Abstract
Meat and nicotinamide acquisition was a defining force during the 2-million-year evolution of the big brains necessary for, anatomically modern, Homo sapiens to survive. Our next move was down the food chain during the Mesolithic ‘broad spectrum’, then horticultural, followed by the Neolithic agricultural revolutions and progressively lower average ‘doses’ of nicotinamide. We speculate that a fertility crisis and population bottleneck around 40 000 years ago, at the time of the Last Glacial Maximum, was overcome by Homo (but not the Neanderthals) by concerted dietary change plus profertility genes and intense sexual selection culminating in behaviourally modern Homo sapiens. Increased reliance on the ‘de novo’ synthesis of nicotinamide from tryptophan conditioned the immune system to welcome symbionts, such as TB (that excrete nicotinamide), and to increase tolerance of the foetus and thereby fertility. The trade-offs during the warmer Holocene were physical and mental stunting and more infectious diseases and population booms and busts. Higher nicotinamide exposure could be responsible for recent demographic and epidemiological transitions to lower fertility and higher longevity, but with more degenerative and auto-immune disease.
Keywords: neolithic, neanderthal, disease transitions, demographic transitions, immune tolerance, ‘K-selection’, ‘r-selection’, fertility, domestication
‘. . . we essay to create a second world within the world of nature’.
Cicero
‘. . . thoughts about food in prehistory are changing with a proper combination of the theoretical and the technical . . .’.
Gosden1
Introduction
Scholars from the 18th century, namely, Turgot, Montesquieu, de Tocqueville, Adam Smith, and later Childe, McNeill, Crosby, and Diamond recognised an interesting set of correlations involving the basic mode of food subsistence (including alcohol, psychedelic and medicinal compounds) and a broad set of human characteristics. Their insights and speculations were derived primarily from observing hunter-gatherers, farmers and pastoralists, the ‘Columbian exchange’, and modernity.2–8 Such characteristics included population density, divisions of labour, the economy and technology, social norms, rituals and institutions, and the emergence of disease and war. How key dietary variables work proximally to effect success or failure through individual metabolism and physiology has not been elucidated, although mitochondria and the need for prodigious amounts of adenosine triphosphate (ATP) get a mention.9–14
Nicotinamide and Evolution
Nicotinamide and nicotinamide adenine dinucleotide (NAD)-based hydrogen (H) metabolism can be traced to the origins of life, specifically multicellular life with the symbiotic acquisition of mitochondria and the preferential use of oxygen (as electron acceptor) for maximal ATP production. NAD can be synthesised from tryptophan, but the preferred source is dietary nicotinamide. Nicotinamide has a detoxification pathway via NNMT that links to methyl metabolism. Nicotinamide adenine dinucleotide consumers control metabolism, and NAD sensors drive the quest for food and construction of an NAD world15–19 (Figure 1).
Figure 1.
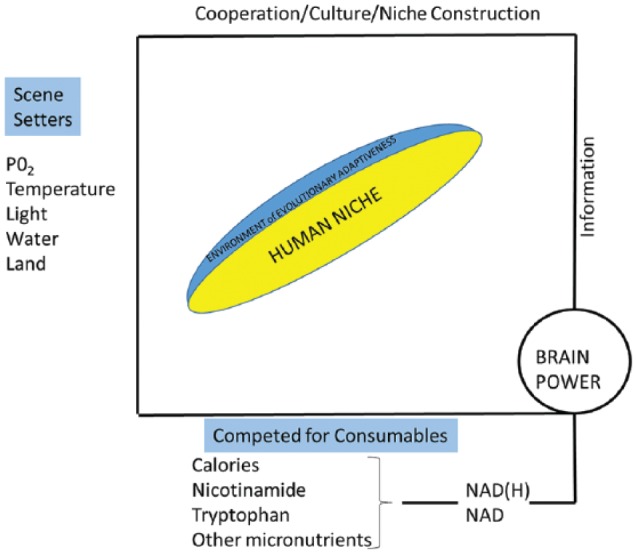
Scene setters are not a given but oxygen varies little (except by altitude) and is free. NAD supplies, by contrast, required brains and overcoming dangers in hunter-gatherer days and now have high (financial) costs that can be prohibitive. NAD indicates nicotinamide adenine dinucleotide.
An evolutionary account of the nicotinamide supply may be an ecological flashlight to explain our history. Brillat-Savarin’s aphorism states ‘The destiny of nations depends upon the manner in which they nourish themselves’ as others have also implied.20–27 Quests for meat and plants, based on communal knowledge and manipulation of nature, is one of our defining features.28–68 Nowadays, money hand-outs to the poor get preferentially spent on meat and trump other welfare programmes, as Engel first pointed out happens whenever wages rise69–73 (Figure 2).
Figure 2.
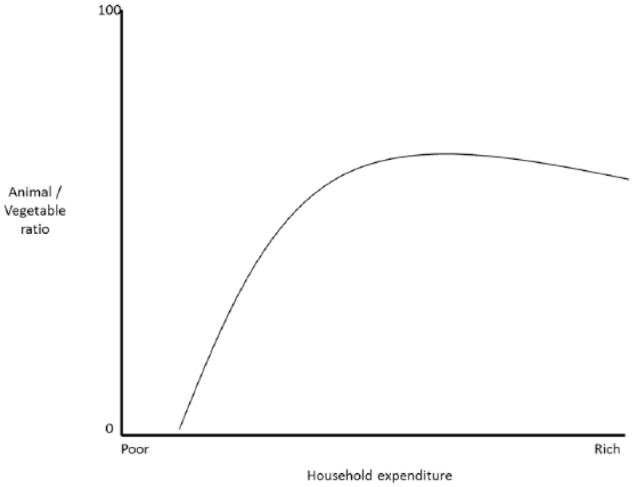
Engel curve showing increased proportion spent on meat as wealth increases.
Early hominids speciated in an unstable climate, driven by solar radiation and tectonic activity, that produced rifts with varied and varying habitats. We left shrinking forests for savannahs, lakes, and shorelines that allow for more game and plant produce. Food drove our global expansion, perhaps at some cost to Megafauna, and later drove empires and colonial settlers for lands to farm to secure dietary balance33,74–96 (Figure 3).
Figure 3.
Early ‘Up’s and Down’s’ and then oscillations on the trophic pyramid. Every move alters nicotinamide/tryptophan metabolism.
If population densities had remained at hunter-gatherer levels of 0.05 per km2, global population would now be 7 million, not 7 billion and rising. Much of the evidence about the fertility (and disease) explosion at the time of the Neolithic is derived from skeletal and genetic data.27,97,98 No form of contraception, infanticide, or extended breast feeding explains easily these low fertility states or, for that matter more recent declines in fertility that tellingly start with high meat intake whether by country or by social strata.99–128 Our trophic level is highly variable. We argue the variances that emerged as we moved down the food chain originally had positive reasons by creating small intellectual classes and larger labour i.e. work-forces with higher fertility, but such variances are now too extreme, no longer necessary, and may be dangerous129–153 (Figure 4).
Figure 4.
Meat: cereal/vegetable variances over our history. These variances are now extreme going beyond homeostatic controls even with the help of symbionts.
Omnivore or Super-Omnivore
We are (super) omnivores as much of our culture, creative minds, behavior, and many local riddles over food preferences and taboos, can be seen in the light of omnivory.154,155 Anthropologists have deciphered that the preferred diet is a very simple A + 2B (meat and 2 vegetables).156 There have always been considerable variances depending on season and latitude being less plant-based at the poles – (economics now, however, being more important than geography). Local conditions favoured a variability package aided by technology and tools, use of fire, fermentation, cooking, and xenobiotic biochemistry to cope with toxins or nutrigenomic developments to deal with lactose in adult life and more starch.157–160 ‘Pharmacophagy’ included hallucinogens and medicines allowed some control of fertility. Triangulating diet with detailed environmental information and social collaboration led to further experiments with fertility and its trade-offs.161,162 Societies created (neuro)chemical and endocrine profiles as nicotinamide and tryptophan affect dopamine, serotonin, immunologic, and reproductive pathways163–167 (Figure 5).
Figure 5.
Prosocial personality and neurochemical evolution involving serotonin and dopaminergic was driven by diet. An early emphasis on ‘K’ selection in our line was later balanced by a change back to a more plant based diet enabling ‘r’ selection when required.
First: Meat Obsessed
Endurance running and technology with problem-solving, social skills, and language helped to capture meat.168–170 Consciousness helped create ‘a second world within the world of nature’ by allowing a sense of purpose in sourcing our supplies. Even recognising the inevitability of death and the need to reproduce for a communal after-life helped to concentrate minds using high-energy interneurons and pyramidal neurones with their oscillating networks (that are lost in cases of pellagra).171–175
Our primate dietary history previously tacked between insectivores, frugivores, herbivores, and near-carnivores back to omnivores. Hunting and sharing meat between the sexes and ages was a feature of all early societies.176
High Intelligence Was Originally Necessary to Survive
Separating foraging cognition, ecological, domain-specific intelligence, and social brain hypotheses may be unhelpful dichotomies as our general intelligence may seamlessly span all functions necessary for food and reproductive needs. Spatial memory, reasoning power, and patiently solving complex ecological challenges to put meat on the shared menu served at a central-place in a risky and fluctuating environment was required.177–181 ‘The Great Acceleration’ putting us in charge of the ‘Anthropocene’ is based on an omnivorous diet and domesticated ‘Walking Larders’ with a central role for adequate nicotinamide and high intelligence.182–185
Nicotinamide Deficiency
Nicotinamide is sourced from meat and milk with contributions through gathering sea-shell foods, eggs, insects, and nuts. Both meat and carbohydrate hungers are accepted, but the real drive may have been for a ‘Goldilocks’ balance between animal and plant produce. Nicotinamide adenine dinucleotide deficiency was recognised as causing arrested evolution and atavistic degeneration by the Italian pellagra specialist Lambroso in 1860 and responsible for down-shifts in culture and behaviour. The earlier move down the food chain in the Neolithic indeed led to a decline in height and the emergence of many infectious diseases and bone and oral ill-health. Both the pellagra epidemics and observations on hunter-gatherers denied their right to hunting demonstrated the profound de-humanisation of human beings and their social networks under these circumstances that also resulted in cases of cannibalism – some sanctioned by states and religions where the meat supply was poor such as the Aztec empire.186–189 Changes from pastoralism to reliance on a maize crop or imports have been recapitulated in many parts of Africa with similar effects on health and behaviour. However, ‘rabbit starvation’ described in Artic explorers shows that meat and excess protein can also be toxic and needs to be balanced with adequate carbohydrates.
Chronic meat and therefore nicotinamide deficiency was likely at the time of the Neolithic transition, allowing the rise of symbionts that can act as back-up, including TB and perhaps leprosy and malaria that later disappear.190–197 Unsuccessful quests may have led to stunting of Homo naledi (not found with evidence of hunting) or Homo floresiensis (as islands have restricted availability of meat), as happens to forest pygmies.198–208
Plague After Plague and Still Plagues of Corn
The pellagra literature includes evocative titles such as ‘A Plague of Corn’ or ‘Maize for the Gods’ suggesting that cereals are a mixed blessing. Defined epidemics of pellagra, diagnosed by a characteristic rash with gut infections and a full gamut of neuropsychiatric disease, occurred from the 18th century, notably in Southern Europe and America, up till the modern day; usually now relating to war and refugees, but all these epidemics may be the tip of an iceberg. Earlier, pellagra may have been responsible for the reports of ‘leprosy’ in the Old and New Testaments on poor ‘manna’ diets – and modern leprosy is of interest as nicotinamide can cure it (as it can also cure TB). Later, pellagra may be underdiagnosed or called ‘environmental encephalopathy’ or simply mental or physical stunting and a poor constitution. Reliance on cereals affects those in (meat) poverty, particularly in Africa where the ‘hidden hunger’ of micronutrient deficiencies is rife that conspires to exacerbate other insults (such as TB and HIV) to cause disease.209–236
Recently, talk has been about integrating diet and the microbiome, concentrating not so much on specific foods or taxonomical identities as their functional competencies, and contribution to providing micronutrients, including vitamin B3, as meta-genomic units of selection and fitness. The epidemics of pellagra are a good example of what can happen when this co-operation goes wrong, leading to dysbioses and uncontrolled autophagy and consequent degeneration. Influential human ecologists have long warned of cereals’ pervasive influence that links population and disease explosions in a series of plagues.237–243
Pellagra Yet Cereal Reliance
On the plus side, fertility goddesses, creation myths, and harvest festivals are linked with cereals, perhaps explaining cereals’ real attraction in encouraging fertility (as does their fermented derivative, alcohol). Meat, however, remains a common aversion when pregnant and is thought to have its adverse effects via the immune system. Later came pastoralism, and with it monotheistic ‘Big Gods’ with (almighty) shepherds and dominion over the animal kingdom, suggesting that attitudes changed in favour of a more balanced diet perhaps once the initial fertility crisis was over. Here, we may have the explanation of why plants became popular despite the costs244–252 (Figure 6).
Figure 6.
Original population bust 30 to 50 kya was on high meat diet and nearly caused our extinction. Neolithic and later booms were cereal dependent with busts triggered by harvest failures, poor constitution leading to plagues or wars over the meat supply. ‘Goldilocks’ mixed diets are the sustainable solution.
Second: Co-evolution of Plant (and Later Animal) Domestication
Selective gathering and plant exploitation harnessed both their sexual and asexual reproduction. Gardening developed with tending, weeding, pruning, and burning and then selection of phenotypic traits, availability, growth, resistance, productivity, and ease of processing or in a word nutritional yield.253 Horticulture, agriculture, and domestication of cereals and later animals are an example of convergent evolution as they happened independently and fairly quickly in different parts of the old world (later in the New World) at cultural and genetic levels, enabling better crops and digestion of starches, lactose, and meat.254–258 Cooking was important as it reduces toxicity of cyanogens in tuberous plants – and in the case of nicotinamide, cooking meat by roasting or boiling helps availability and cooking maize with alkali also increases bioavailability (but the practice was not always imported) as can fermentation by yeasts and germination of foods.259
The evolution of Village life around hearths and Quorn stones, mutual understanding and allo-parenting with sexual division of labour (it takes a village to raise a child) can be seen as enabling a descent down the food chain and increasing fertility. Subsequent horticulture-based populations, such as the Incan or the Songhay empires, were an order of magnitude lower than the cereal-based Roman or Chinese empires, and hunters have lower fertility than those on a more plant-based diet. The Agricultural revolution happened at a time of affluence, and some theoretical advantages such as storage guarding against fluctuations in climate look dubious given frequent crop failures and raids or pests attacking granaries. Farming was hard work with less leisure and many disadvantages for health other than perhaps a reduction in trauma from hunting, but overall may also have increased given intergroup warfare over meat-related resources. Evidence for poor health comes from bone and tooth pathology and is attributed to little animal protein and micronutrient deficiency, including pellagra.260–267
The obsessive interest with death, ancestors, deliberate burial with valuable supplies, and the after-life suggests much interest in life history and the need to procreate. Matriarchal societies may have been common reflecting that women bear the main costs of reproduction. Women may have driven both the need and best use of plant resources. Neanderthals (discussed later) could change to a more plant-based diet as fall-back foods and self-medication with similar cultural starts but with little signs of active gardening, and they may not have moved fast enough to the best sites.
Such sites enabled niche construction using stands of grasses and (tuber) plots leading to early population increases. Plant-based diets included hazelnuts, chestnuts, and acorns. Later came ‘domus’ settlements with gardens, then hydraulic systems, pottery, grinding stones, and cooking. Some used shellfish and other seafood rather than terrestrial meat, although fishing later declined, even in coastal areas, contributing to lower meat consumption. The pull towards plants is clear with much microlithic technology being for plant processing and use of vegetation for manufacture of textiles, cordery, dwellings, and basketry as part of the new culture. These old world data are replicated in the New World where hunter populations settled to horticulture and fishing and only then agriculture.262,268–278 However, the driving force for domestication has never really been explained and must be strong as it evolved independently yet diffusely in heterogeneous societies in interactive mosaics.279–281
‘r-selection’ – Population Size Matters
Evolutionary theory has not been applied in the form of a discussion over whether the Neolithic agricultural revolution led to ‘r-selection’ for quantity over ‘K’ selection for quality.282–285 Formerly favoured reasons involve a response to, rather than the cause of, population pressure despite much evidence to the contrary as populations were, in fact, low with no sign of consistent food shortages. Other species can opt for quantity or quality depending on environmental context.104,105,108,286–290 We argue that a lower nicotinamide dose increases immune tolerance of the foetus and fertility and that is why the move to a higher plant diet was a survival mechanism (Figure 5). The tools that we developed reflected ‘r’ selection with sickles, scythes, and grinding stones. This also explains why we moved further down the biodiversity and food chain to the restricted number that we domesticated as these cereals, particularly maize, are of even lower tryptophan/nicotinamide value than garden plants. The rise of Bronze and Iron Age populations may have been driven by further ‘r’ selected tools whether ploughs, metal scythes, and flails, axes to fell trees or cauldrons for cooking. Our environment can be intrinsically ‘inadequate’ in nicotinamide, but this may have been ‘on purpose’, leading to a decline in individual human capital but an increase in human numbers. Cereals that became staples, such as maize and rice, may have started off as luxury items, but then became linked with remarkably convergent creation and fertility myths.291,292
Reproductive Control of Domesticates and Ourselves
Many have considered the Neolithic in terms of controlling reproduction of domesticates rather than ourselves, other than suggesting that sedentism with less need to transport children or more calories allowed women to have more children. We suggest that the change in diet increased fertility through this low nicotinamide biochemical-immunologic mechanism (Figure 7). Small increases in biological fertility compounded over thousands of years lead to very different trajectories. The fundamental question is why did cultures change their mode of subsistence? The clue, we feel, is that these events are all linked with nicotinamide metabolism – pellagra being the archetypal ‘disease of agriculture’ and maize being the archetypal crop of ‘ecological imperialism’ and population explosions.
Figure 7.

The nicotinamide switch: higher doses, we speculate, switch off the ‘de novo’ tryptophan to NAD pathway increasing a bias to immune intolerance with consequences for diseases and fertility. NAD indicates nicotinamide adenine dinucleotide.
High Meat: Low Fertility
Low fertility on luxurious high meat diets was noted in ancient folklore and was a theory in preindustrial prefamily planning societies with documented correlations within countries and across the globe. Correlations of low fertility and high meat were subsequently noted in hunter-gatherers.125,293–295 Natural regulation of other animal populations has intrinsic controls, not reliant on starvation or predation, even if the mechanism is unknown. Human population growth may depend on the balance between cereals and meat – get this wrong and subgroups flirt with degeneration or extinction. Recent evidence, whether from genetic, cemetery skeletal, or radiocarbon data, suggests very small early populations that we emerged from before the advent of horticulture. Sustained increases can be surprisingly modest with slight changes in fertility, linked with survival, decreasing doubling of populations from thousands to hundreds of years (Figure 6). Despite earlier suggestions that sedentism increased fertility, there is little support from recent studies. Contemporary accounts of reduced fertility in affluent populations in men as well as women may also have a dietary rather than toxic explanation.
An Archaic Fertility Crisis Triggered the Dawn of Civilisation?
The common belief that the Neolithic agricultural revolution was a mistake with cultural evolution being at odds with biological evolution is unlikely given the speed at which it was adopted at independent sites – and that agriculturalists outreproduced hunter-gatherers, even if it came at a price. The price included hard work, deteriorating health and height, and even brain size with retrenchment on symbolic skills and language; even the spiritual connection with nature gained during the Mesolithic, as the first signs of scientific thought with shamans as ‘ecological brokers’, whether of natural history of flora and fauna or of astronomy may have reversed.296–299 These events all date to around 40 000 years in Europe and a population bottleneck that has been attributed to volcanic catastrophe followed by a beneficial neurological mutation, although no such thing has ever been identified. A similar scenario may have unfolded in Southern Africa much earlier also involving a population bottleneck, an adverse climate, and a move to a broader but more reliable coastal diet.300–304 We think it is plausible that a high level of carnivory was involved, adversely affecting fertility that we solved in different geographies and time-frames through a combination of dietary and cultural approaches leading to behavioural modernity.
Sex, Conception, and Care Obsessions Underpin Creative Explosions
We propose that a fertility crisis occurred that was solved by initially a broad-based then a lower biodiversity dietary response with progressive lowering of nicotinamide intakes. A parallel profertility and promating culture is suggested by ‘Gravettian Venuses’ as fertility symbols and body ornamentation (‘Venus-wear’) and even the dawn of conversational languages and stories.305 Hunting and incipient domestication was, for a while, illustrated in cave drawings combining meat quests with sexual symbolism.306–342 Such art was only made over a few thousand years and miles; the obesity often depicted reflecting or perhaps advertising the reproductive advantages of a high carbohydrate diet.343–346 The first musical instruments, flutes, and rituals involving psychedelics and alcohol encouraging social and sexual interactions simultaneously evolved. Our conscious and emotional minds became weighted towards mating and child-care and, as long as reproduction and food were catered for, a wide variety of cultural and religious responses that could all work.347–353
Climate at the time of the Last Glacial Maximum 27 000 years ago triggering flora and fauna change contributed by forcing dietary experimentation after the golden age of big game hunting and Megafaunal extinctions in a restructuring of the food web. ‘Over-kill’ and ‘Over-chill’ may both be implicated in the now seasonal and more plant-based food web. Caves and settlements were super-sites often by seashores (expanded by record low sea levels) or rivers and woods with varied ecologies and formed interconnected networks extending social reach and choice of both cultures and partners.278,339,354–361 Sexual selection for a mating brain perhaps took over from selection for survival. Matriarchal societies and goddesses (in a controversial literature confused with feminist debates) may have even begun before the connection between coitus and births was recognised. The first constructed settlements were temples, not houses, used for meeting, mating, feasting, and worship of fertility gods. Female depictions concentrated on reproductive apparatus. Later, the fertility goddesses, Ceres, and many equivalents across the world, represented cereals, rites of passage, and women commonly of the plebeian and more fertile class.362–366
The Mesolithic has emerged as a crucial pre-adaptation to the Neolithic. Unexploited plant and small animal/shellfish were used with multistep collecting, storage, and processing using grinding and pounding stones as their technocomplex, producing flours from both oats and underground plant storage organs as part of a behavioural package. Higher fertility overcame the localised extinctions, preceded by contractions in to refugia particularly in areas where biodiversity was low, that could have heralded the complete extinction of Homo sapiens, as happened to other hominins.115,367–375
Fate of the Neanderthals Add to the Argument
Population implosions extinguished the Neanderthals (and Denisovans) nearly led to our demise (perhaps as low as 600-10 000 breeding pairs worldwide at times); but in the eventuality we somehow demographically and genetically swamped all other hominids. The near simultaneous timing of their demise and our creative and population explosion would be an extraordinary coincidence favouring a common mechanism. The Neanderthals were successful, outside the tropics, but were always ‘thin on the ground’, suggesting a fertility constraint also making cumulative cultural development more difficult. Their population mini-explosions have been linked with climate changes that allowed for more plants though still low by our standards (5%-30% of caloric intake) and did not stop them developing meat-related hypervitaminosis A. Interbreeding with Homo suggests high fertility drives, but this became their genes, but not their individual, mechanism for survival as their diet and cultural change were, as it turned out we say, not radical enough.262,276,376–397
Critical Masses: ‘Foecunda virorum/Paupertas fugitur’
Population increases and sedentism encouraged division of labour and social stratification based on meat elites. Meat elites drove innovation and controlled power over the more fertile classes in a fragile social contract. The rich elite was an intellectual force, not a primate dominance structure: meat was not another material good to show off, but a need, not just a want – just as a lack of meat enabled a more fertile and labour class (who even may appreciate their children more).398–401 Observations that ‘proles’ (by definition) outreproduced the rich go back to at least Roman times in writings by Pliny and laws by Augustus entreating the rich to produce more children.402–404
Insight has been poor with intellectuals often deriding the proletarian masses let alone being the breeding ground for eugenics and other forms of class warfare, including opposing rather than appeasing revolts by the poor when starvation threatened. Such hierarchies are most obvious where meat was scarce such as South America and Africa, whether from lack of domesticates or cattle diseases. High population sizes with functioning classes develop and maintain new ideas and technology – Tasmania’s isolation and low poulation being the classic study of civilisations moving backward.405–408 Fecund agriculturalists and their cultures prevailed. Such cultures included writing that aided cereal collection, taxing, and trading. Writing may have been triggered partly by poorer memory even as higher collective intelligence overcame some loss of individual skills. Agriculturalist languages in general are intimately linked with specific cereals. Arithmetic and trading meat for cereals is the basis of many ethnic groups and plural societies: the main surviving Indo-European languages were pastoralist inventions. Even a modern metropolis, such as Chicago, was founded on grain and meat markets and good physical communications – so little has changed with the recipe for our success.409–411
Demographic Transitions Spawn Civilisation
Demographically speaking, the gap as diets descend the trophic food chain between fertility rising and disease rising later (a mirror image when diets ascend) is critical to population explosions – we argue that these demographic patterns relate to the slope and direction of the meat/nicotinamide dose.412–422 (Figure 8).
Figure 8.

Dose of nicotinamide, above a minimal starvation level, we propose, negatively correlates with fertility but positively with longevity up to a point. Variable times to changing average doses, largely dependent on the meat supply, is responsible for the degree of any population boom. As the dose falls as in the Neolithic fertility rises followed by a delay then a rise in the death rate. As the dose rises, as in recent transitions, death rates fall followed after a variable delay by fertility.
Hygiene or Diet?
The interlinked epidemiological transition and ‘Hygiene’ hypothesis though much modified has enduring confirmation that it is important in understanding disease transitions from infections such as TB to allergies.423–433 However, a convincing metabolic explanation has not been developed. The tryptophan/IDO pathway and links to TB that excretes nicotinic acid may be key to this puzzle for these transitions (reversed at times such as war when increases in TB coincide with drops in allergy).434–436 The symbiont microbiome (rather than childhood acute infection) and the key involvement of Tregs mediated by tryptophan metabolism in the switch to auto-immune disease are likely players422,437–441 (Figure 5). Undernutrition, after all, is the commonest cause of immune-deficiency relevant to many infections, including TB with nicotinamide having anti-microbial activity against many classes of chronic infections; and overnutrition may be the driver and intersection for dysbioses and immune intolerance affecting Tregs interacting with metabolic, energy, and amino-acid sensors.442–462
Conclusions
The ‘Chinese Malthus’ Hong Liangji (1793) noted high fertility on the American import, maize. In retrospect, all population explosions and increases in biological ‘fitness’ (even as health deteriorated) were probably caused by a move down the food chain. Many original hunter-gatherer populations may have ‘tottered’, and to prosper the (more fertile) egg needed to come first. Sowed cereals and agriculture were not ‘invented’ because of population pressures, as there was no pressure, but the exact opposite of relative infertility. Now, we are reaping the downside as some populations, such as in Africa, continue to boom but will bust, or exacerbate climate change, but if the pendulum swings too far towards meat infertile populations, such as many in Europe, become equally problematic for healthy economics and the need for migration.463–481
Meat originally drove brain evolution and survival, but at the price of low fertility. The move to more plants and then more cereals solved low biological fertility by steadily increasing immune tolerance of the foetus. This came with profertility cultures influencing our unusual life history and the way people farmed, mated, cooked, recorded, interacted, bonded, and believed.442,482,483 The ‘sapient paradox’ of the delay before behavioural modernity explained by the need get the dietary, life-history, and social base, along with genetic modifications, to drive multifaceted profertility cultures in a concerted evolutionary approach with exponential consequences that shape the modern world.484–489
Our genome includes profertility genes dating from this time. These genetic signatures are as close as we are likely to get to proof that an early fertility crisis drove our dietary, nutrigenomic, and cultural evolution.490–492 Such genes now show up as examples of ‘antagonistic pleiotropy’ aiding fertility and growth early in life, but later are risk factors for cancer and neurodegeneration. Alongside preventing extremes in nicotinamide dosage and boosting the dose at times of stress, these trade-offs may be preventable by personalising nicotinamide dose depending on genomic and environmental background, and age-arguments we develop in our companion article.394,493–501
Footnotes
Funding:The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by QEHB Charity, Birmingham, UK.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: AW and LJH drafted, proofed and submitted the final manuscript.
ORCID iD: Lisa J Hill  https://orcid.org/0000-0001-8431-7029
https://orcid.org/0000-0001-8431-7029
References
- 1. Gosden C, Hather JG. The Prehistory of Food: Appetites for Change. Abingdon, UK: Routledge; 2004. [Google Scholar]
- 2. Cohen MN. The Food Crisis in Prehistory: Overpopulation and the Origins of Agriculture. New Haven, CT: Yale University Press; 1977. [Google Scholar]
- 3. de Secondat baron de, Montesquieu C, Cohler AM, Miller BC, Stone HS. Montesquieu: The Spirit of the Laws. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- 4. McNeill WH. The Rise of the West: A History of the Human Community. Chicago, IL: University of Chicago Press; 1992. [Google Scholar]
- 5. Clark G. A Farewell to Alms: A Brief Economic History of the World. Princeton, NJ: Princeton University Press; 2008. [Google Scholar]
- 6. Crosby AW. Ecological Imperialism: The Biological Expansion of Europe, 900–1900. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- 7. Diamond JM. Guns, Germs, and Steel: The Fates of Human Societies. New York, NY: W.W. Norton; 1999. [Google Scholar]
- 8. Childe VG. Man Makes Himself. London, England: Watts; 1936. [Google Scholar]
- 9. Romer PM. The origins of endogenous growth. J Econ Perspect. 1994;8:3–22.10136763 [Google Scholar]
- 10. Citarelli M, Teotia S, Lamb RS. Evolutionary history of the poly (ADP-ribose) polymerase gene family in eukaryotes. BMC Evol Biol. 2010;10:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lunzer M, Miller SP, Felsheim R, Dean AM. The biochemical architecture of an ancient adaptive landscape. Science. 2005;310:499–501. [DOI] [PubMed] [Google Scholar]
- 12. Ternes CM, Schonknecht G. Gene transfers shaped the evolution of de novo NAD+ biosynthesis in eukaryotes. Genome Biol Evol. 2014;6:2335–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu G, Golding GB, Dean AM. The selective cause of an ancient adaptation. Science. 2005;307:1279–1282. [DOI] [PubMed] [Google Scholar]
- 14. Lane N. Life Ascending: The Ten Great Inventions of Evolution. London, England: Profile; 2010. [Google Scholar]
- 15. Elhassan YS, Philp AA, Lavery GG. Targeting NAD+ in metabolic disease; new insights into an old molecule. J Endocr Soc. 2017;1:816–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+. Cell. 2007;129:473–484. [DOI] [PubMed] [Google Scholar]
- 17. Makarov MV, Trammell SAJ, Migaud ME. The chemistry of the vitamin B3 metabolome. Biochem Soc Trans. 2019;47:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kulikova VA, Gromyko DV, Nikiforov AA. The regulatory role of NAD in human and animal cells. Biochemistry (Mosc). 2018;83:800–812. [DOI] [PubMed] [Google Scholar]
- 19. Jacobson MK, Jacobson EL. Vitamin B3 in health and disease: toward the second century of discovery. Methods Mol Biol. 2018;1813:3–8. [DOI] [PubMed] [Google Scholar]
- 20. Huntington E. Mainsprings of Civilization. New York, NY: New American Library/Mentor Books; 1959. [Google Scholar]
- 21. Fernandez-Armesto F. Civilizations: Culture, Ambition, and the Transformation of Nature. New York, NY: Free Press; 2001. [Google Scholar]
- 22. DeCasien AR, Williams SA, Higham JP. Primate brain size is predicted by diet but not sociality. Nat Ecol Evol. 2017;1:112. [DOI] [PubMed] [Google Scholar]
- 23. McNeill JR, Engelke P. The Great Acceleration. Cambridge, MA: Harvard University Press; 2016. [Google Scholar]
- 24. Barker G, Goucher C. The Cambridge World History: Volume 2, a World with Agriculture, 12,000 BCE-500 CE. Cambridge, UK: Cambridge University Press; 2015. [Google Scholar]
- 25. Clark G. Mesolithic Prelude: The Palaeolithic-Neolithic Transition in Old World Prehistory. Edinburgh, UK: Edinburgh University Press; 1980. [Google Scholar]
- 26. Rosas A, Ríos L, Estalrrich A, et al. The growth pattern of Neandertals, reconstructed from a juvenile skeleton from El Sidrón (Spain). Science. 2017;357:1282–1287. [DOI] [PubMed] [Google Scholar]
- 27. Cohen MN, Armelagos GJ, Larsen CS. Paleopathology at the Origins of Agriculture. Gainesville, FL: University Press of Florida; 2013. [Google Scholar]
- 28. Armelagos GJ, Harper KN. Genomics at the origins of agriculture, part two. Evol Anthropol. 2005;14:109–121. [Google Scholar]
- 29. Kiple KF. A Movable Feast: Ten Millennia of Food Globalization. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 30. Lenski G. Ecological-Evolutionary Theory: Principles and Applications. Boulder, CO: Paradigm Publishers; 2005. [Google Scholar]
- 31. Brüssow H. The Quest for Food: A Natural History of Eating. New York, NY: Springer; 2007. [Google Scholar]
- 32. Pasternak CA. Quest: The Essence of Humanity. Hoboken, NJ: Wiley; 2003. [Google Scholar]
- 33. Gillis RE, Kovačiková L, Bréhard S, et al. The evolution of dual meat and milk cattle husbandry in Linearbandkeramik societies. Proc Biol Sci. 2017;284:20170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kelly RL. The Foraging Spectrum. Washington, DC: Smithsonian Institution Press; 1995. [Google Scholar]
- 35. Nettle D. Language and genes: a new perspective on the origins of human cultural diversity. Proc Natl Acad Sci U S A. 2007;104:10755–10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allen JS. The Omnivorous Mind: Our Evolving Relationship with Food. Cambridge, MA: Harvard University Press; 2012. [Google Scholar]
- 37. Cerulli T. The Mindful Carnivore: A Vegetarian’s Hunt for Sustenance. New York, NY: Pegasus Books; 2013. [Google Scholar]
- 38. Crowe I. The Quest for Food: Its Role in Human Evolution & Migration. Stroud, UK: Tempus Pub Limited; 2000. [Google Scholar]
- 39. Dwyer PD. The price of protein: five hundred hours of hunting in the New Guinea highlands. Oceania. 1974;44:278–293. [Google Scholar]
- 40. Dwyer PD. Choice and constraint in a Papua New Guinean food quest. Hum Ecol. 1985;13:49–70. [Google Scholar]
- 41. Eaton SB, Konner MJ, Cordain L. Diet-Dependent Acid Load, Paleolithic Nutrition, and Evolutionary Health Promotion. Oxford, UK: Oxford University Press; 2009. [DOI] [PubMed] [Google Scholar]
- 42. Etkin NL. Eating on the Wild Side: The Pharmacologic, Ecologic and Social Implications of Using Noncultigens. Tucson, AZ: University of Arizona Press; 2000. [Google Scholar]
- 43. Gamble C. Timewalkers: The Prehistory of Global Colonization. Cambridge, MA: Harvard University Press; 1994. [Google Scholar]
- 44. Goodall J, Berman P. Reason for Hope: A Spiritual Journey. New York, NY: Grand Central Publishing; 1999. [Google Scholar]
- 45. Hardy K, Brand-Miller J, Brown KD, Thomas MG, Copeland L. The importance of dietary carbohydrate in human evolution. Q Rev Biol. 2015;90:251–268. [DOI] [PubMed] [Google Scholar]
- 46. Harris DR, Hillman GC. Foraging and Farming: The Evolution of Plant Exploitation. Abingdon, UK: Routledge; 2014. [Google Scholar]
- 47. Hill K. Macronutrient modifications of optimal foraging theory: an approach using indifference curves applied to some modern foragers. Hum Ecol. 1988;16:157–197. [Google Scholar]
- 48. Hobhouse H. Seeds of Change: Five Plants That Transformed Mankind. New York, NY: Perennial Library; 1987. [Google Scholar]
- 49. Hood B. The Domesticated Brain: A Pelican Introduction. London, England: Penguin Books; 2014. [Google Scholar]
- 50. Johns T. With Bitter Herbs They Shall Eat It: Chemical Ecology and the Origins of Human Diet and Medicine. Tucson, AZ: University of Arizona Press; 1990. [Google Scholar]
- 51. Kuhn SL, Stiner MC. The antiquity of hunter-gatherers. In: Panter-Brick C, Layton RH, Rowley-Conwy PA. eds. Another Day, Another Camp: An Interdisciplinary View of Hunter-gatherers. Cambridge, UK: Cambridge University Press; 2001:99–142. [Google Scholar]
- 52. Laland KN. Darwin’s Unfinished Symphony: How Culture Made the Human Mind. Princeton, NJ: Princeton University Press; 2018. [Google Scholar]
- 53. Meltzer DJ. First Peoples in a New World: Colonizing Ice Age America. Berkeley, CA: University of California Press; 2009. [Google Scholar]
- 54. Milton K. Ecological foundations for subsistence strategies among the Mbuti Pygmies. Hum Ecol. 1985;13:71–78. [Google Scholar]
- 55. Rindos D. The Origins of Agriculture: An Evolutionary Perspective. Amserdam, The Netherlands: Elsevier Science; 2013. [Google Scholar]
- 56. Shryock A, Smail DL, Earle T, Feeley-Harnik G. Deep History: The Architecture of Past and Present. Berkeley, CA: University of California Press; 2012. [Google Scholar]
- 57. Smail DL. On Deep History and the Brain. Berkeley, CA: University of California Press; 2008. [Google Scholar]
- 58. Tudge C. Neanderthals, Bandits and Farmers: How Agriculture Really Began. New Haven, CT: Yale University Press; 1999. [Google Scholar]
- 59. Wells S. Pandora’s Seed: The Unforeseen Cost of Civilization. London, England: Penguin Books; 2010. [Google Scholar]
- 60. Bellwood P. First Farmers: The Origins of Agricultural Societies. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- 61. Price TD. Europe’s First Farmers. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 62. Dyble M, Thompson J, Smith D, et al. Networks of food sharing reveal the functional significance of multilevel sociality in two hunter-gatherer groups. Curr Biol. 2016;26:2017–2021. [DOI] [PubMed] [Google Scholar]
- 63. Mace R. Evolutionary ecology of human life history. Anim Behav. 2000;59:1–10. [DOI] [PubMed] [Google Scholar]
- 64. Sauer CO. Agricultural Origins and Dispersals – Primary Source Edition. Charleston, SC: BiblioLife; 2014. [Google Scholar]
- 65. Barton H, Denham T. Vegecultures and the social–biological transformations of plants and people. Quatern Int. 2018;489:17–25. [Google Scholar]
- 66. Brown TA. The role of humans in a protracted transition from hunting-gathering to plant domestication in the Fertile Crescent. Front Plant Sci. 2018;9:1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lefebvre L, Reader SM, Sol D. Brains, innovations and evolution in birds and primates. Brain Behav Evol. 2004;63:233–246. [DOI] [PubMed] [Google Scholar]
- 68. Ofek H. Second Nature: Economic Origins of Human Evolution. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 69. Attanasio O, Mesnard A. The impact of a conditional cash transfer programme on consumption in Colombia. Fisc Stud. 2006;27:421–442. [Google Scholar]
- 70. Hulme D, Joseph Hanlon ABDH, Hanlon J, Barrientos A. Just Give Money to the Poor: The Development Revolution from the Global South. Boulder, CO: Lynne Rienner Publishers; 2012. [Google Scholar]
- 71. Prais SJ, Houthakker HS. The Analysis of Family Budgets. Cambridge, UK: Cambridge University Press; 1971. [Google Scholar]
- 72. Steensland B. The Failed Welfare Revolution: America’s Struggle over Guaranteed Income Policy. Princeton, NJ: Princeton University Press; 2011. [Google Scholar]
- 73. Szreter S. The idea of demographic transition and the study of fertility change: a critical intellectual history. Popul Dev Rev. 1993;19:659–701. [Google Scholar]
- 74. Cox CB, Moore PD, Ladle R. Biogeography: An Ecological and Evolutionary Approach. Hoboken, NJ: Wiley; 2016. [Google Scholar]
- 75. Burroughs WJ. Climate Change in Prehistory: The End of the Reign of Chaos. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- 76. Demenocal PB. African climate change and faunal evolution during the Pliocene–Pleistocene. Earth Planet Sc Lett. 2004;220:3–24. [Google Scholar]
- 77. King G, Bailey G. Tectonics and human evolution. Antiquity. 2006;80:265–286. [Google Scholar]
- 78. Maslin MA, Christensen B. Tectonics, orbital forcing, global climate change, and human evolution in Africa: introduction to the African paleoclimate special volume. J Hum Evol. 2007;53:443–464. [DOI] [PubMed] [Google Scholar]
- 79. Sepulchre P, Ramstein G, Fluteau F, Schuster M, Tiercelin J-J, Brunet M. Tectonic uplift and Eastern Africa aridification. Science. 2006;313:1419–1423. [DOI] [PubMed] [Google Scholar]
- 80. Grine FE. Evolutionary History of the Robust Australopithecines. Abingdon, UK: Routledge; 2017. [Google Scholar]
- 81. Cartmill M. A View to a Death in the Morning: Hunting and Nature through History. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- 82. Martin PS, Wright HE. Pleistocene Extinctions: The Search for a Cause. New Haven, CT: Yale University Press; 1967. [Google Scholar]
- 83. Panter-Brick C, Layton RH, Rowley-Conwy P. Hunter-Gatherers: An Interdisciplinary Perspective. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 84. Trauth MH, Larrasoaña JC, Mudelsee M. Trends, rhythms and events in Plio-Pleistocene African climate. Quaternary Sci Rev. 2009;28:399–411. [Google Scholar]
- 85. Pickering T. Rough and Tumble: Aggression, Hunting, and Human Evolution. Berkeley, CA: University of California Press; 2013. [Google Scholar]
- 86. Lee RB, DeVore I. Man the Hunter. New York, NY: Routledge; 2017. [Google Scholar]
- 87. Speth JD, Spielmann KA. Energy source, protein metabolism, and hunter-gatherer subsistence strategies. J Anthropol Archaeol. 1983;2:1–31. [Google Scholar]
- 88. Bellwood P, Oxenham M. The expansions of farming societies and the role of the neolithic demographic transition. In: Bocquet-Appel J-P, Bar-Yosef O, eds. The Neolithic Demographic Transition and its Consequences. Dordrecht, The Netherlands: Springer; 2008:13–34. [Google Scholar]
- 89. Hanks LM. Rice and Man: Agricultural Ecology in Southeast Asia. Honolulu, HI: University of Hawaii Press; 1992. [Google Scholar]
- 90. Standage T. An Edible History of Humanity. New York, NY: Atlantic Books; 2012. [Google Scholar]
- 91. Allen MW, Bettinger RL, Codding BF, Jones TL, Schwitalla AW. Resource scarcity drives lethal aggression among prehistoric hunter-gatherers in central California. Proc Natl Acad Sci U S A. 2016;113:12120–12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Martin PS, Klein RG. Quaternary Extinctions: A Prehistoric Revolution. Tucson, AZ: University of Arizona Press; 1989. [Google Scholar]
- 93. Turvey ST. Holocene Extinctions. Oxford, UK: Oxford University Press; 2009. [Google Scholar]
- 94. Algaze G. Ancient Mesopotamia at the Dawn of Civilization: The Evolution of an Urban Landscape. Chicago, IL: University of Chicago Press; 2009. [Google Scholar]
- 95. Postgate N. Early Mesopotamia: Society and Economy at the Dawn of History. Abingdon, UK: Routledge; 2017. [Google Scholar]
- 96. Staller J, Carrasco M. Pre-Columbian Foodways: Interdisciplinary Approaches to Food, Culture, and Markets in Ancient Mesoamerica. New York, NY: Springer; 2009. [Google Scholar]
- 97. Aufderheide AC, Rodríguez-Martín C. The Cambridge Encyclopedia of Human Paleopathology. Cambridge, UK: Cambridge University Press; 1998. [Google Scholar]
- 98. Waldron T. Palaeopathology. Cambridge, UK: Cambridge University Press; 2008. [Google Scholar]
- 99. Gregg SA. Foragers and Farmers: Population Interaction and Agricultural Expansion in Prehistoric Europe. Chicago, IL: University of Chicago Press; 1988. [Google Scholar]
- 100. Handwerker WP. The first demographic transition: an analysis of subsistence choices and reproductive consequences. Am Anthropol. 1983;85:5–27. [Google Scholar]
- 101. Hill K, Kaplan H. Tradeoffs in male and female reproductive strategies among the Ache: part 1. In: Mulder MB, Betzig LL, Turke P, eds. Human Reproductive Behavior: A Darwinian Perspective. Cambridge, UK: Cambridge University Press; 1988:277–290. [Google Scholar]
- 102. Shennan S. Demography and cultural innovation: a model and its implications for the emergence of modern human culture. Camb Archaeol J. 2001;11:5–16. [Google Scholar]
- 103. Ammerman AJ, Cavalli-Sforza LL. The Neolithic Transition and the Genetics of Populations in Europe. Princeton, NJ: Princeton University Press; 2014. [Google Scholar]
- 104. Borgerhoff Mulder M. Optimizing offspring: the quantity-quality tradeoff in agropastoral Kipsigis. Evol Hum Behav. 2000;21:391–410. [DOI] [PubMed] [Google Scholar]
- 105. Gillespie DO, Russell AF, Lummaa V. When fecundity does not equal fitness: evidence of an offspring quantity versus quality trade-off in pre-industrial humans. Proc Biol Sci. 2008;275:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hassett B. Built on Bones: 15,000 Years of Urban Life and Death. London, England: Bloomsbury Publishing; 2017. [Google Scholar]
- 107. Pinhasi R, Stock JT. Human Bioarchaeology of the Transition to Agriculture. Hoboken, NJ: Wiley; 2011. [Google Scholar]
- 108. Lambert PM. Health versus fitness: competing themes in the origins and spread of agriculture? Curr Anthropol. 2009;50:603–608. [DOI] [PubMed] [Google Scholar]
- 109. Lawson DW, Alvergne A, Gibson MA. The life-history trade-off between fertility and child survival. Proc Biol Sci. 2012;279:4755–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wenke RJ. Patterns in Prehistory: Humankind’s First Three Million Years. Oxford, UK: Oxford University Press; 1999. [Google Scholar]
- 111. Bocquet-Appel J-P. When the world’s population took off: the springboard of the Neolithic Demographic Transition. Science. 2011;333:560–561. [DOI] [PubMed] [Google Scholar]
- 112. Cohen MN. Introduction: rethinking the origins of agriculture. Curr Anthropol. 2009;50:591–595. [DOI] [PubMed] [Google Scholar]
- 113. Eshed V, Gopher A, Gage TB, Hershkovitz I. Has the transition to agriculture reshaped the demographic structure of prehistoric populations? New evidence from the Levant. Am J Phys Anthropol. 2004;124:315–329. [DOI] [PubMed] [Google Scholar]
- 114. Gignoux CR, Henn BM, Mountain JL. Rapid, global demographic expansions after the origins of agriculture. Proc Natl Acad Sci U S A. 2011;108:6044–6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Shennan S, Downey SS, Timpson A, et al. Regional population collapse followed initial agriculture booms in mid-Holocene Europe. Nat Commun. 2013;4:2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Warden L, Moros M, Neumann T, et al. Climate induced human demographic and cultural change in northern Europe during the mid-Holocene. Sci Rep. 2017;7:15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zahid HJ, Robinson E, Kelly RL. Agriculture, population growth, and statistical analysis of the radiocarbon record. Proc Natl Acad Sci U S A. 2016;113:931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zheng H-X, Yan S, Qin Z-D, Jin L. MtDNA analysis of global populations support that major population expansions began before Neolithic Time. Sci Rep. 2012;2:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bailey G, Spikins P. Mesolithic Europe. Cambridge, UK: Cambridge University Press; 2008. [Google Scholar]
- 120. Divale WT. Migration, external warfare, and matrilocal residence. Behav Sci Res. 1974;9:75–133. [Google Scholar]
- 121. Sahlins MD. Stone Age Economics. Chicago, IL: Aldine; 1972. [Google Scholar]
- 122. Sahlins M. The original affluent society. In: Gowdy J. ed. Limited Wants, Unlimited Means: A Reader on Hunter-Gatherer Economics and the Environment. Washington, DC: Island Press; 1998:5–41. [Google Scholar]
- 123. Seddon C. Humans: From the Beginning: From the First Apes to the First Cities. Cambridge, UK: Glanville Publications; 2015. [Google Scholar]
- 124. Oppenheimer S. The Real Eve: Modern Man’s Journey Out of Africa. New York, NY: Carroll & Graf; 2003. [Google Scholar]
- 125. Bevan A, Colledge S, Fuller D, Fyfe R, Shennan S, Stevens C. Holocene fluctuations in human population demonstrate repeated links to food production and climate. Proc Natl Acad Sci U S A. 2017;114:E10524–E10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Boserup E. Population and Technological Change: A Study of Long-Term Trends. Chicago, IL: University of Chicago Press; 1983. [Google Scholar]
- 127. Downey SS, Haas WR, Jr, Shennan SJ. European Neolithic societies showed early warning signals of population collapse. Proc Natl Acad Sci U S A. 2016;113:9751–9756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Page AE, Chaudhary N, Viguier S, et al. Hunter-gatherer social networks and reproductive success. Sci Rep. 2017;7:1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Stefansson V. Not by Bread Alone. New York, NY: Macmillan; 1946. [Google Scholar]
- 130. Johnston BF, Mellor JW. The role of agriculture in economic development. Am Econ Rev. 1961;51:566–593. [Google Scholar]
- 131. Baez-Mendoza R, Harris CJ, Schultz W. Activity of striatal neurons reflects social action and own reward. Proc Natl Acad Sci U S A. 2013;110:16634–16639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bergey CM, Phillips-Conroy JE, Disotell TR, Jolly CJ. Dopamine pathway is highly diverged in primate species that differ markedly in social behavior. Proc Natl Acad Sci U S A. 2016;113:6178–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Brodie BB, Pletscher A, Shore PA. Evidence that serotonin has a role in brain function. Science. 1955;122:968. [DOI] [PubMed] [Google Scholar]
- 134. Caravaggio F, Chung JK, Gerretsen P, et al. Exploring the relationship between social attachment and dopamine D2/3 receptor availability in the brains of healthy humans using [11C]-(+)-PHNO. Soc Neurosci. 2017;12:163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Curtis JT, Liu Y, Aragona BJ, Wang Z. Dopamine and monogamy. Brain Res. 2006;1126:76–90. [DOI] [PubMed] [Google Scholar]
- 136. Edsinger E, Dolen G. A conserved role for serotonergic neurotransmission in mediating social behavior in octopus. Curr Biol. 2018;28:3136–3142.e4. [DOI] [PubMed] [Google Scholar]
- 137. Fam BSO, Pare P, Felkl AB, et al. Oxytocin and arginine vasopressin systems in the domestication process. Genet Mol Biol. 2018;41:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Finkenwirth C, Martins E, Deschner T, Burkart JM. Oxytocin is associated with infant-care behavior and motivation in cooperatively breeding marmoset monkeys. Horm Behav. 2016;80:10–18. [DOI] [PubMed] [Google Scholar]
- 139. Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Leyton M, Okazawa H, Diksic M, et al. Brain regional α-[11C] methyl-L-tryptophan trapping in impulsive subjects with borderline personality disorder. Am J Psychiat. 2001;158:775–782. [DOI] [PubMed] [Google Scholar]
- 141. Lovejoy CO. Reexamining human origins in light of Ardipithecus ramidus. Science. 2009;326:74–74e8. [PubMed] [Google Scholar]
- 142. Matsui S, Sasaki T, Kohno D, et al. Neuronal SIRT1 regulates macronutrient-based diet selection through FGF21 and oxytocin signalling in mice. Nat Commun. 2018;9:4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Passamonti L, Crockett MJ, Apergis-Schoute AM, et al. Effects of acute tryptophan depletion on prefrontal-amygdala connectivity while viewing facial signals of aggression. Biol Psychiatry. 2012;71:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pearce E, Wlodarski R, Machin A, Dunbar RI. Variation in the β-endorphin, oxytocin, and dopamine receptor genes is associated with different dimensions of human sociality. Proc Natl Acad Sci U S A. 2017;114:5300–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rilling JK, Scholz J, Preuss TM, Glasser MF, Errangi BK, Behrens TE. Differences between chimpanzees and bonobos in neural systems supporting social cognition. Soc Cogn Affect Neurosci. 2012;7:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Robbins TW. Opinion on monoaminergic contributions to traits and temperament. Philos Trans R Soc Lond B Biol Sci. 2018;373:20170153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Stephenson AR, Edler MK, Erwin JM, et al. Cholinergic innervation of the basal ganglia in humans and other anthropoid primates. J Comp Neurol. 2017;525:319–332. [DOI] [PubMed] [Google Scholar]
- 148. Stimpson CD, Barger N, Taglialatela JP, et al. Differential serotonergic innervation of the amygdala in bonobos and chimpanzees. Soc Cogn Affect Neurosci. 2015;11:413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Tanaka SC, Schweighofer N, Asahi S, et al. Serotonin differentially regulates short-and long-term prediction of rewards in the ventral and dorsal striatum. PLoS ONE. 2007;2:e1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. van de, Giessen E, Rosell DR, Thompson JL, et al. Serotonin transporter availability in impulsive aggressive personality disordered patients: a PET study with [11C] DASB. J Psychiat Res. 2014;58:147–154. [DOI] [PubMed] [Google Scholar]
- 151. Wrangham R. The Goodness Paradox: How Evolution Made Us Both More and Less Violent. London, England: Profile; 2019. [Google Scholar]
- 152. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048. [DOI] [PubMed] [Google Scholar]
- 153. Zhong J, Amina S, Liang M, et al. Cyclic ADP-ribose and heat regulate oxytocin release via CD38 and TRPM2 in the hypothalamus during social or psychological stress in mice. Front Neurosci. 2016;10:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bonhommeau S, Dubroca L, Le Pape O, et al. Eating up the world’s food web and the human trophic level. Proc Natl Acad Sci U S A. 2013;110:20617–20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Counihan C, Van Esterik P. Food and Culture: A Reader. New York, NY: Routledge; 2013. [Google Scholar]
- 156. Mead M, Textor RB. The World Ahead: An Anthropologist Anticipates the Future. New York, NY: Berghahn Books; 2005. [Google Scholar]
- 157. Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Gerbault P, Liebert A, Itan Y, et al. Evolution of lactase persistence: an example of human niche construction. Philos Trans R Soc Lond B Biol Sci. 2011;366:863–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Itan Y, Jones BL, Ingram CJ, Swallow DM, Thomas MG. A worldwide correlation of lactase persistence phenotype and genotypes. BMC Evol Biol. 2010;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Salque M, Bogucki PI, Pyzel J, et al. Earliest evidence for cheese making in the sixth millennium bc in northern Europe. Nature. 2012;493:522. [DOI] [PubMed] [Google Scholar]
- 161. Speth JD. Seasonality, resource stress, and food sharing in so-called ‘egalitarian’ foraging societies. J Anthropol Archaeol. 1990;9:148–188. [Google Scholar]
- 162. Sear R, Lawson DW, Kaplan H, Shenk MK. Understanding variation in human fertility: what can we learn from evolutionary demography? Philos Trans R Soc Lond B Biol Sci. 2016;371:20150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Raghanti MA, Edler MK, Stephenson AR, et al. A neurochemical hypothesis for the origin of hominids. Proc Natl Acad Sci U S A. 2018;115:E1108–E1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wei H, Liu S, Lian R, et al. Abnormal expression of indoleamine 2, 3-dioxygenase in human recurrent miscarriage [published online ahead of print March 4, 2019]. Reprod Sci. doi: 10.1177/1933719119833788. [DOI] [PubMed] [Google Scholar]
- 165. Mesoudi A. Cultural Evolution: How Darwinian Theory Can Explain Human Culture and Synthesize the Social Sciences. Chicago, IL: University of Chicago Press; 2011. [Google Scholar]
- 166. Roth G, Dicke U. Evolution of the brain and intelligence. Trends Cogn Sci. 2005;9:250–257. [DOI] [PubMed] [Google Scholar]
- 167. Scarre C. The Human Past: World Prehistory and the Development of Human Societies. London, England: Thames & Hudson; 2005. [Google Scholar]
- 168. Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345. [DOI] [PubMed] [Google Scholar]
- 169. Wheeler PE. The evolution of bipedality and loss of functional body hair in hominids. J Hum Evol. 1984;13:91–98. [Google Scholar]
- 170. Fogarty L, Creanza N. The niche construction of cultural complexity: interactions between innovations, population size and the environment. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Butler AB. Evolution of brains, cognition, and consciousness. Brain Res Bull. 2008;75:442–449. [DOI] [PubMed] [Google Scholar]
- 172. Donald M. A Mind So Rare: The Evolution of Human Consciousness. New York, NY: W.W. Norton; 2002. [Google Scholar]
- 173. Eccles JC. Evolution of consciousness. Proc Natl Acad Sci U S A. 1992;89:7320–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Kann O, Huchzermeyer C, Kovacs R, Wirtz S, Schuelke M. Gamma oscillations in the hippocampus require high complex I gene expression and strong functional performance of mitochondria. Brain. 2011;134:345–358. [DOI] [PubMed] [Google Scholar]
- 175. Tomasello M. The Cultural Origins of Human Cognition. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- 176. Bowles S, Gintis H. A Cooperative Species: Human Reciprocity and Its Evolution. Princeton, NJ: Princeton University Press; 2011. [Google Scholar]
- 177. Clayton NS, Emery NJ. Avian models for human cognitive neuroscience: a proposal. Neuron. 2015;86:1330–1342. [DOI] [PubMed] [Google Scholar]
- 178. Rosati AG. Foraging cognition: reviving the ecological intelligence hypothesis. Trends Cogn Sci. 2017;21:691–702. [DOI] [PubMed] [Google Scholar]
- 179. Dunbar R. Grooming, Gossip, and the Evolution of Language. Cambridge, MA: Harvard University Press; 1998. [Google Scholar]
- 180. Jaynes J. The Origin of Consciousness in the Breakdown of the Bicameral Mind. Boston, MA: Houghton Mifflin Harcourt; 2000. [Google Scholar]
- 181. Plotkin H. Evolution in Mind: An Introduction to Evolutionary Psychology. Cambridge, MA: Harvard University Press; 1997. [Google Scholar]
- 182. Vigne J-D. The origins of animal domestication and husbandry: a major change in the history of humanity and the biosphere. C R Biol. 2011;334:171–181. [DOI] [PubMed] [Google Scholar]
- 183. Clutton-Brock J. The Walking Larder: Patterns of Domestication, Pastoralism, and Predation. Abingdon, UK: Routledge; 2014. [Google Scholar]
- 184. Perren R. The Meat Trade in Britain, 1840-1914. London, England: Routledge and Kegan Paul; 1978. [Google Scholar]
- 185. Rixson D. The History of Meat Trading. Nottingham, UK: Nottingham University Press; 2000. [Google Scholar]
- 186. Avramescu C, Blyth AI. An Intellectual History of Cannibalism. Princeton, NJ: Princeton University Press; 2011. [Google Scholar]
- 187. Diehl D, Donnelly MP. Eat Thy Neighbour: A History of Cannibalism. Stroud, UK: History Press; 2012. [Google Scholar]
- 188. Turnbull C. Mountain People. New York, NY: Simon & Schuster; 1987. [Google Scholar]
- 189. de Almeida RM, Cabral JC, Narvaes R. Behavioural, hormonal and neurobiological mechanisms of aggressive behaviour in human and nonhuman primates. Physiol Behav. 2015;143:121–135. [DOI] [PubMed] [Google Scholar]
- 190. McKeown T. The Role of Medicine: Dream, Mirage, or Nemesis? Princeton, NJ: Princeton University Press; 2014. [Google Scholar]
- 191. London D, Hruschka D. Helminths and human ancestral immune ecology: what is the evidence for high helminth loads among foragers? Am J Hum Biol. 2014;26:124–129. [DOI] [PubMed] [Google Scholar]
- 192. Leles D, Reinhard KJ, Fugassa M, Ferreira LF, Iñiguez AM, Araújo A. A parasitological paradox: why is ascarid infection so rare in the prehistoric Americas? J Archaeol Sci. 2010;37:1510–1520. [Google Scholar]
- 193. Humphrey LT, De Groote I, Morales J, et al. Earliest evidence for caries and exploitation of starchy plant foods in Pleistocene hunter-gatherers from Morocco. Proc Natl Acad Sci U S A. 2014;111:954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Hugot JP, Reinhard KJ, Gardner SL, Morand S. Human enterobiasis in evolution: origin, specificity and transmission. Parasite. 1999;6:201–208. [DOI] [PubMed] [Google Scholar]
- 195. Hotez PJ, Bethony J, Bottazzi ME, Brooker S, Buss P. Hookworm: ‘The Great Infection of Mankind’. PLoS Med. 2005;2:e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Hershkovitz I, Donoghue HD, Minnikin DE, et al. Detection and molecular characterization of 9,000-year-old Mycobacterium tuberculosis from a Neolithic settlement in the Eastern Mediterranean. PLoS ONE. 2008;3:e3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Rasmussen S, Allentoft ME, Nielsen K, et al. Early divergent strains of Yersinia pestis in Eurasia 5,000 years ago. Cell. 2015;163:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Baab KL. The place of Homo floresiensis in human evolution. J Anthropol Sci. 2016;94:5–18. [DOI] [PubMed] [Google Scholar]
- 199. Harcourt-Smith W. Early hominin diversity and the emergence of the genus Homo. J Anthropol Sci. 2016;94:19–27. [DOI] [PubMed] [Google Scholar]
- 200. Hawks J, Elliott M, Schmid P, et al. New fossil remains of Homo naledi from the Lesedi Chamber, South Africa. eLife. 2017;6:e24232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Stringer C. Human evolution: the many mysteries of Homo naledi. eLife. 2015;4:e10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Perry GH, Dominy NJ. Evolution of the human pygmy phenotype. Trends Ecol Evol. 2009;24:218–225. [DOI] [PubMed] [Google Scholar]
- 203. Cartwright FF, Biddiss MD. Disease & History. Stroud, UK: Sutton; 2004. [Google Scholar]
- 204. Dormandy T. The White Death: A History of Tuberculosis. New York, NY: Hambledon and London; 2002. [Google Scholar]
- 205. Hardy A. Health and Medicine in Britain since 1860. Basingstoke, UK: Palgrave; 2001. [Google Scholar]
- 206. Harrison M. Disease and the Modern World: 1500 to the Present Day. Hoboken, NJ: Wiley; 2013. [Google Scholar]
- 207. Hays JN. The Burdens of Disease: Epidemics and Human Response in Western History. New Brunswick, NJ: Rutgers University Press; 2009. [Google Scholar]
- 208. McNeill W. Plagues and Peoples. New York, NY: Knopf Doubleday Publishing Group; 2010. [Google Scholar]
- 209. Fussell BH. The Story of Corn. Albuquerque, NM: University of New Mexico Press; 1992. [Google Scholar]
- 210. Giles D. Singing Valleys: The Story of Corn. New York, NY: Random House; 1940. [Google Scholar]
- 211. Cockburn TA. Infectious diseases in ancient populations. Curr Anthropol. 1971;12:45–62. [DOI] [PubMed] [Google Scholar]
- 212. Karlen A. Plague’s Progress: A Social History of Man and Disease. London, England: Phoenix; 2001. [Google Scholar]
- 213. Nisbett RE. Culture of Honor: The Psychology of Violence in the South. New York, NY: Routledge; 2018. [Google Scholar]
- 214. Pick D. Faces of Degeneration: A European Disorder, C.1848-1918. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- 215. Berry SS. The food crisis and agrarian change in Africa: a review essay. Afr Stud Rev. 1984;27:59–112. [Google Scholar]
- 216. Blake M. Maize for the Gods: Unearthing the 9,000-Year History of Corn. Berkeley, CA: University of California Press; 2015. [Google Scholar]
- 217. Carpenter KJE. Pellagra. Vol. 2 London, England: Hutchinson Ross Publishing Company; 1981. [Google Scholar]
- 218. Etheridge EW. The Butterfly Caste: A Social History of Pellagra in the South. Westport, CT: Greenwood Publishing Company; 1972. [Google Scholar]
- 219. Glantz MH. Drought and Hunger in Africa. Cambridge, UK: Cambridge University Press; 1988. [Google Scholar]
- 220. Goldberger J. Goldberger on Pellagra. Baton Rouge, LA: Louisiana State University Press; 1964. [Google Scholar]
- 221. Harris S. Clinical Pellagra. Maryland Heights, MO: C. V. Mosby Company; 1941. [Google Scholar]
- 222. Iliffe J. The African Poor: A History. Cambridge, UK: Cambridge University Press; 1987. [Google Scholar]
- 223. Katz SH, Hediger ML, Valleroy LA. Traditional Maize Processing Techniques in the New World. Washington, DC: American Association for the Advancement of Science; 1974. [DOI] [PubMed] [Google Scholar]
- 224. Kraut AM. Goldberger’s War: The Life and Work of a Public Health Crusader. New York, NY: Farrar, Straus and Giroux; 2004. [Google Scholar]
- 225. Little PD. The Elusive Granary: Herder, Farmer, and State in Northern Kenya. Cambridge, UK: Cambridge University Press; 1992. [Google Scholar]
- 226. Lombroso C. Trattato profilattico e clinico della pellagra. Turin, Italy: Bocca; 1892. [Google Scholar]
- 227. Marie A. Pellagra. Sydney, NSW, Australia: Wentworth Press; 2016. [Google Scholar]
- 228. Niles GMC. Pellagra: An American Problem. Philadelphia, PA: W.B. Saunders Company; 1912. [Google Scholar]
- 229. Roberts SR. Pellagra; History, Distribution, Diagnosis, Prognosis, Treatment, Etiology. Sydney, NSW, Australia: Wentworth Press; 2016. [Google Scholar]
- 230. Roe DA. A Plague of Corn: The Social History of Pellagra. Ithaca, NY; Cornell University Press; 1973. [Google Scholar]
- 231. Salzman PC, Sadala E. When Nomads Settle: Processes of Sedentarization as Adaptation and Response. Westport, CT: Praeger; 1980. [Google Scholar]
- 232. Starling AP, Stock JT. Dental indicators of health and stress in early Egyptian and Nubian agriculturalists: a difficult transition and gradual recovery. Am J Phys Anthropol. 2007;134:520–528. [DOI] [PubMed] [Google Scholar]
- 233. Watts MJ. Silent Violence: Food, Famine, and Peasantry in Northern Nigeria. Athens, GA: University of Georgia Press; 2013. [Google Scholar]
- 234. Hilliard SB. Hog Meat and Hoecake: A Geographical View of Food Supply in the Heart of the Old South, 1840-1860. Madison, WI: University of Wisconsin–Madison; 1966. [Google Scholar]
- 235. Lucas WM. Perspectives in human malnutrition: a contribution to the biology of disease from a clinical and pathological study of chronic malnutrition and pellagra in the African. J Natl Med Assoc. 1952;44:241–242. [Google Scholar]
- 236. Pearce-Duvet JM. The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease. Biol Rev Camb Philos Soc. 2006;81:369–382. [DOI] [PubMed] [Google Scholar]
- 237. Doolittle WF, Booth A. It’s the song, not the singer: an exploration of holobiosis and evolutionary theory. Biol Philos. 2017;32:5–24. [Google Scholar]
- 238. Warman A. Corn & Capitalism: How a Botanical Bastard Grew to Global Dominance. Chapel Hill, NC: University of North Carolina Press; 2003. [Google Scholar]
- 239. McCann J, McCann J. Maize and Grace: Africa’s Encounter with a New World Crop, 1500-2000. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- 240. White TCR. The Inadequate Environment: Nitrogen and the Abundance of Animals. Berlin, Germany: Springer; 2012. [Google Scholar]
- 241. Fogel RW. The Escape from Hunger and Premature Death, 1700-2100: Europe, America, and the Third World. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- 242. Brenton BP, Paine RR. Reevaluating the health and nutritional status of maize-dependent populations: evidence for the impact of pellagra on human skeletons from South Africa. Ecol Food Nutr. 2007;46:345–360. [Google Scholar]
- 243. Mann CC. 1491: New Revelations of the Americas before Columbus. New York, NY: Vintage Books; 2006. [Google Scholar]
- 244. Fessler DM. Reproductive immunosupression and diet. An evolutionary perspective on pregnancy sickness and meat consumption. Curr Anthropol. 2002;43:19–61. [DOI] [PubMed] [Google Scholar]
- 245. Anderson M, Michael A. British Population History: From the Black Death to the Present Day. Cambridge, UK: Cambridge University Press; 1996. [Google Scholar]
- 246. Bashford A, Chaplin JE. The New Worlds of Thomas Robert Malthus: Rereading the Principle of Population. Princeton, NJ: Princeton University Press; 2016. [Google Scholar]
- 247. Camiscioli E. Reproducing the French Race: Immigration, Intimacy, and Embodiment in the Early Twentieth Century. Durham, NC: Duke University Press; 2009. [Google Scholar]
- 248. Coulmas F. Population Decline and Ageing in Japan – The Social Consequences. Abingdon, UK: Routledge; 2007. [Google Scholar]
- 249. Kaufmann EP. Shall the Religious Inherit the Earth?: Demography and Politics in the Twenty-First Century. London, England: Profile; 2010. [Google Scholar]
- 250. Kuznets S. Population and economic growth. P Am Philos Soc. 1967;111:170–193. [Google Scholar]
- 251. Poston DL, Yaukey D. The Population of Modern China. New York, NY: Springer; 2013. [Google Scholar]
- 252. Wilson C. Fertility below replacement level. Science. 2004;304:207–209. [DOI] [PubMed] [Google Scholar]
- 253. Fuller DQ, Denham T, Arroyo-Kalin M, et al. Convergent evolution and parallelism in plant domestication revealed by an expanding archaeological record. Proc Natl Acad Sci U S A. 2014;111:6147–6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Edwards PC. Revising the Broad Spectrum Revolution: and its role in the origins of Southwest Asian food production. Antiquity. 1989;63:225–246. [Google Scholar]
- 255. Flannery KV. Origins and ecological effects of early domestication in Iran and the Near East. In: Ucko PJ, Dimbleby GW, eds. The Domestication and Exploitation of Plants and Animals. London, England: Gerald Duckworth & Co.; 1969:73–100. [Google Scholar]
- 256. Hublin JJ, Richards MP. The Evolution of Hominin Diets: Integrating Approaches to the Study of Palaeolithic Subsistence. Dordrecht, The Netherlands: Springer; 2009. [Google Scholar]
- 257. Jones EL. In Search of the Broad Spectrum Revolution in Paleolithic Southwest Europe. Cham, Switzerland: Springer International Publishing; 2015. [Google Scholar]
- 258. Weiss E, Wetterstrom W, Nadel D, Bar-Yosef O. The broad spectrum revisited: evidence from plant remains. Proc Natl Acad Sci U S A. 2004;101:9551–9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Pierre J, Appel B, Naji S. Testing the hypothesis of a worldwide neolithic demographic transition: corroboration from American cemeteries. Curr Anthropol. 2006;47:341–365. [Google Scholar]
- 260. Jacob HE, Winston C, Winston R. Six Thousand Years of Bread: Its Holy and Unholy History. New York, NY: Skyhorse Publishing Company, Incorporated; 2007. [Google Scholar]
- 261. Berbesque JC, Marlowe FW, Shaw P, Thompson P. Hunter–gatherers have less famine than agriculturalists. Biol Lett. 2014;10:20130853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Mellars P. Rethinking the Human Revolution: New Behavioural and Biological Perspectives on the Origin and Dispersal of Modern Humans. Cambridge, UK: McDonald Institute for Archaeological Research; 2007. [Google Scholar]
- 263. Karkanas P, Koumouzelis M, Kozlowski JK, et al. The earliest evidence for clay hearths: Aurignacian features in Klisoura Cave 1, southern Greece. Antiquity. 2015;78:513–525. [Google Scholar]
- 264. Tilley CY. A Phenomenology of Landscape: Places, Paths, and Monuments. Oxford, UK: Berg; 1994. [Google Scholar]
- 265. Shennan S. The First Farmers of Europe: An Evolutionary Perspective. Cambridge, UK: Cambridge University Press; 2018. [Google Scholar]
- 266. Becker E. The Denial of Death. New York, NY: Free Press; 2007. [Google Scholar]
- 267. Nowak M, Highfield R. SuperCooperators: Altruism, Evolution, and Why We Need Each Other to Succeed. New York, NY: Free Press; 2011. [Google Scholar]
- 268. Braidwood RJ, Braidwood L. Jarmo: a village early farmers in Iraq. Antiquity. 1950;24:189–195. [Google Scholar]
- 269. Fort J. Synthesis between demic and cultural diffusion in the Neolithic transition in Europe. Proc Natl Acad Sci U S A. 2012;109:18669–18673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Miles D. The Tale of the Axe: How the Neolithic Revolution Transformed Britain. London, England: Thames & Hudson; 2016. [Google Scholar]
- 271. Redman CL. The Rise of Civilization: From Early Farmers to Urban Society in the Ancient near East. New York, NY: W. H. Freeman; 1978. [Google Scholar]
- 272. Whittle A, Cummings V, Academy B. Going Over: The Mesolithis-Neolithic Transition in North West Europe. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 273. Henrich JP. Foundations of Human Sociality: Economic Experiments and Ethnographic Evidence from Fifteen Small-Scale Societies. Oxford, UK: Oxford University Press; 2004. [Google Scholar]
- 274. Kaplan HS, Hooper PL, Gurven M. The evolutionary and ecological roots of human social organization. Philos Trans R Soc Lond B Biol Sci. 2009;364:3289–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Yudkin J. Diet of Man, Needs and Wants. London, England: Applied Science Publishers; 1978. [Google Scholar]
- 276. Powell A, Shennan S, Thomas MG. Late Pleistocene demography and the appearance of modern human behavior. Science. 2009;324:1298–1301. [DOI] [PubMed] [Google Scholar]
- 277. Rutherford A. The Book of Humans: The Story of How We Became Us. London, England: Orion; 2018. [Google Scholar]
- 278. Mariotti Lippi M, Foggi B, Aranguren B, Ronchitelli A, Revedin A. Multistep food plant processing at Grotta Paglicci (Southern Italy) around 32,600 cal B.P. Proc Natl Acad Sci U S A. 2015;112:12075–12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Hodder I. The Domestication of Europe. London, England: Blackwell; 1990. [Google Scholar]
- 280. Wilson PJ. The Domestication of the Human Species. New Haven, CT: Yale University Press; 1991. [Google Scholar]
- 281. Ucko PJ, Tringham R, Dimbleby GW. Man, Settlement and Urbanism. London, England: Duckworth; 1972. [Google Scholar]
- 282. Notestein FW. Intrinsic factors in population growth. P Am Philos Soc. 1939;80:499–511. [Google Scholar]
- 283. Hagen EH, Barrett HC, Price ME. Do human parents face a quantity-quality tradeoff?: evidence from a Shuar community. Am J Phys Anthropol. 2006;130:405–418. [DOI] [PubMed] [Google Scholar]
- 284. Gaskins AJ, Chavarro JE. Diet and fertility: a review. Am J Obstet Gynecol. 2018;218:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Frisch RE. Population food intake and fertility. Science. 1978;199:22–30. [DOI] [PubMed] [Google Scholar]
- 286. James Reynolds S, Schoech SJ, Bowman R. Diet quality during pre-laying and nestling periods influences growth and survival of Florida scrub-jay (Aphelocoma coerulescens) chicks. J Zool. 2003;261:217–226. [Google Scholar]
- 287. Pianka ER. Evolutionary Ecology. New York, NY: HarperCollins; 1994. [Google Scholar]
- 288. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. [DOI] [PubMed] [Google Scholar]
- 289. Promislow DE, Harvey PH. Living fast and dying young: a comparative analysis of life-history variation among mammals. J Zool. 1990;220:417–437. [Google Scholar]
- 290. McLaren IA. Natural Regulation of Animal Populations. Abingdon, UK: Routledge; 2017. [Google Scholar]
- 291. Cauvin J, Watkins T, Ashmore W, Gamble C, O’Shea J, Renfrew C. The Birth of the Gods and the Origins of Agriculture. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 292. Atran S, Medin DL. The Native Mind and the Cultural Construction of Nature. Cambridge, MA: A Bradford Book; 2008. [Google Scholar]
- 293. Doubleday T. The True Law of Population Shewn to be Connected with the Food of the People. London, England: Simpkin, Marshall; 1842. [Google Scholar]
- 294. Aime C, Verdu P, Segurel L, et al. Microsatellite data show recent demographic expansions in sedentary but not in nomadic human populations in Africa and Eurasia. Eur J Hum Genet. 2014;22:1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Suzman J. Affluence without Abundance: The Disappearing World of the Bushmen. London, England: Bloomsbury Publishing; 2017. [Google Scholar]
- 296. Hawks J, Hunley K, Lee S-H, Wolpoff M. Population bottlenecks and Pleistocene human evolution. Mol Biol Evol. 2000;17:2–22. [DOI] [PubMed] [Google Scholar]
- 297. Klein RG. The Human Career: Human Biological and Cultural Origins. 3rd ed: Chicago, IL: University of Chicago Press; 2009. [Google Scholar]
- 298. Mithen S. After the Ice: A Global Human History, 20,000-5000 BC. Cambridge, MA: Harvard University Press; 2006. [Google Scholar]
- 299. Takahata N, Satta Y, Klein J. Divergence time and population size in the lineage leading to modern humans. Theor Popul Biol. 1995;48:198–221. [DOI] [PubMed] [Google Scholar]
- 300. Silvertown J. Dinner with Darwin: Food, Drink, and Evolution. Chicago, IL: University of Chicago Press; 2017. [Google Scholar]
- 301. Marean CW. The transition to foraging for dense and predictable resources and its impact on the evolution of modern humans. Philos Trans R Soc Lond B Biol Sci. 2016;371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Marean CW. Pinnacle Point Cave 13B (Western Cape Province, South Africa) in context: the Cape Floral kingdom, shellfish, and modern human origins. J Hum Evol. 2010;59:425–443. [DOI] [PubMed] [Google Scholar]
- 303. Smith EI, Jacobs Z, Johnsen R, et al. Humans thrived in South Africa through the Toba eruption about 74,000 years ago. Nature. 2018;555:511–515. [DOI] [PubMed] [Google Scholar]
- 304. Esteban I, Marean CW, Fisher EC, Karkanas P, Cabanes D, Albert RM. Phytoliths as an indicator of early modern humans plant gathering strategies, fire fuel and site occupation intensity during the Middle Stone Age at Pinnacle Point 5-6 (south coast, South Africa). PLoS ONE. 2018;13:e0198558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Gibson MA, Mace R. Helpful grandmothers in rural Ethiopia: a study of the effect of kin on child survival and growth. Evol Hum Behav. 2005;26:469–482. [Google Scholar]
- 306. Balter M. The Goddess and the Bull: Çatalhöyük: An Archaeological Journey to the Dawn of Civilization. Abingdon, UK: Routledge; 2016. [Google Scholar]
- 307. Eller C. The Myth of Matriarchal Prehistory: Why an Invented past Won’t Give Women a Future. Boston, NA: Beacon Press; 2000. [Google Scholar]
- 308. Fisher HE. Anatomy of Love: A Natural History of Mating, Marriage, and Why We Stray. New York, NY: Fawcett Columbine; 1994. [Google Scholar]
- 309. Goodison L, Morris C. Ancient Goddesses: The Myths and the Evidence. Madison, WI: University of Wisconsin Press; 1998. [Google Scholar]
- 310. Hodder I. Archaeology beyond Dialogue. Salt Lake City, UT: University of Utah Press; 2003. [Google Scholar]
- 311. Mellaart J. The Archaeology of Ancient Turkey. Lanham, MD: Rowman & Littlefield; 1978. [Google Scholar]
- 312. MacGregor N. Living with the Gods: On Beliefs and Peoples. London, England: Penguin Books; 2018. [Google Scholar]
- 313. Stearns SC. The Evolution of Life Histories. Oxford, UK: Oxford University Press; 1992. [Google Scholar]
- 314. Henshilwood CS, d’Errico F, Marean CW, Milo RG, Yates R. An early bone tool industry from the Middle Stone Age at Blombos Cave, South Africa: implications for the origins of modern human behaviour, symbolism and language. J Hum Evol. 2001;41:631–678. [DOI] [PubMed] [Google Scholar]
- 315. Miller G. The Mating Mind: How Sexual Choice Shaped the Evolution of Human Nature. New York, NY: Vintage Books; 2001. [Google Scholar]
- 316. Bahn PG. Images of the Ice Age. Oxford, UK: Oxford University Press; 2016. [Google Scholar]
- 317. Gimbutas M. The Goddesses and Gods of Old Europe, 6500-3500 BC, Myths and Cult Images. Berkeley, CA: University of California Press; 1982. [Google Scholar]
- 318. Graziosi P. Palaeolithic Art. New York, NY: McGraw-Hill; 1960. [Google Scholar]
- 319. Laming A. Lascaux: Paintings and Engravings. London, England: Penguin Books; 1959. [Google Scholar]
- 320. Leroi-Gourhan A. Gesture and Speech. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- 321. Buss DM. The Evolution of Desire: Strategies of Human Mating. New York, NY: Basic Books; 2016. [Google Scholar]
- 322. Cronin H, Smith JM. The Ant and the Peacock: Altruism and Sexual Selection from Darwin to Today. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- 323. De Block A, Dewitte S. Mating games: cultural evolution and sexual selection. Biol Philos 2007;22:475–491. [Google Scholar]
- 324. Gayon J. Sexual selection: another Darwinian process. C R Biol. 2010;333:134–144. [DOI] [PubMed] [Google Scholar]
- 325. Prum RO. The Evolution of Beauty: How Darwin’s Forgotten Theory of Mate Choice Shapes the Animal World – and Us. New York, NY: Knopf Doubleday Publishing Group; 2017. [Google Scholar]
- 326. Puts DA. Beauty and the beast: mechanisms of sexual selection in humans. Evol Hum Behav. 2010;31:157–175. [Google Scholar]
- 327. Richards E. Darwin and the Making of Sexual Selection. Chicago, IL: University of Chicago Press; 2017. [Google Scholar]
- 328. Shlain L. Sex, Time, and Power: How Women’s Sexuality Shaped Human Evolution. London, England: Penguin Books; 2004. [Google Scholar]
- 329. Allport S. The Primal Feast: Food, Sex, Foraging, and Love. New York, NY: Open Road Integrated Media; 2016. [Google Scholar]
- 330. Symons D. The Evolution of Human Sexuality. Oxford, UK: Oxford University Press; 1979. [Google Scholar]
- 331. Thornhill R, Gangestad SW. The Evolutionary Biology of Human Female Sexuality. Oxford, UK: Oxford University Press; 2008. [Google Scholar]
- 332. Becker GS, Becker GS. A Treatise on the Family. Enlarged Edition. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- 333. Blakemore SJ. The developing social brain: implications for education. Neuron. 2010;65:744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Bowlby J. Attachment and Loss. New York, NY: Basic Books; 1980. [Google Scholar]
- 335. Carroll Mc CP. The Westermarck hypothesis and first cousin marriage: the cultural modification of negative sexual imprinting. J Anthropol Res. 1986;42:573–586. [Google Scholar]
- 336. Chagnon NA, Irons W, Association AA. Evolutionary Biology and Human Social Behavior: An Anthropological Perspective. Duxbury Press; 1979. [Google Scholar]
- 337. Dunbar RI. The social brain hypothesis and its implications for social evolution. Ann Hum Biol. 2009;36:562–572. [DOI] [PubMed] [Google Scholar]
- 338. Hare B. Survival of the friendliest: Homo sapiens evolved via selection for prosociality. Annu Rev Psychol. 2017;68:155–186. [DOI] [PubMed] [Google Scholar]
- 339. Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: diet, intelligence, and longevity. Evol Anthropol. 2000;9:156–185. [Google Scholar]
- 340. Parker ST. A sexual selection model for hominid evolution. Hum Evol. 1987;2:235–253. [Google Scholar]
- 341. Pulliam HR, Dunford C. Programmed to Learn: An Essay on the Evolution of Culture. New York, NY: Columbia University Press; 1980. [Google Scholar]
- 342. Rosenthal GG. Mate Choice: The Evolution of Sexual Decision Making from Microbes to Humans. Princeton, NJ: Princeton University Press; 2017. [Google Scholar]
- 343. Dutton D. The Art Instinct: Beauty, Pleasure, and Human Evolution. London, England: Bloomsbury; 2010. [Google Scholar]
- 344. Collins D. The Human Revolution: From Ape to Artist. Oxford, UK: Phaidon; 1976. [Google Scholar]
- 345. Guthrie RD. The Nature of Paleolithic Art. Chicago, IL: University of Chicago Press; 2005. [Google Scholar]
- 346. Soffer O, Adovasio JM, Hyland DC, et al. The ‘Venus’ figurines: textiles, basketry, gender, and status in the Upper Paleolithic. Curr Anthropol. 2000;41:511–537. [Google Scholar]
- 347. Marshack A. The Roots of Civilization: The Cognitive Beginnings of Man’s First Art, Symbol and Notation. New York, NY: Moyer Bell; 1991. [Google Scholar]
- 348. Sorokowski P, Sorokowska A, Butovskaya M, et al. Love influences reproductive success in humans. Front Psychol. 2017;8:1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Haselton MG, Mortezaie M, Pillsworth EG, Bleske-Rechek A, Frederick DA. Ovulatory shifts in human female ornamentation: near ovulation, women dress to impress. Horm Behav. 2007;51:40–45. [DOI] [PubMed] [Google Scholar]
- 350. d’Errico F, Henshilwood C, Lawson G, et al. Archaeological evidence for the emergence of language, symbolism, and music – an alternative multidisciplinary perspective. J World Prehist. 2003;17:1–70. [Google Scholar]
- 351. Neal L. The Earliest Instrument: Ritual Power and Fertility Magic of the Flute in Upper Paleolithic Culture. Hillsdale, NY: Pendragon Press; 2015. [Google Scholar]
- 352. Geist V. Life Strategies, Human Evolution, Environmental Design: Toward a Biological Theory of Health. New York, NY: Springer; 2013. [Google Scholar]
- 353. Hrdy SB. Mothers and Others. Cambridge, MA: Harvard University Press; 2011. [Google Scholar]
- 354. Isler K, van Schaik CP. Allomaternal care, life history and brain size evolution in mammals. J Hum Evol. 2012;63:52–63. [DOI] [PubMed] [Google Scholar]
- 355. Johnson AW, Earle TK. The Evolution of Human Societies: From Foraging Group to Agrarian State. Stanford, CA: Stanford University Press; 2000. [Google Scholar]
- 356. El Zaatari S, Hublin JJ. Diet of upper paleolithic modern humans: evidence from microwear texture analysis. Am J Phys Anthropol. 2014;153:570–581. [DOI] [PubMed] [Google Scholar]
- 357. Piperno DR, Weiss E, Holst I, Nadel D. Processing of wild cereal grains in the Upper Palaeolithic revealed by starch grain analysis. Nature. 2004;430:670–673. [DOI] [PubMed] [Google Scholar]
- 358. Revedin A, Aranguren B, Becattini R, et al. Thirty thousand-year-old evidence of plant food processing. Proc Natl Acad Sci U S A. 2010;107:18815–18819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Ehlers J, Hughes P, Gibbard PL. The Ice Age. Hoboken, NJ: Wiley; 2016. [Google Scholar]
- 360. Dixson AF. Sexual Selection and the Origins of Human Mating Systems: Oxford, UK: Oxford University Press; 2009. [Google Scholar]
- 361. Soffer O, Gamble C. The World at 18,000 BP: Low Latitudes. London, England: Unwin Hyman; 1990. [Google Scholar]
- 362. Barber EW. Women’s Work: The First 20,000 Years Women, Cloth, and Society in Early Times. New York, NY: W.W. Norton; 1995. [Google Scholar]
- 363. Hager L. Women in Human Evolution. London, England: Routledge; 2005. [Google Scholar]
- 364. Hoquet T. Current Perspectives on Sexual Selection: What’s left after Darwin? Dordrecht, The Netherlands: Springer; 2015. [Google Scholar]
- 365. Kappeler PM, van Schaik CP. Sexual Selection in Primates: New and Comparative Perspectives. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- 366. Taylor T. The Prehistory of Sex: Four Million Years of Human Sexual Culture. London, England: Fourth Estate; 1997. [Google Scholar]
- 367. Renfrew C. The Explanation of Culture Change. Models in Prehistory: Meeting, Sheffield, December 1971, Proceedings. Pittsburgh, PA: University of Pittsburgh Press; 1973. [Google Scholar]
- 368. Mcbrearty S, Brooks AS. The revolution that wasn’t: a new interpretation of the origin of modern human behavior. J Hum Evol. 2000;39:453–563. [DOI] [PubMed] [Google Scholar]
- 369. Ward RH, Weiss KM. The Demographic Evolution of Human Populations. Cambridge, MA: Academic Press; 1976. [Google Scholar]
- 370. Roebroeks W. Hunters of the Golden Age: The Mid Upper Palaeolithic of Eurasia, 30,000 - 20,000 BP. Leiden, The Netherlands: University of Leiden; 1999. [Google Scholar]
- 371. Richards MP, Pettitt PB, Stiner MC, Trinkaus E. Stable isotope evidence for increasing dietary breadth in the European mid-Upper Paleolithic. Proc Natl Acad Sci U S A. 2001;98:6528–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Starkovich BM. Paleolithic subsistence strategies and changes in site use at Klissoura Cave 1 (Peloponnese, Greece). J Hum Evol. 2017;111:63–84. [DOI] [PubMed] [Google Scholar]
- 373. Puleston C, Tuljapurkar S, Winterhalder B. The invisible cliff: abrupt imposition of Malthusian Equilibrium in a natural-fertility, Agrarian Society. PLoS ONE. 2014;9:e87541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Brody H. Other Side of Eden: Hunters, Farmers and the Shaping of the World. Madeira Park, BC, Canada: D & M Publishers; 2009. [Google Scholar]
- 375. Lev E, Kislev ME, Bar-Yosef O. Mousterian vegetal food in Kebara cave, Mt. Carmel. J Archaeol Sci. 2005;32:475–484. [Google Scholar]
- 376. Mithen S. The Singing Neanderthals: The Origins of Music, Language, Mind and Body. London, England: Orion; 2011. [Google Scholar]
- 377. Hardy BL, Moncel MH. Neanderthal use of fish, mammals, birds, starchy plants and wood 125-250,000 years ago. PLoS ONE. 2011;6:e23768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Henry AG, Brooks AS, Piperno DR. Plant foods and the dietary ecology of Neanderthals and early modern humans. J Hum Evol. 2014;69:44–54. [DOI] [PubMed] [Google Scholar]
- 379. Richards MP, Trinkaus E. Out of Africa: modern human origins special feature: isotopic evidence for the diets of European Neanderthals and early modern humans. Proc Natl Acad Sci U S A. 2009;106:16034–16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Marean CW, Assefa Z. Zooarcheological evidence for the faunal exploitation behavior of Neandertals and early modern humans. Evol Anthropol. 1999;8:22–37. [Google Scholar]
- 381. Finlayson C. Neanderthals and Modern Humans: An Ecological and Evolutionary Perspective. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- 382. Chikhi L, Destro-Bisol G, Bertorelle G, Pascali V, Barbujani G. Clines of nuclear DNA markers suggest a largely Neolithic ancestry of the European gene pool. Proc Natl Acad Sci U S A. 1998;95:9053–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Deevey ES., Jr The human population. Sci Am. 1960;203:195. [PubMed] [Google Scholar]
- 384. Berger TD, Trinkaus E. Patterns of trauma among the Neandertals. J Archaeol Sci. 1995;22:841–852. [Google Scholar]
- 385. Pettitt P, White M. The British Palaeolithic: Hominin Societies at the Edge of the Pleistocene World. New York, NY: Routledge; 2012. [Google Scholar]
- 386. Rogers AR, Bohlender RJ, Huff CD. Early history of Neanderthals and Denisovans. Proc Natl Acad Sci U S A. 2017;114:9859–9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Weyrich LS, Duchene S, Soubrier J, et al. Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature. 2017;544:357–361. [DOI] [PubMed] [Google Scholar]
- 388. Lahr MM. The Evolution of Modern Human Diversity: A Study of Cranial Variation. Cambridge, UK: Cambridge University Press; 1996. [Google Scholar]
- 389. Otto G. Human evolution: archaic admixture with Denisovans. Nat Rev Genet. 2018;19:251. [DOI] [PubMed] [Google Scholar]
- 390. Paabo S. The diverse origins of the human gene pool. Nat Rev Genet. 2015;16:313. [DOI] [PubMed] [Google Scholar]
- 391. Sanchez-Quinto F, Lalueza-Fox C. Almost 20 years of Neanderthal palaeogenetics: adaptation, admixture, diversity, demography and extinction. Philos Trans R Soc Lond B Biol Sci. 2015;370:20130374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Walker A, Zimmerman MR, Leakey RE. A possible case of hypervitaminosis A in Homo erectus. Nature 1982;296:248–250. [DOI] [PubMed] [Google Scholar]
- 393. Wynn T, Coolidge FL. How to Think Like a Neandertal. New York, NY: Oxford University Press; 2011. [Google Scholar]
- 394. Barrett CB, Garg T, McBride L. Well-Being Dynamics and Poverty Traps. Leeds, UK: Centre for Climate Change Economics and Policy; 2016. [Google Scholar]
- 395. Gokhman D, Lavi E, Prufer K, et al. Reconstructing the DNA methylation maps of the Neandertal and the Denisovan. Science. 2014;344:523–527. [DOI] [PubMed] [Google Scholar]
- 396. Gokhman D, Malul A, Carmel L. Inferring past environments from ancient epigenomes. Mol Biol Evol. 2017;34:2429–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Pennisi E. Epigenetics. Ancient DNA holds clues to gene activity in extinct humans. Science. 2014;344:245–246. [DOI] [PubMed] [Google Scholar]
- 398. Bentley GR, Jasienska G, Goldberg T. Is the fertility of agriculturalists higher than that of nonagriculturalists? Curr Anthropol. 1993;34:778–785. [Google Scholar]
- 399. Mace R. Reproducing in cities. Science. 2008;319:764–766. [DOI] [PubMed] [Google Scholar]
- 400. Senior AM, Lihoreau M, Buhl J, Raubenheimer D, Simpson SJ. Social network analysis and nutritional behavior: an integrated modeling approach. Front Psychol. 2016;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Kohler TA, Smith ME, Bogaard A, et al. Greater post-Neolithic wealth disparities in Eurasia than in North America and Mesoamerica. Nature. 2017;551:619–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Foley R, Gamble C. The ecology of social transitions in human evolution. Philos Trans R Soc Lond B Biol Sci. 2009;364:3267–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Gamble C, Gowlett J, Dunbar R. The social brain and the shape of the Palaeolithic. Camb Archaeol J. 2011;21:115–136. [Google Scholar]
- 404. Wrangham RW. An ecological model of female-bonded primate groups. Behaviour. 1980;75:262–300. [Google Scholar]
- 405. Diamond J, Bellwood P. Farmers and their languages: the first expansions. Science. 2003;300:597–603. [DOI] [PubMed] [Google Scholar]
- 406. Ehret C. Language family expansions: broadening our understandings of cause from an African perspective. In: Bellwood PS, Renfrew C, eds. Examining the Farming/Language Dispersal Hypothesis. Cambridge, UK: University of Cambridge; 2002:163–176. [Google Scholar]
- 407. Puchner M. The Written World: How Literature Shaped History. London, England: Granta Publications; 2017. [Google Scholar]
- 408. Renfrew C. Archaeology and Language: The Puzzle of Indo-European Origins. Cambridge, UK: Cambridge University Press; 1990. [Google Scholar]
- 409. Price TD, Feinman GM. Foundations of Social Inequality. New York, NY: Springer; 2013. [Google Scholar]
- 410. Henrich J. Demography and cultural evolution: how adaptive cultural processes can produce maladaptive losses – the Tasmanian case. Am Antiquity. 2004;69:197–214. [Google Scholar]
- 411. Cronon W. Nature’s Metropolis: Chicago and the Great West. New York, NY: W.W. Norton; 2009. [Google Scholar]
- 412. Burger O, DeLong JP. What if fertility decline is not permanent? The need for an evolutionarily informed approach to understanding low fertility. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Colleran H. The cultural evolution of fertility decline. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Dribe M, Breschi M, Gagnon A, et al. Socio-economic status and fertility decline: insights from historical transitions in Europe and North America. Popul Stud (Camb). 2017;71:3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Hruschka DJ, Burger O. How does variance in fertility change over the demographic transition? Philos Trans R Soc Lond B Biol Sci. 2016;371:20150155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416. Page AE, Viguier S, Dyble M, et al. Reproductive trade-offs in extant hunter-gatherers suggest adaptive mechanism for the Neolithic expansion. Proc Natl Acad Sci U S A. 2016;113:4694–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417. Wood JW. Fertility in anthropological populations. Annu Rev Anthropol. 1990;19:211–242. [DOI] [PubMed] [Google Scholar]
- 418. Coleman D, Schofield R. The State of Population Theory: Forward from Malthus. Oxford, UK: Basil Blackwell; 1986. [Google Scholar]
- 419. Colleran H, Jasienska G, Nenko I, Galbarczyk A, Mace R. Fertility decline and the changing dynamics of wealth, status and inequality. Proc Biol Sci. 2015;282:20150287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Lamba S, Mace R. Demography and ecology drive variation in cooperation across human populations. Proc Natl Acad Sci U S A. 2011;108:14426–14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Page AE, Chaudhary N, Viguier S, et al. Hunter-gatherer social networks and reproductive success. Sci Rep. 2017;7:1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Chang RQ, Li DJ, Li MQ. The role of indoleamine-2,3-dioxygenase in normal and pathological pregnancies. Am J Reprod Immunol. 2018;79:e12786. [DOI] [PubMed] [Google Scholar]
- 423. Bach JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol. 2018;18:105–120. [DOI] [PubMed] [Google Scholar]
- 424. de Laval B, Sieweke MH. Trained macrophages support hygiene hypothesis. Nat Immunol. 2017;18:1279. [DOI] [PubMed] [Google Scholar]
- 425. De Rosa V, La Cava A, Matarese G. Metabolic pressure and the breach of immunological self-tolerance. Nat Immunol. 2017;18:1190–1196. [DOI] [PubMed] [Google Scholar]
- 426. Ege MJ. The hygiene hypothesis in the age of the microbiome. Ann Am Thorac Soc. 2017;14:S348–S353. [DOI] [PubMed] [Google Scholar]
- 427. Isolauri E, Huurre A, Salminen S, Impivaara O. The allergy epidemic extends beyond the past few decades. Clin Exp Allergy. 2004;34:1007–1010. [DOI] [PubMed] [Google Scholar]
- 428. Khan TK, Palmer DJ, Prescott SL. In-utero exposures and the evolving epidemiology of paediatric allergy. Curr Opin Allergy Clin Immunol. 2015;15:402–408. [DOI] [PubMed] [Google Scholar]
- 429. Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18:1076–1083. [DOI] [PubMed] [Google Scholar]
- 430. Miftahussurur M, Nusi IA, Graham DY, Yamaoka Y. Helicobacter, hygiene, atopy, and asthma. Front Microbiol. 2017;8:1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Peters SE, Gaines RR. Formation of the ‘Great Unconformity’ as a trigger for the Cambrian explosion. Nature. 2012;484:363–366. [DOI] [PubMed] [Google Scholar]
- 432. Santiago HC, Nutman TB. Human helminths and allergic disease: the hygiene hypothesis and beyond. Am J Trop Med Hyg. 2016;95:746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Sokolowska M, Akdis CA. Highlights in immune response, microbiome and precision medicine in allergic disease and asthma. Curr Opin Immunol. 2017;48:iv–ix. [DOI] [PubMed] [Google Scholar]
- 434. Ehlers S, Kaufmann SH. Infection, inflammation, and chronic diseases: consequences of a modern lifestyle. Trends Immunol. 2010;31:184–190. [DOI] [PubMed] [Google Scholar]
- 435. Gerriets VA, Kishton RJ, Johnson MO, et al. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nat Immunol. 2016;17:1459–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. La Rocca C, Carbone F, De Rosa V, et al. Immunometabolic profiling of T cells from patients with relapsing-remitting multiple sclerosis reveals an impairment in glycolysis and mitochondrial respiration. Metabolism. 2017;77:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Ban Y, Chang Y, Dong B, Kong B, Qu X. Indoleamine 2, 3-dioxygenase levels at the normal and recurrent spontaneous abortion fetal–maternal interface. J Int Med Res. 2013;41:1135–1149. [DOI] [PubMed] [Google Scholar]
- 438. McKenzie C, Tan J, Macia L, Mackay CR. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol Rev. 2017;278:277–295. [DOI] [PubMed] [Google Scholar]
- 439. Mellor AL, Lemos H, Huang L. Indoleamine 2,3-dioxygenase and tolerance: where are we now? Front Immunol. 2017;8:1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology. 2009;126:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441. Dietert R. The Human Superorganism: How the Microbiome Is Revolutionizing the Pursuit of a Healthy Life. London, England: Penguin Books; 2016. [Google Scholar]
- 442. Frodeman R, Klein JT, Pacheco RCDS. The Oxford Handbook of Interdisciplinarity. Oxford, UK: Oxford University Press; 2017. [Google Scholar]
- 443. Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Blaser M. Missing Microbes. London, England: Oneworld Publications; 2014. [Google Scholar]
- 445. Bollrath J, Powrie F. Feed your tregs more fiber. Science. 2013;341:463–464. [DOI] [PubMed] [Google Scholar]
- 446. von Bubnoff D, Bieber T. The indoleamine 2, 3-dioxygenase (IDO) pathway controls allergy. Allergy. 2012;67:718–725. [DOI] [PubMed] [Google Scholar]
- 447. Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448. Ciprandi G, Fuchs D. Tryptophan metabolic pathway, airway nitric oxide, and allergy. Ann Allergy Asthma Immunol. 2017;119:395–396. [DOI] [PubMed] [Google Scholar]
- 449. Davis DM. The Beautiful Cure: Harnessing Your Body’s Natural Defences. New York, NY: Random House; 2018. [Google Scholar]
- 450. Gostner JM, Becker K, Kofler H, Strasser B, Fuchs D. Tryptophan metabolism in allergic disorders. Int Arch Allergy Immunol. 2016;169:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Li Z, Li X, Lin S, et al. Nicotinic acid receptor GPR109A exerts anti-inflammatory effects through inhibiting the Akt/mTOR signaling pathway in MIN6 pancreatic beta cells. Ann Clin Lab Sci. 2017;47:729–737. [PubMed] [Google Scholar]
- 452. Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. [DOI] [PubMed] [Google Scholar]
- 453. Melnik BC. Milk – a nutrient system of mammalian evolution promoting mTORC1-dependent translation. Int J Mol Sci. 2015;16:17048–17087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454. Sakaguchi S, Wing K, Miyara M. Regulatory T cells – a brief history and perspective. Eur J Immunol. 2007;37:S116–S123. [DOI] [PubMed] [Google Scholar]
- 455. Shevach EM. The resurrection of T cell-mediated suppression. J Immunol. 2011;186:3805–3807. [DOI] [PubMed] [Google Scholar]
- 456. Thornton AM, Shevach EM. CD4+ CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457. Villeneuve C, Kou HH, Eckermann H, et al. Evolution of the hygiene hypothesis into biota alteration theory: what are the paradigms and where are the clinical applications? Microbes Infect. 2018;20:147–155. [DOI] [PubMed] [Google Scholar]
- 458. Wu Z, Wang L, Tang Y, Sun X. Parasite-derived proteins for the treatment of allergies and autoimmune diseases. Front Microbiol. 2017;8:2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459. Carbone F, De Rosa V, Carrieri PB, et al. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med. 2014;20:69–74. [DOI] [PubMed] [Google Scholar]
- 460. Choi IY, Piccio L, Childress P, et al. A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 2016;15:2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461. Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M. Role of ‘Western diet’ in inflammatory autoimmune diseases. Curr Allergy Asthma Rep. 2014;14:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. Xing X, Liao Z, Tan F, Zhu Z, Jiang Y, Cao Y. Effect of nicotinamide against Candida albicans. Front Microbiol. 2019;10:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463. Barkow JH, Burley N. Human fertility, evolutionary biology, and the demographic transition. Ethol Sociobiol. 1980;1:163–180. [Google Scholar]
- 464. Bookman MZ. The Demographic Struggle for Power: The Political Economy of Demographic Engineering in the Modern World. Abingdon, UK: Routledge; 2013. [Google Scholar]
- 465. Bremmer I. The J Curve: A New Way to Understand Why Nations Rise and Fall. New York, NY: Simon & Schuster; 2006. [Google Scholar]
- 466. Caldwell JC, Caldwell P. The cultural context of high fertility in sub-Saharan Africa. Popul Dev Rev. 1987;13:409–437. [Google Scholar]
- 467. Clark G. In defense of the Malthusian interpretation of history. Eur Rev Econ Hist. 2008;12:175–199. [Google Scholar]
- 468. Coole D. Should We Control World Population? Hoboken, NJ: Wiley; 2018. [Google Scholar]
- 469. Cullather N. The Hungry World: America’s Cold War Battle against Poverty in Asia. Cambridge, MA: Harvard University Press; 2013. [Google Scholar]
- 470. Deaton A. The Great Escape: Health, Wealth, and the Origins of Inequality. Princeton, NJ: Princeton University Press; 2013. [Google Scholar]
- 471. Easterly W, Easterly WR. The White Man’s Burden: Why the West’s Efforts to Aid the Rest Have Done So Much Ill and So Little Good. London, England: Penguin Books; 2006. [Google Scholar]
- 472. Kenny C. Getting Better: Why Global Development Is Succeeding – And How We Can Improve the World Even More. New York, NY: Basic Books; 2012. [Google Scholar]
- 473. Lee R. The demographic transition: three centuries of fundamental change. J Econ Perspect. 2003;17:167–190. [Google Scholar]
- 474. Lesthaeghe R. The unfolding story of the second demographic transition. Popul Dev Rev. 2010;36:211–251. [DOI] [PubMed] [Google Scholar]
- 475. Li H, Durbin R. Inference of human population history from individual whole-genome sequences. Nature. 2011;475:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Lutz W, Butz WP, Samir KC. World Population and Human Capital in the Twenty-First Century: An Overview. Oxford, UK: Oxford University Press; 2017. [Google Scholar]
- 477. Morland P. The Human Tide: How Population Shaped the Modern World. London, England: Hodder & Stoughton; 2019. [Google Scholar]
- 478. Pearson CS. On the Cusp: From Population Boom to Bust. Oxford, UK: Oxford University Press; 2015. [Google Scholar]
- 479. Sen A. Development as Freedom. Oxford, UK: Oxford University Press; 2001. [Google Scholar]
- 480. Van Den Bergh JCJM, Rietveld P. Reconsidering the limits to world population: meta-analysis and meta-prediction. BioScience. 2004;54:195–204. [Google Scholar]
- 481. Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth’s ecosystems. Science. 1997;277:494–499. [Google Scholar]
- 482. Katz SH. Food and biocultural evolution: a model for the investigation of modern nutritional problems. In: Johnston FE. ed. Nutritional Anthropology. New York, NY: Alan R. Liss; 1987:41–63. [Google Scholar]
- 483. Cochran G, Harpending H. The 10,000 Year Explosion: How Civilization Accelerated Human Evolution. New York, NY: Basic Books; 2009. [Google Scholar]
- 484. Bauman Z. Mortality, Immortality and Other Life Strategies. Hoboken, NJ: Wiley; 2013. [Google Scholar]
- 485. Anthony DW. The Horse, the Wheel, and Language: How Bronze-Age Riders from the Eurasian Steppes Shaped the Modern World. Princeton, NJ: Princeton University Press; 2010. [Google Scholar]
- 486. Cunliffe B. By Steppe, Desert, and Ocean: The Birth of Eurasia. Oxford, UK: Oxford University Press; 2015. [Google Scholar]
- 487. Dunbar R. The Human Story. London, England: Faber & Faber; 2011. [Google Scholar]
- 488. Riley JC. Rising Life Expectancy: A Global History. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 489. Shah NM, Herasimtschuk AA, Boasso A, et al. Changes in T cell and dendritic cell phenotype from mid to late pregnancy are indicative of a shift from immune tolerance to immune activation. Front Immunol. 2017;8:1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490. Everman ER, Morgan TJ. Antagonistic pleiotropy and mutation accumulation contribute to age-related decline in stress response. Evolution. 2018;72:303–317. [DOI] [PubMed] [Google Scholar]
- 491. Patten MM. Selfish X chromosomes and speciation. Mol Ecol. 2018;27:3772–3782. [DOI] [PubMed] [Google Scholar]
- 492. Presgraves DC. Evaluating genomic signatures of ‘the large X-effect’ during complex speciation. Mol Ecol. 2018;27:3822–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493. Gibney MJ, Walsh MC. The future direction of personalised nutrition: my diet, my phenotype, my genes. Proc Nutr Soc. 2013;72:219–225. [DOI] [PubMed] [Google Scholar]
- 494. Gorman U, Mathers JC, Grimaldi KA, Ahlgren J, Nordstrom K. Do we know enough? A scientific and ethical analysis of the basis for genetic-based personalized nutrition. Genes Nutr. 2013;8:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495. Stover PJ. Influence of human genetic variation on nutritional requirements. Am J Clin Nutr. 2006;83:436S–442S. [DOI] [PubMed] [Google Scholar]
- 496. Stromland O, Niere M, Nikiforov AA, VanLinden MR, Heiland I, Ziegler M. Keeping the balance in NAD metabolism. Biochem Soc Trans. 2019;47:119–130. [DOI] [PubMed] [Google Scholar]
- 497. Yaku K, Okabe K, Hikosaka K, Nakagawa T. NAD metabolism in cancer therapeutics. Front Oncol. 2018;8:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498. Neelakantan H, Brightwell CR, Graber TG, et al. Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle. Biochem Pharmacol. 2019;163:481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499. Poddar SK, Sifat AE, Haque S, Nahid NA, Chowdhury S, Mehedi I. Nicotinamide mononucleotide: exploration of diverse therapeutic applications of a potential molecule. Biomolecules. 2019;9:E34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. Rieff D. The Reproach of Hunger: Food, Justice, and Money in the Twenty-First Century. New York, NY: Simon & Schuster; 2016. [Google Scholar]
- 501. Zechner U, Wilda M, Kehrer-Sawatzki H, Vogel W, Fundele R, Hameister H. A high density of X-linked genes for general cognitive ability: a run-away process shaping human evolution? Trends Genet. 2001;17:697–701. [DOI] [PubMed] [Google Scholar]






