Abstract
The kynurenine pathway is important in cellular energy generation and limiting cellular ageing as it degrades about 90% of dietary tryptophan into the essential co-factor NAD+ (nicotinamide adenine dinucleotide). Prior to the production of NAD+, various intermediate compounds with neuroactivity (kynurenic acid, quinolinic acid) or antioxidant activity (3-hydroxykynurenine, picolinic acid) are synthesized. The kynurenine metabolites can participate in numerous neurodegenerative disorders (Alzheimer disease, amyotrophic lateral sclerosis, Huntington disease, and Parkinson disease) or other diseases such as AIDS, cancer, cardiovascular diseases, inflammation, and irritable bowel syndrome. Recently, the role of gut in affecting the emotional and cognitive centres of the brain has attracted a great deal of attention. In this review, we focus on the bidirectional communication between the gut and the brain, known as the gut-brain axis. The interaction of components of this axis, namely, the gut, its microbiota, and gut pathogens; tryptophan; the kynurenine pathway on tryptophan availability; the regulation of kynurenine metabolite concentration; and diversity and population of gut microbiota, has been considered.
Keywords: gut microbiota; gut-brain axis; indoleamine 2,3-dioxygenase; kynurenine pathway; neurodegenerative disorder; tryptophan
Background
Microorganisms are capable of producing a wide range of metabolites from antibiotics,1–3 pigments,4 and vitamins, to antioxidants,5,6 enzymes,7 and toxins.8 They can exert positive or negative effects through the production of some of these metabolites when they live in the human body as microbiota.9 For instance, the human microbiota, particularly gastrointestinal (GI) tract microbiota and their metabolites, influence both the physiology and immunity states of the body. In recent decades, many efforts have been devoted to understand the complex roles of GI microorganisms in several health complications in respect of diversity and population. For example, the microbiota-brain communication and its mechanism of action on the various organs are under investigation.10–12 The kynurenine pathway (KP) is a major pathway of l-tryptophan catabolism, contributing to the production of various neuroactive compounds.13 It is well documented that the KP metabolites are associated with inflammatory responses, neurological disorders, and immunity regulation.14 Similarly, the metabolism of l-tryptophan in the GI tract by the host cells and microbiota may be involved in intestinal homeostasis and various health complications through influencing the biosynthesis of some compounds such as neurotransmitters, neurotoxicity, and antimicrobial metabolites. This review discusses the role of GI bacteria in tryptophan metabolism and explains how tryptophan metabolism, particularly through the KP, can affect the diversity and population of microbiota in the GI. We begin by describing tryptophan catabolism by the KP and its impact on health status. This is followed by discussion on the interactions between GI bacteria and tryptophan metabolism and their effects on various immunity responses and disorders. Understanding the complex communication between GI microbes and tryptophan metabolism can be helpful in exploring new treatment targets in various human diseases.
Tryptophan and Its Importance in Human Body
Tryptophan is an essential amino acid, which cannot be synthesized by the human body. Therefore, it must be provided through nutritional sources. Tryptophan is found in 2 forms in the body: either bound to albumin or in free form. Both are in equilibrium. The transportation of tryptophan across the blood-brain barrier occurs only in the free form through non-specific transporters of l-type amino acids. The amount of l-tryptophan in the tissue of the human body is lower than other amino acids,15 and minute concentrations of tryptophan are essential for maintaining health.16,17 The recommended amount of tryptophan is approximately 250 to 425 mg/d/adult, but usually a higher dose of 900 to 1000 mg/d for each individual is received from the diet.16 The most common sources of tryptophan in the human diet are fish, bananas, milk, oats, chicken, chocolate, and peanuts.15
In the human body, tryptophan is mainly consumed for proteins synthesis and is produced in smaller amounts than other amino acids. It, therefore, may play a rate-limiting role in the synthesis of proteins.16,18 In addition, tryptophan is a precursor of 2 metabolic pathways, including KP and serotonin pathway.19,20 Approximately 90% of tryptophan catabolism occurs through KP,21 which produces various metabolites with kynurenic acid and quinolinic acid as the most important intermediates. The former is recognized as glutamate receptor antagonist, whereas the latter is a glutamate receptor agonist.22 In mammals, approximately 95% of the serotonin is found in the GI tract, and only 3% of dietary tryptophan is used for serotonin synthesis.15 The amount of 1% of dietary tryptophan is involved in serotonin synthesis in the brain,23 which functions as a neurotransmitter and neuromodulator. In addition to protein, kynurenine, and serotonin synthesis, tryptophan is used for the synthesis of tryptamine, which is a neuromodulator of serotonin. In vivo studies have indicated that tryptamine can control the balance between the excitatory and inhibitory activity of serotonin.24 Moreover, tryptamine is recognized as an independent neurotransmitter with a specific receptor.24 Melatonin is another metabolite produced in the serotonin pathway and influences the digestive, immune, and reproductive systems and diurnal rhythms.25,26 Tryptophan can also act as a substrate for the synthesis of coenzymes nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). Nicotinamide adenine dinucleotide participates in cellular energy (oxidation-reduction reactions) and ageing.
KP: The Major Route of Tryptophan Catabolism
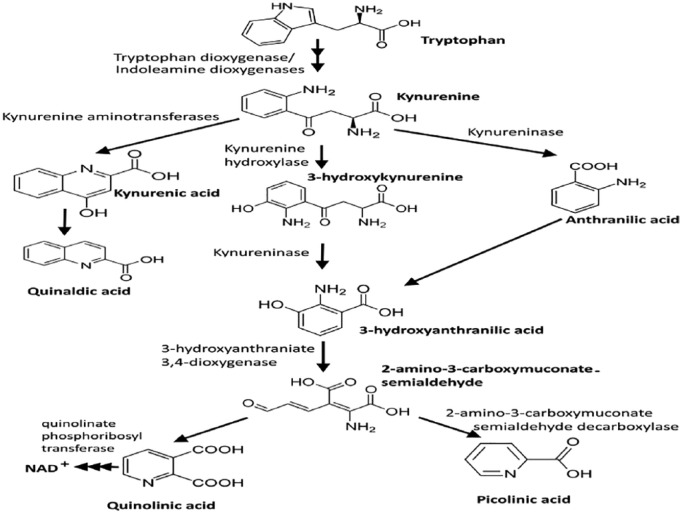
Nicotinamide adenine dinucleotide and several neuroactive intermediates are produced during the KP, that is, the main route of l-tryptophan catabolism (Figure 1).13 The intermediates include kynurenine, kynurenic acid, 3-hydroxykynurenine, 3-hydroxyanthranilic acid, picolinic acid, and quinolinic acid.27 In the first step, tryptophan is cleaved by tryptophan 2,3-dioxygenase (TDO), indoleamine 2,3-dioxygenase (IDO) 1, or IDO2. TDO is mainly present in liver cells, whereas IDO1 is found in other type of cells, including microglia, macrophages, and neurons.28 IDO1 can be upregulated by cytokines and inflammatory molecules such as interferon γ (IFN-γ) (the most potent inducer), amyloid peptides, and lipopolysaccharides. Kynurenine is produced from tryptophan by the catalytic activity of IDO or TDO and is converted into kynurenic acid, 3-hydroxy kynurenine, and anthranilic acid, respectively. 3-hydroxy kynurenine and anthranilic acid are converted into 3-hydroxyanthranilic acid and finally can form either picolinic acid or quinolinic acid in enzymatic (2-amino-3-carboxymuconate-semialdehyde decarboxylase) or nonenzymatic reactions, respectively (Figure 1).
Figure 1.
Kynurenine pathway, the main route of tryptophan metabolism. NAD indicates nicotinamide adenine dinucleotide.
KP and Its Role in Human Health Status
The KP has been tracked in various diseases of the central nervous system (CNS) such as Alzheimer disease, amyotrophic lateral sclerosis, AIDS dementia complex, and Huntington disease.14,29-31 Under various CNS disorders, the metabolism of tryptophan increases and the concentration of KP metabolites arises in the serum and cerebrospinal fluid (CSF) (Table 1). Here, we describe the impact of various intermediates of the KP on human health status.
Table 1.
Measurement of kynurenine pathway metabolites in plasma/serum and CSF of CNS patients.
| Disease | Origin of sample | Compound | Patient | Control | References |
|---|---|---|---|---|---|
| Anxiety | Plasma/serum | KYN (μM) | 9.32 ± 0.2 | 4.32 ± 0.3 | Orlikov et al32 |
| Alzheimer disease | Plasma/serum | KYN (μM) KYNA (nM) |
2.5 ± 0.1 15.82.31 ± 1.1 |
2.01 ± 0.2 23.13 ± 2.2 |
Hartai et al33 |
| ALS | Plasma/serum | KYNA (nM) | 57.8 ± 35.0 81.6 ± 41.2a (m.c.s.) 39.9 ± 14.7 (s.c.s.) |
59.6 ± 20.5 | Ilzecka et al34 |
| ALS | Plasma/serum | TRP (μM) KYN (μM) QUIN (mM) PIC (mM) K/T (×103) |
143.28 ± 5.64 4.02 ± 0-2 0.37 ± 0.018 1.42 ± 0.087 37 ± 2.5 |
75.0 ± 10.5 2.52 ± 0.19 0.30 ± 0.026 2.38 ± 0.37 39 ± 4 |
Chen et al35 |
| Chronic brain injury | Plasma/serum | TRP (μM) KYN (μM) KYNA (μM) 3-HK (μM) 3-HAA (μM) QUIN (μM) K/T (×103) |
Data obtained from graph: No difference Higher Lower Lower Lower No difference Higher |
Mackay et al36 | |
| CNS pathology | CSF | QUIN (nM) KYN (nM) |
31 ± 5 (Hy) 200 ± 113 (H) 282 ± 82 (T) 1084 ± 549 (C) 185 ± 40 (Hy) 254 ± 128 (H) 1698 ± 589 (T) 2610 ± l067 (C) |
20 ± 2 54 ± 7 |
Heyes et al37 |
| HD | Plasma/serum | TRP (µM) KYN (µM) KYNA (µM) 3-HK (µM) 3-HAA (µM) QUIN (µM) K/T (×103) |
Data obtained from graph: No difference Higher No difference Lower Lower No difference Higher |
Stoy et al38 | |
| Major depression | Plasma/serum | TRP (μM) KYN (μM) KYNA (nM) 3-HAA (nM) K/T (×103) |
65.8 ± 15.57 1.81 ± 0.56 24.29 ± 8.09 24.53 ± 11.91 25 ± 12 |
69.71 ± 13.65 1.87 ± 0.43 35.95 ± 13.4 24.12 ± 7.3 17 ± 14 |
Myint et al39 |
| MS | CSF | KYNA (nM) | 0.41 (MS) 0.67 (OND) 1.7 (ID) |
Rejdak et al40 | |
| MS and cerebrovascular disease (CVD) | CSF | TRP (nM) 5-HT (pM) 5-HIAA (pM) |
1.25 ± 0.14 (MS) 3.34 ± 0.54 (CVD) 5 ± 1 (MS) 7 ± 2 (CVD) 116 + 15 (MS) 299 ± 50 (CVD) |
2.02 ± 0.34 7 ± 2 173 ± 20 |
Baig et al41 |
| Schizophrenia | CSF | KYN (nM) | 1.67 ± 0.027 | 0.97 ± 0.07 | Erhardt et al42 |
| Schizophrenia | CSF | KYNA (nM) | 1.45 ± 0.10 (grp) 1.53 ± 0.19 (1st) 1.53 ± 0.17 (T) 1.16 ± 0.06 (noD) |
1.06 ± 0.06 | Nilsson et al43 |
| Attention deficit hyperactivity disorder | Serum | KYNA (ng/mL) TRP (ng/mL) KYN (ng/mL) |
3.2 ± 0.9 8914.9 ± 1776.3 440.3 ± 158.6 |
3.6 ± 1.4 8038.6 ± 2219.6 296.0 ± 148.7 |
Evangelisti et al44 |
Abbreviations: 1st, drug-naïve first-episode patients; ALS, amyotrophic lateral sclerosis; C, CSF infection; CNC, central nervous system; CSF, cerebrospinal fluid; grp, all patients; H, haemorrhage; Hy, hydrocephalus; ID, infectious inflammatory disease; m.c.s/s.c.s., mild clinical status/severe clinical status; MS, patients with relapsing multiple sclerosis (MS) during remission or not progressing for at least 2 months; noD, patients who had been treated but are now drug-free; OND, non-inflammatory neurological disorders; T, patients undergoing treatment with anti-psychotic drugs; T, tumour.
Significantly lower KYNA in s.c.s. compared with m.c.s.
Kynurenic acid
Kynurenic acid is a neuroprotective molecule which can act as an antagonist of quinolinic acid on both glycine and glutamate modulatory sites of N-methyl-d-aspartate (NMDA) receptor at low and high concentrations, respectively. This molecule also is an antagonist for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate and alpha7 (α7) nicotinic acetylcholine receptors.45 High levels of kynurenic acid in the brain have anticonvulsant, sedative, and protective effects against brain ischaemia.46 On the contrary, increases in kynurenic acid in CSF of patients with schizophrenia have been reported, suggesting the effect of this molecule on the glutamatergic and dopaminergic systems as well as its possible activity in the development of schizophrenia.42 In vivo studies have shown that in a septic shock mouse model, a significant decrease in the release of tumour necrosis factor α, nitric oxide, and high-mobility group box 1 protein occurred in the presence of kynurenic acid.47 Unexpectedly, kynurenic acid can suppress the release of fibroblastic growth factor 1. This compound mainly facilitates the recovery of damaged cells and promotes glial cell proliferation.47
3-hydroxyanthranilic acid
This neurotoxin compound is produced through 3-hydroxykyurenine hydrolysis or anthranilic acid oxidation. The presence of copper and 3-hydroxyanthranilic acid causes free radical generation. However, it has been found that this compound can also act as an antioxidant and radical scavenger.48 Inhibition of nuclear factor κB and inducible nitric oxide synthase has been observed in murine macrophages after treatment with 3-hydroxyanthranilic acid at sub-millimolar concentrations.49 Studies of the lines of tumorigenesis have indicated that 3-hydroxyanthranilic acid is non-toxic at low concentrations and could not affect the T-cell receptor–induced CD8+ T-lymphocyte proliferation. However, it can significantly inhibit the cell proliferation triggered by antigen-independent cytokine.50 Moreover, 3-hydroxyanthranilic acid has a selective apoptotic activity on murine thymocytes and TH1 cells. This selective apoptosis is triggered through the activation of caspase 8 and release of cytochrome c from mitochondria. Notably, this activity is observed at lower concentrations in those cells that are needed for the apoptosis of macrophages, representing a significant role of this compound in peripheral immunoregulation.51
Picolinic acid
This endogenous neuroprotectant compound is recognized as an iron and zinc chelator. Various activities such as antifungal, antiviral, antitumoural, and cell growth regulatory activities have been reported for picolinic acid. In vitro studies have exhibited that picolinic acid can potentially arrest the cells in the G1 phase through interaction with NAD+.52 It has been found that the SK-N-SH neuroblastoma cell line is not capable of synthesizing picolinic acid.53 Therefore, the absence of this antitumour activity may lead to tumour persistence.
Picolinic acid exerts its antifungal activity through a synergetic effect with IFN-γ to boost the inhibitory activity of neutrophils against Candida albicans.54,55 At concentrations of 1.5 to 3 mM, picolinic acid showed antiviral and cytotoxic activity against HIV-1 and human herpes simplex virus 2, which could be due to the upregulation of the inflammatory protein (MIP-1α) in macrophages and expression of MIP-1β messenger RNA (mRNA).56,57 It is important to note that this effect of picolinic acid on MIP-1α and MIP-1β is counteracted by IFN-γ, showing the significant role of picolinic acid-IFN-γ complex in the regulation of inflammatory responses.58
Similar to kynurenic acid, but to a lesser extent, picolinic acid can also inhibit the neurotoxic effect of quinolinic acid through a different mechanism, that is, possibly through chelating zinc and/or decreasing the calcium-dependent glutamate release.59,60
Quinolinic acid
Quinolinic acid can exert neurotoxic activity by activating NMDA receptors in neurons. In the brain, most tryptophan molecules are metabolized into 5-hydroxytryptamine rather than to formylkynurenine, leading to lower concentrations of quinolinic acid than in blood and systemic tissues.61 Interestingly, during a systemic or central immune response, the IDO1 activity and quinolinic acid concentration increase. The reason for this is unclear.62 Infiltrating macrophages, dendritic cells, and microglia provide the main sources of quinolinic acid in the brain during inflammation.63 Astrocytes are unable to synthesize the quinolinic acid due to the fact that these cells do not have the essential enzyme, kynurenine hydroxylase. Quinolinic acid can play a neuroprotective role in low concentration, which could be catabolized to NAD+. However, quinolinic acid levels beyond this point cause saturation of the catabolic system, resulting in neurotoxic effects.64 In vitro, quinolinic acid can induce the TH1 target cells’ apoptosis and can selectively suppress the proliferation of killer cells, and CD4+ and CD8+ T lymphocytes that are overstated under the absence of tryptophan.65,66 These studies have revealed that the sub-millimolar concentration of quinolinic acid has a toxic effect on neuronal culture and causes cell death.67,68 Moreover, in vivo investigations have shown that injection of quinolinic acid to the brain of rats generates axon-sparing lesions.69 According to the several studies, quinolinic acid contributes to various neurodegenerative diseases such as Alzheimer disease, AIDS dementia complex, Huntington disease, and multiple sclerosis.27,70
The Gut Microbiota-Brain Communication
The microbiota, a complex dynamic community of microorganisms with a rich pool of genetic materials, has significant roles in the physiological and developmental processes of humans.71,72 Microbiota is present in various organs of body such as the skin, oral cavity, respiratory and GI tracts, and vagina. Composition of microbiota is an important factor in determining their positive or negative roles in the human host’s health.9 The microbiota is a dynamic community evolving during the lifespan of the host, particularly in the first 3 years of life, to form a relatively stable population of microbiota.73 The GI tract harbours the largest community of microbes with a total population of about 1014 from 1000 microbial species containing 4 × 106 genes.74,75 The gut microbiota of a typical healthy adult consists of 4 main phyla, including Actinobacteria, Bacteroidetes, Firmicutes, and Verrucomicrobia. A number of factors such as age, antibiotic intake, diet, genes, infection, and stress affect the community of microbiota in the GI tract.76 For example, the population of a microbial community shifts in favour of Bacteroidetes species in the gut of old people, compared with young individuals.77
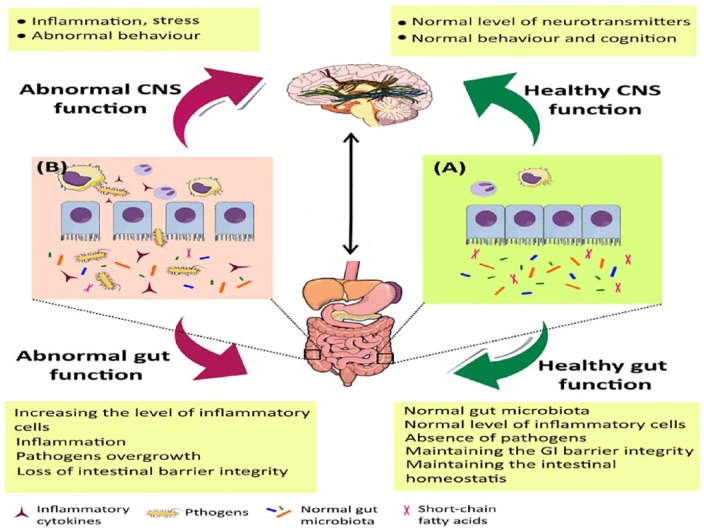
The significant regulatory and modulating roles of microbiota in the gut-brain axis and the brain function, as well as developing a bidirectional communication between the brain and gut, have been extensively studied.11,72,78 The communication between GI tract and CNS involves the CNS, enteric nervous system, neuroendocrine and neuroimmune pathways, sympathetic and parasympathetic nerve systems, and the gut microbial community.79 The homeostasis preservation which results from the normal gut-brain communication may be disrupted in various diseases. Moreover, any disturbance in the brain-GI bidirectional communication reduces the functions of digestive system, that is, digestion and absorption of food (Figure 2).79,80
Figure 2.
Communication between the gut microbiota and the CNS. (A) Normal microbial community in the GI controls the intestinal homeostasis and contributes to better immunity responses in the intestine and the brain. In contrast, (B) intestinal dysbiosis alters the normal microbial community in the GI and adversely affects immunity regulation in both organs as well as the CNS function. CNS indicates central nervous system; GI, gastrointestine.
The GI microbiota can adjust neurotransmitter levels through either production of the neurotransmitter or regulation of the production of its precursors. For example, it has been reported that the neurotransmitter γ-aminobutyric acid is produced by Bifidobacterium and Lactobacillus species.78 In addition, Bacillus, Escherichia, and Saccharomyces species have the ability to produce the neurotransmitter norepinephrine.78 The effects of GI microbiota on neurogenesis have been investigated using in vivo studies in mice.81 Accordingly, germ-free (GF) mice showed higher hippocampal neurogenesis in the dorsal hippocampus. Post-weaning of microbiota in GF mice could not reverse the changes, emphasizing the significance of the pre-weaning microbial colonization in GI.81
A number of investigations have concluded that the GI microbiota can influence behaviour, mood, and anxiety.82-84 These findings have been reported through using various approaches such as GF animal models, antibiotic treatment, probiotic treatment, and GI microbiota transplantation.79 A more recent study has reported that individuals with depressive-like behaviour have a different GI microbiota composition compared with healthy controls.85 It is worth mentioning that gut microbiota may also contribute to social behaviour. It has been reported that the faecal microbiota transplantation from depressive-like patients to GF models could induce the depressive-like phenotype and behaviour in the latter group.83 In addition, in vivo studies have indicated that GF mice exhibited different social behaviours, compared with normal mice.84 This altered behaviour can be normalized through post-weaning the gut microbiota.
Effect of Microbiota on Tryptophan Metabolism and KP
Serotonin is one of the important compounds produced during tryptophan metabolism. The enterochromaffin cells of the mucosa and the nerve terminals of the enteric nervous system neurons are the major serotonin sources in the gut.82 Serotonin is mainly responsible for controlling GI motility and secretion in the gut and plays an important role in mood and cognition in the brain.86,87 Any changes to the production or amount of this neurotransmitter may cause GI and brain disorders. The availability of tryptophan strongly determines the ability of the brain in serotonin synthesis. The necessary amount of tryptophan in the brain is solely supplied through the diet. Therefore, the peripheral level of tryptophan in GI is essential for serotonin synthesis in the CNS. Interestingly, in the brains of GF animals, alteration in the level of serotonin synthesis was correlated with the availability of tryptophan.88 In another study, it has been reported that tryptophan depletion due to a low-tryptophan diet causes low concentration of serotonin in the CNS of human and animal models.89 The inhibition of IDO1 that is responsible for the conversion of tryptophan to kynurenine decreases serotonin concentration in the brains of rats and the subsequent alteration in behaviour.90
About 90% of tryptophan is metabolized by the KP,91 and its conversion rate is determined by the expression of IDO1 (in all tissues) and TDO (in liver only). Inflammatory molecules and corticosteroids can affect the activity of these 2 enzymes, respectively. In vitro and in vivo studies have shown that when INF-γ activates the IDO1, more tryptophan is converted to kynurenine than serotonin, and depressive symptoms are observed.92 Desbonnet et al93 reported that probiotic Bifidobacterium infantis can affect the tryptophan metabolism, and hence gut-brain axis communication. The inflammatory response decreased and serotonergic precursor increased when animal models were orally given B infantis. Consequently, GI inflammation was attenuated, and depression was treated. However, this finding could not be generalized to all members of the genus Bifidobacterium, for example, Bifidobacterium longum did not alter tryptophan metabolism and KP in examined animal models.79 In the absence of gut microbiota, an increase in the concentration of tryptophan in plasma leads to a reduction in the kynurenine-to-tryptophan ratio, which can be attributed to the activities of IDO and TDO. Notably, administration of microbiota could restore the normal activity of these enzymes.79
Among the metabolites produced in the KP, quinolinic acid and kynurenic acid are the most important compounds in neurogastroenterology studies. These metabolites target NMDA and α7 nicotinic acetylcholine receptors in the CNS and the enteric nervous system. The role of quinolinic acid and kynurenic acid in GI has been poorly understood. However, it seems that both of these compounds are immunoregulators. To date, the anti-inflammatory role of kynurenic acid in the GI and its inhibitory effect on colon cancer cells have been confirmed through the in vitro and in vivo studies.94
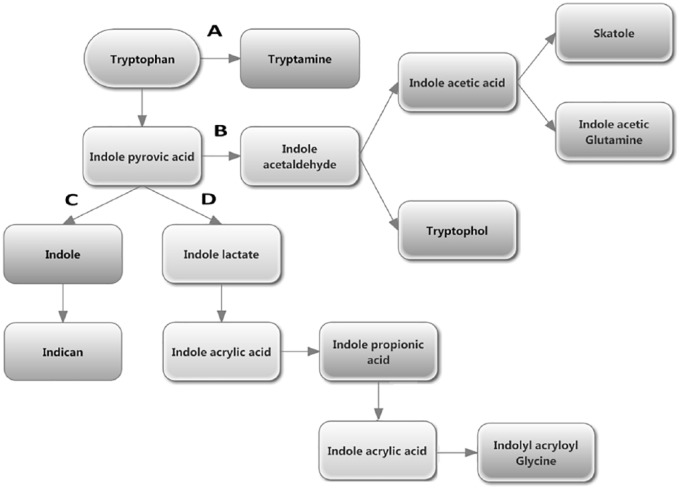
Gut microbiota is able to metabolize the tryptophan directly and change the tryptophan availability in their host. About 4% to 6% of tryptophan is metabolized into indican, indole or indole acid derivatives, skatole, and tryptamine by the gut bacteria (Figure 3).95 Although the definition of tryptophan metabolism by the gut bacteria is relatively easy at the molecular level, it is complicated to determine which type of metabolite gut bacteria produce. This is due to the diverse population of microbial community and their complex ecology.
Figure 3.
Tryptophan metabolism in the gastrointestine by microbiota. (A) Decarboxylation of tryptophan to tryptamine by Clostridium sporogenes and Ruminococcus gnavus. (B) Conversion of tryptophan into indole pyruvic acid by tryptophanase followed by its pyruvic acid decarboxylation produces the precursor of tryptophol and indole acetic acid, that is, indole acetaldehyde. Indole acetic acid is converted to skatole by Lactobacillus, Clostridium, Bacteroides, and others.95 (C) Production of indole and indican from indole pyruvic acid. (D) Catabolism of indole pyruvic acid into indole acrylic acid and its further conversion into indolyl acryloyl glycine after incorporation of glycine molecule.
The Role of Bacteria in Activation of IDO1
IDO1, the rate-limiting enzyme of KP, can influence the adaptive immunity of hosts.96 Tissue damage causes overexpression of IDO1 by intestinal mononuclear cells. This enzyme can induce anti-inflammatory and immunosuppressive responses on the intestinal mucosa through regulation of the host immunomodulatory activities by kynurenine production, mucosal immune reactivity, and metabolism of gut microbes.97,98 In addition, IDO1 can exert its effect on T cells to establish the balance of anti-inflammatory and pro-inflammatory status in the gut. IDO1 can also regulate the host-microbiota relationships by affecting tryptophan metabolism.99,100 On the contrary, the gut microbiota can alter the tryptophan degradation rate as well as its availability, and hence influences the tryptophan metabolism through the KP in the host. In vivo studies have shown that in GF animals, KP activity and kynurenine-to-tryptophan ratio are reduced due to the lack of microbial population. However, this deficiency is eliminated after administration of normal microbiota in GF animals.88 Importantly, in animal models with microbiota deficiency, the KP is not detected in the CNS. Infection of rodents with Toxoplasma gondii increased the metabolites of the KP in the brain, such as kynurenine, kynurenic acid, quinolinic acid, and 3-hydroxykynurenine.101 In contrast, transferring the Bifidobacterium infantis in rodents increased the tryptophan level and kynurenic acid-to-kynurenine ratio and reduced both the kynurenine-to-tryptophan ratio and IDO1 activity.93 Colonization of Lactobacillus johnsonii in rats decreased the levels of IDO1 mRNA and kynurenine concentrations in serum. Similarly, cell-free suspension of L johnsonii decreased the IDO1 activity by 47% in HT-29 intestinal epithelial cells.102,103 The main mechanism for the decrease in IDO1 activity after colonization with L johnsonii is the production enhancement of H2O2 in ileum lumen. H2O2 acts as a signalling molecule and inhibits the activity of IDO1 via affecting the host-microbiota symbiotic interactions.95
The high activity of KP enzymes contributes to increasing the kynurenine-to-tryptophan ratio, which may be considered as a biomarker for irritable bowel syndrome.104 In addition, the high ratio of kynurenine to tryptophan has been reported in other inflammatory diseases and cancers in which the concentration of IFN-γ is higher than the normal status. The inflammatory process that causes IDO1 activation and subsequent larger kynurenine-to-tryptophan ratio may have a microbial origin. Brottveit et al105 reported an increase of IFN-γ in duodenal biopsies of gluten-sensitive patients with no coeliac disease after eating gluten-containing bread. Gluten is a compound that is not completely digested by humans, so a high amount of intact gluten remains in the small intestinal cells, which can be toxic to human cells and has antimicrobial and immunomodulatory properties. Moreover, the undigested gluten can be used as a food source for gut microbes, leading to the activation of IFN-γ, followed by IDO1 activation.
Kynurenines have antimicrobial activity and can affect the microbial community of the GI tract. However, microbiota can influence the tryptophan metabolism via KP in the host in association with the immune system. Some investigations showed that GF animals possessed deficient immune systems and low rates of tryptophan metabolism through the KP. After administration of microbiota into the GF animals, tryptophan metabolism and KP were normalized.88 Toll-like receptors (TLRs) act as important junctions for identification of the microbial components in the GI tract. In GF animals, the expression of TLRs decreases and KP metabolism increases, which can be in association with IFN-γ–mediated IDO1 activity elevation.106 Aryl hydrocarbon receptor (AhR) is a cytosolic transcription factor that acts as an immunity and inflammation regulator and mediates intestinal homeostasis and carcinogenesis.107 The KP metabolites such as kynurenic acid, xanthurenic acid, and cinnabarinic acid can attach to the AhR and induce the expression of AhR-dependent gene and lead to intestinal homeostasis. In addition, AhR itself can modulate the expression of IDO1 and TDO and is considered a main mediator of GI microbiota, KP, and the host immune system. Briefly, interactions between the gut microbiota, KP metabolites, and immune responses are complex and are controlled by various mechanisms. Gut microbiota has significant roles in KP and its metabolites and controls the host’s immunity through tryptophan metabolism adjustment.95
Conclusion
Tryptophan is one of the essential amino acids for humans, which contributes to controlling emotional states, that is, happiness, well-being, as well as the cellular ageing process and energy production through its conversion into serotonin or NAD+. These compounds are the end products of the serotonin pathway and KP, respectively. Despite potential therapeutic effects of supplying excessive amounts of NAD+, KP can divert tryptophan away from serotonin pathways and cause depression. Moreover, enhanced activity of KP may lead to some neurodegenerative disorders through the production and accumulation of neurotoxic intermediates such as quinolinic acid. Generally, a large kynurenine-to-tryptophan ratio due to the overexpression of KP enzymes is observed in different human health complications, including irritable bowel syndrome, inflammatory diseases, cancer, and neurodegenerative disorders. Gut microbiota can also consume tryptophan and subsequently decrease its availability for both pathways because the human body can receive this amino acid from diet only. Moreover, the KP rate-limiting enzymes such as IDO1 and gut microbiota have mutual effects on each other. Some gut microbiota and gut pathogens can enhance IDO1 activity. In contrast, gut microbiota may produce some compounds such as H2O2 to suppress IDO1. On the contrary, IDO1 can make tryptophan unavailable for gut microbiota consumption and convert it into potential antimicrobial intermediates, such as kynurenine, resulting in killing some of the microbiota. In addition, the diversity, population, and ecology of gut microbiota are also determined by antibiotic intake, certain types of food ingredients (such as gluten), diseases, age, and stress, which influence tryptophan availability and KP rate. As the result of these interactions, a sophisticated and highly interconnected loop involving gut microbiota, KP, and tryptophan availability is drawn. Currently, our insight into this loop is very poor. By careful investigation, therapeutic or preventive use(s) of gut microbiota in some human diseases by regulating the tryptophan availability and/or the KP rate may be understood. Alternatively, gut microbiota may have the potential to reduce the concentration of some neurotoxic KP intermediates, lowering the severity/pathogenicity of neurodegenerative disorders.
Acknowledgments
The technical and language editing was provided by Red Fern Communication, Australia (www.redferncommunication.com).
Footnotes
Funding:Prof Gilles J Guillemin is supported by the National Health and Medical Research Council (NHMRC) and the Australian Research Council (ARC).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Dr. Dehhahgi and M. Kazemi Shariat Panahi both wrote the manuscript and Prof Guillemin edited, approved and finalized it.
ORCID iD: Gilles J Guillemin  https://orcid.org/0000-0001-8105-4470
https://orcid.org/0000-0001-8105-4470
References
- 1. Mohammadipanah F, Panahi HKS, Imanparast F, Hamedi J. Development of a reversed-phase liquid chromatographic assay for the quantification of total persipeptides in fermentation broth. Chromatographia. 2016;79:1325–1332. [Google Scholar]
- 2. Panahi HKS, Mohammadipanah F, Dehhaghi M. Optimization of extraction conditions for liquid-liquid extraction of persipeptides from Streptomyces zagrosensis fermentation broth. Eur Chem Bull. 2016;5:408–415. [Google Scholar]
- 3. Dehhaghi M, Mohammadipanah F. Evaluation of growth inhibition activity of myxobacterial extracts against multi-drug resistant Acinetobacter baumannii. Prog Biol Sci. 2017;6:181–187. [Google Scholar]
- 4. Hamedi J, Mohammadipanah F, Panahi HKS. Biotechnological exploitation of actinobacterial members. In: Maheshwari D, Saraf M, eds. Halophiles. Cham, Switzerland: Springer International Publishing; 2015:57–143. [Google Scholar]
- 5. Dehhaghi M, Mohammadipanah F, Guillemin GJ. Myxobacterial natural products: an under-valued source of products for drug discovery for neurological disorders. Neurotoxicology. 2018;66:195–203. [DOI] [PubMed] [Google Scholar]
- 6. Dehhaghi M, Tan V, Heng B, Mohammadipanah F, Guillemin GJ. Protective effects of myxobacterial extracts on hydrogen peroxide-induced toxicity on human primary astrocytes. Neuroscience. 2019;399:1–11. [DOI] [PubMed] [Google Scholar]
- 7. Mohammadipanah F, Hamedi J, Dehhaghi M. Halophilic bacteria: potentials and applications in biotechnology. In: Maheshwari D, Saraf M, eds. Halophiles. Cham, Switzerland: Springer International Publishing; 2015:277–321. [Google Scholar]
- 8. Dehhaghi M, Kazemi Shariat Panahi H, Holmes EC, Hudson BJ, Schloeffel R, Guillemin GJ. Human tick-borne diseases in Australia. Front Cell Infect Microbiol. 2019;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dehhaghi M, Kazemi Shariat Panahi H, Guillemin GJ. Microorganisms’ footprint in neurodegenerative diseases. Front Cell Neurosci. 2018;12:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bauer KC, Huus KE, Finlay BB. Microbes and the mind: emerging hallmarks of the gut microbiota–brain axis. Cell Microbiol. 2016;18:632–644. [DOI] [PubMed] [Google Scholar]
- 12. Mohajeri MH, La Fata G, Steinert RE, Weber P. Relationship between the gut microbiome and brain function. Nutr Rev. 2018;76:481–496. [DOI] [PubMed] [Google Scholar]
- 13. Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012;279:1356–1365. [DOI] [PubMed] [Google Scholar]
- 14. Guillemin GJ, Brew BJ. Implications of the kynurenine pathway and quinolinic acid in Alzheimer’s disease. Redox Rep. 2002;7:199–206. [DOI] [PubMed] [Google Scholar]
- 15. Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-tryptophan: basic metabolic functions, behavioral research and therapeutic indications. Int J Tryptophan Res. 2009;2:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sainio E-L, Pulkki K, Young SN. L-Tryptophan: biochemical, nutritional and pharmacological aspects. Amino Acids. 1996;10:21–47. [DOI] [PubMed] [Google Scholar]
- 17. Rambali B, Van Andel I, Schenk E, Wolterink G, Stevenson H, Vleeming W. The contribution of cocoa additive to cigarette smoking addiction. https://www.rivm.nl/bibliotheek/rapporten/650270002.pdf. Up-dated 2003.
- 18. Wurtman RJ, Hefti F, Melamed E. Precursor control of neurotransmitter synthesis. Pharmacol Rev. 1980;32:315–335. [PubMed] [Google Scholar]
- 19. Fernstrom JD. Role of precursor availability in control of monoamine biosynthesis in brain. Physiol Rev. 1983;63:484–546. [DOI] [PubMed] [Google Scholar]
- 20. Marsh DM, Dougherty DM, Moeller FG, Swann AC, Spiga R. Laboratory-measured aggressive behavior of women: acute tryptophan depletion and augmentation. Neuropsychopharmacology. 2002;26:660. [DOI] [PubMed] [Google Scholar]
- 21. Dougherty DM, Marsh-Richard DM, Mathias CW, et al. Comparison of 50-and 100-g L-tryptophan depletion and loading formulations for altering 5-HT synthesis: pharmacokinetics, side effects, and mood states. Psychopharmacology (Berl). 2008;198:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moroni F. Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur J Pharmacol. 1999;375:87–100. [DOI] [PubMed] [Google Scholar]
- 23. Sandyk R. L-tryptophan in neuropsychiatry disorders: a review. Int J Neurosci. 1992;67:127–144. [DOI] [PubMed] [Google Scholar]
- 24. Jones R. Tryptamine: a neuromodulator or neurotransmitter in mammalian brain? Prog Neurobiol. 1982;19:117–139. [DOI] [PubMed] [Google Scholar]
- 25. Szczepanik M. Melatonin and its influence on immune system. J Physiol Pharmacol. 2007;58:115–124. [PubMed] [Google Scholar]
- 26. Thor P, Krolczyk G, Gil K, Zurowski D, Nowak L. Melatonin and serotonin effects. J Physiol Pharmacol. 2007;58:97–105. [PubMed] [Google Scholar]
- 27. Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res. 2009;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takikawa O. Biochemical and medical aspects of the indoleamine 2, 3-dioxygenase-initiated L-tryptophan metabolism. Biochem Biophys Res Commun. 2005;338:12–19. [DOI] [PubMed] [Google Scholar]
- 29. Guillemin GJ, Kerr SJ, Brew BJ. Involvement of quinolinic acid in AIDS dementia complex. Neurotox Res. 2005;7:103–123. [DOI] [PubMed] [Google Scholar]
- 30. Beal MF, Matson WR, Swartz KJ, Gamache PH, Bird ED. Kynurenine pathway measurements in Huntington’s disease striatum: evidence for reduced formation of kynurenic acid. J Neurochem. 1990;55:1327–1339. [DOI] [PubMed] [Google Scholar]
- 31. Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. [DOI] [PubMed] [Google Scholar]
- 32. Orlikov AB, Prakhye IB, Ryzov IV. Kynurenine in blood plasma and DST in patients with endogenous anxiety and endogenous depression. Biol Psychiatry. 1994;36:97–102. [DOI] [PubMed] [Google Scholar]
- 33. Hartai Z, Juhasz A, Rimanoczy A, et al. Decreased serum and red blood cell kynurenic acid levels in Alzheimer’s disease. Neurochem Int. 2007;50:308–313. [DOI] [PubMed] [Google Scholar]
- 34. Ilzecka J, Kocki T, Stelmasiak Z, Turski WA. Endogenous protectant kynurenic acid in amyotrophic lateral sclerosis. Acta Neurol Scand. 2003;107:412–418. [DOI] [PubMed] [Google Scholar]
- 35. Chen Y, Stankovic R, Cullen KM, et al. The kynurenine pathway and inflammation in amyotrophic lateral sclerosis. Neurotox Res. 2010;18:132–142. [DOI] [PubMed] [Google Scholar]
- 36. Mackay G, Forrest C, Stoy N, et al. Tryptophan metabolism and oxidative stress in patients with chronic brain injury. Eur J Neurol. 2006;13:30–42. [DOI] [PubMed] [Google Scholar]
- 37. Heyes MP, Saito K, Milstien S, Schiff SJ. Quinolinic acid in tumors, hemorrhage and bacterial infections of the central nervous system in children. J Neurol Sci. 1995;133:112–118. [DOI] [PubMed] [Google Scholar]
- 38. Stoy N, Mackay G, Forrest C, et al. Tryptophan metabolism and oxidative stress in patients with Huntington’s disease. J Neurochem. 2005;93:611–623. [DOI] [PubMed] [Google Scholar]
- 39. Myint A-M, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007;98:143–151. [DOI] [PubMed] [Google Scholar]
- 40. Rejdak K, Bartosik-Psujek H, Dobosz B, et al. Decreased level of kynurenic acid in cerebrospinal fluid of relapsing-onset multiple sclerosis patients. Neurosci Lett. 2002;331:63–65. [DOI] [PubMed] [Google Scholar]
- 41. Baig S, Halawa I, Qureshi GA. High performance liquid chromatography as a tool in the definition of abnormalities in monoamine and tryptophan metabolites in cerebrospinal fluid from patients with neurological disorders. Biomed Chromatogr. 1991;5:108–112. [DOI] [PubMed] [Google Scholar]
- 42. Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–98. [DOI] [PubMed] [Google Scholar]
- 43. Nilsson L, Linderholm K, Engberg G, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80:315–322. [DOI] [PubMed] [Google Scholar]
- 44. Evangelisti M, De Rossi P, Rabasco J, et al. Changes in serum levels of kynurenine metabolites in paediatric patients affected by ADHD. Eur Child Adolesc Psychiatry. 2017;26:1433–1441. [DOI] [PubMed] [Google Scholar]
- 45. Stone TW, Addae JI. The pharmacological manipulation of glutamate receptors and neuroprotection. Eur J Pharmacol. 2002;447:285–296. [DOI] [PubMed] [Google Scholar]
- 46. Cozzi A, Carpenedo R, Moroni F. Kynurenine hydroxylase inhibitors reduce ischemic brain damage: studies with (m-nitrobenzoyl)-alanine (mNBA) and 3, 4-dimethoxy-[-N-4-(nitrophenyl) thiazol-2yl]-benzenesulfonamide (Ro 61–8048) in models of focal or global brain ischemia. J Cereb Blood Flow Metab. 1999;19:771–777. [DOI] [PubMed] [Google Scholar]
- 47. Di Serio C, Cozzi A, Angeli I, et al. Kynurenic acid inhibits the release of the neurotrophic fibroblast growth factor (FGF)-1 and enhances proliferation of glia cells, in vitro. Cell Mol Neurobiol. 2005;25:981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci U S A. 1990;87:2506–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sekkai D, Guittet O, Lemaire G, Tenu JP, Lepoivre M. Inhibition of nitric oxide synthase expression and activity in macrophages by 3-hydroxyanthranilic acid, a tryptophan metabolite. Arch Biochem Biophys. 1997;340:117–123. [DOI] [PubMed] [Google Scholar]
- 50. Weber WP, Feder-Mengus C, Chiarugi A, et al. Differential effects of the tryptophan metabolite 3-hydroxyanthranilic acid on the proliferation of human CD8+ T cells induced by TCR triggering or homeostatic cytokines. Eur J Immunol. 2006;36:296–304. [DOI] [PubMed] [Google Scholar]
- 51. Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069. [DOI] [PubMed] [Google Scholar]
- 52. Fernandez-Pol JA, Bono VH, Jr, Johnson GS. Control of growth by picolinic acid: differential response of normal and transformed cells. Proc Natl Acad Sci U S A. 1977;74:2889–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Guillemin GJ, Cullen KM, Lim CK, et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27:12884–12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Varesio L, Clayton M, Blasi E, Ruffman R, Radzioch D. Picolinic acid, a catabolite of tryptophan, as the second signal in the activation of IFN-gamma-primed macrophages. J Immunol. 1990;145:4265–4271. [PubMed] [Google Scholar]
- 55. Ruffmann R, Schlick R, Chirigos M, Budzynsky W, Varesio L. Antiproliferative activity of picolinic acid due to macrophage activation. Drugs Exp Clin Res. 1987;13:607–614. [PubMed] [Google Scholar]
- 56. Fernandez-Pol JA, Klos DJ, Hamilton PD. Antiviral, cytotoxic and apoptotic activities of picolinic acid on human immunodeficiency virus-1 and human herpes simplex virus-2 infected cells. Anticancer Res. 2001;21:3773–3776. [PubMed] [Google Scholar]
- 57. Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. [DOI] [PubMed] [Google Scholar]
- 58. Rapisarda A, Pastorino S, Massazza S, Varesio L, Bosco M. Antagonistic effect of picolinic acid and interferon-gamma on macrophage inflammatory protein-1a/b production. Cell Immunol. 2002;220:7080. [DOI] [PubMed] [Google Scholar]
- 59. Jhamandas K, Boegman R, Beninger R, Flesher S. Role of zinc in blockade of excitotoxic action of quinolinic acid by picolinic acid. Amino Acids. 1998;14:257–261. [DOI] [PubMed] [Google Scholar]
- 60. Cockhill J, Jhamandas K, Boegman R, Beninger RJ. Action of picolinic acid and structurally related pyridine carboxylic acids on quinolinic acid-induced cortical cholinergic damage. Brain Res. 1992;599:57–63. [DOI] [PubMed] [Google Scholar]
- 61. Heyes MP, Chen CY, Major EO, Saito K. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem J. 1997;326:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Heyes M, Saito K, Crowley J, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115:1249–1273. [DOI] [PubMed] [Google Scholar]
- 63. Brew BJ, Corbeil J, Pemberton L, et al. Quinolinic acid production is related to macrophage tropic isolates of HIV–1. J Neurovirol. 1995;1:369–374. [DOI] [PubMed] [Google Scholar]
- 64. Guillemin GJ, Brew B, Noonan C, Takikawa O, Cullen KM. Indoleamine 2, 3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol. 2005;31:395–404. [DOI] [PubMed] [Google Scholar]
- 65. Belladonna ML, Grohmann U, Guidetti P, et al. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J Immunol. 2006;177:130–137. [DOI] [PubMed] [Google Scholar]
- 66. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2, 3-dioxygenase. J Exp Med. 2002;196:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim J, Choi DW. Quinolinate neurotoxicity in cortical cell culture. Neuroscience. 1987;23:423–432. [DOI] [PubMed] [Google Scholar]
- 68. Whetsell WO, Jr, Schwarcz R. Prolonged exposure to submicromolar concentrations of quinolinic acid causes excitotoxic damage in organotypic cultures of rat corticostriatal system. Neurosci Lett. 1989;97:271–275. [DOI] [PubMed] [Google Scholar]
- 69. Schwarcz R, Whetsell WO, Jr, Mangano RM. Quinolinic acid: an endogenous metabolite that produces axon-sparing lesions in rat brain. Science. 1983;219:316–318. [DOI] [PubMed] [Google Scholar]
- 70. Dehhaghi M, Tan V, Heng B, Braidy N, Mohammadipanah F, Guillemin GJ. Neuroprotective effect of myxobacterial extracts on quinolinic acid-induced toxicity in primary human neurons. Neurotox Res. 2019;35:281–290. [DOI] [PubMed] [Google Scholar]
- 71. Diamond B, Huerta PT, Tracey K, Volpe BT. It takes guts to grow a brain: increasing evidence of the important role of the intestinal microflora in neuro- and immune-modulatory functions during development and adulthood. Bioessays. 2011;33:588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bengmark S. Gut microbiota, immune development and function. Pharmacol Res. 2013;69:87–113. [DOI] [PubMed] [Google Scholar]
- 73. Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. The application of ecological theory toward an understanding of the human microbiome. Science. 2012;336:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rodriguez JM, Murphy K, Stanton C, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis. 2015;26:26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178. [DOI] [PubMed] [Google Scholar]
- 78. Lyte M. Microbial endocrinology: host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes. 2014;5:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701. [DOI] [PubMed] [Google Scholar]
- 80. Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144:36–49. [DOI] [PubMed] [Google Scholar]
- 81. Luczynski P, Whelan SO, O’Sullivan C, et al. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur J Neurosci. 2016;44:2654–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moloney RD, Desbonnet L, Clarke G, Dinan TG, Cryan JF. The microbiome: stress, health and disease. Mamm Genome. 2014;25:49–74. [DOI] [PubMed] [Google Scholar]
- 83. Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21:786. [DOI] [PubMed] [Google Scholar]
- 84. Arentsen T, Raith H, Qian Y, Forssberg H, Diaz Heijtz R. Host microbiota modulates development of social preference in mice. Microb Ecol Health Dis. 2015;26:29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. [DOI] [PubMed] [Google Scholar]
- 86. Costedio MM, Hyman N, Mawe GM. Serotonin and its role in colonic function and in gastrointestinal disorders. Dis Colon Rectum. 2007;50:376–388. [DOI] [PubMed] [Google Scholar]
- 87. Wrase J, Reimold M, Puls I, Kienast T, Heinz A. Serotonergic dysfunction: brain imaging and behavioral correlates. Cogn Affect Behav Neurosci. 2006;6:53–61. [DOI] [PubMed] [Google Scholar]
- 88. Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666. [DOI] [PubMed] [Google Scholar]
- 89. Browne CA, Clarke G, Dinan TG, Cryan JF. An effective dietary method for chronic tryptophan depletion in two mouse strains illuminates a role for 5-HT in nesting behaviour. Neuropharmacology. 2012;62:1903–1915. [DOI] [PubMed] [Google Scholar]
- 90. Naslund J, Studer E, Nilsson K, Westberg L, Eriksson E. Serotonin depletion counteracts sex differences in anxiety-related behaviour in rat. Psychopharmacology (Berl). 2013;230:29–35. [DOI] [PubMed] [Google Scholar]
- 91. O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. [DOI] [PubMed] [Google Scholar]
- 92. Wichers MC, Koek G, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-α-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10:538. [DOI] [PubMed] [Google Scholar]
- 93. Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–174. [DOI] [PubMed] [Google Scholar]
- 94. Kaszaki J, Erces D, Varga G, Szabo A, Vecsei L, Boros M. Kynurenines and intestinal neurotransmission: the role of N-methyl-D-aspartate receptors. J Neural Transm (Vienna). 2012;119:211–223. [DOI] [PubMed] [Google Scholar]
- 95. Gao J, Xu K, Liu H, et al. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. [DOI] [PubMed] [Google Scholar]
- 97. Wolf AM, Wolf D, Rumpold H, et al. Overexpression of indoleamine 2, 3-dioxygenase in human inflammatory bowel disease. Clin Immunol. 2004;113:47-55. [DOI] [PubMed] [Google Scholar]
- 98. Dai X, Zhu BT. Indoleamine 2, 3-dioxygenase tissue distribution and cellular localization in mice: implications for its biological functions. J Histochem Cytochem. 2010;58:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nino-Castro A, Abdullah Z, Popov A, et al. The IDO1-induced kynurenines play a major role in the antimicrobial effect of human myeloid cells against Listeria monocytogenes. Innate Immun. 2014;20:401–411. [DOI] [PubMed] [Google Scholar]
- 100. Romani L, Zelante T, De Luca A, et al. Microbiota control of a tryptophan–AhR pathway in disease tolerance to fungi. Eur J Immunol. 2014;44:3192–3200. [DOI] [PubMed] [Google Scholar]
- 101. Notarangelo F, Wilson E, Horning K, et al. Evaluation of kynurenine pathway metabolism in Toxoplasma gondii-infected mice: implications for schizophrenia. Schizophr Res. 2014;152:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Freewan M, Rees MD, Plaza TSS, et al. Human Indoleamine 2, 3-Dioxygenase is a catalyst of physiological heme peroxidase reactions: implications for the inhibition of dioxygenase activity by hydrogen peroxide. J Biol Chem. 2013;288:1548–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Valladares R, Bojilova L, Potts AH, et al. Lactobacillus johnsonii inhibits indoleamine 2, 3-dioxygenase and alters tryptophan metabolite levels in BioBreeding rats. FASEB J. 2013;27:1711–1720. [DOI] [PubMed] [Google Scholar]
- 104. Clarke G, McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. A distinct profile of tryptophan metabolism along the kynurenine pathway downstream of toll-like receptor activation in irritable bowel syndrome. Front Pharmacol. 2012;3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Brottveit M, Beitnes Tollefsen S, Bratlie JE, et al. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. Am J Gastroenterol. 2013;108:842. [DOI] [PubMed] [Google Scholar]
- 106. Wang Y, Devkota S, Musch MW, et al. Regional mucosa-associated microbiota determine physiological expression of TLR2 and TLR4 in murine colon. PLoS ONE. 2010;5:e13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Korecka A, Dona A, Lahiri S, et al. Bidirectional communication between the Aryl hydrocarbon Receptor (AhR) and the microbiome tunes host metabolism. NPJ Biofilms Microbiomes. 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]