. Neuropsychology 2009; 23(1): 20–39. [DOI] [PubMed] [Google Scholar]
- 7. Cicerone KD, Langenbahn DM, Braden C, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil 2011; 92(4): 519–530. [DOI] [PubMed] [Google Scholar]
- 8. Maas AIR, Menon DK, Adelson PD, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol 2017; xvi(12): 987–1048. [DOI] [PubMed] [Google Scholar]
- 9. Das Nair R, Lincoln NB, Ftizsimmons D, et al. Rehabilitation of Memory following Brain Injury (ReMemBrIn): study protocol for a randomised controlled trial. Trials 2015; 16: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sunderland A, Harris JE, Baddeley AD. Do laboratory tests predict everyday memory? A neuropsychological study. J Verb Learn Verb Behav 1983; 22: 341–357. [Google Scholar]
- 11. Wilson BA, Greenfield E, Clare L, et al. Rivermead Behavioural Memory Test – Third Edition (RBMT-3). London: Pearson, 2008. [Google Scholar]
- 12. Syder D, Body R, Parker M. Sheffield screening test for acquired language disorder. Windsor: NferNelson, 1993. [Google Scholar]
- 13. Hoffmann TC, Glasziou PP, Milne R, et al. Better reporting of interventions: Template for Intervention Description and Replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organisation. International Classification of Functioning, Disability and Health. Geneva: World Health Organisation, 2001. [Google Scholar]
- 15. Bowen A, Knapp P, Gillespie D, et al. Non-pharmacological interventions for perceptual disorders following stroke and other adult-acquired, non-progressive brain injury. Cochrane Database Syst Rev 2011; 4: CD007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Das Nair R, Cogger H, Worthington E, et al. Cognitive rehabilitation for memory deficits after stroke. Cochrane Database Syst Rev 2016; 9: CD002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldberg RJ, Williams PA. User’s guide to the General Health Questionnaire. Windsor, ON, Canada: NferNelson, 1988. [Google Scholar]
- 18. Bateman A, Teasdale TW, Willmes K. Assessing construct validity of the self-rating version of the European Brain Injury Questionnaire (EBIQ) using Rasch analysis. Neuropsychol Rehabil 2009; 19(6): 941–954. [DOI] [PubMed] [Google Scholar]
- 19. EuroQol Group. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990; 16(3): 199–208. [DOI] [PubMed] [Google Scholar]
- 20. Das Nair R, Bradshaw LE, Carpenter H, et al. A group memory rehabilitation programme for people with traumatic brain injuries: the ReMemBrIn RCT. Health Technol Assess in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trigg E. Therapist competence and clinical outcome in the rehabilitation of memory following traumatic brain injury (ReMemBrIn) Trial. MSc Dissertation, University of Nottingham, Nottingham, 2018. [DOI] [PubMed] [Google Scholar]
- 22. Raudenbush SW. Optimal design software for multi-level and longitudinal research. Version 3.01, www.wtgrantfoundation.org (2011, accessed November 2017).
- 23. Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials 2005; 2(2): 152–162. [DOI] [PubMed] [Google Scholar]
- 24. Shrier I, Steele RJ, Verhagen E, et al. Beyond intention to treat: what is the right question. Clin Trials 2014; 11(1): 28–37. [DOI] [PubMed] [Google Scholar]
- 25. Pearson Assessment. Rivermead Behavioural Memory Test – Third Edition (RBMT-3) – frequently asked questions. London: Pearson Assessment, http://www.pearsonclinical.co.uk/Psychology/AdultCognitionNeuropsychologyandLanguage/AdultMemory/RivermeadBehaviouralMemoryTestThirdEditionRBMT3/ForThisProduct/FrequentlyAskedQuestions.aspx (accessed September 2017). [Google Scholar]
- 26. Devlin NJ, Shah KK, Feng Y, et al. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2016; 27: 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Institute for Health and Care Excellence (NICE). Quality statement 7: community rehabilitation services for people (aged 16 and over) with traumatic brain injury, https://www.nice.org.uk/guidance/qs74/chapter/Quality-statement-7-Community-rehabilitation-services-for-people-aged-16-and-over-with-traumatic-brain-injury (2014, accessed September 2017).
- 28. Cicerone KD, Dahlberg C, Malec JF, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Arch Phys Med Rehabil 2005; 86(8): 1681–1692. [DOI] [PubMed] [Google Scholar]
- 29. Elliott M, Parente F. Efficacy of memory rehabilitation therapy: a meta-analysis of TBI and stroke cognitive rehabilitation literature. Brain Inj 2014; 28(12): 1610–1616. [DOI] [PubMed] [Google Scholar]
- 30. Gray DS, Burnham R. Preliminary outcome analysis of a long-term rehabilitation program for severe acquired brain injury. Arch Phys Med Rehabil 2000; 81(11): 1447–1456. [DOI] [PubMed] [Google Scholar]
- 31. Das Nair R, Lincoln NB. Evaluation of rehabilitation of memory in neurological disabilities (ReMiND): a randomized controlled trial. Clin Rehabil 2012; 26: 894–903. [DOI] [PubMed] [Google Scholar]
- 32. Das Nair R, Lincoln NB. The effectiveness of memory rehabilitation following neurological disabilities: a qualitative inquiry of patient perspectives. Neuropsychol Rehabil 2013; 23(4): 528–545. [DOI] [PubMed] [Google Scholar]
- 33. Chiaravalloti ND, Sandry J, Moore NB, et al. An RCT to treat learning impairment in traumatic brain injury: the TBI-MEM trial. Neurorehabil Neural Repair 2016; 30(6): 539–550. [DOI] [PubMed] [Google Scholar]
- 34. Sander AM, Clark AN, van Veldhoven LM, et al. Factor analysis of the everyday memory questionnaire in persons with traumatic brain injury. Clin Neuropsychol 2017; 32: 495–509. [DOI] [PubMed] [Google Scholar]
- 35. Ossher L, Flegal KE, Lustig C. Everyday memory errors in older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2013; 20(2): 220–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Byrne C, Coetzer B, Addy K. Investigating the discrepancy between subjective and objective cognitive impairment following acquired brain injury: the role of psychological affect. NeuroRehabilitation 2017; 41(2): 501–512. [DOI] [PubMed] [Google Scholar]
- 37. Carr SE, das Nair R, Schwartz AF, et al. Group memory rehabilitation for people with multiple sclerosis: a feasibility randomized controlled trial. Clin Rehabil 2014; 28(6): 552–561. [DOI] [PubMed] [Google Scholar]
- 38. Martin KJ, Lincoln NB, das Nair R. Group-based memory rehabilitation for people with multiple sclerosis: subgroup analysis of the ReMiND trial. Int J Ther Rehabil 2014; 21(12): 590–596. [Google Scholar]
- 39. Goodwin RA, Lincoln NB, das Nair R, et al. Evaluation of NeuroPage as a memory aid for people with multiple sclerosis: a randomised controlled trial. Neuropsychol Rehabil. Epub ahead of print 20 March 2018. DOI: 10.1080/09602011.2018.1447973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Material for Clinical and cost effectiveness of memory rehabilitation following traumatic brain injury: a pragmatic cluster randomized controlled trial by Roshan das Nair, Lucy E Bradshaw, Florence EC Day, Avril Drummond, Shaun RS Harris, Deborah Fitzsimmons, Alan A Montgomery, Gavin Newby, Catherine Sackley and Nadina B Lincoln in Clinical Rehabilitation