Abstract
Background:
Several studies have reported positive efficacy outcomes for patients with inflammatory bowel disease treated with CT-P13, an infliximab biosimilar. Data from follow-up periods longer than 1 year are still scarce. Here, we assessed the long-term efficacy data, loss of response and safety after switching from infliximab to CT-P13 in patients with inflammatory bowel disease.
Methods:
This was a prospective single-center observational study involving patients with moderate-to-severe Crohn’s disease and ulcerative colitis switched from infliximab to CT-P13 treatment and reviewed up to 24 months. Efficacy and loss of response were measured using the Harvey–Bradshaw (HB) index and partial Mayo score for patients with Crohn’s disease and ulcerative colitis respectively. C-reactive protein, infliximab drug levels, adverse events and antidrug antibodies were also monitored throughout the study.
Results:
A total of 64 patients with Crohn’s disease and 36 patients with ulcerative colitis were included. Most of them (72%) remained on CT-P13. Overall, 28% of patients discontinued the therapy due to loss of response, adverse events or long-lasting clinical remission. Remission at 18 and 24 months occurred in 69.9% and 68.5% of patients, respectively. Dose increase was performed in 22% of patients, with remission being reached in 60% of them. HB index, partial Mayo score, C-reactive protein and infliximab drug levels did not show significant changes. Serious adverse events were reported in 14% of patients. Overall, two patients developed low levels of antidrug antibodies.
Conclusions:
Most of the patients switching from original infliximab were maintained on CT-P13 at 2 years of follow up with a good profile of efficacy and safety.
Keywords: Crohn’s disease, CT-P13, ulcerative colitis
Introduction
Infliximab (IFX) was the first monoclonal antibody against tumor necrosis factor alpha (TNF-α) approved by the United States Food and Drug Administration (US FDA) and European Medicines Agency (EMA) for the treatment of Crohn’s disease (CD) and ulcerative colitis (UC), almost two decades ago.1,2 Despite an undoubted efficacy of these biological products in achieving remission,3–6 these agents are much more expensive than traditional treatments and have a considerable burden on the national health system. The expiration of the patent of certain anti-TNF agents (e.g. IFX branded as Remicade® and adalimumab branded as Humira®) has triggered the development of biosimilars.
The introduction of the new concept of ‘biosimilar therapy’ is reducing the financial burden on patient care, making this treatment more accessible.7,8 However, there is debate about its effectiveness in inflammatory bowel disease (IBD), because the approval for the biosimilar therapy is derived from clinical trials in rheumatological diseases (PLANETRA and PLANETA studies).9,10 In 2013, the EMA approved the first IFX biosimilar, CT-P13 (Remsima® and Inflectra®), for the same indications as the original product, including IBD, based on the extrapolation of results.11,12 Since then, most medical societies around the world have issued position statements regarding the use of biosimilars for IBD, supporting the efficacy and safety of CT-P13 in both naïve and switched patients based on results from published observational studies.13,14 The European Crohn’s Colitis Organization (ECCO) published a position statement on the use of biosimilars for IBD in December 2016: ‘when a biosimilar product is registered in the European Union, it is considered to be as efficacious as the reference product when its use is in accordance with the information provided in the Summary of Product Characteristics’.15 The British Society of Gastroenterology recommended the maintenance of pharmacovigilance for this new biological product.16
All published observational post-marketing IBD studies have reported positive efficacy outcomes for patients with CD and UC treated with CT-P13, irrespective of prior anti-TNF treatment.17–26 However, data about the efficacy and safety for follow-up periods longer than 1 year are limited. We have previously presented our 1-year follow-up results.27 Here, we present our 24-month follow-up results and describe an observational study in a real-life cohort assessing the efficacy and safety when switching from the IFX reference product (RP) to CT-P13 in patients with IBD.
Methods
Study design
This was a prospective observational study conducted at the Virgen Macarena University Hospital (Seville, Spain) from 2015 to 2017. The study was approved by the Research Ethics Committee of Virgen Macarena University Hospital, Seville, Spain (SAP-INF-2015), and was conducted following good clinical practice guidelines. All study participants, or their legal guardian, provided informed written consent prior to study enrollment. Follow-up parameters after the switch were prospectively recorded using a specific inflammatory unit database in the hospital.
Patients analysis
The cohort included all patients with a previous diagnosis of moderate-to-severe CD or UC, whose treatment was switched from original IFX (Remicade®) to CT-P13 in March 2015 and had completed 24 months of follow up. Therefore, 100 patients were included in this study. The switch was done because of financial reasons.
We recorded the Montreal classification status as well as previous and concomitant therapies for all patients before enrollment. All patients received intravenous premedication as usual in our unit prior to the IFX therapy (corticosteroids and antihistamines).
The primary endpoint was to analyze the loss of response to IFX after switching. When at least one of the following was present, a loss of response was considered: Harvey–Bradshaw (HB) index >4 for CD, partial Mayo score >2 for UC, steroid use or surgery related to the activity of the disease, IFX dose increase during the follow up.
The secondary endpoints were:
- To analyze safety during 24 months of follow up.
- To compare changes in biochemical markers such as C-reactive protein (CRP) at 0, 12, 18 and 24 months.
- To compare IFX trough levels at 12, 18 and 24 months after switching medications.
For patients with CD and UC who were in remission at the time of the switch, remission was considered to be sustained if the HB index was ⩽4 and the partial Mayo score was ⩽2 after switching, respectively, without the need for steroids, surgery, or dosage increase. For those patients who were not in remission at the time of the switch, remission was considered to be achieved if a HB index was ⩽4 (patients with CD) or a partial Mayo score was ⩽2 (patients with UC).
Medication changes during follow up were allowed at the discretion of the treating physician and were registered in a database. Medication changes in most of the patients were based on clinical symptoms and not on IFX drug levels (because drug monitoring could be done only after the first year of treatment with CT-P13). Adverse events (AEs) from the first infusion of CT-P13 till the end of the study were recorded according to the requirements of the Office of Human Research Protection.
IFX trough levels
We measured the IFX serum level and antidrug antibodies (ADAs) before the IFX infusion, using an enzyme-linked immunosorbent assay using Progenika kits (PROMONITOR®, Progenika Biopharma, Spain) from 2016 onwards. We included only patients who remained on CT-P13 for 12 months after the switch, because theses kits were introduced in our hospital in 2016. IFX levels between 3.0 and 7.0 µg/ml were considered to be in the therapeutic range, as shown in the TAXIT28 study.
Statistical analysis
Demographic and nominal results were reported in percentages and frequencies. Numerical results were reported as the mean and standard deviation in cases of normal distribution, and as the median and interquartile range (IQR) in cases of skewed distribution. The Cochrane’s Q test and the Friedman test were used to analyze the evolution of the clinical scores (HB index and partial Mayo score), the CRP values and the IFX serum drug levels. Confidence intervals (CIs) were calculated at 95% and a p value of 0.05 was used as the statistical significance level.
Patients who discontinued treatment due to AEs, remission/mucosa healing within 6 months after the switch and those who changed treatment for other medical indication were excluded from the efficacy analysis (n = 8). The data of this study were analyzed by protocol. The analysis was performed using SPSS 25 (IBM Corporation).
Results
Baseline characteristics
A total of 100 patients with IBD (64 with CD and 36 with UC) were included. Of these, 51 were men (51%) and 49 were women (49%) with a median age of 41 (32–50) years. The principal characteristics of the patients are shown in Table 1.
Table 1.
Baseline demographic and clinical characteristics.
| Crohn’s disease patients | Ulcerative colitis patients | |
|---|---|---|
| n | 64 | 36 |
| Females, n (%) | 31 (48.4) | 18 (50) |
| Males, n (%) | 33 (51.6) | 18 (50) |
| Age, median years (range) | 40.5 (18–77) | 44.0 (37–53) |
| Age at diagnosis, median years (IQR) | 26 (19–37) | 29 (25–44) |
| Smoking status, n (%) | ||
| Never smoked | 43 (67.2) | 25 (69.4) |
| Ex-smoker | 11 (17.2) | 7 (19.4) |
| Current smoker | 10 (15.6) | 4 (11.2) |
| Extraintestinal manifestations, n (%) | 20 (31.3) | 10 (27.7) |
| Prior medical exposure, n (%) | ||
| Thiopurines | 33 (51.6) | 19 (52.8) |
| Methotrexate | 20 (31.3) | 5 (13.9) |
| Concomitant medication use | ||
| Thiopurines | 27 (42.2) | 13 (36.1) |
| Methotrexate | 14 (21.8) | 3 (8.3) |
| Steroids | 10 (15.6) | 5 (13.9) |
| Time from diagnosis to end of follow up in years (median, IQR) | 11 (6–14) | 10 (6–13) |
| Duration of original IFX therapy in months (median, IQR) | 70 (44–85) | 50 (22–76) |
| Crohn’s disease | ||
| Age at diagnosis, n (%) | A1 (diagnosed <17 years of age) | 8 (12.5) |
| A2 (diagnosed 17–40 years of age) | 46 (71.9) | |
| A3 (diagnosed >40 years of age) | 10 (25.6) | |
| Disease location at diagnosis, n (%) | L1 (ileal) | 16 (25) |
| L2 (colonic) | 27 (42.2) | |
| L3 (ileocolonic) | 18 (28.2) | |
| L3 (ileocolonic) + L4 (upper gastrointestinal tract) | 3 (4.7) | |
| Disease behavior, n (%) | B1 (nonstricturing, nonpenetrating) | 39 (60.9) |
| B2 (stricturing) | 12 (18.8) | |
| B3 (penetrating) | 13 (20.3) | |
| Perianal disease, n (%) | 28 (43.8) | |
| Extraintestinal manifestations, n (%) | 20 (31.3) | |
| Median Harvey–Bradshaw index prior to switch (IQR) | 1 (1–3) | |
| Ulcerative colitis | ||
| Extent, n (%) | E1 (proctitis) | 13 (36.1) |
| E2 (left-sided colitis) | 11 (30.6) | |
| E3 (pancolitis) | 12 (33.3) | |
| Severity, n (%) | S1 (mild) | 13 (36.1) |
| S2 (moderate) | 17 (47.2) | |
| S3 (severe) | 6 (16.7) | |
| Extraintestinal manifestations, n (%) | 10 (27.7) | |
| Median partial Mayo score prior to switch (IQR) | 2 (1–3) | |
IFX, infliximab; IQR, interquartile range.
Global analysis
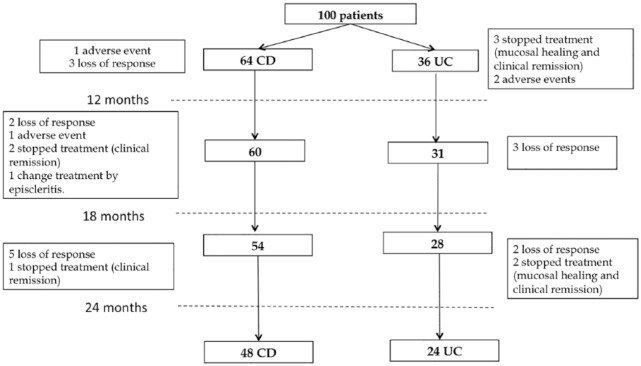
Most patients (72%) remained on CT-P13 during the 2 years of follow up. A total of 28% of them discontinued the therapy due to a loss of response (15%), AEs (4%) or clinical remission (8%). A patient in clinical remission was changed to another anti-TNF therapy because he developed episcleritis (Figure 1). Of the eight patients who discontinued therapy due to clinical remission, five (UC) had histological healing confirmed by colonoscopy, and for the remaining three (CD) the decision to discontinue therapy was based on clinical remission.
Figure 1.
Patient withdrawals during follow up.
CD, Crohn’s disease; UC, ulcerative colitis.
At the beginning of the study, 78% (78/100) of patients were in remission. At 12, 18 and 24 months, 69.6% (65/93), 69.9% (65/93) and 68.5% (63/92) of them were still in remission respectively (p = 0.038).
Patients who lost response had a median (range) disease duration of 11 years (7–19), and previous RP treatment of 46 months (3–71). Finally, the median time of discontinuation was 15 months (8–24).
A total of 22% of the patients (16 CD and 6 UC), needed to have their dose increased during the 2 years of follow up. Overall, two patients were in clinical remission, one had severe activity as detected by small bowel magnetic resonance imaging, and another one had severe arthralgia of rheumatological etiology. After the dose increase, 60% of patients (13/22) were in remission. The CT-P13 dose was reduced in 9% of the patients who were in remission.
CD group
The median time of the disease before starting the follow up was 10.5 years (IQR: 6–14). The median duration of ongoing IFX RP treatment at the beginning of the study was 70 months (43–85). There was concomitant thiopurine use by 42.2% of the patients during the follow up.
At 2 years of follow up, 16/64 (25%) patients discontinued the CT-P13 due to the following reasons: 15.6% (n = 10) loss of response, 4.7% (n = 3) clinical remission, 3.1% (n = 2) AEs and 1.6% (n = 1) other medical indication. Patient withdrawals are shown in Figure 1.
UC group
The median duration of the disease before starting follow up was 9.5 years (IQR: 6–13). The median duration of ongoing IFX original treatment at the beginning of the study was 50 (22–75) months. Before starting the follow up, 51% of the patients had used concomitant thiopurines. The main pathology at diagnosis was proctitis in 36.1% of the cases, with a moderate severity in 47.2% of the patients. A concomitant use of thiopurines during the follow up occurred in 36.1% of the patients.
At 2 years of follow up, 12/36 (33.33%) patients discontinued the CT-P13 due to the following reasons: 13.9% (n = 5) loss of response, 13.9% (n = 5) remission evidenced by mucosal healing and 5.6% (n = 2) due to AE. Patient withdrawals are shown in Figure 1.
Efficacy
CD group
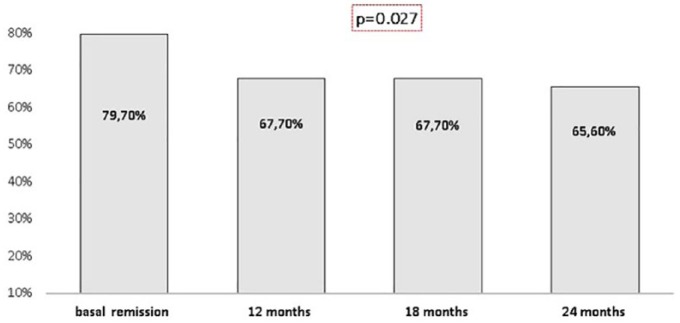
At the beginning of the study, 79.7% (51/64) of the patients with CD were in remission. Regarding the patients, who continued using CT-P13 after the switch, 67.7% (42/62), 67.7% (42/62), and 65.6% (40/61) were in remission at 12, 18 and 24 months, respectively (p = 0.027; Figure 2). During the follow up, 16 out of 64 patients (25%) needed an increase of the CT-P13 dose and 62.5% (10/16) reached remission. After 24 months of follow up, 50 out of 61 patients (82%) were in remission.
Figure 2.
Remission in patients with Crohn’s disease during follow up.
Of the 51 patients who were in remission at the time of the switch, 74% (37/50), 76% (38/50), and 75.5% (37/49) remained in remission at 12, 18 and 24 months, respectively (p = <0.001). Overall, seven patients needed to have their dose increased to achieve remission again.
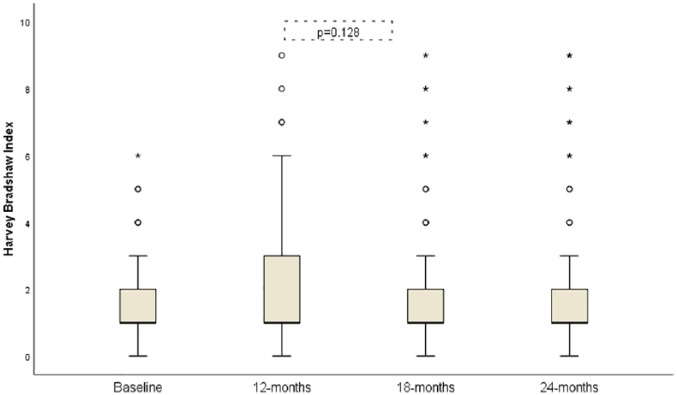
The HB index did not show significant changes over 24 months; the median HB index (95% CI) was: 1 (1–3), 2 (1–4), 1 (1–2), 1 (1–2) at baseline, 12, 18 and 24 months respectively (p = 0.128; Figure 3).
Figure 3.
Evolution of Harvey–Bradshaw index from baseline to 24 months follow up.
No significant changes of the median (95% CI) CRP levels were observed in patients with CD over the same period [1.0 mg/dl (0–5), 0.45 mg/dl (0.21–2), 1.2 mg/dl (1–2.83), 1.2 mg/dl (0.83–3) at 0, 12, 18 and 24 months, respectively; p = 0.224].
UC group
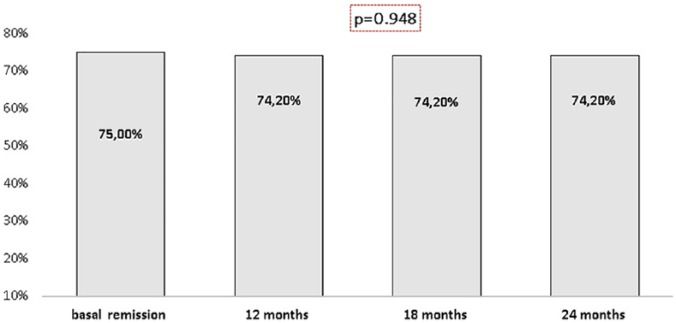
At the beginning of the study, 75% (27/36) of the patients with UC were in remission. When we analyzed the patients, who remained on CT-P13 after the switch, 74.2% (23/31), 74.2% (23/31), and 74.2% (23/31) were in remission at 12, 18 and 24 months, respectively (p = 0.948; Figure 4).
Figure 4.
Remission in patients with ulcerative colitis during follow up.
Of the 27 patients who were in remission at the time of the switch, 87.5% (21/24), 87.5% (21/24), and 87.5% (21/24) remained in remission at 12, 18 and 24 months, respectively (p = 0.145).
During the follow up, six patients (16.7%) needed a dose increase of the CT-P13 and 50% of them (3/6) achieved remission. The remission rate after increasing the dose was 83.9% (26/31).
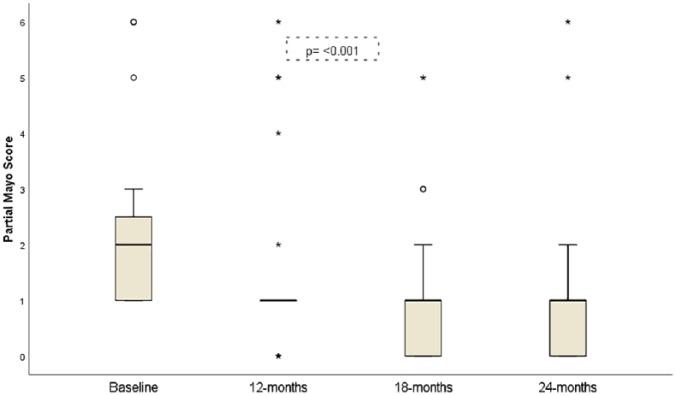
The median (95% CI) of the partial Mayo score decreased significantly during the 24 months of follow up (p ⩽ 0.001; Figure 5). The median partial Mayo score (95% CI) was: 2 (1–3), 2 (1–2), 1 (0–1), 1 (0–1) at baseline, 12, 18 and 24 months respectively. The decrease in the scores was detected between the baseline and 18 months (p = 0.001) and the baseline and 24 months (p = 0.003).
Figure 5.
Evolution of partial Mayo score from baseline to 24 months of follow up.
In contrast, no significant changes in the median CRP level (95% CI) were detected over the same period [2 (1–10), 0.52 (0.13–2.4), 1.2 (1–2.02), 1.5 (1–3.1) at 0, 12, 18 and 24 months, respectively; p = 0.365].
IFX drug levels
No significant changes in the median IFX drug level (95% CI) were observed in patients with CD over the same period [3.2 µg/ml (1–6.6), 3.6 µg/ml (1.2–6.8), 4.8 µg/ml (1.6–6.3)] at 12, 18 and 24 months (p = 0.792). Similarly, no significant changes were found in the median IFX drug level (95% CI) in patients with UC during follow up [6.5 µg/ml (1.9–13.4), 4.8 µg/ml (1.2–13.4), 4 µg/ml (2.8–8.8)] at 12, 18 and 24 months respectively (p = 0.590).
Safety
In 14 (14%) patients the following AEs were observed: 1 skin reaction, 1 asthenia, 2 headaches, 2 paresthesias during infusion, 1 Sweet’s syndrome, 1 polyarthralgia, 1 vaginal infection, 1 Herpes simplex infection, 1 candida infection, 1 psoriasis case, and 2 palpitations cases. In this group, four patients discontinued treatment because of these AEs. A total of six patients had a hospital admission: one patient because of a cerebrovascular accident and five because of a flare of disease. Overall, three out of this last group of five patients needed surgical intervention (two CD, one UC), and the other two were discharged from hospital on steroids.
IFX levels
At 12 months after the switch, the results of IFX levels were obtained in 75 patients: 44% of the patients had normal IFX levels, 30.7% had low IFX levels and 25.3% had high levels. Overall, two patients with CD developed ADAs during the follow up over 18 months, one discontinued treatment due to remission, and one was changed to adalimumab.
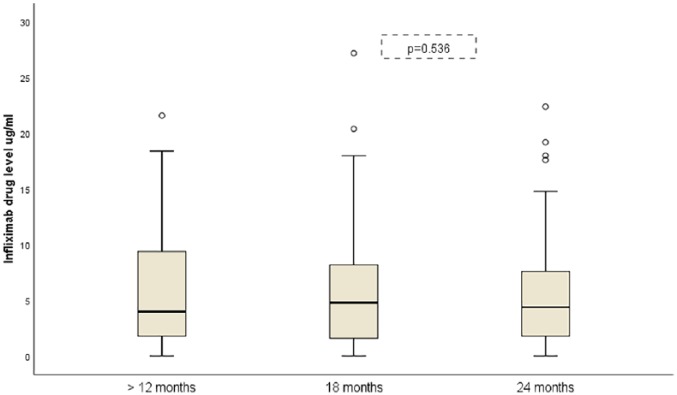
Figure 6 shows the median IFX drug levels (95% CI) in patients who completed 24 months of follow up (n = 51).
Figure 6.
Evolution of infliximab drug levels during follow up.
Discussion
We have previously reported the results of our cohort of patients, whose IFX treatment was switched after a 6 and 12-month follow-up period.24,27 In the present study, we report our results during the second year of follow up. Remarkably, there are few reports about long-term follow up of patients who switched to an IFX biosimilar.28,29 Smits and colleagues28 in a recently published study with 2 years of follow up, observed that 66% of patients who switched to CT-P13 remained on the therapy, 25% needed a dose increase, and 12% discontinued the therapy due to a loss of response. Høivik and colleagues29 included 143 patients, who were switched from the original IFX to CT-P13 and were followed prospectively for 18 months; 91% of the patients remained on CT-P13 and 10 patients discontinued the CT-P13.
In our study, with a median disease time of 10 years, and prior treatment with the original IFX RP for 58 months, 72% of patients remained with the IFX biosimilar after the switch from the RP. The remission after switching was 68.5% at 24 months; 15% of patients discontinued the therapy due to loss of response. Our results are in line with the findings reported by Smits28 and Høivik29 and with historic data about IFX RP.30–33
The present data indicate a statistically significant global loss of remission at the 2-year follow up (10%). These values are consistent with the ones in the literature since the incidence of loss of response varies between 10–20% and 13–30%3,29–31 depending on the clinical trial and clinical practices. The annual risk of loss of response was suggested to be around 13–15% patients/year.34
When we analyzed each disease separately, patients with UC showed a significant decrease in the median of the partial Mayo score at 18 and 24 months compared with baseline. However, the majority of patients who were on clinical remission did not show changes from baseline to 24 months. In patients with CD, there was a significant change in remission during follow up, and loss of response occurred in 14.1% of patients. Høivik and colleagues29 showed that the loss of remission in patients with CD was greater than 12% without statistical significance. In the NOR-SWITCH trial,25 disease deterioration happened more frequently in patients with CD (36.5%) who were switched to an IFX biosimilar at 52 weeks of follow up, without statistically significant difference. It should be kept in mind that the NOR-SWITCH trial was not powered to assess changes within each indication, and so these results must be interpreted with caution.
In the 26-week open-label NOR-SWITCH EXTENSION trial35 safety and immunogenicity were assessed between a CT-P13 treated group throughout the 78-week study period (maintenance group) and a group that switched from IFX original to CT-P13 at week 52 (switch group). Overall, disease deterioration occurred in 16.8% of patients in the maintenance group and in 11.6% in the switch group. In CD, disease deterioration occurred in 20.6% and 11.1% of patients, whereas in the case of UC it was observed in 15.4% and 5.1% of the cases in the maintenance and switch group, respectively.35 Our results are in line with this reported rate of loss of response. We believe that this is due to the heterogeneous cohort of patients, with a median of previous treatment close to 5 years. Therefore, the loss of response cannot be attributed to the medication alone. In addition, 30.7% of the patients in our cohort had low IFX levels during the follow up, and that could explain this loss of response. However, we could not perform an analysis based on IFX levels, as the changes of the IFX doses were based on clinical symptoms.
In the current study, AEs occurred in 14% of the cases, and 5.6% of the patients discontinued treatment because of the AEs. These findings are in agreement with the results of other studies showing a rate of AEs between 10–20%.36,37 Overall, two patients with CD developed ADAs during the follow up over 18 months; one of the two discontinued the treatment because of remission, and the other one changed to adalimumab. These observations are in line with comparable switch studies. Smits28 and colleagues reported two new cases of ADAs in the first year of follow up. Høivik29 and colleagues reported 5.6% ADAs during the follow up; six patients developed transient ADAs at low levels, without clinical significance, and two others developed ADAs at moderate levels and discontinued the therapy.
In addition, when compared with the cost of the original IFX in Spain in 2015, the overall cost savings with the switch to CT-P13 were €3000 per patient per year.
Our study has some limitations. First, the study sample was heterogeneous with a median time of previous treatment of 58 months. It is known that the longer the same treatment lasts, the greater the chances of developing a loss of response are. In fact, only 66% patients had the standard IFX dose at the beginning of the study. Other additional limitations were that we could not measure fecal calprotectin as a biomarker of relapse because we did not have the technique available in our hospital, and we could not measure IFX levels during the first year after the switch. IFX levels measurement was introduced in our unit since 2016, and fecal calprotectin in 2017. This is the reason we could not compare IFX levels during the follow up.
Despite the already discussed limitations, this study provides valuable data regarding the long-term efficacy of CT-P13 treatment after switching from the original IFX and demonstrates that most of the patients maintained the therapy with a good profile of efficacy and safety after 2 years of follow up.
Acknowledgments
The authors would like to thank Dr Ana Saavedra and Pablo Rivas on behalf of Springer Healthcare, who provided writing assistance. Kern Pharma funded the medical writing support.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: M.F. Guerra Veloz, M. Belvis Jiménez, T. Valdez Delgado, L. Castro Laria, B. Maldonado Pérez have received financial support from Kern Pharma to attend scientific meetings. F. Argüelles-Arias has participated in advisory boards and has received financial support from Kern Pharma to attend scientific meetings. R. Perea Amarillo, V. Merino Bohórquez, A. Caunedo Álvarez and A. Vilches Arenas declare that there is no conflict of interest
ORCID iD: María Fernanda Guerra Veloz  https://orcid.org/0000-0001-9708-8245
https://orcid.org/0000-0001-9708-8245
Contributor Information
María Fernanda Guerra Veloz, Gastroenterology Department, University Hospital Virgen Macarena, C/ Dr. Fedriani 3, Seville, 41007, Spain.
María Belvis Jiménez, Gastroenterology Department, Virgen Macarena University Hospital, Seville, Spain.
Teresa Valdes Delgado, Gastroenterology Department, Virgen Macarena University Hospital, Seville, Spain.
Luisa Castro Laria, Gastroenterology Department, Virgen Macarena University Hospital, Seville, Spain.
Belén Maldonado Pérez, Gastroenterology Department, Virgen Macarena University Hospital, Seville, Spain.
Raúl Perea Amarillo, Gastroenterology Department, Virgen Macarena University Hospital, Seville, Spain.
Vicente Merino Bohórquez, Pharmacy Unit, University Hospital Virgen Macarena, Seville, Spain.
Ángel Caunedo Álvarez, Gastroenterology Department, Virgen Macarena University Hospital, Seville, Spain.
Ángel Vilches Arenas, Preventive Medicine and Public Health, University Hospital Virgen Macarena, Seville, Spain.
Federico Argüelles-Arias, Gastroenterology Department, Virgen Macarena University Hospital, Seville, Spain.
References
- 1. Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther 2008; 117: 244–279. [DOI] [PubMed] [Google Scholar]
- 2. European Medicines Agency. European public assessment reports: infliximab, www.ema.europa.eu/documents/product-information/remicade-epar-product-information_en.pdf (2009, accessed 23 January 2018).
- 3. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359: 1541–1549. [DOI] [PubMed] [Google Scholar]
- 4. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004; 350: 876–885. [DOI] [PubMed] [Google Scholar]
- 5. Sands BE, Blank MA, Patel K, et al. Long-term treatment of rectovaginal fistulas in Crohn’s disease: response to infliximab in the ACCENT II study. Clin Gastroenterol Hepatol 2004; 2: 912–920. [DOI] [PubMed] [Google Scholar]
- 6. Rutgeerts P, Sandborn W, Feagan B, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–2476. [DOI] [PubMed] [Google Scholar]
- 7. Haustein R, de Milla C, Höer A, et al. Saving money in the European healthcare systems with biosimilars. GaBI J 2012; 1: 120–126. [Google Scholar]
- 8. Mulcahy A, Hlavka J, Case S. Biosimilar cost saving in the United States. Initial experience and future potential. Rand Health Q 2018; 7: 3. [PMC free article] [PubMed] [Google Scholar]
- 9. Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when co-administered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis 2013; 72: 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis 2013; 72: 1605–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. European Medicines Agency. CHMP assessment report Remsima [EMA/CHMP/589317/2013], www.ema.europa.eu/documents/assessment-report/remsima-epar-public-assessment-report_en.pdf (2013, accessed 23 January 2018).
- 12. European Medicines Agency. New guide on biosimilar medicines for healthcare professionals, www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2017/05/news_detail_002739.jsp&mid=WC0b01ac058004d5c1 (2017, accessed 11 October 2017).
- 13. Feagan BG, Choquette D, Ghosh S, et al. The challenge of indication extrapolation for infliximab biosimilars. Biologicals 2014; 42: 177–183. [DOI] [PubMed] [Google Scholar]
- 14. Argüelles-Arias F, Barreiro-de-Acosta M, Carballo F, et al. Joint position statement by “Sociedad Española de Patología Digestiva” (Spanish Society of Gastroenterology) and “Sociedad Española de Farmacología” (Spanish Society of Pharmacology) on biosimilar therapy for inflammatory bowel disease. Rev Esp Enferm Dig 2013; 105: 37–43. [DOI] [PubMed] [Google Scholar]
- 15. Danese S, Fiorino G, Raine T, et al. ECCO position statement on the use of biosimilars for inflammatory bowel disease-an update. J Crohns Colitis 2017; 11: 26–34. [DOI] [PubMed] [Google Scholar]
- 16. BSG guidance on the use of biosimilar infliximab CT-P13 in inflammatory bowel disease, www.bsg.org.uk/resource/bsg-guidance-on-the-use-of-biosimilar-infliximab-ct-p13-in-ibd.html (2016, accessed 15 September 2018).
- 17. Farkas K, Rutka M, Bálint A, et al. Efficacy of the new infliximab biosimilar CT-P13 induction therapy in Crohn’s disease and ulcerative colitis – experiences from a single center. Expert Opin Biol Ther 2015; 15: 1257–1262. [DOI] [PubMed] [Google Scholar]
- 18. Keil R, Wasserbauer M, Zádorová Z, et al. Clinical monitoring: infliximab biosimilar CT-P13 in the treatment of Crohn’s disease and ulcerative colitis. Scand J Gastroenterol 2016; 51: 1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gecse K, Vegh Z, Kurti Z, et al. Final results on efficacy and safety of biosimilar infliximab after one-year: results from a prospective nationwide cohort. Gastroenterology 2017; 152: S393. [Google Scholar]
- 20. Park S, Kim Y, Lee J, et al. Post-marketing study of biosimilar infliximab (CT-P13) to evaluate its safety and efficacy in Korea. Expert Rev Gastroenterol Hepatol 2015; 9: 35–44. [DOI] [PubMed] [Google Scholar]
- 21. Farkas K, Rutka M, Bálint A, et al. Efficacy of the new infliximab biosimilar CT-P13 induction therapy in Crohn’s disease and ulcerative colitis – experiences from a single center. Expert Opin Biol Ther 2015; 15: 1257–1262. [DOI] [PubMed] [Google Scholar]
- 22. Jahnsen J. Clinical experience with infliximab biosimilar Remsima (CT-P13) in inflammatory bowel disease patients. Therap Adv Gastroenterol 2016; 9: 322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smits L, Grelack A, Derikx L, et al. Long-term clinical outcomes after switching from Remicade® to biosimilar CT-P13 in inflammatory bowel disease. Dig Dis Sci 2017; 62: 3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Argüelles-Arias F, Guerra Veloz M, Perea Amarillo R, et al. Effectiveness and safety of CT-P13 (biosimilar infliximab) in patients with inflammatory bowel disease in real life at 6 months. Dig Dis Sci 2017; 62: 1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jørgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017; 389: 2304–2316. [DOI] [PubMed] [Google Scholar]
- 26. Bergqvist V, Kadivar M, Molin D, et al. Switching from originator infliximab to the biosimilar CT-P13 in 313 patients with inflammatory bowel disease. Therap Adv Gastroenterol 2018; 11: 1756284818801244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Argüelles-Arias F, Guerra Veloz MF, Perea Amarillo R, et al. Switching from reference infliximab to CT-P13 in patients with inflammatory bowel disease: 12 months results. Eur J Gastroenterol Hepatol 2017; 29: 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smits LJT, van Esch AAJ, Derikx LAAP, et al. Drug survival and immunogenicity after switching from Remicade to biosimilar CT-P13 in inflammatory bowel disease patients: two-year follow-up of a prospective observational cohort study. Inflamm Bowel Dis 2019; 25: 172–179. [DOI] [PubMed] [Google Scholar]
- 29. Høivik ML, Buer LCT, Cvancarova M, et al. Switching from originator to biosimilar infliximab - real world data of a prospective 18 months follow-up of a single-centre IBD population. Scand J Gastroenterol 2018; 53: 692–699. [DOI] [PubMed] [Google Scholar]
- 30. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005; 353: 2462–2476. [DOI] [PubMed] [Google Scholar]
- 31. Roda G, Jharap B, Neeraj N, et al. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Trans Gastroenterol 2016; 7: e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sprakes M, Ford A, Warren L, et al. Efficacy, tolerability, and predictors of response to Infliximab therapy for Crohn’s disease: a large single centre experience. J Crohn Colitis 2012; 6: 143–153. [DOI] [PubMed] [Google Scholar]
- 33. Chaparro M, Panes J, Garcia V, et al. Long-term durability of infliximab treatment in Crohn’s disease and efficacy of dose “escalation” in patients losing response. J Clin Gastroenterol 2011; 45: 113–118. [DOI] [PubMed] [Google Scholar]
- 34. Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol 2009; 104: 760–767. [DOI] [PubMed] [Google Scholar]
- 35. Goll GL, Jørgensen KK, Sexton J, et al. Long-term efficacy and safety of biosimilar infliximab (CT-P13) after switching from originator infliximab: Open-label extension of the NOR-SWITCH trial. J Intern Med. Epub ahead of print 14 February 2019. DOI: 10.1111/joim.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fiorino G, Manetti N, Armuzzi A, et al. The PROSIT-BIO cohort: a prospective observational study of patients with inflammatory bowel disease treated with infliximab biosimilar. Inflamm Bowel Dis 2017; 23: 233–243. [DOI] [PubMed] [Google Scholar]
- 37. Komaki Y, Yamada A, Komaki F, et al. Systematic review with meta-analysis: the efficacy and safety of CT-P13, a biosimilar of anti-tumour necrosis factor-α agent (infliximab), in inflammatory bowel diseases. Aliment Pharmacol Ther 2017; 45: 1043–1057. [DOI] [PubMed] [Google Scholar]