Abstract
Clostridium botulinum produces botulinum neurotoxin (BoNT), which is the causative agent of botulism, a rare but serious disease that can result in death if not treated. Infant botulism occurs when C. botulinum colonizes the intestinal tract of infants and produces BoNT. It has been proposed that infants under the age of 1 year are uniquely susceptible to colonization by C. botulinum as their intestinal microbiota is not fully developed and provides little competition, allowing C. botulinum to thrive and produce BoNT in the gut. There are seven well-characterized serotypes (A–G) of BoNT identified by the ability of specific antitoxins to neutralize BoNTs. Molecular technology has allowed researchers to narrow these further into subtypes based on nucleic acid sequences of the botulinum toxin (bont) gene. One of the most recently recognized subtypes for bont/B is subtype bont/B7. We identified through whole genome sequencing five C. botulinum isolates harboring bont/B7 from CDC's strain collection, including patient isolates and an epidemiologically linked isolate from an opened infant formula container. In this study, we report the results of whole genome sequencing analysis of these C. botulinum subtype bont/B7 isolates. Average nucleotide identity and high quality single nucleotide polymorphism (hqSNP) analysis resulted in two major clades. The epidemiologically linked isolates differed from each other by 2–6 hqSNPs, and this clade separated from the other isolates by 95–119 hqSNPs, corroborating available epidemiological evidence.
Keywords: botulism, proteolytic, C. botulinum, subtype B7, bont/B7, infant botulism
Introduction
Clostridium botulinum is a Gram-positive spore-forming obligate anaerobe that is ubiquitous in soil (Hatheway, 1990). C. botulinum produces botulinum neurotoxin (BoNT), a toxin that causes botulism, a rare but serious neuroparalytic disorder that can result in death if untreated. Four naturally occurring forms of botulism have been described: foodborne botulism, infant botulism, wound botulism, and adult intestinal colonization (Sobel, 2005). Foodborne botulism is caused by ingestion of foods contaminated with BoNT. Wound botulism occurs when an open wound is contaminated with C. botulinum spores, which germinate in the anaerobic environment of the wound and produce BoNT. Adult intestinal colonization is caused by colonization of the intestines by C. botulinum spores, which germinate and produce BoNT in the intestinal tract of persons older than 1 year. Infant botulism, also known as “floppy baby syndrome,” occurs when spores of BoNT-producing clostridia colonize the gut of infants under 1 year of age. Clinicians treat infants with BabyBIG®, containing human-derived anti-BoNT antibodies, and frequently the recovery is uneventful, if protracted. It has been proposed that these colonization cases originate with ingestion of spores from the environment, including dust or soil exposure within the home (Chin et al., 1979; Takahashi et al., 1988; Nevas et al., 2005; Derman et al., 2014).
To date, seven BoNT serotypes (A–G) have been defined, according to the ability to neutralize the toxin in vivo with polyclonal antibodies. Additional serotypes have been proposed in the literature, but consensus has not been achieved regarding them in the scientific community (Barash and Arnon, 2014; Maslanka et al., 2016; Doxey et al., 2018). In addition, several bivalent strains have been reported that produce two toxin types, such as A and B and B and F, as well as chimeric BoNT that exhibits properties from two different serotypes, such as C and D (Kalb et al., 2015; Maslanka et al., 2016). Nucleotide sequences of the botulinum toxin genes (bont) also show diversity within serotypes. These bont gene variants, denominated toxin subtypes, are identified as distinct subtypes if they encode a BoNT amino acid sequence that differs from the reference sequence by at least 2.6% (Peck et al., 2017). To date, eight subtypes have been identified within serotype B, denoted as bont/B1-B8 (Peck et al., 2017). The gene encoding components of the BoNT complexes can also differ in sequence and organization; bont/B genes are reported to be associated with ntnh, ha70, ha17, ha33, and botR genes (Rossetto et al., 2014).
Kalb et al. (Kalb et al., 2012) identified bont/B7 by mass spectrometric analysis of the toxin produced by strain Bac-04-07755 (accession no. JQ354985), isolated from a stool sample from an infant botulism case in New York. Strain NCTC 3807, isolated from soil from the Blue Ridge Mountains of Virginia, was also identified as harboring the bont/B7 gene (accession no. JN120760) (Kalb et al., 2012). To date, these are the only two strains reported to produce BoNT/B7. Through whole genome sequencing, we identified five other C. botulinum isolates in CDC's strain collection that contained the bont/B7 gene. Among those isolates harboring bont/B7 gene, we identified an isolate from an opened container of ready-to-eat infant formula and two isolates from stool samples from an infant botulism patient, who had consumed the formula. Clinical and food isolates had nearly indistinguishable pulsotype (CDC, unpublished data) indicating a close relationship between them. To date, this is one of few instances of recovery of C. botulinum organism from infant formula (McHugh et al., 2017) and the only instance of recovery from liquid ready-to-eat formula. In this study, we analyzed whole genome sequencing data to further characterize these C. botulinum isolates harboring bont/B7.
Materials and Methods
Strains
Strains used in this study are described in Table 1. Strains were grown at 35°C in Chopped Meat Glucose Starch (CMGS) broth overnight anaerobically. Growth from the CMGS broth was streaked to Egg Yolk Agar for isolation to confirm purity. Single colonies were inoculated into Trypticase Peptone Glucose Yeast broth and incubated overnight anaerobically at 35°C. The presence of C. botulinum genes and toxin in the isolate from New York (BAC04- 07755) were detected by multiplex polymerase chain reaction and mass spectrometry as described previously (Davis et al., 2016; Perry et al., 2017).
Table 1.
Clostridium Botulinum Subtype B7 Strains Used in This Study
| Strain ID | Location | Source | Year |
|---|---|---|---|
| CDC37496 | District of Columbia | Stool | 1982 |
| CDC37498 | District of Columbia | Infant formula (opened container) | 1982 |
| CDC37513 | District of Columbia | Stool | 1982 |
| BAC-04-07755 | New York | Stool | 2004 |
| CDC68158 | Virginia | Stool | 2013 |
| CDC69068 | New Jersey | Stool | 2014 |
All strains were associated with infant botulism cases.
Genomic DNA extraction
Genomic DNA was extracted from 8 to 9 mL of 16–24 h anaerobic growth using Epicentre (Illumina; Madison, WI) MasterPure Complete DNA and RNA Purification Kit, with a modified method. Briefly, cells were pelleted at 4°C for 10 min at 4000 rpm, supernatant discarded and pellets resuspended in Lysozyme Stock Solution (25 mM Tris-HCl, pH 8.0, 2.5 mM 0.5 M EDTA, 10 mL Triton X-100, 20 mg/mL Lysozyme from chicken egg white), and incubated for 15 min in a preheated 37°C water bath. A measure of 300 μL of undiluted 2X T& C buffer and 3 μL of RNase A (Qiagen; Redwood City, CA) were added to the cell suspension and incubated for 10 min in a preheated 57°C water bath, followed by addition of 3 μL of Proteinase K (Invitrogen/Thermo Fisher; Waltham, MA) and another 10 min incubation in the 57°C water bath. To extract DNA, 350 μL of MPC Protein Precipitation Buffer was added to the cell solution and then centrifuged at 4°C for 10 min at 4000 rpm. Supernatants were collected and each added to 1 mL of 99% isopropanol. Solutions were gently mixed and DNA precipitates were collected, washed with 1 mL of 70% ethanol, and rehydrated overnight in 10 mM Tris. The DNA extracts were then filtered through 0.1 μM centrifugal filters (MilliporeSigma; Burlington, MA).
Whole genome shotgun sequencing
Libraries were constructed with the Kapa Biosciences 200 Base Pair Kit (Roche; Wilmington, MA), pooled and diluted to obtain an equimolar total concentration of 100 pM, and then were templated and enriched using the Ion Torrent Chef (Thermo Fisher; Waltham, MA) automated system and Ion Hi-Q (Thermo Fisher; Waltham, MA) chemistry. Sequencing was completed for CDC68158, CDC69068, CDC37496, CDC37498, and CDC37513 using the Ion Torrent Personal Genome machine (Thermo Fisher; Waltham, MA) and resulted in an average coverage of 49.5. × (24 × –94 × ). The New York Department of Health isolated BAC-04-07755, performed DNA extractions, created Illumina sequencing libraries using the Nextera XT Kit (Illumina; San Diego, CA), and sequenced the isolated on the MiSeq (Illumina) instrument using 2 × 250 chemistry.
Bioinformatics
Read quality was assessed using FastQCv.11.3 (Leggett et al., 2013), and reads were assembled using SPAdes v.3.1.0 (Bankevich et al., 2012). Assemblies were assessed using Quast v. 4.3 (Gurevich et al., 2013). The bont gene subtype was determined using the “map reads to reference” feature in CLC Genomics Workbench v.9 (Qiagen; Redwood City, CA). The read coverage across the reference may be seen in the Supplementary Figure S1. We determined the nearest available reference sequence using Average Nucleotide Identity (ANI) (Goris et al., 2007). We used Mashtree to create a neighbor-joining tree with the sequences and type B reference strains. Then, we used Lyve-SET (Katz et al., 2017) version 1.1.4f (settings were for single ended reads, with minimum coverage of 10, the minimum alt fraction was set to 0.75 and the allowed flanking set to 5, phages and cliffs in the pileup were masked); CG-pipeline (Kislyuk et al., 2010) was used to clean the reads, and smalt was used to map them to identify high quality single nucleotide polymorphisms (hqSNPs) common to all 6 sequences and to approximate a phylogeny with bootstrap support. The sequence type (ST) of the 6 C. botulinum isolates harboring bont/B7 was determined by querying the Center for Genomic Epidemiology's (Denmark Technical University; Lyngby, Denmark) multilocus sequence typing (MLST) tool.
Results
Resulting reads and assembly and mapping statistics can be found in Table 2.
Table 2.
Sequencing Statistics for Clostridium Botulinum Subtype B7 Strains Used in This Study
| Strain ID | Total length | GC% | No. of reads | Average read length (bp) | De novo coverage | No. of reads mapped to chromosome Okra B1 | No. of reads mapped to plasmid Okra B1 | No. of unmapped reads |
|---|---|---|---|---|---|---|---|---|
| CDC37496 | 4,024,675 | 28.1 | 1,933,230 | 194 | 93.8 | 1,830,273 | 83,943 | 19,014 |
| CDC37498 | 3,905,902 | 28.1 | 1,375,102 | 208 | 71.5 | 1,370,930 | 1655 | 2517 |
| CDC37513 | 3,998,101 | 28.0 | 639,392 | 201 | 32.1 | 610,995 | 23,553 | 4844 |
| BAC-04-07755 | 4,015,174 | 28.1 | R1 1,474,900 | 250 | 184.4 | 2,843,494 | 81,031 | 25,275 |
| R2 1,474,900 | ||||||||
| CDC68158 | 4,050,546 | 28.2 | 505,860 | 188 | 23.8 | 479,275 | 22,901 | 3684 |
| CDC69068 | 4,019,721 | 28.1 | 491,333 | 217 | 26.7 | 37,995 | 651 | 452,687 |
Seven-gene MLST is a profiling methodology that can be used to characterize and group strains of C. botulinum into ST profiles (Jacobson et al., 2008a). All six isolates harboring bont/B7 were identified as ST-34.
We also analyzed the genes encoding components of the BoNT complexes: ntnh, ha70, ha17, ha33, and botR genes. Sequences from two isolates had sufficient coverage to reconstruct the sequences from these accessory genes. We used the CLC genomics map reads to reference feature to extract the consensus sequence for the genes from the short reads and the NCBI BLAST (NCBI; Bethesda, MD) feature to find other similar sequences. All three ha genes, botR gene, and ntnh gene were 100% similar to the corresponding genes in reference strain Okra, subtype bont/B1. The bont gene clusters were found on plasmids similar to pCLD rather than the chromosome.
We used pairwise ANI to determine homology between the draft assemblies, as well as to C. botulinum references CDC67086, ATCC3502, and Okra. ANI values among sequences of C. botulinum isolates containing bont/B7 gene were >99% (99.24–99.90%). ANI values between genome assemblies containing bont/B7 gene and the serotype B reference sequence Okra, which harbors a bont/B1 gene, were >99% (99.23–99.98%), while ANI values for CDC67086 and ATCC3502 were <98%.
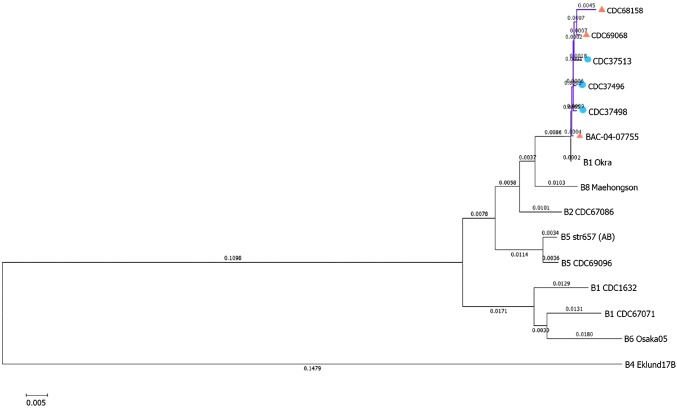
A neighbor-joining tree generated with MASH distances (Ondov et al., 2016), which included C. botulinum type B reference sequences revealed a single clade containing the six C. botulinum subtype B7 sequences and the reference strain Okra (subtype B1) (Fig. 1). This tree also shows the close relationship between the two C. botulinum subtype B7 isolated from stool specimens (CDC37496 and CDC37513) and the associated isolate CDC37498 obtained from infant formula.
FIG. 1.
Neighbor-joining tree drawn from Mash distances containing well-characterized Clostridium botulinum reference strains and the six subtype B7 isolates used in this study. Circles represent the formula isolate and clinical isolates. Triangles represent the type strain and unrelated infant cases that produced an isolate harboring bont/B7.
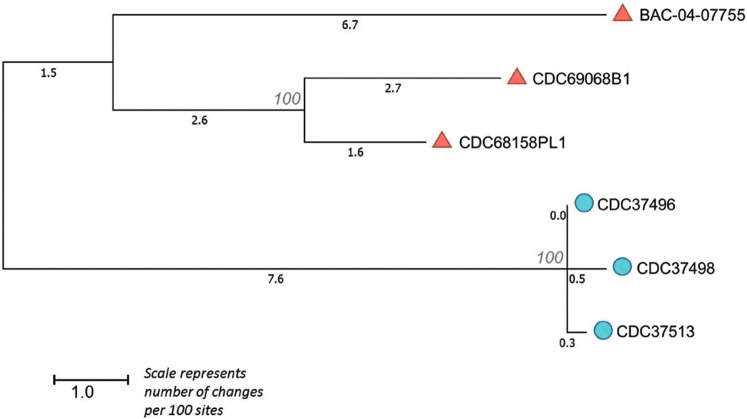
To further characterize these isolates, we used Lyve-SET to identify hqSNPs. Lyve-SET identified 180 hqSNPs that were used to approximate a phylogenetic tree with bootstrap support (Fig. 2). The resulting tree contained two clades, one of which contained CDC37496, CDC37498, and CDC37513. There were 2–6 hqSNP differences between the three isolates. By contrast, there were between 95 and 119 hqSNP differences between the two clades and 32–87 hqSNP differences within the second clade.
FIG. 2.
High quality SNP tree generated by Lyve-SET with bootstrap support. Circles represent the formula isolate and clinical isolates. Triangles represent the type strain and unrelated infant cases that produced an isolate harboring bont/B7. SNP, single nucleotide polymorphism.
Draft assemblies and reads were uploaded to GenBank and the sequence read archive under the following accessions: SRR6451451, SRR6451452, SRR6451450, SRR6462918, SRR6462919, SRR8444826, POTI00000000, POTJ00000000, POTK00000000, POTG00000000, POTH00000000, SCKF00000000.
Discussion
Since Kalb et al. reported subtype bont/B7 in 2012 (Kalb et al., 2012), there have been no new descriptions in the literature of strains harboring this toxin gene subtype. We undertook this study to assess the diversity of C. botulinum strains harboring the bont/B7 gene. All of the isolates described herein were obtained from stool or food specimens associated with infant botulism cases. Of note, these botulism cases occurred in the east coast of the United States, where serotype A is more prevalent (Centers for Disease Control and Prevention, 1998).
Traditional 7-gene MLST (Jacobson et al., 2008b) results indicated that all six C. botulinum subtype B7 isolates were members of ST-34. The PubMLST database has only three C. botulinum type B strains in this group: strain 407 isolated in Japan in 1984, the reference strain Okra from the United States, and strain NCTC7273 from the United Kingdom isolated in 1947. Interestingly, these three strains harbor bont/B1 gene rather than bont/B7. Both of these toxin subtypes are carried on plasmids; thus, it is not surprising that these strains share the same ST. Strains Okra and 407 have a nearly identical pulsotype as well and have been shown to share a multilocus variable number tandem repeat profile (Umeda et al., 2013).
We were able to reconstruct the three ha genes, botR gene, and ntnh gene from the short read sequence data for CDC37496 and CDC37513. These were all most closely related to bont/B1 accessory genes from CDC1632 (CP013243.1) with 99% identity and did not display any unusual characteristics.
The neighbor-joining tree generated from MASH distances (Ondov et al., 2016) confirmed the relationships seen with other methods (Fig. 1). All six isolates described herein that harbor the bont/B7 gene formed a single clade with the reference strain Okra, which harbors bont/B1 gene. However, the underlying genetic backbone of these strains seems to be somewhat diverse, with a wide range of hqSNP differences between the two major clades (Fig. 2).
Isolates CDC37496 and CDC37513 were nearly indistinguishable by hqSNP analysis from the epidemiologically linked isolate CDC37498, recovered from an open container of liquid infant formula. C. botulinum has been isolated from containers of opened powder infant formula from the home of patients with infant botulism; however, dairy powders have not been satisfactorily implicated as the cause of a case of infant botulism (McHugh et al., 2017). Although the three isolates showed high similarity by hqSNP analysis, indicating a single strain or common ancestor, because the container had been opened, it is not possible to determine whether the formula was contaminated during manufacture or during use in the home. Moreover, the infant could have acquired the spores from the environment independently from ingesting the formula.
Conclusions
Whole genome sequence analysis provides a fast and thorough mechanism for exploring characteristics of newly identified subtypes. The three epidemiologically linked clinical and infant formula isolates (CDC37496, CDC37498, and CDC37513) were distinguishable from the isolates with no epidemiological link, indicating that hqSNPs revealed by the Lyve-SET algorithm can be a useful tool for analyzing closely related C. botulinum strains.
Supplementary Material
Acknowledgments
Centers for Disease Control and Prevention, Office of Public Health Preparedness and Response kindly provided support for this project. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012;19:455–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash JR, Arnon SS. A novel strain of Clostridium botulinum that produces type B and type H botulinum toxins. J Infect Dis 2014;209:183–191 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Botulism in the United States (1899–1996). Handbook for Epidemiologists, Clinicians, and LAboratory Workers. U.S. Department of Health and Human Services, CDC, (ed.). Atlanta, GA: U.S. Department of Health and Human Services, CDC, 1998 [Google Scholar]
- Chin J, Arnon SS, Midura TF. Food and environmental aspects of infant botulism in California. Rev Infect Dis 1979;1:693–697 [DOI] [PubMed] [Google Scholar]
- Davis SW, Kelly-cirino CD, Cirino NM, Hannett GE, Musser KA, Egan C. A 10 year analysis of the use of multiplex real-time PCR screening for botulinum neurotoxin-producing clostridium species. J Bacteriol Mycol 2016;3:1030–1038 [Google Scholar]
- Derman Y, Korkeala H, Salo E, Lonnqvist T, Saxen H, Lindstrom M. Infant botulism with prolonged faecal excretion of botulinum neurotoxin and Clostridium botulinum for 7 months. Epidemiol Infect 2014;142:335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxey AC, Mansfield MJ, Montecucco C. Discovery of novel bacterial toxins by genomics and computational biology. Toxicon 2018;147:2–12 [DOI] [PubMed] [Google Scholar]
- Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 2007;57:81–91 [DOI] [PubMed] [Google Scholar]
- Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013;29:1072–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev 1990;3:66–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MJ, Lin G, Whittam TS, Johnson EA. Phylogenetic analysis of Clostridium botulinum type A by multi-locus sequence typing. Microbiology 2008a;154:2408–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MJ, Lin G, Whittam TS, Johnson EA. Phylogenetic Analysis of Clostridium botulinum type A by multi-locus sequence typing. Microbiology (Reading, England) 2008b;154:2408–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb SR, Baudys J, Raphael BH, Dykes JK, Luquez C, Maslanka SE, Barr JR. Functional characterization of botulinum neurotoxin serotype H as a hybrid of known serotypes F and A (BoNT F/A). Anal Chem 2015;87:3911–3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb SR, Baudys J, Rees JC, Smith TJ, Smith LA, Helma CH, Hill K, Kull S, Kirchner S, Dorner MB, Dorner BG, Pirkle JL, Barr JR. De novo subtype and strain identification of botulinum neurotoxin type B through toxin proteomics. Anal Bioanal Chem 2012;403:215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LS, Griswold T, Williams-Newkirk AJ, Wagner D, Petkau A, Sieffert C, Van Domselaar G, Deng X, Carleton HA. A comparative analysis of the Lyve-SET phylogenomics pipeline for genomic epidemiology of foodborne pathogens. Front Microbiol 2017;8:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislyuk AO, Katz LS, Agrawal S, Hagen MS, Conley AB, Jayaraman P, Nelakuditi V, Humphrey JC, Sammons SA, Govil D, Mair RD, Tatti KM, Tondella ML, Harcourt BH, Mayer LW, Jordan IK. A computational genomics pipeline for prokaryotic sequencing projects. Bioinformatics 2010;26:1819–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett RM, Ramirez-Gonzalez RH, Clavijo BJ, Waite D, Davey RP. Sequencing quality assessment tools to enable data-driven informatics for high throughput genomics. Front Genet 2013;4:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslanka SE, Luquez C, Dykes JK, Tepp WH, Pier CL, Pellett S, Raphael BH, Kalb SR, Barr JR, Rao A, Johnson EA. A novel botulinum neurotoxin, previously reported as serotype H, has a hybrid-like structure with regions of similarity to the structures of serotypes A and F and is neutralized with serotype A Antitoxin. J Infect Dis 2016;213:379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh AJ, Feehily C, Hill C, Cotter PD. Detection and enumeration of spore-forming bacteria in powdered dairy products. Front Microbiol 2017;8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevas M, Lindstrom M, Virtanen A, Hielm S, Kuusi M, Arnon SS, Vuori E, Korkeala H. Infant botulism acquired from household dust presenting as sudden infant death syndrome. J Clin Microbiol 2005;43:511–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondov BD, Treangen TJ, Melsted P, Mallonee AB, Bergman NH, Koren S, Phillippy AM. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol 2016;17:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck MW, Smith TJ, Anniballi F, Austin JW, Bano L, Bradshaw M, Cuervo P, Cheng LW, Derman Y, Dorner BG, Fisher A, Hill KK, Kalb SR, Korkeala H, Lindstrom M, Lista F, Luquez C, Mazuet C, Pirazzini M, Popoff MR, Rossetto O, Rummel A, Sesardic D, Singh BR, Stringer SC. Historical perspectives and guidelines for botulinum neurotoxin subtype nomenclature. Toxins 2017;9:38–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MJ, Centurioni DA, Davis SW, Hannett GE, Musser KA, Egan CT. Implementing the Bruker MALDI biotyper in the public health laboratory for C. botulinum neurotoxin detection. Toxins 2017;9:94–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetto O, Pirazzini M, Montecucco C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat Rev Microbiol 2014;12:535–549 [DOI] [PubMed] [Google Scholar]
- Sobel J. Botulism. Clin Infect Dis 2005;41:1167–1173 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Shimizu T, Ooi K, Noda H, Nasu T, Sakaguchi G. Quantification of Clostridium botulinum type A toxin and organisms in the feces of a case of infant botulism and examination of other related specimens. Jpn J Med Sci Biol 1988;41:21–26 [DOI] [PubMed] [Google Scholar]
- Umeda K, Wada T, Kohda T, Kozaki S. Multi-locus variable number tandem repeat analysis for Clostridium botulinum type B isolates in Japan: Comparison with other isolates and genotyping methods. Infect Genet Evol 2013;16:298–304 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.