Abstract
Background: The rapid emergence of antimicrobial resistance among Gram-positive organisms, especially staphylococci, has become a serious clinical challenge. Efflux machinery and biofilm formation are considered two of the main causes of antimicrobial resistance and therapy failure.
Aim: Our study aims to evaluate the antibiofilm and efflux pump inhibitory activity of the antifungal ketoconazole against multidrug-resistant (MDR) Staphylococcus aureus.
Methods: Ketoconazole was tested for its effect on the following: minimum inhibitory concentrations (MICs) of ciprofloxacin, norfloxacin, levofloxacin, and ethidium bromide (EtBr) by the broth microdilution method, the efflux of EtBr by NorA-positive MDR S. aureus, and the relative expression of NorA, NorB, and NorC efflux pump genes. Docking studies of ketoconazole were performed using 1PW4 (glycerol-3-phosphate transporter from Escherichia coli which was the representative structure from the major facilitator superfamily).
Results: Ketoconazole significantly decreased the MICs of levofloxacin, ciprofloxacin, norfloxacin, and EtBr (a substrate for efflux pump) by 8 to 1024-fold (P<0.01) and decreased the efflux of EtBr. Furthermore, a time-kill assay revealed that combinations of levofloxacin with ketoconazole or carbonyl cyanide m-chlorophenylhydrazone showed no growth for the tested strains after 24 h in comparison to the effect of levofloxacin alone. Docking studies and the ability of ketoconazole to diminish the relative expression of NorA gene in comparison to control (untreated strains) confirmed its action as an efflux pump inhibitor. Conclusion: The findings showed that the antifungal ketoconazole has no antibacterial activity but can potentiate the activity of the fluroquinolones against MDR S. aureus via inhibiting efflux pump and biofilm formation in vitro.
Keywords: ketoconazole, efflux pump, biofilm, Nor genes
Introduction
Staphylococci are one of the most common human pathogens, and are responsible for a variety of community and hospital-acquired infections. Staphylococcus aureus resistance is one of the major challenges that lead to therapy failure. Because of the widespread use and the misuse of antimicrobials for treatment of different infectious diseases of human and animals, resistance to antibiotics has arisen and this can be to one or more classes of antibiotic. In addition, the appearance of different patterns of resistance such as multidrug resistant (MDR), extensively drug resistant, or pandrug resistant has become common among certain isolated strains.1
The difficulty and failure of staphylococcal infection treatments is due to the representation of several antibiotic resistance mechanisms, such as their ability to form a biofilm (a community of microorganisms enclosed in glycocalyx), enzymatic degradation of antimicrobials, modification of the target site, and decreasing the intracellular concentration of antibiotics by decreasing their permeability or by the expression of energy-dependent or active efflux.2
A biofilm or glycocalyx consists of DNA, polysaccharides, and proteins. Biofilm layers represent a barrier to the access of antibiotics to the embedded bacteria. In addition, bacteria in the biofilm layer are in the dormant state. So, they do not respond to the actions of antibiotics.3,4
Efflux mechanisms have been considered one of the most important mechanisms of resistance to various classes of antimicrobials. Some efflux pumps can export certain antibiotics and some other pumps can export more than one antibiotic (known as multidrug efflux pumps). Hence, the inhibition of efflux pumps may improve the clinical performance of various antibiotics. Five families of efflux transporter are known. Four families use the proton motive force as an energy source – major facilitator superfamily (MFS), small multidrug resistance family, multi-antimicrobial extrusion family, and resistance–nodulation–division family – while the ATP binding cassette family is energized by ATP hydrolysis.5
Considerable research has been undertaken during the past two decades searching for efflux inhibitors. Many natural and synthetic compounds were shown to have efflux inhibitory activity. Capsaicin,6 caffeoylquinic acids,7 reserpine, and vitamin K8 are examples of discovered efflux pump inhibitors. In addition, the energy decoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP), which is used for in-vitro testing of the expression of bacterial efflux pumps because it is highly toxic to eukaryotic cells9 and proton pump inhibitors,10 was also discovered.
Previous studies on the action of imidazoles on bacterial growth, especially S. aureus, have also been undertaken. Depending on the ability of imidazoles to inhibit ergosterol synthesis and their ability to cause direct damage to fungal membranes at high concentrations, many researchers thought that imidazoles have good bactericidal activity by targeting the bacterial plasma membrane. These studies showed that miconazole has a bactericidal effect on S. aureus at low concentrations by inducing K+ release while ketoconazole has no effect, even at high concentrations showing very little effect on K+ release.11 Another study was performed on the ability of caspofungin (an antifungal agent) to increase S. aureus biofilm susceptibility to fluoroquinolones by affecting the expression of icaA operon (shares homology with β-1,3-glucan synthase) which is responsible for synthesizing poly-N-acetyl-glucosamine polymer.4 Based on these findings we thought that ketoconazole may have other mechanisms which can potentiate the antimicrobial activity of some antibiotics of different classes, such as the efflux mechanism that may be responsible for resistance against many unrelated groups of antimicrobials. This was done by testing the effect of ketoconazole on the minimum inhibitory concentration (MIC) of different antibiotics, ethidium bromide (EtBr) accumulation as it is a substrate for efflux pump, studying its effect on the killing time of the tested antibiotic, and biofilm formation which acts as a barrier for the diffusion of antibiotics. Finally, the effect of ketoconazole was confirmed by studying its activity on the relative gene expression of the Nor efflux pump system and undergoing docking studies.
Materials and methods
Chemicals
Levofloxacin, ciprofloxacin, norfloxacin, and ketoconazole powders were obtained from Sigma-Aldrich Co. (St Louis, MO, USA). Stock solutions of the tested antimicrobials were prepared in dimethyl sulfoxide (Merck & Co., Inc., Whitehouse Station, NJ, USA) and then diluted with sterile water. EtBr powder was obtained from Sigma (Madrid, Spain). The antibiotic discs were ciprofloxacin, tetracycline, clindamycin, oxacillin, cefoxitin, ceftazidime, levofloxacin, amoxicillin/clavulanic acid, gentamicin, ceftriaxone, and norfloxacin. All discs were purchased from Oxoid (Thermo Fisher Scientific, Waltham, MA, USA). CCCP in powder form was purchased from Sigma-Aldrich Co. A stock solution of CCCP was prepared in 50% methanol.
Bacterial strains
Staphylococcus aureus (ATCC 6538) was obtained from the MIRCEN culture collection of the Faculty of Agriculture, Ain Shams University, Cairo, Egypt. S. aureus (ATCC 6538) was resistant to beta-lactam antibiotics which were amoxicillin, ampicillin, oxacillin, amoxicillin/clavulanic acid, and methicillin (MRSA). One fluoroquinolone-susceptible S. aureus strain and two MDR isolates of S. aureus (R strain and 5ʹ strain) positive for efflux pump genes (NorA) (Table 1) were obtained from the Department of Microbiology, Faculty of Pharmacy, Minia University, Minia, Egypt. All cultures were stored as agar slants at 4 °C. These three strains were isolated from clinical samples and MDR S. aureus was completely resistant to more than three antimicrobial classes including fluoroquinolones.
Table 1.
Resistance pattern of the tested Staphylococcus aureusstrains
| Strain | Resisance pattern |
|---|---|
| S. aureus ATCC6538 | OX, AK, DA, CRO, CAZ, CIP, LEV, CN, NOR, FOX, SAM, AMC, CFR, TE, AM, AZM |
| S. aureus(quinolone-sensitive strain) | AM, FOX, CN, CRO, CAZ, E, CFR, CEC, CAZ, CL, AMC, SXT |
| S. aureus R | OX, AK, DA, FOX, CN, CRO, CAZ, CIP, LEV, NOR, SAM, AMC, CFR, TE, E, NA, S, OFX, PEF |
| S. aureus 5ʹ | OX, DA, CRO, CAZ, CIP, LEV, NOR, CFP, SAM, AMC, E, DO, CN, FOX |
Abbreviations: AK, amikacin; AM, ampicillin; AMC, amoxicillin/clavulanic; AZM, azithromycin; CAZ, ceftazidime; CEC, cefaclor; CFP, cefoperazone; CIP, ciprofloxacin; CN, gentamicin; CRO, ceftriaxone; CTX, cefotaxime; DA, clindamycin; DO, doxycycline; FOX, cefoxitin; LEV, levofloxacin; NA, nalidixic acid; NOR, norfloxacin; OFX, ofloxacin; OX, oxacillin; PEF, pefloxacin; S, streptomycin; SAM, ampicillin/sulbactam; SXT, sulfamethoxazole/trimethoprim; TE, tetracycline.
Determination of efflux pump inhibition activity by MIC reduction
The MICs and MBCs (MBC is defined as the lowest concentration of antibacterial agent that reduces the viability of the initial bacterial inoculum by ≥99.9%) of ketoconazole, levofloxacin, ciprofloxacin, norfloxacin, CCCP, and EtBr were determined according to Clinical and Laboratory Standards Institute guidelines12 by the broth microdilution method. All assays were performed in triplicate and results were averaged.
To test the ability of ketoconazole to alter efflux pump action, the capacity of ketoconazole to decrease the MICs of EtBr, ciprofloxacin, norfloxacin, and levofloxacin in comparison to the action of CCCP (efflux inhibitor) was assessed. The action of CCCP and ketoconazole as efflux pump inhibitors was tested at ½×MIC of CCCP and 500 μg/mL of ketoconazole. Controls were performed using the MICs of the antibiotics and EtBr each alone. All assays were performed in triplicate and results were averaged. A reduction of an antibiotic MIC by 2-fold or more by an EPI is an indication that the main resistance mechanism is expression of the efflux pump.13
Antibiotic–efflux pump inhibitor disc synergy test
Antibiotic–efflux pump inhibition using the disc diffusion method was performed as previously described for the tested isolates.14 Three antibiotic discs were used per plate. One disc was saturated with 10 μL of CCCP, the second disc was saturated with 10 μL of ketoconazole, and the third disc was the antibiotic disc alone. A difference of ≥5 mm in zone diameter between antibiotic discs alone and antibiotic with ketoconazole discs was considered a positive result. Results for antibiotic discs with CCCP were taken as positive controls for efflux pump–antibiotic-mediated resistance.15
Ethidium bromide accumulation assay
The effect of levofloxacin alone and in combination with CCCP (½×MIC) or ketoconazole (500 μg/ml) on cell membrane permeability of two MDR S. aureus isolates to EtBr (excitation at 518 nm, emission at 605 nm) was evaluated by the method as described by Paixao et al.16 Relative final fluorescence was calculated by subtracting values for the respective blank controls. Differences in relative final fluorescence between compound-containing samples and control assays (samples containing EtBr alone and in combination with levofloxacin) were indicative of activity of the compound to inhibit efflux of EtBr.
Time-kill assays
Of three different S. aureus isolates, one strain was levofloxacin susceptible and the other two were levofloxacin resistant. The three isolates were inoculated in (8 ml) MHB tubes containing different concentrations of levofloxacin (¼×MIC, ½×MIC, MIC, and 2×MIC) and/or ½×MIC CCCP or 500 µg/mL ketoconazole. Tubes containing MHB alone were used as control. The bacterial suspensions were incubated at 37 °C with gentle shaking for defined times (0, 3, 12, 18, and 24 h). One milliliter of bacterial suspension was serially diluted in MHB tubes. Of each dilution, 25 µL were plated on Mueller Hinton agar plates. After overnight incubation of the plates at 37 °C, colony forming units (CFUs) were counted.17
Biofilm susceptibility assay
The biofilms of S. aureusstrains (ATCC 6538 and two clinical strains) were prepared in 96-well polystyrene microtiter plates (Tarson, India), using the method of Wei et al18 with a few modifications. The microbial suspensions were prepared from the overnight grown culture and the turbidity of the suspension was adjusted to 0.7 OD610 (1×109 CFU/ml). Different concentrations of levofloxacin (¼×MIC, ½×MIC, MIC, 2×MIC) and ketoconazole at a concentration of 500 µg/mL each alone and in combination were prepared in tryptone soy broth (TSB) (Difco Laboratories) supplemented with 0.5% glucose. Forty microliters of fresh TSB with 0.5% glucose was added to each well, followed by the addition of 60 μL of the above microbial suspension. Then, different dilutions of the tested drugs were added and the plates were incubated for 18 h at 37 °C. The plates were washed by PBS and biofilms were fixed with methanol for 15–30 min, stained with Crystal Violet (Sigma-Aldrich Co.). Biofilm formation was quantified by the addition of 95% ethanol to the crystal violet-stained wells and recording the absorbance at 595 nm using a microplate reader (Multiskan spectrum; Finland).
Real-time reverse transcription PCR analysis for the tested agents on S. aureus efflux pump-related genes
For RNA extraction, strains were cultured in TSB media containing one of the following: levofloxacin, EtBr, or CCCP at ½×MIC and ketoconazole at 500 μg/mL. In addition, S. aureus strains were cultured in TSB media containing 1/8×MIC of levofloxacin or EtBr in combination with one of either 500 µg/ml ketoconazole or ¼×MIC of CCCP for each strain or in drug-free TSB, and grown until an OD600 nm of 0.6. The RNeasy Mini Kit (Qiagen NV, Venlo, the Netherlands) was used for total RNA extraction after treating bacterial cultures with RNA protect bacterial reagent (Qiagen). RNase-free DNase (Qiagen) was used for the removal of DNA contamination.
The expression of Nor A, B, C genes in the tested MDR S. aureus isolates was determined by quantitative real-time reverse transcription PCR according to the method previously described by DeMarco et al 200719 using the QuantiTect SYBR Green RT-PCR Kit (Qiagen), the 7500 Fast Real Time PCR (Applied Biosystems; Thermo Fisher Scientific) and primers described in Table 2. The comparative threshold cycle (CT) method was used to determine the relative quantity of mRNA corresponding to genes NorA, NorB, and NorC. Relative expression of the tested efflux pump genes was determined by: 1) comparing the relative quantity of the mRNA in the tested S. aureus isolates to that present in the reference strain ATCC 6538; and 2) comparing the relative quantity of the respective mRNA in the presence of levofloxacin or EtBr (at ½×MIC) and that in their presence in combination with CCCP or ketoconazole to the relative quantity of the respective mRNA in drug-free conditions. For each strain, three assays were performed, corresponding to three independent total RNA extractions using 16S rDNA as a reference. Negative controls were included. An increase in gene expression of at least 4-fold, when compared to the drug-free condition, was considered to be overexpressed.19
Table 2.
Primer sequence of the tested genes
| Primer | Sequences 5ʹ→3ʹ | Product size (bp) |
|---|---|---|
| NorA_Fw NorA_Rv |
TTCACCAAGCCATCAAAAAG CTTGCCTTTCTCCAGCAATA |
620 |
| NorB_Fw NorB_Rv |
GCAGGTGGTCTTGCTGATAA AGCGCGTTGTCTATCTTTCC |
213 |
| NorC_Fw NorC_Rv |
ATACCTGAAGCAACGCCAAC AATGGGTTCTAAGCGACCAA |
216 |
Docking study
The automated docking simulation study was performed using Molecular Operating Environment (MOE) version 2014.09 at Assuit University Faculty of Pharmacy, Chemical Computing Group Inc., Montreal, Canada. The X-ray crystallographic structures of 1PW4 (glycerol-3-phosphate transporter from Escherichia coli which was the representative structure from the MFS) was obtained from the Protein Data Bank (PDB). Using the builder interface of the MOE program, ketoconazole and 4ʹ,5ʹ-O-dicaffeoylquinic acid (4ʹ,5ʹ-ODCQA) 3D models were constructed. Then, their structures and the formal charges on atoms were checked by 2D depiction, which is followed by a conformational search for the target compounds and energy minimization for all conformers using MOE until a root mean square difference gradient of 0.01 kcal/mol and a root mean square distance of 0.1 Å with an MMFF 94X force field, and the partial charges were automatically calculated. Preparing efflux pump proteins for docking studies was done by adding hydrogen atoms to the system with their standard geometry, checking for any errors in the atom connection and type with automatic correction, and fixing the selection of the receptor and their atom potential. The active site search in the tested proteins was done using the MOE alpha site finder. Dummy atoms were created from the obtained alpha spheres.
Statistical analysis
Each experiment was done in triplicate. Data were represented as mean±SD. The Student’s t-test was performed using SPSS 17 statistical software (SPSS Inc., Chicago, IL, USA), where P<0.05 or P<0.01 was considered statistically significant.
Results
Evaluation of efflux pump inhibition by MIC reduction
First, the MICs and MBCs of the tested agents were determined. The MIC of CCCP was found to be 8 µg/mL while tubes containing ketoconazole showed growth at a concentration up to 2000 µg/mL. Then, the effect of ½×MIC of CCCP and 500 µg/mL of ketoconazole on the MICs of levofloxacin and EtBr were tested (Table 3). In the presence of CCCP at 4 µg/ml, the MICs and MBCs of the tested fluoroquinolone antibiotics and EtBr were decreased by 2 to 1024-fold. Ketoconazole showed no antibacterial activity on all tested bacterial strains. On the other hand, ketoconazole showed a significant decrease in the MICs and MBCs of the tested antibiotics against S. aureus strains by 8 to 1024-fold. For the susceptible strains, there is a slight change in the MICs in the presence of CCCP which may be due to its antibacterial activity. On the other hand, no change in the MICs of the tested antibiotics was observed in the presence of ketoconazole (Table 3).
Table 3.
MICs/MBCs (µg/mL) of norfloxacin, levofloxacin, and ciprofloxacin alone and in combination with CCCP or ketoconazole for the tested bacterial isolates
| Strain | Norfloxacin (MIC/MBC) (∆) | Levofloxacin (MIC/MBC) (∆) | Ciprofloxacin (MIC/MBC) (∆) | Ethidium bromide (MIC/MBC) (∆) |
|---|---|---|---|---|
S. aureus sensitive strain
|
1/1 0.5/0.5 1/1 |
0.5/1 0.25/0.5 0.5/1 |
0.25/0.5 0.25/0.25 0.25/0.25 |
2/4 2/2 2/4 |
S. aureus ATCC6538
|
16/32 1/1 (16) 1/1 (16) |
16/32 0.25/0.5 (64) 0.25/0.25 (64) |
64/64 0.25/0.5 (256) 0.25/0.5 (256) |
512/1024 0.5/0.5 (1024) 0.5/0.5 (1024) |
S. aureus R
|
16/32 0.5/1 (32) 0.125/0.25 (128) |
16/16 1/2 (16) 0.5/1 (32) |
16/32 2/4 (8) 1/2 (16) |
64/128 0.125/0.25 (512) 0.125/0.25 (512) |
S. aureus 5ʹ
|
16/32 8/16 (2) 2/4 (8) |
16/32 8/16 (2) 2/2 (8) |
16/32 2/4 (8) 0.5/1 (32) |
8/16 4/8 (2) 0.25/0.5 (32) |
Notes: (∆) MIC fold change between antibiotic alone and antibiotic+CCCP, ie ratio of MIC of antibiotic alone to that of antibiotic+CCCP.
Abbreviations: CCCP, carbonyl cyanide m-chlorophenylhydrazone; MBC, lowest concentration of antibacterial agent that reduces the viability of the initial bacterial inoculum by ≥99.9%; MIC, minimum inhibitory concentration; S. aureus, Staphylococcus aureus.
Antibiotic–efflux pump inhibitor disc synergy test
We compared the effect of ketoconazole upon its addition to the antibiotic discs and the effect obtained by the addition of CCCP to the antibiotic discs as a positive control. According to Huang et al,15 a difference of ≥5 mm in zone diameter between antibiotics discs alone and antibiotic discs with CCCP was taken as a positive result for efflux pump-mediated resistance. Ketoconazole was found to increase the zone of inhibition diameters of the tested antibiotic discs against MDR S. aureus strains by more than ≥5 mm. By using a sensitive S. aureus strain, zone of inhibition diameters of the tested discs with ketoconazole were not changed in comparison with the disc of antibiotic alone but CCCP showed a slight increase in the zone of inhibition diameters due to its antibacterial activity (Table 4 and Figure 1).
Table 4.
Average of zone inhibition diameters of the tested antibiotic discs with and without ketoconazole or CCCP
| Strain | Inhibition Zone diameter in mm ± S.D | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CIP | TE | DA | OX | FOX | CAZ | LEV | AMC | CN | CRO | |
|
S. aureus ATCC6538 Ab Ab+ketoconazole$$ Ab+CCCP$ |
6±0.5
26±0.3 36±0.21 |
18±0.4 30±0.6 35±0.62 |
10±0.7 26±0.45 30±0.32 |
5±0.44 28±0.63 35±0.46 |
6±0.46 20±0.53 23±34 |
5±0.72 20±0.76 50±0.82 |
10±0.34 27±0.52 35±0.33 |
11±0.42 29±0.35 36±0.7 |
8±0.28 22±0.45 34±0.37 |
6±0.64 27±0.74 34±0.82 |
|
S. aureus R Ab Ab+ketoconazole$$ Ab+CCCP$ |
6±0.42 20±0.61 22±0.48 |
28±0.65(s) 28±0.33 30±0.8 |
30±0.52(s) 30±0.73 34±0.62 |
5±0.21 17±0.31 26±0.5 |
5±0.66 28±0.47 28±0.55 |
5±0.42 14±0.52 28±0.49 |
13±0.7 21±0.5 25±0.7 |
10±0.67 26±0.81 32±0.34 |
8±0.35 20±0.46 24±0.77 |
6±0.52 24±0.62 28±0.43 |
|
S. aureus 5ʹ Ab Ab+ketoconazole$$ Ab+CCCP$ |
12±0.52 23±0.42 28±0.38 |
12±0.66 20±0.34 30±0.69 |
28±0.52(s) 28±0.64 31±0.82 |
22±0.43(s) 22±0.4 24±0.71 |
5±0.37 22±0.42 40±0.61 |
6±0.34 26±0.88 34±0.6 |
10±0.3 22±0.6 26±0.32 |
6±0.54 24±0.6 34±0.34 |
10±0.6 23±0.4 30±0.4 |
8±0.51 25±0.34 29±0.62 |
S. aureus sensitive strain
|
30±0.25 32±0.3 30±0.21 |
24±0.29 25±0.16 24±0.62 |
21±0.7 24±0.45 21±0.31 |
29±0.42 28±0.63 29±0.46 |
26±0.5 26±0.3 27±0.21 |
25±0.22 25±0.37 25±0.62 |
26±0.76 26±0.45 26±0.32 |
31±0.44 33±0.23 31±0.46 |
16±0.5 16±0.3 16±0.21 |
28±0.4 30±0.6 28±0.12 |
Notes: $Combination of antibiotic and CCCP or ketoconazole (as efflux inhibitor). A difference of >5 mm between the antibiotic discs and Ab–CCCP zone diameters was interpreted as positive efflux-mediated resistance. $$Ab–ketoconazole disc combinations which give a difference of >5 mm between the antibiotic discs.
Abbreviations: Ab, antibiotic; AMC, amoxicillin/clavulanic acid; CAZ, ceftazidime; CCCP, carbonyl cyanide m-chlorophenylhydrazone; CIP, ciprofloxacin; CN, gentamicin; CRO, ceftriaxone; DA, clindamycin; FOX, cefoxitin; LEV, levofloxacin; OX, oxacillin; (s), susceptible; S. aureus, Staphylococcus aureus; TE, tetracycline.
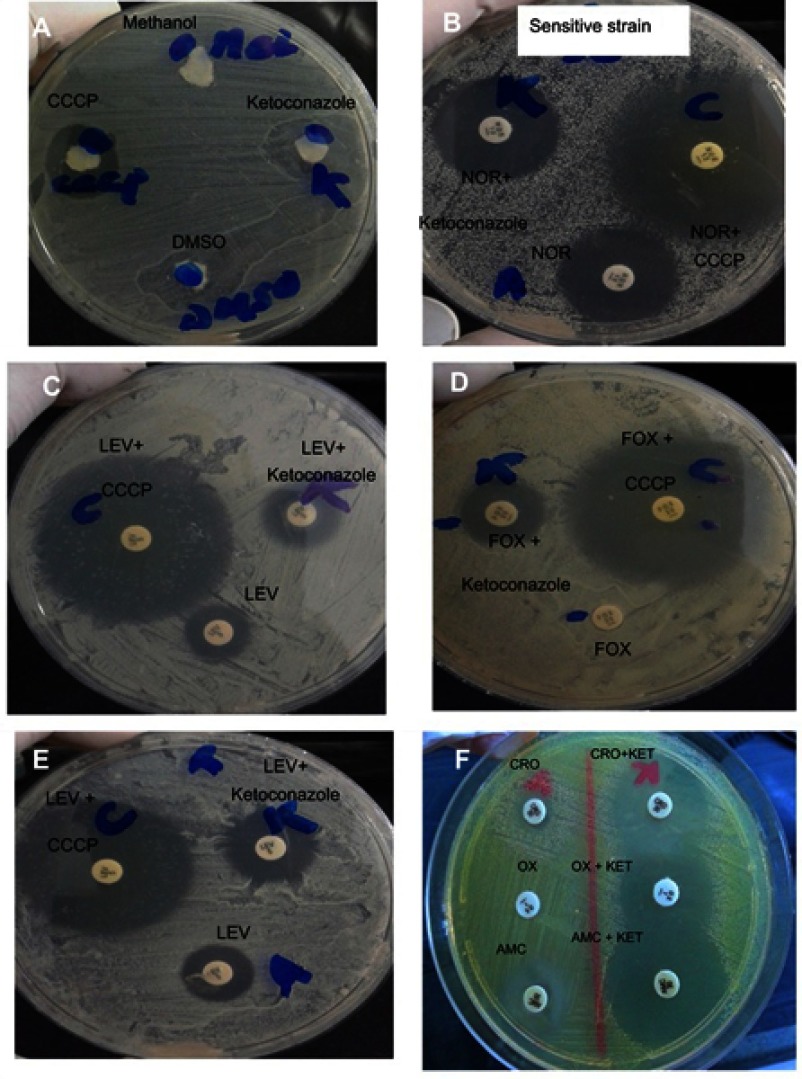
Figure 1.
Antibiotic–EPI disc synergy test using antibiotic discs combined with CCCP or ketoconazole as EPIs. (A) Antibacterial activity of methanol (used for dissolving CCCP), of dimethyl sulfoxide (DMSO) used as a solvent for ketoconazole, of CCCP, and of ketoconazole (each alone). (B) Norfloxacin (NOR)-sensitive Staphylococcus aureusshowing no change in zone diameter in combination with ketoconazole. Addition of CCCP to antibiotic disc showed an increase in zone diameter due to its antibacterial activity. (C–E) CCCP and ketoconazole increase the activity of the tested antibiotics. (F) Effect of ketoconazole on the addition of ceftriaxone (CRO), oxacillin (OX), and amoxicillin/clavulanic acid (AMC) discs.
Abbreviations: CCCP, carbonyl cyanide m-chlorophenylhydrazone; FOX, cefoxitin; KET, ketoconazole; LEV, levofloxacin.
Ethidium bromide accumulation assay
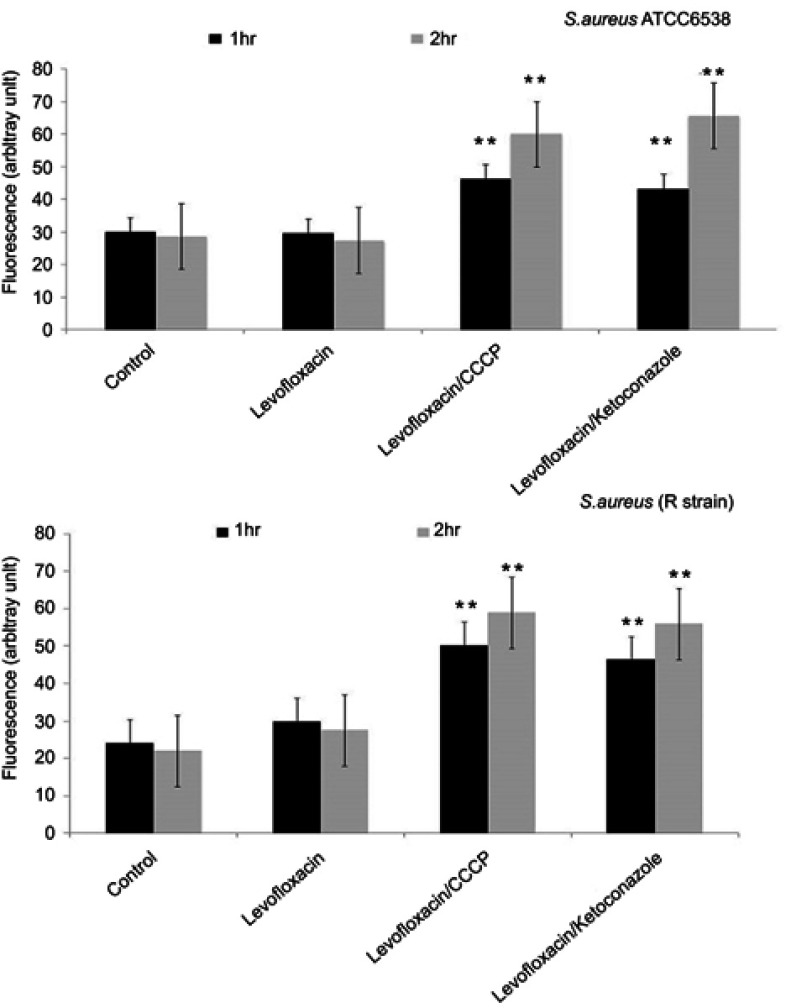
In the presence of levofloxacin, no increase in the accumulation of EtBr happened in comparison to control (untreated strain). With the addition of CCCP, we monitored an increase in the EtBr fluorescence due to an increase of penetration and the accumulation of EtBr inside the cell in comparison to control (untreated strain). Similar results were shown by ketoconazole increasing the fluorescence by more than 2-fold (Figure 2).
Figure 2.
Effect of the tested antibiotic (levofloxacin) alone or in combination with ketoconazole or CCCP on the accumulation of ethidium bromide by Staphylococcus. aureus ATCC 6538 and S. aureus R strain. Data represent the mean±SD of two different experiments performed in triplicate. **Highly significant, P<0.001 (Student’s t-test).
Abbreviation: CCCP, carbonyl cyanide m-chlorophenylhydrazone.
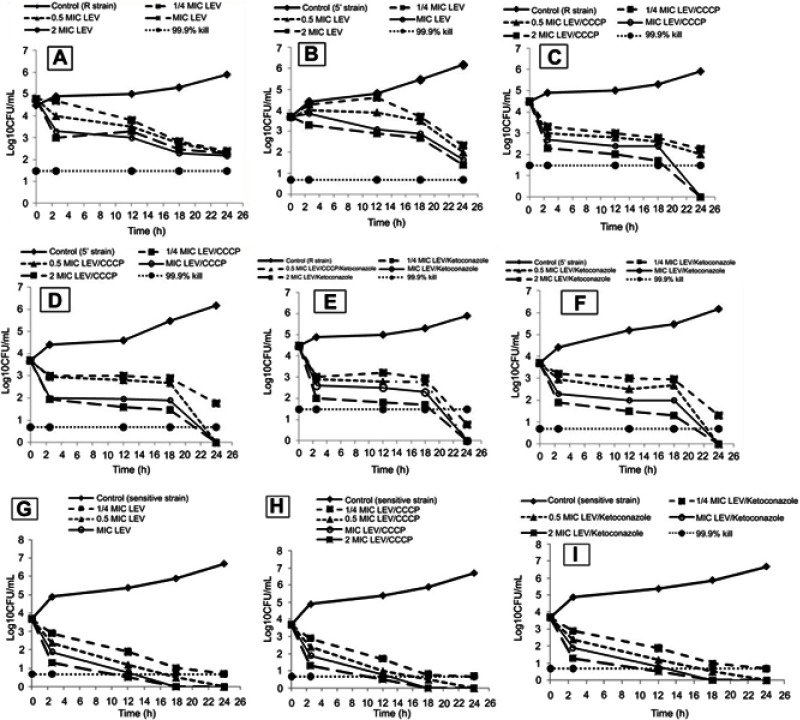
Time-kill assay
A time-kill assay was conducted to study the growth and kill pattern of levofloxacin in the presence and absence of ketoconazole or CCCP. Time-kill curves (Figure 3) showed a lag phase up to 3 h and then a slow decline phase at all concentrations of levofloxacin alone and in combination with ketoconazole or CCCP after 3 h. A significant decrease in CFU counts was observed after 18 h while the maximum killing effect was achieved in the presence of CCCP and ketoconazole at 24 h at all tested concentrations in comparison to that obtained by levofloxacin alone (Figure 3). In addition, no decrease in CFUs of the susceptible strain was observed in the presence of CCCP or ketoconazole.
Figure 3.
Time-kill curves of levofloxacin against two MDR Staphylococcus aureus strains: (A) R strain and (B) 5ʹ strain. (G) The sensitive strain levofloxacin+CCCP (½×MIC) against R strain (C) and 5ʹ strain (D). (H) The sensitive strain and levofloxacin+ketoconazole (500 µg/ml) against (E) R strain and (F) 5ʹ strain.
Abbreviations: CCCP, carbonyl cyanide m-chlorophenylhydrazone; CFU, colony forming unit; LEV, levofloxacin; MIC, minimum inhibitory concentration.
Biofilm susceptibility assay
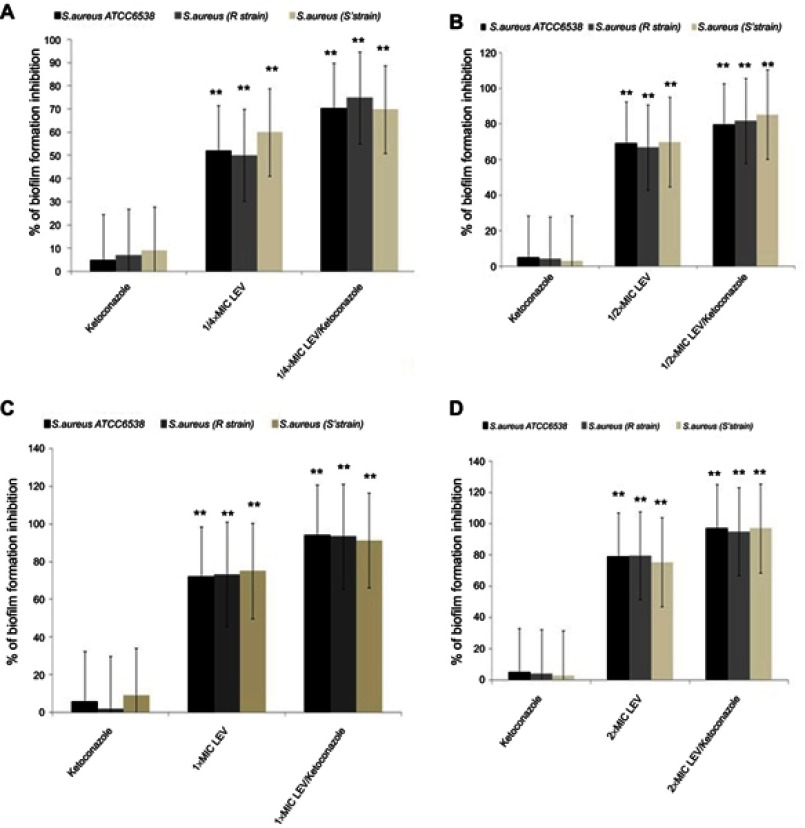
Biofilm formation by the tested strains was tested in the absence and the presence of different concentrations of levofloxacin alone or in combination with a fixed concentration of ketoconazole (500 µg/mL). The inhibitory effect of levofloxacin alone on biofilm formation of the tested isolates ranged from 52 to 75.3%, while in combination with ketoconazole the biofilm formation inhibition ranged from 79.5 to 97% (Figure 4).
Figure 4.
Inhibitory effect of ketoconazole and levofloxacin each alone and in combination (using different concentrations of levofloxacin) on biofilm formation by the tested Staphylococcus aureus strains. (A) Effect of ketoconazole, ¼×MIC of levofloxacin each alone and in combination. (B) Effect of ketoconazole, ½×MIC levofloxacin each alone and in combination. (C) Effect of ketoconazole, 1×MIC levofloxacin each alone and in combination. (D) Effect of ketoconazole, 2×MIC levofloxacin each alone and in combination. Data represent the mean±SD of two different experiments performed in triplicate. **Highly significant, P<0.001 (Student’s t-test).
Abbreviations: LEV, levofloxacin; MIC, minimum inhibitory concentration.
NorA, NorB, NorC gene expression analysis in the presence of the tested agents
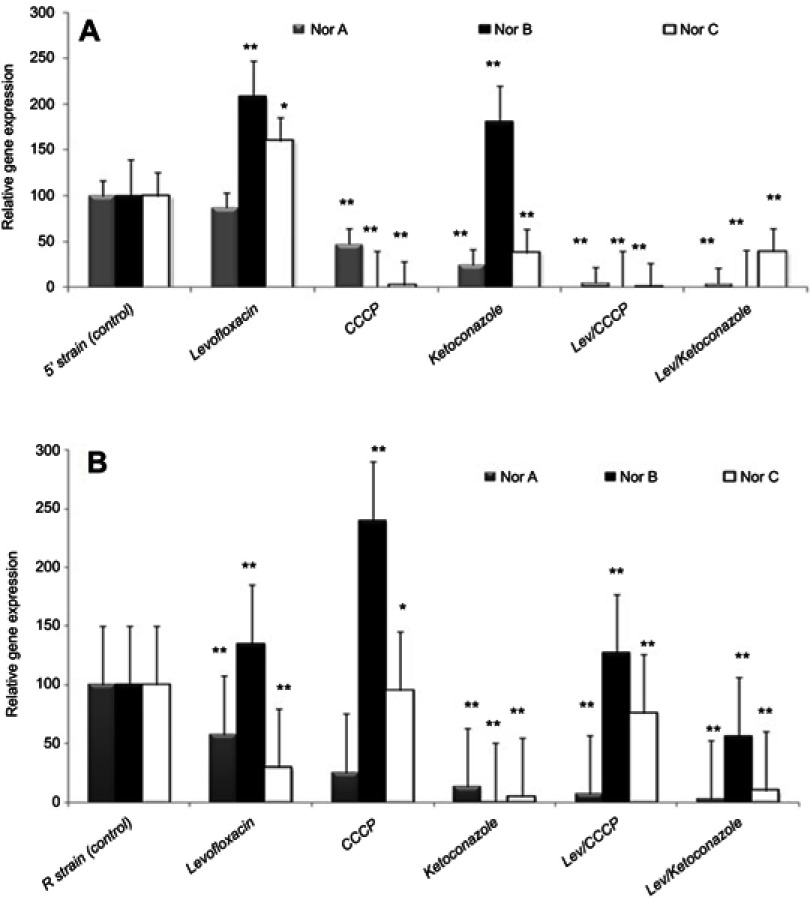
Gene expression analysis for NorA, NorB, and NorC among S. aureus strains positive for MDR efflux pump genes in the presence of the tested agents was done using real-time reverse transcription PCR. Ketoconazole significantly decreased NorA gene relative expression in R and 5ʹ strains by 75–87%, while levofloxacin, CCCP, and EtBr each alone caused limited decrease in the relative expression of the NorA gene (11.5–42.2%) in comparison to control (Figures 5 and 6).
Figure 5.
Effect of levofloxacin, ketoconazole, CCCP, and levofloxacin combination with ketoconazole or CCCP on the relative expression of efflux pump genes of Staphylococcus aureus (A) 5’ strain and (B) R strain. Error bars indicate the SD of three independent experiments. *Significant, P<0.05; **highly significant, P<0.01.
Abbreviations: CCCP, carbonyl cyanide m-chlorophenylhydrazone; LEV, levofloxacin; Nor, norfloxacin.
Figure 6.

Effect of ethidium bromide, ketoconazole, CCCP, and ethidium bromide combination with ketoconazole or CCCP on the relative expression of efflux pump genes of Staphylococcus aureus (A) 5’ strain and (B) R strain. Error bars indicate the SD of three independent experiments. *Significant, P<0.05; **highly significant, P<0.01.
Abbreviations: CCCP, carbonyl cyanide m-chlorophenylhydrazone; EtBr, ethidium bromide; Nor, norfloxacin.
Using ketoconazole in combination with levofloxacin or EtBr significantly reduced the relative expression of the NorA gene (reduction ranged from 91.8 to 97.1%) that resulted in the termination of NorA gene action. In addition, the inhibitory effect of ketoconazole was close to or higher than the results for CCCP which agreed with the results of MIC reduction by EPIs, antibiotic–EPI disc synergy test, and time-kill curve assay. The effects of both CCCP and ketoconazole on the expression of NorB and NorC were variable, which indicates that ketoconazole as well as CCCP showed more specificity for the NorA gene than for the other efflux pump genes (Figures 5 and 6).
Docking studies
The compound 4ʹ,5ʹ-ODCQA obtained from artemisia absinthium, previously studied by Fiamegos et al,7 was taken as a reference for our docking study on ketoconazole as an efflux pump inhibitor targeting efflux systems in Gram-positive S. aureus. Two biologically active isomers of ketoconazole, 2S,4R and 2R,4S, were tested in the active site glycerol-3-phosphate transporter from E. coli which was the representative structure from the MFS (PDB ID: 1PW4) using MOE package version 2016.08. The tested compounds have high binding affinity to 1PW4 as the binding free energy values were −6.33 kJ/mol and −8.81 kJ/mol for 2S,4R and 2R,4S, respectively, while the reference 4ʹ,5ʹ-ODCQA compound showed a binding energy score of −7.13 kJ/mol. This indicates that these compounds can successfully enter the binding site of the efflux system.
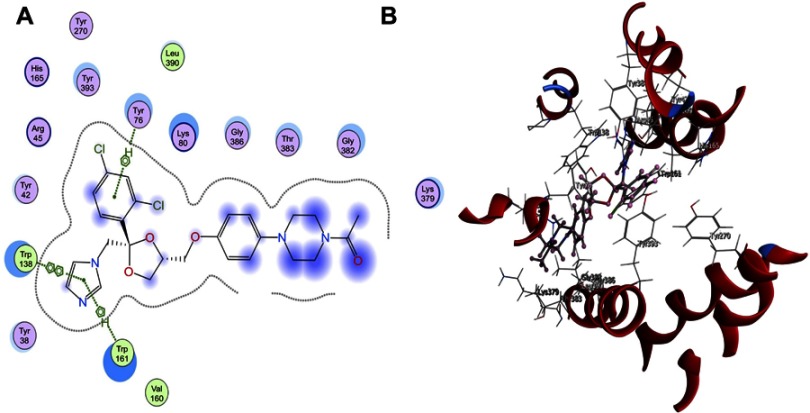
Docking of the most active stereoisomer of ketoconazole, 2S,4R and 2R,4S in the active site of glycerol-3-phosphate transporter protein (PDB ID: 1PW4) showed that the binding mode of the reference 4ʹ,5ʹ-ODCQA was through a hydrophobic interaction between the catechol–type rings with the amino acid Lys80. In addition, an important hydrogen bonding of the carboxylic group of 4ʹ,5ʹ-ODCQA with the amino acid Arg269 and a hydrogen bond with the adjacent β-OH with TrP161 were observed. Another hydrogen bonding was assigned between the 3-OH group of the catechol ring and amino acid Asn162 (Figure 7). On the other hand, the 2S,4R diastereoisomer of ketoconazole showed good fit with the active site of the protein (PDB ID: 1PW4) target. Further, it showed a hydrophobic π–π interaction between the 2,4-dichloro ring and the amino acid Tyr76. Further hydrophobic bonding was detected between the imidazole carbon skeleton and both Trp161 and Trp138 (Figure 8). The other isomer, 2R,4S, showed a hydrophobic π–π interaction between both the phenoxy group and the imidazole skeleton with the amino acid Tyr393. Another hydrogen bond was formed between the donor imidazole N and the amino acid Arg45 (Figure 8). It is obvious that the reference 4ʹ,5ʹ-ODCQA, the 2S,4R isomer, and the 2R,4S isomer can bind with the active site of the protein (PDB ID: 1PW4) spontaneously with different binding modes.
Figure 7.
(A) 2D and (B) 3D molecular interaction of 4ʹ,5ʹ-O-dicaffeoylquinic acid into the active site (Protein Data Bank ID: 1PW4).
Abbreviation: 1PW4, glycerol-3-phosphate transporter from Escherichia coli which was the representative structure from the major facilitator superfamily.
Figure 8.
(A) 2D and (B) 3D molecular interaction of 2S,4R and 2R,4S diastereoisomers into the active site (Protein Data Bank ID: 1PW4).
Abbreviation: 1PW4, glycerol-3-phosphate transporter from Escherichia coli which was the representative structure from the major facilitator superfamily.
Discussion
The emergence of MDR bacterial clinical isolates may be due to a combination of chromosomal and plasmid-mediated R or due to the overexpression of efflux pumps. When patients are subjected to increasing concentrations of one member of a class of antibiotic, activation of the genes of regulators and transporters of the pathogen occurs. However, if the dose of the antibiotic was adjusted and kept constant for a long period of time, the bacteria start to mutate and gain resistance to many classes of antibiotics, becoming MDR strains.20 So, there is a need for new compounds or testing compounds which were used for different therapeutic purposes for their antimicrobial activity or their ability to potentiate other antimicrobials. Many studies reported the effect of some newly synthesized compounds, such as Kalia et al,6 Fiamegos et al,7 and Saeed et al21 who found that guanidine derivative bearing adamantane-1-carbonyl and 2-bromo-4,6-difluoro-phenyl substituents (H-BDF) had bactericidal activity against MDR strains of S. aureus and some Gram-negative bacilli.
Resistance of S. aureus to fluoroquinolones was found to increase rapidly, limiting their use in the treatment of problematic pathogens. Fluoroquinolone resistance occurs mainly due to the following mechanisms: mutation in the gyr genes that affect DNA gyrase, mutation in grl genes that alter topoisomerase IV, and an increase in NorA expression leading to the presence of membrane-associated NorA efflux protein in high quantities.22 Presence of the NorA efflux pump permits S. aureus to extrude and remove the toxic compounds nonselectively, which may be natural as with chloramphenicol or synthetic as with fluoroquinolones and EtBr. Although resistance of S. aureus to fluoroquinolones develops in a stepwise manner and the overproduction of NorA may not be observed initially, transient upregulation allows S. aureus to grow in the presence of these drugs and the grl and gyr genes to mutate. So, inhibition of NorA activity can improve fluoroquinolone activity, increase their intracellular accumulation, decrease the MICs, decrease the time required for their killing activity, and prevent the emergence of resistant mutants.23
In this study, ketoconazole did not show any antibacterial effects even at high concentrations (2000 µg/mL). This is in keeping with Sud and Feingold,11 who reported that azoles like miconazole exhibit good anti-staphylococcal activity while ketoconazole showed no activity. Modifying antibacterial activity by decreasing their MICs is used for testing the ability of some compounds to increase or reverse the bacterial resistance of certain antibiotics. A reduction of an antibiotic MIC by 2-fold or more by an EPI is an indication that the main resistance mechanism is expression of the efflux pump. Also, using EtBr as a substrate for the efflux pump has been studied previously in the literature, as well as in our work. The decrease of the MICs of EtBr and antibiotics using EPI compounds is an indication of a similar mechanism of inhibition.24
Our results showed that ketoconazole has potent EPI activity on the tested MDR S. aureus strains. Many studies have reported that a number of compounds show moderate to high EPI activity on Gram-positive bacteria, especially S. aureus, while fewer compounds showed activity against E. coli and other Gram-negative bacteria.25,26 Although many efflux pump inhibitors have been discovered showing promising activity in vitro, their use for medical purposes is considered a major challenge. Many of these compounds (reserpine, omeprazole, phenothiazines) show significant EPI activity at very high concentrations which are toxic to eukaryotic cells. Another challenge is that these compounds can affect eukaryotic transporters because they mainly target several pumps in a given organism, or they target a transporter of a given class of antibiotic. In addition, by testing antibiotic–EPI disc synergy using antibiotics (fluoroquinolones, cefoxitin, ceftriaxone, tetracycline, aztreonam) and EPIs (CCCP and ketoconazole), the zone of inhibition diameters of the tested antibiotics were enlarged by more than 5 mm. This increase is an indication of the ability of ketoconazole to potentially reverse resistance and inhibit efflux pumps that are able to extrude antibiotics of different classes, facilitating the treatment of MDR S. aureus infections in comparison to that obtained by CCCP. The antibiotic–EPI disc synergy test has been used in many studies to investigate the ability of some compounds to inhibit the efflux pump and reverse the resistance.27,28
In our research, significant killing activity was obtained by levofloxacin combination with CCCP or ketoconazole in comparison to levofloxacin alone after 18 h, while the maximum activity was obtained by the tested combinations at 24 h. Aeschlimann et al10 studied the effect of reserpine, omeprazole, and lansoprazole on the time-kill curve of quinolones. Here they found that reserpine caused an improvement of fluoroquinolone activity until 8 h followed by regrowth within 24 h. A similar study conducted by Kalia et al6 showed that a combination of capsaicin and ciprofloxacin exhibited a bactericidal effect and a >3 log10 reduction in CFU was observed in 8 h but regrowth was observed within 24 h.
The effect of ketoconazole on biofilm formation by S. aureus was studied as described previously. Levofloxacin increased biofilm formation from 52 to 75.3% while in the presence of ketoconazole the biofilm formation inhibition ranged from 79.5 to 97%. In the study by Siala et al,4 caspofungin increased the activity of fluoroquinolones against S. aureus biofilm by inhibiting N-acetylglucosamine transferase. The expression of efflux pump systems plays an important role in S. aureus resistance to quinolones especially efflux pump members of the MFS transporters. These pumps use proton motive forces to energize the export of antimicrobials across the cell membrane (H+:drug antiport mechanism). So, Nor gene-mediated efflux was found to be affected by protonophores, such as CCCP.29 Ketoconazole downregulated expression of the NorA gene in a manner resembling that shown by CCCP but a variable effect was shown on the expression of both NorB and NorC. The variation in the relative gene expression level among those genes was also reported by Huet et al.30 They found that after exposure to fluoroquinolones or dyes, the expression levels of the three genes were different. NorA showed overexpression after single exposure to fluoroquinolones and multiple exposure to EtBr, while NorC showed overexpression only after multiple exposure to the tested dye (rhodamine) and no increase in the expression of NorB was observed. The researchers explained the variable response of these genes by the presence of some factors controlling their response. First, the three genes differ in their substrates. EtBr (dyes), hydrophilic fluoroquinolones, and quaternary ammonium compounds are substrates for NorA.31 Hydrophilic and hydrophobic fluoroquinolones, tetracycline, quaternary ammonium compounds, and EtBr (dyes) are substrates for NorB.32 Fluoroquinolones and dyes such as rhodamine are substrates for NorC but EtBr is a poor substrate for it.33 Second, their expression is affected by the environmental conditions as shown by Hassan et al.34 Finally, the regulation of these transporter proteins is controlled by a variety of regulatory systems such as MgrAregulators (positively regulate NorA but negatively regulates NorB and NorC) and NorG (activates both NorA and NorB expression but decreases NorC gene expression),32,33 which makes the determination of the effect of Nor pump expression difficult and confusing on interpretation of the data.
Many studies tested the ability of CCCP and 2,4-dinitrophenol to inhibit efflux pumps energized by proton motive force in Salmonella enterica, Mycobacterium tuberculosis, and Pseudomonas aeruginosaby testing their effect on the MICs of the tested antibiotics and EtBr accumulation assay.35–37 So, CCCP was used as a positive control in our study for testing the effect of ketoconazole as an EPI. It was found that ketoconazole reduced the MICs of the tested antibiotics by 8 to 1024-fold, increased the accumulation of EtBr, and decreased the relative gene expression of NorA gene, which confirmed its effect on the proton motive force.
Molecular modeling studies showed strong binding of 2R,4S ketoconazole and 2S,4R ketoconazole into the active site of glycerol-3-phosphate transporter protein (PDB ID: 1PW4). The favored binding free energies indicated spontaneous binding between the tested ketoconazole isomers and the reference 4ʹ,5ʹ-ODCQA into the active site of 1PW4. In conclusion, the binding energy and binding mode of 2R,4S ketoconazole and 2S,4R ketoconazole showed that ketoconazole could potentially inhibit the glycerol-3-phosphate transporter protein (PDB ID: 1PW4) active site.
Limitations of the study and future perspectives
The strains used in this study were not tested for the presence of topoisomerase mutation. Although ketoconazole (500 µg/mL) may be not suitable for clinical use at this concentration, our study provides proof that ketoconazole (500 µg/mL) is bioactive and we believe that this will open the door for researchers in the future to do additional work to bring down the active concentration of this molecule via structure remodeling using a bioinformatics approach or using nanotechnology. Also, we think that ketoconazole could be used in the detection of efflux pump in MDR S. aureus.
Conclusion
Ketoconazole was found to decrease the MIC of the tested antibiotics by 8 to 1024-fold. Synergistic activity of ketoconazole and the tested antibiotics was confirmed by an increase in their inhibition zone diameters of >5 mm in comparison to the effects observed by CCCP. It increased the accumulation of EtBr (substrate for the NorA efflux pump), which was confirmed by increasing fluorescence by more than 2-fold. Ketoconazole in combination with levofloxacin caused significant killing activity after 24 h in comparison with that shown by levofloxacin alone. Also, it decreased the relative expression of NorA by 75–87% but showed variable effects on NorB and NorC gene expression. Docking studies showed that ketoconazole can effectively bind with the active site of 1PW4 and block binding of quinolones with the efflux binding cavity. No effect was shown by ketoconazole on the MICs of antibiotics or time-kill assay of the tested susceptible strains. Thus, ketoconazole can potentiate the activity of the fluoroquinolones against MDR S. aureus via inhibiting efflux pump and biofilm formation in vitro.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 2.Kumar A, Schweizer HP. Bacterial resistance to antibiotics: active efflux and reduced uptake. Adv Drug Deliv Rev. 2005;57(10):1486–1513. doi: 10.1016/j.addr.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 3.Fernandes A, Dias M. The microbiological profiles of infected prosthetic implants with an emphasis on the organisms which form biofilms. J Clin Diagn Res. 2013;7(2):219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siala W, Kucharíková S, Braem A, et al. The antifungal caspofungin increases fluoroquinolone activity against Staphylococcus aureus biofilms by inhibiting N-acetylglucosamine transferase. Nat Commun. 2016;7:13286. doi: 10.1038/ncomms13286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Bambeke F, Glupczynski Y, Plesiat P, Pechere JC, Tulkens PM. Antibiotic efflux pumps in prokaryotic cells: occurrence, impact on resistance and strategies for the future of antimicrobial therapy. J Antimicrob Chemother. 2003;51(5):1055–1065. [DOI] [PubMed] [Google Scholar]
- 6.Kalia NP, Mahajan P, Mehra R, et al. Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. J Antimicrob Chemother. 2012;67(10):2401–2408. doi: 10.1093/jac/dks232 [DOI] [PubMed] [Google Scholar]
- 7.Fiamegos YC, Kastritis PL, Exarchou V, et al. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from artemisia absinthium against gram-positive pathogenic bacteria. PLoS One. 2011;6(4):e18127. doi: 10.1371/journal.pone.0018127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tintino SR, Oliveira-Tintino CDM, Campina FF, et al. Vitamin K enhances the effect of antibiotics inhibiting the efflux pumps of Staphylococcus aureus strains. Med Chem Res. 2018;27(1):261–267. doi: 10.1007/s00044-017-2063-y [DOI] [Google Scholar]
- 9.Zgurskaya HI, Nikaido H. Multidrug resistance mechanisms: drug efflux across two membranes. Mol Microbiol. 2000;37(2):219–225. doi: 10.1046/j.1365-2958.2000.01926.x [DOI] [PubMed] [Google Scholar]
- 10.Aeschlimann JR, Dresser LD, Kaatz GW, Rybak MJ. Effects of NorA inhibitors on in vitro antibacterial activities and postantibiotic effects of levofloxacin, ciprofloxacin, and norfloxacin in genetically related strains of staphylococcus aureus. Antimicrob Agents Chemother. 1999;43(2):335–340. doi: 10.1128/AAC.43.2.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sud IJ, Feingold DS. Action of antifungal imidazoles on Staphylococcus aureus. Antimicrob Agents Chemother. 1982;22(3):470–474. doi: 10.1128/AAC.22.3.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CLSI. Performance standards of antimicrobial disk susceptibility test: ninth Informational Supplement. NCCLS document M100-S9. National Committee for Clinical Laboratory Standards, 2008:120–126; Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 13.Kristiansen JE, Thomsen VF, Martins A, Viveiros M, Amaral L. Non-antibiotics reverse resistance of bacteria to antibiotics. In Vivo. 2010;24(5):751–754. [PubMed] [Google Scholar]
- 14.CLSI CaLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard. 8th ed. Broth Microdilution Method Wayne, PA: CLSI; 2009. [Google Scholar]
- 15.Huang L, Sun L, Xu G, Xia T. Differential susceptibility to carbapenems due to the AdeABC efflux pump among nosocomial outbreak isolates of acinetobacter baumannii in a Chinese hospital. Diagn Microbiol Infect Dis. 2008;62(3):326–332. doi: 10.1016/j.diagmicrobio.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 16.Paixao L, Rodrigues L, Couto I, et al. Fluorometric determination of ethidium bromide efflux kinetics in escherichia coli. J Biol Eng. 2009;3:18. doi: 10.1186/1754-1611-3-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa SS, Falcao C, Viveiros M, et al. Exploring the contribution of efflux on the resistance to fluoroquinolones in clinical isolates of Staphylococcus aureus. BMC Microbiol. 2011;11:241. doi: 10.1186/1471-2180-11-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei GX, Campagna AN, Bobek LA. Effect of MUC7 peptides on the growth of bacteria and on streptococcus mutans biofilm. J Antimicrob Chemother. 2006;57(6):1100–1109. doi: 10.1093/jac/dkl120 [DOI] [PubMed] [Google Scholar]
- 19.DeMarco CE, Cushing LA, Frempong-Manso E, Seo SM, Jaravaza TA, Kaatz GW. Efflux-related resistance to norfloxacin, dyes, and biocides in bloodstream isolates of Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(9):3235–3239. doi: 10.1128/AAC.00430-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pages JM, Amaral L. Mechanisms of drug efflux and strategies to combat them: challenging the efflux pump of Gram-negative bacteria. Biochim Biophys Acta. 2009;1794(5):826–833. doi: 10.1016/j.bbapap.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 21.Saeed A, Bosch A, Bettiol M, Nossa González DL, Erben MF, Lamberti Y. Novel guanidine compound against multidrug-resistant cystic fibrosis-associated bacterial species. Molecules. 2018;23(5):1158. doi: 10.3390/molecules23051158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka M, Zhang YX, Ishida H, Akasaka T, Sato K, Hayakawa I. Mechanisms of 4-quinolone resistance in quinolone-resistant and methicillin-resistant staphylococcus aureus isolates from Japan and China. J Med Microbiol. 1995;42(3):214–219. doi: 10.1099/00222615-42-3-214 [DOI] [PubMed] [Google Scholar]
- 23.Ng EY, Trucksis M, Hooper DC. Quinolone resistance mediated by norA: physiologic characterization and relationship to flqB, a quinolone resistance locus on the Staphylococcus aureus chromosome. Antimicrob Agents Chemother. 1994;38(6):1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel D, Kosmidis C, Seo SM, Kaatz GW. Ethidium bromide MIC screening for enhanced efflux pump gene expression or efflux activity in Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54(12):5070–5073. doi: 10.1128/AAC.01058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pages JM, Amaral L, Fanning S. An original deal for new molecule: reversal of efflux pump activity, a rational strategy to combat gram-negative resistant bacteria. Curr Med Chem. 2011;18(19):2969–2980. [DOI] [PubMed] [Google Scholar]
- 26.Takacs D, Cerca P, Martins A, et al. Evaluation of forty new phenothiazine derivatives for activity against intrinsic efflux pump systems of reference escherichia coli, salmonella enteritidis, enterococcus faecalis and staphylococcus aureus strains. In Vivo. 2011;25(5):719–724. [PubMed] [Google Scholar]
- 27.Choudhury D, Talukdar AD, Maurya AP, et al. Contribution of efflux pumps in fluroquinolone resistance in multi-drug resistant nosocomial isolates of pseudomanas aeruginosa from a tertiary referral hospital in north east India. Indian J Med Microbiol. 2015;33(1):84–86. doi: 10.4103/0255-0857.148388 [DOI] [PubMed] [Google Scholar]
- 28.Osei Sekyere J, Amoako DG. Carbonyl Cyanide m-Chlorophenylhydrazine (CCCP) reverses resistance to colistin, but not to carbapenems and tigecycline in multidrug-resistant enterobacteriaceae. Front Microbiol. 2017;8:228. doi: 10.3389/fmicb.2017.00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaatz GW, Seo SM, Ruble CA. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37(5):1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huet AA, Raygada JL, Mendiratta K, Seo SM, Kaatz GW. Multidrug efflux pump overexpression in Staphylococcus aureus after single and multiple in vitro exposures to biocides and dyes. Microbiology. 2008;154(Pt 10):3144–3153. doi: 10.1099/mic.0.2008/021188-0 [DOI] [PubMed] [Google Scholar]
- 31.Yoshida H, Bogaki M, Nakamura S, Ubukata K, Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990;172(12):6942–6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Truong-Bolduc QC, Dunman PM, Strahilevitz J, Projan SJ, Hooper DC. MgrA is a multiple regulator of two new efflux pumps in staphylococcus aureus. J Bacteriol. 2005;187(7):2395–2405. doi: 10.1128/JB.187.7.2395-2405.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Truong-Bolduc QC, Strahilevitz J, Hooper DC. NorC, a new efflux pump regulated by MgrA of staphylococcus aureus. Antimicrob Agents Chemother. 2006;50(3):1104–1107. doi: 10.1128/AAC.50.3.1104-1107.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan KA, Skurray RA, Brown MH. Active export proteins mediating drug resistance in staphylococci. J Mol Microbiol Biotechnol. 2007;12(3–4):180–196. doi: 10.1159/000099640 [DOI] [PubMed] [Google Scholar]
- 35.Rushdy AA, Mabrouk MI, Abu-Sef FA, Kheiralla ZH, Mohamed Abdel-All S, Saleh NM. Contribution of different mechanisms to the resistance to fluoroquinolones in clinical isolates of Salmonella enterica. Braz J Infect Dis. 2013;17(4):431–437. doi: 10.1016/j.bjid.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdi-Ali A, Rahmani-Badi A, Falsafi T, Nikname V. Study of antibiotic resistance by efflux in clinical isolates of Pseudomonas aeruginosa. Pak J Biol Sci. 2007;10(6):924–927. [DOI] [PubMed] [Google Scholar]
- 37.Singh M, Jadaun GP, Ramdas SK, et al. Effect of efflux pump inhibitors on drug susceptibility of ofloxacin resistant Mycobacterium tuberculosis isolates. Indian J Med Res. 2011;133:535–540. [PMC free article] [PubMed] [Google Scholar]