Abstract
Objective:
The objective of this study was to prospectively analyze the effectiveness of a treatment protocol in patients diagnosed with rhinocerebral mucormycosis.
Materials and Methods:
This series included ten patients who reported with suspicious clinical signs of mucormycosis. The diagnosis was established by histopathology, and computed tomography imaging was used to assess the extent of spread. All patients were treated with immediate radical surgical debridement and antifungal chemotherapy with amphotericin B. Simultaneous correction of the underlying immunosuppressive condition was carried out. The primary outcome assessed was disease-free survival, and the patients were followed up for up to 6 months after discharge.
Results:
In this series, the cause of immunosuppression was uncontrolled diabetes mellitus in all patients. All the patients responded to the treatment protocol and were free of the disease up to 6 months of follow-up.
Conclusion:
Mucormycosis is an invasive and potentially fatal disease. Prognosis can be improved by early recognition and prompt treatment with aggressive resection, intravenous amphotericin B, and control of the patient's underlying systemic condition.
Keywords: Amphotericin B, diabetic ketoacidosis, mucormycosis, opportunistic fungi
INTRODUCTION
Mucormycosis is an opportunistic, life-threatening disease, caused by fungi that belong to the order Mucorales and subphylum Mucormycotina.[1] Although this fungus is commonly present in soil and organic matter, inhalation of the spores causes disease only in immunocompromised hosts, and is commonly seen in patients with hematological malignancies or transplants, who are undergoing immunosuppressive therapy.[2] However, in India, the most common cause of mucormycosis appears to be uncontrolled diabetes mellitus.[3]
The most common variant of mucormycosis is rhinocerebral mucormycosis, which predominantly affects the nasomaxillary complex. This disease is extremely aggressive and can quickly spread from the sinus to the orbit and brain, resulting in vision and neural deficit.[4] Despite there being several case reports and retrospective series in the literature describing rhinocerebral mucormycosis, there is still insufficient evidence to guide therapy of this condition,[5] and the mortality rate remains high, ranging from 25% to almost 80% in recent reports.[6]
The aim of the current study was to prospectively analyze the effectiveness of a treatment protocol, in patients diagnosed with rhinocerebral mucormycosis.
MATERIALS AND METHODS
The current study was a prospective case series. We included patients who reported to us with the suspicious clinical presentation of mucormycosis, such as swelling of the midface and palatal ulceration. The underlying disease responsible for immunosuppression was assessed through case history and clinical examination. Blood glucose levels on presentation were obtained for all patients.
The diagnosis of mucormycosis was confirmed by histopathology of biopsy specimens obtained from the palate. Computed tomography (CT) imaging was also obtained to assess the extent of the disease in the bony maxilla and to assess orbital soft-tissue changes.
Once the diagnosis and disease extent were confirmed, the treatment was instituted immediately, within 48 h of presentation.
Treatment protocol
The treatment protocol that was followed in these patients had three arms. Written informed consent was obtained from all patients. First, immediately on diagnosis, the patient was taken up for radical surgery, which included maxillectomy and wound debridement; second, slow and sustained antifungal chemotherapy was instituted; and third, simultaneous correction of the underlying disease was done.
The surgical procedures that were carried out are summarized in Table 1. The maxilla was exposed using the Weber Ferguson approach and an intraoral vestibular incision. Maxillectomy was performed, preserving the orbital floor in nine patients. In one patient, due to orbital involvement, orbital exenteration was performed [Figure 1]. In all patients, we attempted to include at least 1 cm of uninvolved tissue to achieve proper clearance. Silicon putty or tincture benzoin gauze was placed in the wound, and the defect was closed with a custom-made acrylic obturator fixed in the hard palate with screws and wires.
Table 1.
Summary of clinical presentation and surgical treatment done
| Number | Age/gender | Side | RBS at presentation | Clinical presentation | Surgical procedure |
|---|---|---|---|---|---|
| 1 | 32/female | Right | 428 | Swelling, right-sided facial nerve palsy, and palatal ulceration | Maxillectomy with orbital floor preservation |
| 2 | 59/male | Left | 416 | Swelling of cheek, parotid and periorbital region, and proptosis | Maxillectomy with orbital exenteration |
| 3 | 43/male | Left | 534 | Swelling over the left cheek and palate | Subtotal maxillectomy |
| 4 | 32/male | Right | 440 | Swelling, ulceration of palate, and oro-antral communication | Maxillectomy with orbital floor preservation |
| 5 | 60/male | Right | 510 | Extensive ulceration of palate | Maxillectomy with orbital floor preservation |
| 6 | 52/female | Left | 534 | Bone sequestration and exposure in relation to the left maxillary alveolus | Partial maxillectomy |
| 7 | 45/male | Left | 600 | Swelling of the left cheek | Maxillectomy with orbital floor preservation |
| 8 | 48/female | Bilateral | 828 | Swelling and pain over the paranasal region | Bilateral maxillectomy with orbital floor preservation |
| 9 | 60/male | Left | 446 | Swelling of the cheek | Maxillectomy with orbital floor preservation |
| 10 | 63/male | Right | 663 | Swelling over the right check, palatal ulceration | Maxillectomy with orbital floor preservation |
RBS=Random blood sugar
Figure 1.
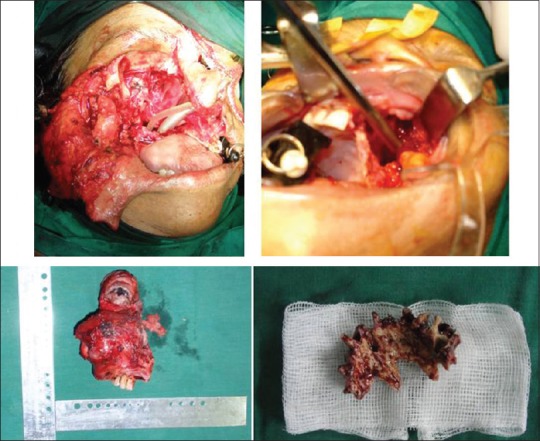
Extent of resection performed, with specimens
Immediately after surgery, antifungal chemotherapy was instituted. Fifty milligrams of amphotericin B was given as a slow intravenous infusion once daily over 12 h. This was continued for 4 weeks. Serum electrolytes, urea, and creatinine were evaluated at 24 h, 48 h, 1 week, and 4 weeks to assess renal function.
Diabetic ketoacidosis was corrected in consultation with the physician. Glucose levels were brought under control using insulin therapy and maintained by an adjusted dose of oral hypoglycemic medication and human insulin, which was continued after discharge.
Patient follow-up was done at 1 week, 3 weeks, 1, 3, and 6 months after discharge. The main outcome assessed was disease-free survival.
RESULTS
In this prospective series, ten patients were treated for mucormycosis, of which seven were male and three were female. The age of the patients ranged from 32 to 63 years (mean age, 49.4 years).
All the patients presented with a chief complaint of swelling of the face, with the duration of symptoms ranging from 2 to 4 months. One patient, a 32-year-old female, presented with a complaint of right-sided facial nerve palsy [Figure 2a]. On examination, all these patients had an extraoral, very tender swelling over the affected maxilla [Figure 2b]. Bleeding from the nose was present. Intraorally, there was ulceration of the hard palate, which ranged from minor bone exposure to extensive sequestration [Figure 3] and bony sequestrum was noticed. Proptosis was seen in one patient.
Figure 2.
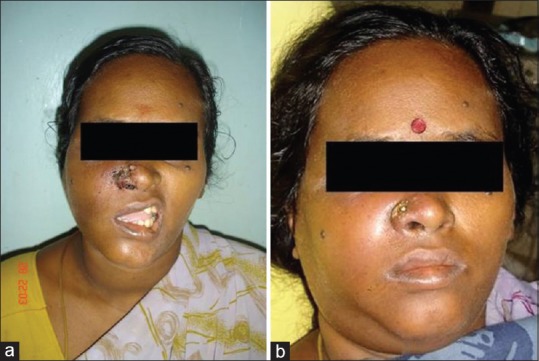
(a) Mucormycosis presenting with facial nerve palsy. (b) Mucormycosis presenting with facial swelling
Figure 3.
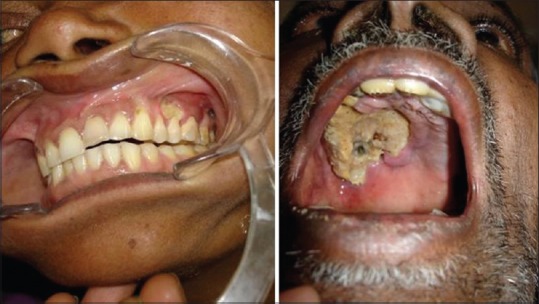
Extent of palatal ulceration
All the patients presented with uncontrolled diabetes mellitus. The blood glucose level varied from 416 to 828 mg/dl at the time of presentation, and ketoacidosis was uniformly present in all patients. One patient additionally had bronchial asthma, which had been treated with steroids for 1 year.
Histopathological examination revealed aseptate hyphae, and areas of necrosis and hemorrhage, which confirmed the diagnosis of mucormycosis in all ten patients. CT imaging revealed the erosion of the palatal wall and obliteration of the maxillary sinus [Figure 4a], which extended up to the right frontal sinus in two patients [Figure 4b]. Retrobulbar soft-tissue changes were visible in the patient who had presented with proptosis [Figure 4c].
Figure 4.
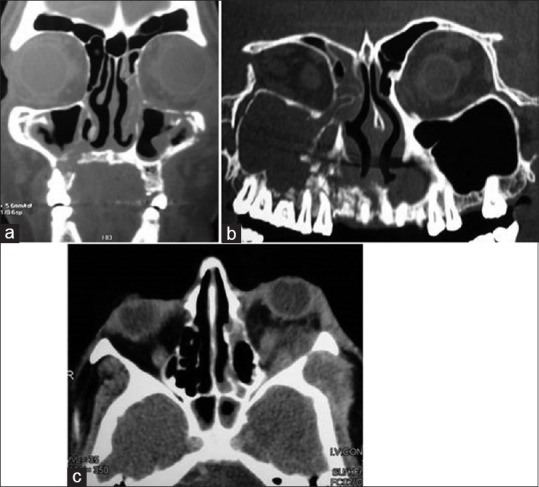
Computed tomography presentation of mucormycosis. (a) Erosion of maxillary sinus. (b) Extension into frontal sinus. (c) Retrobulbar soft-tissue involvement
The clinical features and management for all ten patients are summarized in Table 1.
The postoperative recovery was uneventful in all patients, except one who died after 2 weeks due to uncontrolled systemic sepsis. Wound healing was good and progressive, and the patients were discharged after 4 weeks’ inpatient hospital care. All patients having successful treatment of this potentially lethal condition were free of the disease after 6 months of follow-up.
DISCUSSION
Mucormycosis is a fast spreading, potentially lethal disease that requires prompt diagnosis and aggressive therapy for its effective control. The rapid progression that occurs is due to the unique pathogenesis. The fungal hyphae invade the walls of the blood vessels, causing endothelial damage. This leads to intravascular thrombus formation and vascular occlusion, which in turn results in ischemia and necrosis of host tissues [Figure 5]. The organism thrives in necrotic tissues and continues to invade adjacent areas through blood vessels. In rhinocerebral mucormycosis, thrombosis occurs in the sphenopalatine and internal maxillary arteries, which causes necrosis of the maxilla.[7] Through these vessels, it can quickly spread to the orbit and brain and lead to death.
Figure 5.
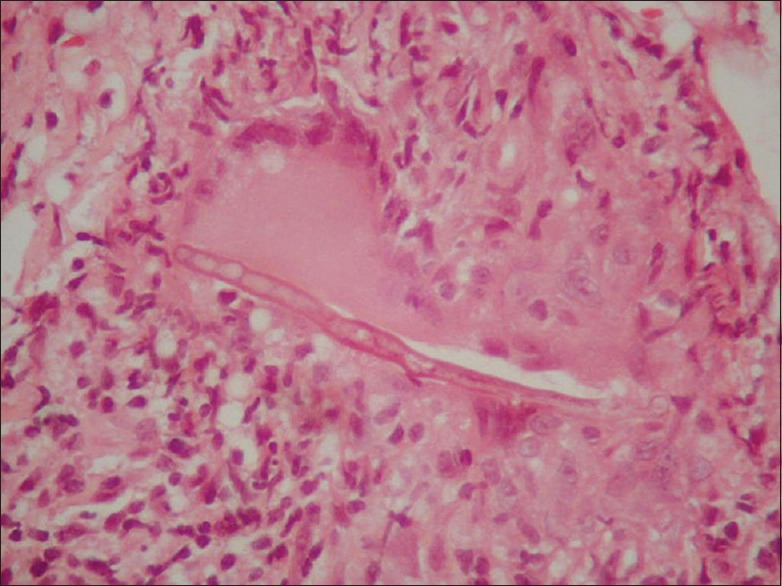
Histopathological picture
This disease is most commonly found in patients with hematological malignancies and those who have undergone solid organ transplants. However, in India, poorly controlled diabetes mellitus has been established as the leading risk factor and is believed to account for 25%–64% of cases seen here.[3] A possible explanation for this is that India not only has an extremely high prevalence of diabetes but also has a record of poor glycemic control. A study by Raheja et al. showed that almost 50% of diabetic patients had poor glycemic control.[8] In the current case series, uncontrolled diabetes was found to be the risk factor in all the patients, which highlights the need for blood glucose tests in patients suspected with mucormycosis.
There are other conditions which are clinically similar to mucormycosis, including bacterial cellulitis, aspergillosis, and rapidly growing malignancies.[9] The first two conditions may also be associated with high blood sugar levels; aspergillosis, being another fungal disease, also carries a poor prognosis. All these conditions can be ruled out by histopathological staining, which is why the authors advocate an immediate biopsy for confirmation.
Evidence-based guidelines for the management of mucormycosis are poor. Roden et al. reviewed 929 cases of mucormycosis that were reported in the literature,[10] and they concluded that combining surgical debridement with antifungal chemotherapy appeared to have a better survival rate (70%) than surgery alone (57%) or antifungal chemotherapy alone (61%). More recent studies have followed the combined approach for therapy; however, the mortality rate has been significant, ranging from 14.2%[11] to almost 83%.[6] The current protocol had a mortality rate of 10%, owing to the death of one patient in whom disseminated spread could not be controlled.
None of the studies reported in the literature have addressed the timing of therapy; one study by Chamilos et al.[12] stated that initiating antifungal chemotherapy within 5 days of diagnosis markedly improved survival (83%) as opposed to initiation after the 6th day (49%). Amphotericin B is the only antifungal agent that has been proven to be consistently effective against mucormycosis; however, the ideal dose of antifungal therapy is not defined. The current study used a dose of 50 mg/day, which was effective and was tolerated by all patients. This is slightly less than the dose of 1 mg/kg/day recommended by other authors.[13] Liposomal amphotericin B is believed to be superior, as it is less toxic and therefore allows for higher doses; however, the lack of availability of this formulation and the higher cost led us to use plain amphotericin B.[5]
Radical surgical debridement serves to physically remove the nidus of infection. The ideal timing of surgical debridement has not been defined in the literature. We were able to take up cases for surgery within 48 h of presentation, which could have limited the spread and thereby enhanced survival rates. Prompt surgery depends on the early diagnosis; since the clinical signs are nonspecific, it is imperative to maintain a high index of suspicion in the presence of known risk factors and do an immediate biopsy for histopathological confirmation. Although we used CT imaging to assess the extent of the disease, CT findings are nonspecific, and even invasive disease can sometimes present with a normal sinus CT.[14] In the current series, we were able to appreciate both bony erosion and soft-tissue spread in most cases. CT imaging is useful for assessing the extent of involvement, which would help in planning the extent of surgical debridement. CT imaging revealed retrobulbar soft-tissue changes in one patient in the current series; this led us to perform orbital exenteration as part of the surgical debridement, to control disease spread.
Treatment and reversal of the underlying immunosuppression are of paramount importance in the management of mucormycosis. Diabetic ketoacidosis was managed in our series by instituting a regime of insulin, followed by oral hypoglycemic agents. For the patient who was taking steroids for bronchial asthma, steroids were stopped immediately. However, patients who present with hematological malignancies would have pancytopenia, which is more difficult to address. Chemotherapy and immunosuppressant drugs may be discontinued temporarily or reduced to the minimum until the disease is under control. Gonzalez et al. suggested the use of granulocyte colony-stimulating factor to enhance leukocyte production, thereby improving immunity in such patients.[15]
CONCLUSION
Mucormycosis is a rapidly progressing, potentially fatal disease, and surviving this condition largely depends on early identification and immediate treatment. The early diagnosis is facilitated by maintaining a high index of suspicion and the correct correlation of clinical and histological features. Management of this condition must include simultaneous aggressive surgical debridement, antifungal therapy with amphotericin B, and correction of the underlying condition.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, et al. A higher-level phylogenetic classification of the fungi. Mycol Res. 2007;111:509–47. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–69. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chakrabarti A, Singh R. Mucormycosis in India: Unique features. Mycoses. 2014;57(Suppl 3):85–90. doi: 10.1111/myc.12243. [DOI] [PubMed] [Google Scholar]
- 4.Huang JS, Kok SH, Lee JJ, Hsu WY, Chiang CP, Kuo YS, et al. Extensive maxillary sequestration resulting from mucormycosis. Br J Oral Maxillofac Surg. 2005;43:532–4. doi: 10.1016/j.bjoms.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014;20(Suppl 3):5–26. doi: 10.1111/1469-0691.12371. [DOI] [PubMed] [Google Scholar]
- 6.Abu El-Naaj I, Leiser Y, Wolff A, Peled M. The surgical management of rhinocerebral mucormycosis. J Craniomaxillofac Surg. 2013;41:291–5. doi: 10.1016/j.jcms.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Gosavi DK. Acute necrosis of the maxilla due to mucormycosis. J Laryngol Otol. 1978;92:365–9. [PubMed] [Google Scholar]
- 8.Raheja BS, Kapur A, Bhoraskar A, Sathe SR, Jorgensen LN, Moorthi SR, et al. DiabCare Asia – India study: Diabetes care in India – Current status. J Assoc Physicians India. 2001;49:717–22. [PubMed] [Google Scholar]
- 9.Arndt S, Aschendorff A, Echternach M, Daemmrich TD, Maier W. Rhino-orbital-cerebral mucormycosis and aspergillosis: Differential diagnosis and treatment. Eur Arch Otorhinolaryngol. 2009;266:71–6. doi: 10.1007/s00405-008-0692-y. [DOI] [PubMed] [Google Scholar]
- 10.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin Infect Dis. 2005;41:634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 11.Khanna JN, Natarajan S, Galinde J. Rhinocerebral mucormycosis – An emerging threat. J Oral Maxillofac Surg Med Pathol. 2015;27:550. [Google Scholar]
- 12.Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47:503–9. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]
- 13.Spellberg B, Walsh TJ, Kontoyiannis DP, Edwards J, Jr, Ibrahim AS. Recent advances in the management of mucormycosis: From bench to bedside. Clin Infect Dis. 2009;48:1743–51. doi: 10.1086/599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhiwakar M, Thakar A, Bahadur S. Improving outcomes in rhinocerebral mucormycosis – Early diagnostic pointers and prognostic factors. J Laryngol Otol. 2003;117:861–5. doi: 10.1258/002221503322542854. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez CE, Couriel DR, Walsh TJ. Disseminated zygomycosis in a neutropenic patient: Successful treatment with amphotericin B lipid complex and granulocyte colony-stimulating factor. Clin Infect Dis. 1997;24:192–6. doi: 10.1093/clinids/24.2.192. [DOI] [PubMed] [Google Scholar]


