Abstract
Objectives: Reduced reward responsiveness, as measured by the event-related potential (ERP) component, the reward positivity (RewP), has been shown to play a role in the development of internalizing disorders, but implications for treatment remain unclear. In adult patients with anxiety and/or depression, reduced RewP has emerged as a predictor of greater change in symptoms following cognitive behavior therapy (CBT) or selective serotonin reuptake inhibitor (SSRI) treatment. The objectives of this preliminary study were to extend these findings to children and adolescents with anxiety disorders by evaluating RewP to reward as a predictor of change in anxiety severity or depressive symptoms following treatment with CBT or SSRI and to explore whether RewP differentially predicts response to one type of treatment.
Methods: Patients (7–19 years old) with social and/or generalized anxiety disorder (N = 27) completed baseline measures of anxiety severity and depressive symptoms, as well as an ERP monetary reward anticipation and feedback task. RewP was measured in response to reward and breaking even feedback. Patients were then randomly assigned to CBT or SSRI treatment, and completed measures of anxiety and depressive symptom severity at the last treatment session.
Results: Reduced reward responsiveness, as measured by RewP to rewards, predicted greater change in depressive symptoms following treatment, adjusting for baseline symptoms, age, and RewP to breaking even. RewP was not a significant predictor of change in anxiety symptoms. Although preliminary, exploratory analyses suggested that among anxious youth, RewP specifically predicted change in depressive symptoms following CBT, rather than SSRI.
Conclusion: Results provide preliminary support for the utility of ERP measures of reward responsiveness in predicting treatment response in youth. With further research and standardization, ERP assessments could potentially be implemented in clinical settings to inform prognosis and treatment planning for youth with internalizing disorders.
Keywords: reward, event-related potentials, anxiety, depression, cognitive behavior therapy, youth
Introduction
Anxiety disorders are relatively common psychiatric disorders in youth and are associated with long-term impairment across domains of functioning, as well as high rates of comorbid depression (Beesdo et al. 2010; Cummings et al. 2014). Cognitive behavior therapy (CBT) and pharmacotherapy (i.e., selective serotonin reuptake inhibitors [SSRIs]) are generally considered efficacious for youth internalizing disorders, but up to 40%–50% of youth show limited treatment response (e.g., March et al. 2004; Walkup et al. 2008). Predictors of treatment response that could be used to identify those most likely to benefit, and ultimately, prescriptive indicators for selecting between treatment options may improve outcomes and success rates.
There are few established clinical or demographic predictors of response to treatment for pediatric internalizing disorders. Although several clinical and family environmental factors, including comorbid psychopathology and family dysfunction, have been shown to predict treatment response (Brent et al. 1998; Crawford and Manassis 2001), demographic factors like age and socioeconomic status do not appear to be consistent predictors (Berman et al. 2000; Layne et al. 2003). Promising evidence suggests that measures of brain function can be integrated with clinical indicators to improve prediction of treatment response in adult internalizing disorders (Ball et al. 2014). For example, in one neuroimaging study of adults with social anxiety disorder (SAD), neural measures of social threat processing and clinical predictors combined accounted for 40% of the variance in response to CBT (Doehrmann et al. 2013).
At the neural level, altered reward responsiveness is thought to be a core process involved in the development of internalizing disorders (Zisner and Beauchaine 2016; Nusslock and Alloy 2017). As such, individual differences in reward responsiveness are relevant to consider with regard to treatment response and targets, but very little work has evaluated reward responsiveness and treatment, particularly across development. In addition to reducing negative emotions, treatments for anxiety and depression likely have effects on the experiences of positive emotions and availability of rewards in the environment. For example, both psychotherapy and pharmacotherapy involve establishing a positive alliance with the therapist or psychiatrist. In addition, exposure therapy, a key component of CBT for anxiety, and SSRIs reduce avoidance of activities and relationships (Connor et al. 2006; Olatunji et al. 2010), potentially increasing rewarding experiences. Finally, treatments for depression directly target low motivation and aim to increase engagement in pleasant events (Dimidjian et al. 2011). Thus, the extent to which individuals exhibit deficits in reward responsiveness before treatment might predict how likely they are to respond to treatment and might ultimately be useful for selecting among treatment options.
Event-related potentials (ERPs) derived from the electroencephalogram (EEG) provide a relatively economical neural measure that can be integrated into clinical settings and applied across development. In particular, an ERP indicator of reward responsiveness, reward positivity (RewP), has demonstrated good test-retest reliability and internal consistency across childhood and adolescence, suggesting that it could be reliably applied in intervention research (Bress et al. 2015a; Kujawa et al. 2018). RewP presents as a relative positivity in the ERP wave over frontocentral electrode sites beginning ∼250 ms following the receipt of reward or positive feedback and has been correlated with activation of the ventral striatum and medial prefrontal cortex (Carlson et al. 2011). Reduced reward responsiveness, as measured by RewP, has consistently been shown to predict the emergence of depressive symptoms in adolescence (for a review, Kujawa and Burkhouse 2017). There is also some evidence that RewP may predict CBT or SSRI response among adults with internalizing disorders (Burkhouse et al. 2016, 2018), but these methods have yet to be extended to pediatric treatment studies.
RewP may be particularly relevant for consideration in predicting change in depressive symptoms with treatment. Consistent concurrent and prospective associations have been observed between attenuated RewP and depressive symptoms in clinical and community samples of youth and adults (Liu et al. 2014; Bress et al. 2015a; Belden et al. 2016; Nelson et al. 2016; Burkhouse et al. 2017a; Kujawa et al. 2019). That is, reduced RewP magnitude prospectively predicts increases in depressive symptoms across time and development (Bress et al. 2015a; Nelson et al. 2016; Kujawa et al. 2019), suggesting that rather than a state marker, RewP might reflect relatively stable individual differences in reward responsiveness that predict the potential for later changes in depressive symptoms. Fewer studies have examined RewP in anxiety. There is some evidence to suggest that reward responsiveness is also altered in anxiety disorders, although directions of associations may differ from that observed in depression and vary across studies. For example, one previous study found an enhanced RewP among children with elevated symptoms of SAD, and a reduced RewP among children with elevated symptoms of generalized anxiety disorder (GAD; Kessel et al. 2015), but other smaller studies have failed to find significant effects of anxiety symptoms on RewP (Foti et al. 2009; Bress et al. 2015b).
Intriguingly, two previous studies of adults have indicated that individual differences in reward responsiveness, as measured by RewP, predict change in depressive symptoms following treatment. Surprisingly, both studies indicated that a reduced RewP before treatment predicted greater change in symptoms following treatment (i.e., better treatment response). In a study of adults with anxiety disorders with and without comorbid depression, a more attenuated RewP in a reward anticipation and feedback task administered at baseline predicted greater likelihood of response to CBT and greater reductions in depressive symptoms, specifically (Burkhouse et al. 2016). In a follow-up study of adults with anxiety or depressive disorders, a reduced RewP in a reward feedback task at baseline predicted greater depressive symptom reduction, particularly for patients treated with SSRIs (Burkhouse et al. 2018). We have previously argued that CBT and/or SSRI treatment might be best serving the needs of patients with reduced reward responsiveness, leading to greater symptom change, whereas those with depressive symptoms, but intact or elevated reward responsiveness, might benefit from alternative or combined treatments.
Taken together, evidence suggests the RewP might be a useful predictor of response to CBT or SSRI for internalizing disorders, but a number of questions remain and replication is needed. Most relevant to this study is the extent to which these findings generalize to children and adolescents. Reward circuits undergo significant developmental changes through adolescence and into young adulthood (Casey et al. 2008; Galvan 2010), raising the need for empirical research extending treatment response findings in adults to childhood and adolescence. There is some preliminary evidence that functional magnetic resonance imaging (fMRI) measures of reward responsiveness predict treatment response in pediatric internalizing disorders (Forbes et al. 2010a). To our knowledge, only one recent study has examined ERP measures of reward responsiveness as a predictor of treatment response in pediatric samples. In very young children with depression completing parent-child interaction therapy, change in RewP pre-treatment to post-treatment corresponded with improvement in depressive symptoms, although baseline RewP did not significantly predict treatment response (Barch et al. 2018). However, the extent to which RewP might be a predictor of response to CBT or SSRI in older youth, similar to our observations in adults (Burkhouse et al. 2016, 2018), remains unexplored.
In our initial study of RewP using a task that included a lengthy anticipation period before monetary reward feedback, a reduced RewP predicted responses to CBT in adults with depression and anxiety (Burkhouse et al. 2016). Yet, in a follow-up study of adults with depression or anxiety, RewP in a more immediate reward feedback task emerged as a specific predictor of response to SSRI rather than CBT (Burkhouse et al. 2018). One possible explanation for these discrepancies is that individuals who exhibit attenuated sustained reward responses may perform better with CBT, whereas impairments in more immediate reward responsiveness might predict response to SSRI, specifically. Although differences in task design may account for this discrepancy, replication in samples treated with CBT or SSRI is needed. Given these goals, this preliminary study examined RewP, using a task similar to Burkhouse et al. (2016), as a prospective predictor of change in anxiety and/or depressive symptom in a sample of anxious children and adolescents with a range of comorbid depressive symptoms treated with CBT or SSRI (Kujawa et al. 2016; Bunford et al. 2017; Burkhouse et al. 2017b). Consistent with research in adults (Burkhouse et al. 2016, 2018), we hypothesized that a reduced RewP would predict greater changes in depressive, but not change in anxiety severity. Additional exploratory analyses examined RewP as a predictor of treatment response in those treated with CBT versus SSRI.
Methods
Participants
Participants were youth between the ages of 7 and 19 with primary diagnoses of GAD or SAD, participating in a larger pediatric anxiety treatment study at the University of Illinois at Chicago (UIC) and University of Michigan. The study design was modeled after the Child/Adolescent Anxiety Multimodal Study, in that, children and adolescents with relatively common and often co-occurring disorders of GAD, SAD, or separation anxiety disorders were eligible to participate (Compton et al. 2010; Kujawa et al. 2016). The reward responsiveness task was added to the study after recruitment moved to UIC. Consequently, only the UIC cohort is included in this study and none of the participants in the current subsample had primary diagnoses of separation anxiety disorder. The Schedule of Affective Disorders and Schizophrenia for School-Age Children (Kaufman et al. 1997) diagnostic interview was administered by master's- or doctoral-level clinicians (Kujawa et al. 2015). Exclusion criteria included history of bipolar disorder, schizophrenia, intellectual disability, pervasive development disorders, current substance use disorders, severe depression, or suicidal ideation. Participants with comorbid anxiety, depressive, or externalizing disorders were included in the study (see Table 1 for Participant Characteristics). Participants were not taking psychiatric medications or in psychotherapy for at least 4 weeks before the initial assessment.
Table 1.
Demographic and Clinical Characteristics of Participants in Each Treatment Group
| CBT (N = 16) | SSRI (N = 11) | Statistic | |
|---|---|---|---|
| M (SD) | M (SD) | t | |
| Age | 11.63 (4.29) | 15.18 (2.60) | −2.45a |
| Pre-treatment anxiety severity | 25.06 (3.89) | 24.91 (4.13) | 0.10 |
| Post-treatment anxiety severity | 11.45 (6.97) | 11.26 (4.75) | 0.08 |
| Pre-treatment depressive symptoms | 11.19 (6.81) | 16.45 (6.73) | −1.98 |
| Post-treatment depressive symptoms | 7.62 (6.47) | 8.45 (5.94) | −0.34 |
| N (%) | N (%) | χ2 | |
|---|---|---|---|
| Male | 9 (56.3) | 7 (63.6) | 0.15 |
| SAD diagnosis | 6 (37.5) | 8 (72.7) | 3.24 |
| GAD diagnosis | 13 (81.3) | 7 (63.6) | 1.05 |
| Lifetime depression diagnosis | 3 (18.8) | 4 (36.6) | 1.05 |
| ADHD diagnosis | 4 (25.0) | 1 (9.1) | 1.09 |
| Separation anxiety diagnosis | 1 (6.3) | 0 (0.0) | 0.71 |
| Panic disorder diagnosis | 2 (12.5) | 0 (0.0) | 1.49 |
| Specific phobia diagnosis | 4 (25.0) | 3 (27.3) | 0.02 |
p < 0.05.
ADHD, attention-deficit/hyperactivity disorder; CBT, cognitive behavior therapy; GAD, generalized anxiety disorder; SAD, social anxiety disorder; SSRI, selective serotonin reuptake inhibitor.
Twenty-seven patients had acceptable EEG data (defined as at least 13 trials for each condition after artifact rejection) and completed either CBT or SSRI treatment. The final sample was 59.3% male, and had a mean age of 13.07 (SD = 4.05; range: 7–19 years). With regard to race, the sample was 25.9% Caucasian, 37.0% Hispanic or Latino, 7.4% African American, 14.8% Asian, 3.7% Native Hawaiian or Pacific Islander, 7.4% multiracial, and 3.7% other race.
Procedure
This study was approved by the UIC Institutional Review Board. Informed consent was obtained from parents and adult participants and assent obtained from minor participants. Participants completed the clinical interview and pre-treatment symptom measures during an initial intake visit. Participants then completed the EEG assessment before beginning treatment. Participants were initially randomly assigned to receive either SSRI or CBT, but could opt to switch from SSRI to CBT due to side effects (six participants in this sample were initially assigned to SSRI, but opted to complete CBT instead). SSRI treatment consisted of 12 weeks of sertraline prescribed by a child psychiatrist during medication management sessions, beginning with a dose of 12.5 or 25 mg/day and in a flexible dosing design increasing on subsequent visits up to 200 mg/day based on tolerability and treatment response. CBT was delivered through weekly 60-minute sessions (up to a maximum of 18 sessions) by a master's- or doctoral-level therapist. Treatment followed an established manualized CBT intervention (i.e., Coping Cat, C.A.T Project) for pediatric anxiety (Kendall et al. 2002; Kendall and Hedtke 2006).
Measures
Anxiety and depression symptoms
To assess severity of anxiety symptoms pre-treatment and post-treatment, participants were administered the Pediatric Anxiety Rating Scale (PARS; The Research Units on Pediatric Psychopharmacology Anxiety Study Group 2002). The PARS was administered by an interviewer both at the initial screening visit and at the final treatment session. Greater PARS change scores (i.e., pre-treatment minus post-treatment) indicate more improvement in anxiety symptoms following treatment. To assess depressive symptoms, participants completed the self-report Children's Depression Inventory (CDI; Kovacs 1992) at the initial screening visit and the final treatment session. Greater CDI change scores (i.e., pre-treatment minus post-treatment) indicate more improvement in depressive symptoms following treatment.
Monetary reward task
While EEG data were recorded, participants completed a reward task (Burkhouse et al. 2016) based on an fMRI reward task by Forbes and colleagues (Forbes et al. 2010b) that includes both reward anticipation and feedback stages. The task included 60 trials (15 win, 15 loss, 15 no-win, and 15 no-loss), each consisting of a decision, anticipation, and outcome period, separated by an intertrial interval ranging between 4 and 7 seconds (Fig. 1). During the decision period, participants were presented with a question mark (4 seconds) and pressed a button to guess whether a computer-selected number was greater than or less than 5. Following the decision, participants were presented with a circle with the numbers 1–9 and a yellow arrow indicating the range of the “actual” number for 6 seconds. An arrow consistent with the participant's response indicated a correct response and the possibility of winning money. An arrow inconsistent with the participant's response indicated an incorrect response and the possibility of a loss. This design was selected so that performance feedback (correct vs. incorrect) was presented separately from reward feedback (reward vs. even vs. loss). Participants were informed that a correct response indicated the possibility of winning $1 or breaking even, while an incorrect response indicated the possibility of losing 50¢ or breaking even. In reality, 50% of trials were win possible trials and 50% were loss possible, regardless of participant performance, and the order of win possible and loss possible trials was presented pseudo-randomly across the task. During the outcome period, participants were presented with the “actual” number for 500 ms and received feedback for 500 ms in the form of a happy face for wins, sad face for losses, and neutral face for breaking even. Participants saw their total earnings every 20 trials ($2.50, $4.50, and $7.50), and earnings were rounded up to $10 at the end of the task. In addition to RewP, the task was designed to measure neural response in anticipation of reward and loss feedback. However, in our previous work, we failed to find effects of condition or treatment response prediction on anticipatory ERPs from this task. Thus, we limit our analysis in this study to RewP (i.e., feedback stage).
FIG. 1.

Design of the guessing reward task. The task included 60 trials (15 win, 15 loss, 15 no-win/break even, and 15 no-loss/break even), each consisting of a decision period, anticipation period, and outcome period, separated by an intertrial interval. Analyses in this study focused on the win possible condition and the outcome stage of processing.
EEG data acquisition and processing
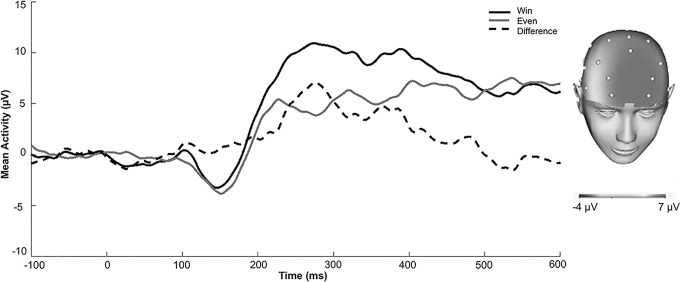
Continuous EEG was recorded using a 34-channel cap (32 channel 10/20 system with the addition of FCz and Iz) and the BioSemi system (BioSemi, Amsterdam, Netherlands). Electrodes were placed on the left and right mastoids, and the electrooculogram was recorded from four facial electrodes. The data were digitized at 24-bit resolution with a Least Significant Bit value of 31.25 nV and a sampling rate of 1024 Hz. The voltage from each active electrode was referenced online with respect to a common mode sense active electrode. Data were processed offline using Brain Vision Analyzer software (Brain Products, Gilching, Germany). Data were converted to a linked mastoid reference and filtered with high- and low-pass filters of 0.1 and 30 Hz. Continuous EEG data were segmented beginning 100 ms before stimulus onset and continuing for 600 ms after onset. Eyeblinks were corrected using the method by Gratton et al. (1983), and semi-automated artifact rejection procedures removed artifacts with the following criteria: voltage step of more than 50 μV between sample points, a voltage difference of 300 μV within a trial, and a maximum voltage difference of less than 0.5 μV within 100 ms intervals. Additional artifacts were removed using visual inspection. Data were baseline corrected to the 100 ms interval before feedback. ERPs were averaged across win, even (win possible), loss, and even (loss possible) trials. RewP was scored where it appeared maximal in the overall sample and consistent with previous work using this task in adults (Burkhouse et al. 2016): 230–300 ms at a pooling of frontal sites (AF3, AF4, and Fz; see Fig. 2). Because these sites are somewhat more anterior than the location RewP tends to be maximal using other types of reward tasks (e.g., Bress et al. 2015a; Kujawa et al. 2018b), we followed up significant effects of RewP with exploratory correlations of RewP scored at individual electrode sites and symptom measures. Given our focus on reward responsiveness, RewP in response to monetary reward feedback was examined as a predictor of change in symptoms following treatment (adjusting for RewP to breaking even feedback when reward was possible).
FIG. 2.
ERPs in response to wins, breaking even feedback (when rewards were possible), and the win minus even difference in the overall sample. The scalp distribution depicts the response to wins minus breaking even when wins were possible. ERP, event-related potential.
Data analyses
Separate hierarchical multiple regression analyses were computed to examine predictors of change in symptoms of anxiety and depression from pre- to post-treatment. For both models, clinical and demographic variables (baseline severity of anxiety and depression and age) were entered into Step 1. To measure additional variance accounted for by neural measures, RewP to reward feedback and RewP to breaking even feedback were entered into Step 2. Additional exploratory analyses examined effects of RewP to loss to evaluate whether effects are driven by responses to reward, specifically.
Results
The majority of the sample (74.1%) had current GAD, and 51.9% had current SAD. With regard to depression, 25.9% of the sample had experienced a depressive episode, including major depressive disorder, dysthymia, or depressive disorder not otherwise specified. No participant was diagnosed with a current comorbid depressive disorder at the initial diagnostic assessment, although subthreshold and clinical levels of symptoms were endorsed on the CDI. Demographic and clinical characteristics for each treatment group are presented in Table 1 and bivariate correlations between all study variables are presented in Supplementary Table S1. Variability in symptoms of both depression and anxiety was observed in the sample (PARS ranged from 19 to 32 pre-treatment and 0 to 24 post-treatment and CDI ranged from 2 to 30 pre-treatment and 0 to 23 post-treatment). With clinical cutoffs of ∼17.5 on PARS and 16 on CDI (Roelofs et al. 2010; Ginsburg et al. 2011), all participants were in the clinical range for anxiety and 29.6% were in the clinical range for depression pre-treatment. Post-treatment, only 11.9% of participants were in the clinical range for anxiety and 7.4% for depression.
At baseline, a reduced RewP to reward feedback predicted greater depressive symptoms, controlling for RewP to breaking even, r(24) = −0.47, p = 0.02, but RewP was not significantly associated with baseline anxiety severity (p = 0.68). RewP to reward feedback was negatively associated with age, r(24) = −0.65, p < 0.001, controlling for RewP to breaking even, and there was a trend such that older youth reported more depressive symptoms, r(25) = 0.35, p = 0.07. RewP to reward feedback was strongly correlated across electrode sites (r's = 0.82 to 0.91).
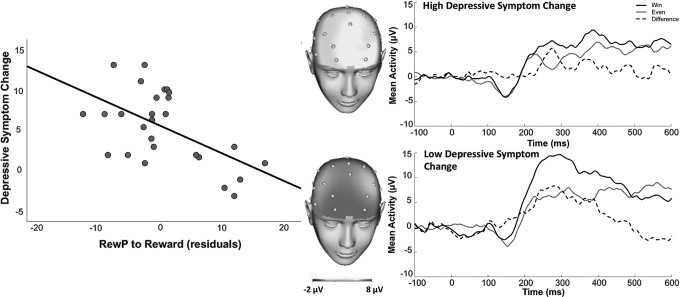
Results of multiple regression analyses predicting change in symptoms following treatment are presented in Table 2. RewP was not a significant predictor of change in anxiety symptoms (p's > 0.14), but a reduced RewP to reward feedback predicted greater change in depressive symptoms, t(21) = −2.10, p < 0.05, over and above baseline symptom severity and age (Fig. 3). These results were driven by RewP specifically to reward feedback, as RewP in response to loss feedback or breaking even when loss was possible were not significant predictors of change in depressive or anxiety symptoms (p's > 0.32). Exploratory partial correlations indicated that change in depressive symptoms, adjusting for baseline depression symptoms, was significantly correlated with RewP at Fz, AF3, and AF4 (p's < 0.05), suggesting results are relatively consistent across these electrode sites.
Table 2.
Hierarchical Regression Analyses Examining Predictors of Change in Anxiety Severity and Depressive Symptoms from Pre-Treatment to Post-Treatment
| Change in anxiety severity | Change in depressive symptoms | |||
|---|---|---|---|---|
| Predictors | b | SE | b | SE |
| Step 1—Demographic/clinical | R2 = 0.22, F(3, 23) = 2.17 | R2 = 0.31, F(3, 23) = 3.42a | ||
| Age | 0.22 | 0.32 | 0.19 | 0.21 |
| Baseline anxiety | 0.73a | 0.32 | −0.09 | 0.20 |
| Baseline depression | −0.24 | 0.18 | 0.29a | 0.12 |
| Step 2—Reward responsiveness | ΔR2 = 0.14, F(2, 21) = 2.31 | ΔR2 = 0.13, F(2, 21) = 2.51b | ||
|---|---|---|---|---|
| RewP to rewards | 0.31 | 0.21 | −0.29a | 0.14 |
| RewP to breaking even | 0.37 | 0.24 | −0.11 | 0.16 |
| Total model | R2 = 0.36, F(5, 21) = 2.38 | R2 = 0.44, F(5, 21) = 3.32a | ||
p < 0.05.
p = 0.11.
b, unstandardized regression coefficients; RewP, reward positivity.
FIG. 3.
Scatterplot depicting RewP to reward feedback (adjusting for responses to breaking even) predicting change in depressive symptoms with treatment, and ERPs and scalp distributions depicting RewP for youth who showed high (top) versus low (bottom) change in depressive symptoms from pre-treatment to post-treatment. Note: A median split of change in depressive symptoms was computed for illustrative purposes only. ERPs, event-related potentials; RewP, reward positivity.
Given the relatively small sample, we were underpowered to test whether type of treatment moderated effects. For an exploratory and preliminary examination of whether RewP differentially predicts response specifically to CBT or SSRI in anxious youth, we repeated hierarchical regression models in each treatment group and compared effect sizes for RewP predicting change in depressive symptoms for each group. The effect of RewP predicting change in depressive symptoms approached significance in the CBT group, partial r = −0.54, t(10) = −2.02, p = 0.07, but not in the SSRI group, partial r = −0.02, t(5) = −0.05, p = 0.96.
Discussion
The goal of this preliminary study was to examine a neurophysiological indicator of reward responsiveness, RewP, as a predictor of treatment response in youth with clinical anxiety. Consistent with prior work in adults (Burkhouse et al. 2016, 2018), reduced reward responsiveness predicted more change in depressive symptoms following treatment (i.e., better treatment response). Importantly, RewP emerged as a relatively specific predictor of change in depressive symptoms, rather than anxiety severity. Moreover, effects were more apparent among youth treated with CBT rather than SSRIs, although these analyses must be interpreted cautiously given the small sample in each treatment group.
Alterations in reward responsiveness have been identified as a core process underlying the development of psychopathology, particularly mood disorders (Zisner and Beauchaine 2016; Kujawa and Burkhouse 2017; Nusslock and Alloy 2017). Although more commonly studied in depression as opposed to anxiety, high rates of comorbidity between mood and anxiety disorders warrant examination of reward responsiveness across emotional or internalizing disorders. Interestingly, in community samples, reduced reward responsiveness as measured by RewP has consistently been shown to predict increases in depressive symptoms across development—suggesting a poorer prognostic course (Bress et al. 2015a; Nelson et al. 2016; Kujawa et al. 2019). Yet, in three independent clinical samples, we have now demonstrated that a blunted RewP predicts more change in depressive symptoms following treatment (Burkhouse et al. 2016, 2018). That is, although reduced reward responsiveness appears to be a vulnerability that increases risk for the development of depression in combination with other risk factors and stressful experiences (Kujawa and Burkhouse 2017), it also appears to predict greater potential for improvement following treatment.
Although further research is needed to elucidate the mechanisms driving these effects, one possibility is that established treatments are better able to meet the needs of individuals with reduced reward responsiveness. For example, elements of CBT for anxiety, including establishing a positive therapeutic alliance and exposures, which could reduce avoidance and lead to engagement in more enjoyable activities and relationships, may be most effective for people with alterations in reward responsiveness. Consistent with this possibility, there is some evidence that behavioral activation for depression can alter activation of the striatum to rewards (Dichter et al. 2009) and patients who show increases in RewP following treatment for depression or anxiety show larger reductions in symptoms (Barch et al. 2018; Burkhouse et al. 2018). That is, treatment can alter reward responsiveness, at least for some individuals, and may be particularly effective for those with tendencies toward reduced reward responsiveness. On the other hand, manualized CBT or SSRI treatment may not directly meet the needs of individual with depressive symptoms, who have intact or enhanced reward responsiveness. As such, further research is needed to evaluate prescriptive indicators that can be applied to differentially predict response to distinct forms of psychotherapy (e.g., interpersonal therapy vs. CBT) or pharmacotherapy.
Although we were underpowered to examine differences between types of treatment in this study, our results provide preliminary evidence that among anxious youth, RewP may predict response to CBT, specifically. This is consistent with our initial work in this area, indicating that reduced RewP predicted change in depressive symptoms following CBT among a sample of adults with comorbid anxiety and depression (Burkhouse et al. 2016). Yet, in a second sample using a doors guessing task to elicit RewP, reduced RewP was a predictor of response to treatment with SSRIs specifically (Burkhouse et al. 2018). Differences in task design between these two studies may be driving these distinct patterns of effects, in that the CBT study used a task similar to this study measuring RewP after a long delay period and the SSRI study used a guessing task in which reward and loss feedback were presented immediately after selecting one of two doors.
In considering this work, it is also likely that stage of development matters. That is, reward-related brain networks develop across adolescence and into young adulthood (Casey et al. 2008; Galvan 2010), and as such, may be more amenable to change from environmental experiences and practice obtained through CBT in childhood and adolescence. On the other hand, adults with reduced reward responsiveness may have more difficulty engaging in CBT—at least in some contexts and depending on comorbidity and chronicity—and might benefit from pharmacotherapy. Nonetheless, differences between treatments in this study must be interpreted cautiously given the small sample size, marginally significant effect of RewP on depressive symptom change in the CBT group, and age difference between the treatment groups, and further work is needed to evaluate these possibilities. It should also be noted that a relatively large proportion of participants initially assigned to SSRI opted to switch to CBT, and we cannot rule out the possibility that preference for CBT might contribute to effects of reward responsiveness on treatment response in this group.
This study is among the first to examine neurophysiological measures of reward responsiveness as predictors of treatment response in children and adolescents with internalizing disorders, and results replicate prior work in adults, but extend these findings earlier in development. Nonetheless, there are a number of limitations to this study. First, the small sample limits our ability to evaluate potential study confounds and/or moderators of treatment response (e.g., type of treatment, diagnosis, age, and comorbidity). Second, although significant results were specific to change in depressive symptoms, the sample was selected and treated for clinical levels of anxiety, and few participants had experienced an episode of clinical depression at the time of the initial assessment. It should be noted that childhood anxiety and subthreshold depressive symptoms are both predictors of the later emergence of depressive symptoms (Keenan et al. 2008; Silk et al. 2012), rates of which should continue to increase into later adolescence and young adulthood. Thus, change in subthreshold depressive symptoms following CBT for anxiety might be clinically meaningful and may reduce risk for future depression (Silk et al. 2019). At the same time, we do not know whether these results extend to youth with clinical depression and engaged in treatment aimed at directly targeting symptoms of depression or anhedonia specifically, such as behavioral activation. Finally, we were unable to examine changes in RewP following treatment and cannot conclude whether change in reward responsiveness is a mechanism of the effects of treatment on depressive symptoms. Although there is evidence in adults that change in reward responsiveness, assessed by ERP and fMRI, might be a mechanism of the effects of CBT and/or SSRI treatment (Dichter et al. 2009; Burkhouse et al. 2018), future work is needed to evaluate this possibility in children and adolescents.
Conclusions
Despite these limitations, this study offers preliminary data supporting the utility of neurophysiological measures of reward responsiveness in predicting response to treatment for internalizing disorders in youth. Results extend previous findings in adults suggesting that a reduced RewP to rewards predicts greater change in depressive symptoms following treatment. With further replication and validation of these approaches in larger samples, as well as improvements in technology, EEG/ERP could potentially be integrated into clinical settings to aid in selecting among treatment options and identifying objective targets for treatment and markers of treatment response in youth with internalizing disorders.
Clinical Significance
Anxiety disorders are relatively common psychiatric disorders in children and adolescents, but many patients fail to respond to the most efficacious treatments. Identifying prescriptive indicators of treatment response is essential for improving outcomes. Results of this study indicate that ERP measures of reduced reward responsiveness predict response to treatment in anxious youth, particularly change in depressive symptoms following CBT. With further research and standardization of measures, ERP measures of reward responsiveness could potentially be integrated into clinical settings to inform prognosis and treatment planning.
Supplementary Material
Acknowledgments
We would like to thank David Simpson, PhD, LCSW, and Sucheta Connolly, MD, for providing treatment to participants. This work was supported by National Institute of Mental Health Grant [R01-MH086517] to C.S.M. and K.L.P. K.L.B. is supported by National Institute of Mental Health Grant [K23-MH113793-01].
Disclosures
The authors have no conflict of interests or financial relationships with pharmaceutical companies to disclose.
Supplementary Material
References
- Ball TM, Stein MB, Paulus MP: Toward the application of functional neuroimaging to individualized treatment for anxiety and depression. Depress Anxiety 31:920–933, 2014 [DOI] [PubMed] [Google Scholar]
- Barch DM, Whalen D, Gilbert K, Kelly D, Kappenman ES, Hajcak G, Luby JL: Neural indicators of anhedonia: Predictors and mechanisms of treatment change in a randomized clinical trial in early childhood depression. Biol Psychiatry 85:863–871, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Beesdo K, Pine DS, Lieb R, Wittchen H-U: Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry 67:47–57, 2010 [DOI] [PubMed] [Google Scholar]
- Belden AC, Irvin K, Hajcak G, Kappenman ES, Kelly D, Karlow S, Luby JL, Barch DM: Neural correlates of reward processing in depressed and healthy preschool-age children. J Am Acad Child Adolesc Psychiatry 55:1081–1089, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SL, Weems CF, Silverman WK, Kurtines WM: Predictors of outcome in exposure-based cognitive and behavioral treatments for phobic and anxiety disorders in children. Behav Ther 31:713–731, 2000 [Google Scholar]
- Brent DA, Kolko DJ, Birmaher B, Baugher M, Bridge J, Roth C, Holder D: Predictors of treatment efficacy in a clinical trial of three psychosocial treatments for adolescent depression. J Am Acad Child Adolesc Psychiatry 37:906–914, 1998 [DOI] [PubMed] [Google Scholar]
- Bress JN, Meyer A, Hajcak G: Differentiating anxiety and depression in children and adolescents: Evidence from event-related brain potentials. J Clin Child Adolesc Psychol 44:238–249, 2015b [DOI] [PubMed] [Google Scholar]
- Bress JN, Meyer A, Proudfit GH: The stability of the feedback negativity and its relationship with depression during childhood and adolescence. Dev Psychopathol 17:1285–1294, 2015a [DOI] [PubMed] [Google Scholar]
- Bunford N, Kujawa A, Fitzgerald KD, Swain JE, Hanna GL, Koschmann E, Simpson D, Connolly S, Monk CS, Phan KL: Neural reactivity to angry faces predicts treatment response in pediatric anxiety. J Abnorm Child Psychol 45:385–395, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Gorka SM, Afshar K, Phan KL: Neural reactivity to reward and internalizing symptom dimensions. J Affect Disord 217:73–79, 2017a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Gorka SM, Klumpp H, Kennedy AE, Karich S, Francis J, Ajilore O, Craske MG, Langenecker SA, Shankman SA: Neural responsiveness to reward as an index of depressive symptom change following cognitive-behavioral therapy and SSRI treatment. J Clin Psychiatry 79:e1–e8, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Kujawa A, Kennedy AE, Shankman SA, Langenecker SA, Phan KL, Klumpp H: Neural reactivity to reward as a predictor of cognitive behavioral therapy response in anxiety and depression. Depress Anxiety 33:281–288, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Kujawa A, Klumpp H, Fitzgerald KD, Monk CS, Phan KL: Neural correlates of explicit and implicit emotion processing in relation to treatment response in pediatric anxiety. J Child Psychol Psychiatry 58:546–554, 2017b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G: Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. Neuroimage 57:1608–1616, 2011 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA: The adolescent brain. Ann N Y Acad Sci 1124:111–126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton SN, Walkup JT, Albano AM, Piacentini JC, Birmaher B, Sherrill JT, Ginsburg GS, Rynn MA, McCracken JT, Waslick BD, Iyengar S, Kendall PC, March JS: Child/Adolescent Anxiety Multimodal Study (CAMS): Rationale, design, and methods. Child Adolesc Psychiatry Ment Health 4:1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, Davidson JRT, Chung H, Yang R, Clary CM: Multidimensional effects of sertraline in social anxiety disorder. Depress Anxiety 23:6–10, 2006 [DOI] [PubMed] [Google Scholar]
- Crawford AM, Manassis K: Familial predictors of treatment outcome in childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry 40:1182–1189, 2001 [DOI] [PubMed] [Google Scholar]
- Cummings CM, Caporino NE, Kendall PC: Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychol Bull 140:816–845, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ: The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry 66:886–897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidjian S, Barrera M, Martell C, Muñoz RF, Lewinsohn PM, Barrera Jr M, Martell C, Muñoz RF, Lewinsohn PM: The origins and current status of behavioral activation treatments for depression. Annu Rev Clin Psychol 7:1–38, 2011 [DOI] [PubMed] [Google Scholar]
- Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F, Keshavan A, Triantafyllou C, Saygin ZM, Whitfield-Gabrieli S, Hofman SG, Pollack M, Gabrieli JD: Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry 70:87–97, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, Dahl RE: Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect Behav Neurosci 10:107–118, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarrillo SR, Dahl RE: Healthy adolescents' neural response to reward: Associations with puberty, positive affect, and depressive symptoms. J Am Acad Child Adolesc Psychiatry 49:162–172, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G: Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biol Psychol 81:1–8, 2009 [DOI] [PubMed] [Google Scholar]
- Galvan A: Adolescent development of the reward system. Front Hum Neurosci 4:1–9, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg GS, Keeton CP, Drazdowski TK, Riddle MA: The utility of clinicians ratings of anxiety using the Pediatric Anxiety Rating Scale (PARS). Child Youth Care Forum 40:93–105, 2011 [Google Scholar]
- Gratton G, Coles MGMG, Donchin E: A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 55:468–484, 1983 [DOI] [PubMed] [Google Scholar]
- Keenan K, Hipwell A, Feng X, Babinski D, Hinze A, Rischall M, Henneberger A: Subthreshold symptoms of depression in preadolescent girls are stable and predictive of depressive disorders. J Am Acad Child Adolesc Psychiatry 47:1433–1442, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall PC, Choudhury S, Hudson J, Webb A: The C.A.T. Project Workbook. Ardmore, PA, Workbook Publishing, 2002 [Google Scholar]
- Kendall PC, Hedtke K: Coping Cat Workbook. Ardmore, PA, Workbook Publishing, 2006 [Google Scholar]
- Kessel EM, Kujawa A, Hajcak Proudfit G, Klein DN: Neural reactivity to monetary rewards and losses differentiates social from generalized anxiety in children. J Child Psychol Psychiatry 56:792–800, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M: Children's Depression Inventory. Toronto, ON, Multi-Health Systems, Inc., 1992 [Google Scholar]
- Kujawa A, Burkhouse KL: Vulnerability to depression in youth: Advances from affective neuroscience. Biol Psychiatry Cogn Neurosci Neuroimaging 2:28–37, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Carroll A, Mumper E, Mukherjee D, Kessel EMM, Olino T, Hajcak G, Klein DN: A longitudinal examination of event-related potentials sensitive to monetary reward and loss feedback from late childhood to middle adolescence. Int J Psychophysiol 132:323–330, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Klein DN: Reduced reward responsiveness moderates the effect of maternal depression on depressive symptoms in offspring: Evidence across levels of analysis. J Child Psychol Psychiatry 60:82–90, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, Phan KL: Enhanced neural reactivity to threatening faces in anxious youth: Evidence from event-related potentials. J Abnorm Child Psychol 43:1493–1501, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Swain JE, Hanna GL, Koschmann E, Simpson D, Connolly S, Fitzgerald KD, Monk CS, Phan KL: Prefrontal reactivity to social signals of threat as a predictor of treatment response in anxious youth. Neuropsychopharmacology 41:1983–1990, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne A, Bernstein GA, Egan EA, Kushner MG: Predictors of treatment response in anxious-depressed adolescents with school refusal. J Am Acad Child Adolesc Psychiatry 42:319–326, 2003 [DOI] [PubMed] [Google Scholar]
- Liu W, Wang L, Shang H, Shen Y, Li Z, Cheung EFC, Chan RCK: The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia 53:213–220, 2014 [DOI] [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J: Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA 292:807–820, 2004 [DOI] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G: Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. Am J Psychiatry 173:1223–1230, 2016 [DOI] [PubMed] [Google Scholar]
- Nusslock R, Alloy LB: Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. J Affect Disord 216:3–16, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji BO, Cisler JM, Deacon BJ: Efficacy of cognitive behavioral therapy for anxiety disorders: A review of meta-analytic findings. Psychiatr Clin 33:557–577, 2010 [DOI] [PubMed] [Google Scholar]
- Roelofs J, Braet C, Rood L, Timbremont B, van Vlierberghe L, Goossens L, van Breukelen G: Norms and screening utility of the dutch version of the children's depression inventory in clinical and nonclinical youths. Psychol Assess 22:866–877, 2010 [DOI] [PubMed] [Google Scholar]
- Silk JS, Davis S, McMakin DL, Dahl RE, Forbes EE: Why do anxious children become depressed teenagers? The role of social evaluative threat and reward processing. Psychol Med 42:2095–2107, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Price RB, Rosen D, Ryan ND, Forbes EE, Siegle GJ, Dahl RE, McMakin DL, Kendall PC, Ladouceur CD: A longitudinal follow-up study examining adolescent depressive symptoms as a function of prior anxiety treatment. J Am Acad Child Adolesc Psychiatry 58:359–367, 2019 [DOI] [PubMed] [Google Scholar]
- The Research Units on Pediatric Psychopharmacology Anxiety Study Group: The Pediatric Anxiety Rating Scale (PARS): Development and psychometric properties. J Am Acad Child Adolesc Psychiatry 41:1061–1069, 2002 [DOI] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, Iyengar S, March JS, Kendall PC: Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 359:2753–2766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zisner A, Beauchaine TP: Neural substrates of trait impulsivity, anhedonia, and irritability: Mechanisms of heterotypic comorbidity between externalizing disorders and unipolar depression. Dev Psychopathol 28:1177–1208, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.