Abstract
Background:
To evaluate the effect of low-level laser therapy (LLLT) on bone healing in patients undergoing bilateral sinus lifting and simultaneous dental implant application.
Methods:
Twelve patients with total/partial posterior maxillary edentulism who needed bilateral sinus bone augmentation were included in the study. Dental implants were inserted in the same session. LLLT (λ = 630–660 nm, 25 mW/cm2, 6 min) was used for one operation side on the 1st, 3rd, 5th, and 7th days, whereas contralateral side served as control side. Preoperative and postoperative 1st, 3rd, and 6th month orthopantomograms were obtained using the aluminum step-wedge technique. Optic density analyses were performed using a Cardinal Health Digital Densitometer (Fluke Biomedical 07-443) with 1 mm diameter. Digital densitometry results were obtained as the equivalent aluminum thickness for each radiograph. These data were used to evaluate the changes in optical bone density and to compare the treatment side with the control side for each patient.
Results:
The LLLT side showed better results than the control side according to the densitometry results. Increase in the bone density at all the postoperative intervals was statistically significant (P < 0.05).
Conclusions:
LLLT enhances bone regeneration in sinus augmentation with simultaneous dental implant placement.
Keywords: Dental implant, low-level laser therapy, maxillary sinus augmentation, radiographic evaluation
INTRODUCTION
Implant treatment of the posterior edentulous maxilla can be complicated because of the resorption of the alveolar ridge or by an aging-related increase in the pneumatization of the maxillary sinus.[1] Maxillary sinus grafting procedures have been used to create adequate bone volume for optimum implant placement.[2] The lateral window approach is commonly used in surgical procedures for maxillary sinus grafting.[3] Many types of grafting materials have been used for sinus floor augmentation, such as autogenous bones, allografts, xenografts, alloplastic materials, and various mixtures of these materials.[4,5] Demineralized bone matrix (DBM) has been used clinically since 1889 and contains bone morphogenetic protein, transforming growth factor-beta, osteogenin, insulin-like growth factor, and fibroblast growth factor. The efficiency of the DBM has been widely studied in previous reports.[6,7,8] The efficiency of bone substitute materials is a critical factor in sinus augmentations for promoting graft maturation and providing long-term support for the implants.[9] Several studies have evaluated the beneficial effects of bone grafts and the quality of the newly formed bones. Platelet-rich plasma, platelet-rich fibrin, and hyaluronic acid combined with bone grafts were used for improving the quality of the newly formed bones.[9,10,11]
Low-level laser therapy (LLLT) is a commonly used alternative therapy for accelerating wound healing. LLLT increases the vascularity and osteoblastic activity as well as improves the organization of the collagen fibers.[12,13] LLLT is a noninvasive therapy for stimulating osteogenesis and accelerating the healing of bone defects.[13,14] The LLLT enhances bone quality and mechanical strength around the implant.[15,16]
The objective of this study was to evaluate the effects of LLLT on bone density in patients undergoing sinus floor augmentations using DBM.
METHODS
Twelve patients who required bilateral sinus floor augmentation for implant prosthetic rehabilitation were included in this study. Nine patients were bilaterally free-end partial edentulous and three patients were totally edentulous with a residual alveolar height between 3 and 6 mm. All patients required bilateral sinus augmentation with simultaneous implant placement.
Patients who had maxillary sinus pathology, previous history of chronic sinus infection, habit of smoking more than ten cigarettes per day, or any systemic disease that interfered with the surgical procedure were excluded from the study.
The study protocol was approved by the Ankara University Dentistry Faculty Research Ethics Committee. All patients were informed about the treatment procedure and gave written consent for this study.
Surgical protocol
Bilateral sinus augmentation and simultaneous implant placement were performed in the same session using the same surgical protocol [Figure 1]. All surgical procedures were performed under local anesthesia (Ultracain D-S Forte; Sanofi-Aventis, Istanbul, Turkey). After the elevation of the mucoperiosteal flap, a hinge-door osteotomy was performed on the lateral wall of the maxillary sinus with a round diamond bur under saline solution irrigation. The Schneiderian membrane was carefully detached from the inferior and lateral walls of the sinus until enough space was created for the placement of bone grafts. Implant osteotomies were performed, and DBM putty bone grafts (Dyna Graft bone putty, Keystone Dental, Burlington, MA, USA) were placed on the medial, posterior, and anterior sides of the implant sockets. Implants (Touareg S, Adin, Afula, Israel) were inserted into the osteotomy site after sufficient grafting. The access window was filled with bone graft after the implant placement. A membrane was not used over the access window, and 3/0 silk sutures were used to close the flap. Antibiotics (amoxicillin thrice per day), analgesics (naproxen sodium, twice per day), and mouthwash (isotonic saline twice per day) were prescribed for postoperative management. The patients were advised to consume a soft diet and to avoid sneezing until 2 weeks. Sutures were removed 1 week postoperatively. No complications occurred during the surgeries, and all surgical wounds healed uneventfully.
Figure 1.
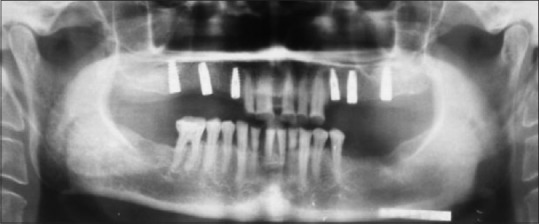
Bilateral sinus lifting and dental implant placement (postoperative 6-month panoramic view)
Low-level laser therapy protocol
In this study, a 630–660-nm aluminum (Al) gallium indium phosphide laser device (Scorpion Dental Optima Model 405-7A; Optica Laser, Sofia, Bulgaria) was used immediately after the surgery, and on the 3rd, 5th, and 7th days postoperatively for the treatment side [Figures 2 and 3]. The treatment side was randomly selected by another surgeon who performed the LLLT. Tissue was irradiated at 25 mW power for a total of 6 min: 2 min for each point (buccal, palatal, and crestal). In addition, 72 J/cm2 energy density was deposited in one session.
Figure 2.
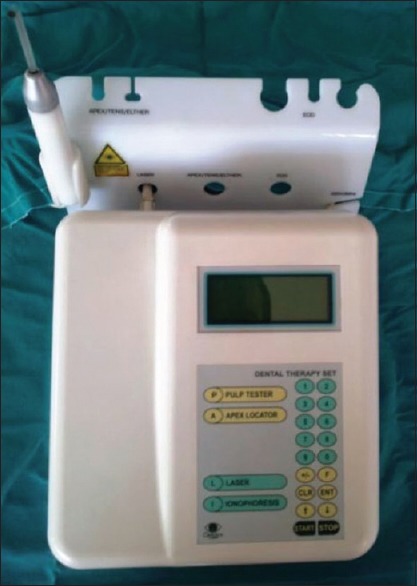
Low-level laser therapy device
Figure 3.
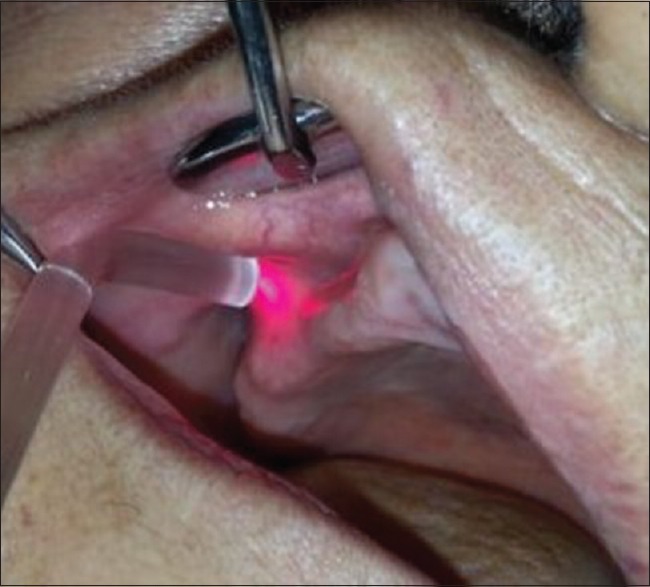
Low-level laser therapy application intraorally
Aluminum step-wedge and radiographic assessment
The aluminum step-wedge method was used as the reference material in the densitometric evaluation of the conventional radiographs. The aluminum wedges were prepared using 99.7% pure aluminum plates; the dimensions of the plates were 1 mm in width and 2, 4, 6, 8, 10, and 12 mm in length. The six prepared aluminum plates were stuck in a row, and a stair-like aluminum wedge was developed.
The prepared aluminum step-wedges were placed in a fixed location (lower left corner) in the film cassettes to maintain the calibration and standardization of the radiographs. Special care was taken to avoid the superposition of the hyoid bone and other bony structures with the step-wedge.
In this research, all radiographic assessments were performed using panoramic radiographs. The radiographic assessments were performed by the same radiology technician from the Oral Diagnosis and Radiology Department of Ankara University. Mediphot X-O/RP, sens: green, 15 × 30 panoramic radiographs were used in this research.
All radiographs were taken using a 80-kVp, 12-mA, 2.5-mm Al total filtrated “PM 2002 CC Proline” panoramic radiography device with the same kV and mA values. Radiographic film processing was performed using automatic radiograph processing device Velopex Extra-XE model (HEXAGON International LTD, UK).
Four panoramic radiographs, one preoperative and three postoperative (in the postoperative 1st, 3rd, and 6th months), were taken from each patient.
Densitometric assessment
The densitometric analysis was performed on 48 radiographs, including those taken preoperatively and those taken on postoperative 1st, 3rd, and 6th months. Densitometric assessments of the radiographs were performed in the Medical Physics Department of the Ankara University Nuclear Sciences Institute. A Cardinal Health Digital Densitometer (Fluke Biomedical 07-443) with 1-mm diameter was used for optic density measurement [Figure 4]. The results were read from the digital screen and immediately recorded.
Figure 4.

Densitometer device
The densitometric measurements were taken three times at different points on each aluminum step-wedge and sinus augmentation zones, and the mean measurements were calculated.
The aluminum-equivalent thickness of the augmented sinus areas was identified. Using this information, the mineralization levels of the graft materials on the radiographs were measured, and the results obtained were assessed statistically.
Statistical assessment
The Shapiro–Wilk test was used to determine whether the data were normally distributed. The Friedman test was used to evaluate preoperative and postoperative follow-up data within two groups. In cases of significant differences among the measurements, the Bonferroni-adjusted Wilcoxon signed-rank test was used for post hoc analysis. In addition, the Bonferroni-adjusted Wilcoxon signed-rank test (paired observations) was used to compare the two groups at the same time points. All tests were performed using the statistical software SPSS (SPSS Inc., version 19.0, Chicago, IL, USA). The median as well as minimum and maximum values was calculated for each parameter. P < 0.05 was considered statistically significant.
RESULTS
Twenty-four sinus augmentations with simultaneous implant placement were performed in twelve patients. No complications occurred during the 6-month healing period. All patients healed uneventfully. Implant failure was not observed in any patient. All patients were treated using precious metal-ceramic alloy bridges approximately 7 months postoperatively.
Data obtained from the radiographs were statistically analyzed [Table 1]. Bone density was increased in the postoperative 1st month; thereafter, it decreased in the 3rd month, and again increased at 6 months in both the groups. The increase in the bone density of the treatment side at the 1st, 3rd, and 6th months was statistically significant (P < 0.05).
Table 1.
Bone density changes in each group and between groups (P<0.05)
| Periods | Laser | Control | P |
|---|---|---|---|
| Baseline | 2.70 (0.002) | 2.63 (0.002) | 0.908 |
| 1 month | 4.02 (0.002) | 3.77 (0.003) | 0.326 |
| 3 months | 3.98 (0.002) | 3.21 (0.002) | 0.002a |
| 6 months | 4.45 (0.002) | 3.99 (0.002) | 0.049a |
All values are statistically significant in each group. Statistical differences were observed between groups at 3rd- and 6th-month intervals. Data are expressed as the mean (P value). aStatistically significant difference between groups (Mann-Whitney U-test) (P<0.05)
DISCUSSION
This study was designed to evaluate the effects of LLLT on bone healing in patients with bilateral sinus augmentation with simultaneous dental implant placement. According to the results of the densitometric evaluation, increase in the bone density of the treatment side at the 1st, 3rd, and 6th months was statistically significant (P < 0.05). The treatment side showed better results than the control side according to the densitometric analysis results.
Improved maxillary sinus lift techniques and diverse bone graft materials have been used for this purpose. Although autogenous bone grafts are considered the gold standard for bone grafting, they have some disadvantages such as the formation of a second surgical region, morbidity in the donor region, and the provision of a limited amount of bone. The introduction of new graft materials such as allografts, xenografts, and alloplastic materials has provided alternatives to the autogenous bone. DBM is the most commonly used allograft that has been clinically used for more than 40 years. In 1975, Libin et al. first used DBM in the field of maxillofacial surgery.[17] Previous studies have demonstrated that allogenic grafts may be successful in oral and maxillofacial surgery.[18,19,20] Several studies have reported that DBM enables successful outcomes in maxillary sinus grafting.[6,7,8,21]
We used the putty form of DBM. According to Üngör who evaluated the ossification of the two forms of DBM putty and powder in maxillary sinus augmentation by using digital densitometry on panoramic radiographs, there was no significant difference between the putty and powder forms of DBM based on the radiographic and clinical examinations.[7] Chesmel et al. showed that human DBM in gel, putty, and sheet forms proved to be as effective as an autograft in a critical size defect in a thymic rat model. There were no significant differences between these three forms.[22] In our study, the putty form of DBM showed good clinical and radiographic results.
In the treatment and control groups, bone density was increased in the 1st month, decreased in the 3rd month, and increased again in the 6th month. These results were similar to those of Üngör's study[7] which reported that the appearance of high bone density in the 1st month may depend on the nonresorption of the graft material and edema. In addition, Fassbender et al. reported that the amount of radiopaque tissue decreased distinctly over time in defects treated with DBM. These results indicate the biodegradation of the implanted graft material rather than an anabolic bone activity.[23]
The cellular effects of LLLT have been demonstrated in in vitro and in vivo studies. LLLT stimulates cellular metabolism by the activation of cytochrome c oxidase that induces adenosine triphosphate (ATP) synthesis in the mitochondria. Increased ATP production enhances the function and metabolism of cells in ischemic and wounded tissues.[24,25] LLLT exerts a positive effect on cell growth and protein synthesis by increasing the synthesis of DNA and RNA.[26] LLLT application induces the proliferation of endothelial cells that induce the formation of new vessels for blood supply to the wound.[27,28] LLLT exerts an anti-inflammatory effect by stimulating the lymphocytes, activating the mast cells, and proliferating various cell types.[29] LLLT therapy also stimulates fibroblasts for collagen synthesis, the precursor for the bone matrix, as well as osteoprogenitor cells, osteoblasts, and osteoclasts that promote bone remodeling.[14,30,31]
Several studies have been published about the effect of LLLT on bone regeneration. Márquez Martínez et al. evaluated the effect of 830-nm wavelength LLLT on healing in rat femur defects grafted with inorganic bovine bone Gen-ox. A positive effect of LLLT on bone healing was observed in the histological evaluation. At the 30th day, there was a marked increase in the number of collagen fibers, osteoblastic activity, and bone trabeculae formation. A previous study has reported that LLLT has a positive effect on the healing of rat femur defect grafted using inorganic bovine bone.[32] Paes et al. assessed the effect of LLLT (830 nm) on morselized bone allograft incorporation in rabbit cranial defects. At 70 days, histologically, the allograft + LLLT group had statistically better bone remodeling and vascularization than that of the allograft control group. The authors concluded that LLLT had a qualitative and quantitative positive effect on the speed of osteogenesis in morselized bone grafts.[33] Monea et al. evaluated the effects of LLLT on socket grafting using a particulate allograft material covered with a resorbable collagen wound dressing. Patients were randomly divided into the following equal groups: the laser-treated group and the control group who did not undergo postoperative laser therapy. Biopsies were taken at the 60th day from the laser-treated group and at the 120th day from the control group. Histological bone assessment in the laser group at the 60th day showed abundant new bone formation without any signs of inflammation; the same result was observed in the control group at the 120th postoperative day. The authors concluded that LLLT photobiomodulation can accelerate bone healing and reduce the healing time after grafting of the extraction socket.[34] Further, Brawn and Kwong-Hing reported that enhanced bone regeneration and faster particle resorption were observed with near-infrared phototherapy in hydroxyapatite-treated extraction sockets.[25] Jakse et al. assessed the effect of LLLT (680 nm) on bone regeneration and osseointegration of dental implants performed in second-stage surgery in a sinus graft model on sheep. Bilateral sinus augmentation was performed on 12 sheep using the cancellous bone from the iliac crest. One side was treated with LLLT, whereas the other side was not. The animals were sacrificed 16 weeks after the surgery. Biopsies of the augmented area were obtained during implant insertion and after the animals were sacrificed. The histological results of the two groups were comparable at 4 and 12 weeks; however, greater bone–implant contact was observed in the laser group. According to the authors, their study did not confirm the positive effect of LLLT on bone regeneration using a cancellous sinus graft; however, LLLT could have a positive effect on implant osseointegration.[16]
According to the results of the densitometric evaluation, increase in the bone density of the LLLT-treated side at the 1st, 3rd, and 6th months was statistically significant (P < 0.05). The results of the present study suggest that LLLT enables higher optical bone density and faster bone healing. Additional studies should be performed to evaluate the effect of LLLT using histological examination and cone-beam computed tomographical densitometric assessment.
CONCLUSIONS
From the results of this study, it is evident that LLLT enhances bone regeneration in sinus augmentation with simultaneous implant placement. Long-term follow-up studies with larger samples are needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Suguimoto RM, Trindade IK, Carvalho RM. The use of negative pressure for the sinus lift procedure: A technical note. Int J Oral Maxillofac Implants. 2006;21:455–8. [PubMed] [Google Scholar]
- 2.Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980;38:613–6. [PubMed] [Google Scholar]
- 3.Moon JW, Sohn DS, Heo JU, Kim JS. Comparison of two kinds of bovine bone in maxillary sinus augmentation: A histomorphometric study. Implant Dent. 2015;24:19–24. doi: 10.1097/ID.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 4.Del Fabbro M, Testori T, Francetti L, Weinstein R. Systematic review of survival rates for implants placed in the grafted maxillary sinus. Int J Periodontics Restorative Dent. 2004;24:565–77. [PubMed] [Google Scholar]
- 5.Chiapasco M, Zaniboni M, Boisco M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implants Res. 2006;17(Suppl 2):136–59. doi: 10.1111/j.1600-0501.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 6.Won YH, Kim SG, Oh JS, Lim SC. Clinical evaluation of demineralized bone allograft for sinus lifts in humans: A clinical and histologic study. Implant Dent. 2011;20:460–4. doi: 10.1097/ID.0b013e31823541e7. [DOI] [PubMed] [Google Scholar]
- 7.Ungör C. Radiologic evaluation of putty versus powder form of demineralized bone matrix in sinus floor elevation. J Oral Implantol. 2012;38:337–43. doi: 10.1563/AAID-JOI-D-10-00017. [DOI] [PubMed] [Google Scholar]
- 8.Irinakis T. Efficacy of injectable demineralized bone matrix as graft material during sinus elevation surgery with simultaneous implant placement in the posterior maxilla: Clinical evaluation of 49 sinuses. J Oral Maxillofac Surg. 2011;69:134–41. doi: 10.1016/j.joms.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 9.Taschieri S, Testori T, Corbella S, Weinstein R, Francetti L, Di Giancamillo A, et al. Platelet-rich plasma and deproteinized bovine bone matrix in maxillary sinus lift surgery: A split-mouth histomorphometric evaluation. Implant Dent. 2015;24:592–7. doi: 10.1097/ID.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 10.Göçmen G, Atalı O, Aktop S, Sipahi A, Gönül O. Hyaluronic acid versus ultrasonic resorbable pin fixation for space maintenance in non-grafted sinus lifting. J Oral Maxillofac Surg. 2016;74:497–504. doi: 10.1016/j.joms.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Tangl S, Huber CD, Lin Y, Qiu L, Rausch-Fan X, et al. Effects of Choukroun's platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: A histological and histomorphometric study. J Craniomaxillofac Surg. 2012;40:321–8. doi: 10.1016/j.jcms.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Barbosa D, de Souza RA, Xavier M, da Silva FF, Arisawa EA, Villaverde AG, et al. Effects of low-level laser therapy (LLLT) on bone repair in rats: Optical densitometry analysis. Lasers Med Sci. 2013;28:651–6. doi: 10.1007/s10103-012-1125-0. [DOI] [PubMed] [Google Scholar]
- 13.de Almeida AL, Medeiros IL, Cunha MJ, Sbrana MC, de Oliveira PG, Esper LA, et al. The effect of low-level laser on bone healing in critical size defects treated with or without autogenous bone graft: An experimental study in rat calvaria. Clin Oral Implants Res. 2014;25:1131–6. doi: 10.1111/clr.12239. [DOI] [PubMed] [Google Scholar]
- 14.da Silva RV, Camilli JA. Repair of bone defects treated with autogenous bone graft and low-power laser. J Craniofac Surg. 2006;17:297–301. doi: 10.1097/00001665-200603000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Lopes CB, Pinheiro AL, Sathaiah S, Da Silva NS, Salgado MA. Infrared laser photobiomodulation (lambda 830 nm) on bone tissue around dental implants: A Raman spectroscopy and scanning electronic microscopy study in rabbits. Photomed Laser Surg. 2007;25:96–101. doi: 10.1089/pho.2006.2030. [DOI] [PubMed] [Google Scholar]
- 16.Jakse N, Payer M, Tangl S, Berghold A, Kirmeier R, Lorenzoni M, et al. Influence of low-level laser treatment on bone regeneration and osseointegration of dental implants following sinus augmentation. An experimental study on sheep. Clin Oral Implants Res. 2007;18:517–24. doi: 10.1111/j.1600-0501.2007.01369.x. [DOI] [PubMed] [Google Scholar]
- 17.Libin BM, Ward HL, Fishman L. Decalcified, lyophilized bone allografts for use in human periodontal defects. J Periodontol. 1975;46:51–6. doi: 10.1902/jop.1975.46.1.51. [DOI] [PubMed] [Google Scholar]
- 18.Glowacki J, Kaban LB, Murray JE, Folkman J, Mulliken JB. Application of the biological principle of induced osteogenesis for craniofacial defects. Lancet. 1981;1:959–62. doi: 10.1016/s0140-6736(81)91730-x. [DOI] [PubMed] [Google Scholar]
- 19.Kaban LB, Mulliken JB, Glowacki J. Treatment of jaw defects with demineralized bone implants. J Oral Maxillofac Surg. 1982;40:623–6. doi: 10.1016/0278-2391(82)90109-4. [DOI] [PubMed] [Google Scholar]
- 20.Mulliken JB, Glowacki J, Kaban LB, Folkman J, Murray JE. Use of demineralized allogeneic bone implants for the correction of maxillocraniofacial deformities. Ann Surg. 1981;194:366–72. doi: 10.1097/00000658-198109000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dellavia C, Tartaglia G, Sforza C. Histomorphometric analysis of human maxillary sinus lift with a new bone substitute biocomposite: A preliminary report. Clin Implant Dent Relat Res. 2009;11(Suppl 1):e59–68. doi: 10.1111/j.1708-8208.2009.00203.x. [DOI] [PubMed] [Google Scholar]
- 22.Chesmel KD, Branger J, Wertheim H, Scarborough N. Healing response to various forms of human demineralized bone matrix in athymic rat cranial defects. J Oral Maxillofac Surg. 1998;56:857–63. doi: 10.1016/s0278-2391(98)90015-5. [DOI] [PubMed] [Google Scholar]
- 23.Fassbender M, Minkwitz S, Thiele M, Wildemann B. Efficacy of two different demineralised bone matrix grafts to promote bone healing in a critical-size-defect: A radiological, histological and histomorphometric study in rat femurs. Int Orthop. 2014;38:1963–9. doi: 10.1007/s00264-014-2321-2. [DOI] [PubMed] [Google Scholar]
- 24.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: Reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B. 2005;81:98–106. doi: 10.1016/j.jphotobiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Brawn PR, Kwong-Hing A. Histologic comparison of light emitting diode phototherapy-treated hydroxyapatite-grafted extraction sockets: A same-mouth case study. Implant Dent. 2007;16:204–11. doi: 10.1097/ID.0b013e318065a84c. [DOI] [PubMed] [Google Scholar]
- 26.Karu T, Pyatibrat L, Kalendo G. Irradiation with he-ne laser increases ATP level in cells cultivated in vitro . J Photochem Photobiol B. 1995;27:219–23. doi: 10.1016/1011-1344(94)07078-3. [DOI] [PubMed] [Google Scholar]
- 27.Schlager A, Kronberger P, Petschke F, Ulmer H. Low-power laser light in the healing of burns: A comparison between two different wavelengths (635 nm and 690 nm) and a placebo group. Lasers Surg Med. 2000;27:39–42. doi: 10.1002/1096-9101(2000)27:1<39::aid-lsm5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Trelles MA, Mayayo E. Bone fracture consolidates faster with low-power laser. Lasers Surg Med. 1987;7:36–45. doi: 10.1002/lsm.1900070107. [DOI] [PubMed] [Google Scholar]
- 29.Stadler I, Evans R, Kolb B, Naim JO, Narayan V, Buehner N, et al. In vitro effects of low-level laser irradiation at 660 nm on peripheral blood lymphocytes. Lasers Surg Med. 2000;27:255–61. doi: 10.1002/1096-9101(2000)27:3<255::aid-lsm7>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 30.Freitas I, Baranauskas V, Cruz-Höfling M. Laser effects on osteogenesis. Appl Surf Sci. 2000;154:548–54. [Google Scholar]
- 31.Garavello-Freitas I, Baranauskas V, Joazeiro PP, Padovani CR, Dal Pai-Silva M, da Cruz-Höfling MA, et al. Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J Photochem Photobiol B. 2003;70:81–9. doi: 10.1016/s1011-1344(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 32.Márquez Martínez ME, Pinheiro AL, Ramalho LM. Effect of IR laser photobiomodulation on the repair of bone defects grafted with organic bovine bone. Lasers Med Sci. 2008;23:313–7. doi: 10.1007/s10103-007-0488-0. [DOI] [PubMed] [Google Scholar]
- 33.Paes JV, Valiati R, de Sá V, Paes FL, de Moraes JFD, Dedavid BA, Gava A. Effects of laser therapy on morselized bone allograft incorporation laser therapy and bone grafts incorporation. J Surg Transplant Sci. 2014;2:1004–11. [Google Scholar]
- 34.Monea A, Beresescu G, Boeriu S, Tibor M, Popsor S, Antonescu DM, et al. Bone healing after low-level laser application in extraction sockets grafted with allograft material and covered with a resorbable collagen dressing: A pilot histological evaluation. BMC Oral Health. 2015;15:134. doi: 10.1186/s12903-015-0122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


