Short abstract
The epidermal growth factor receptor (EGFR) located in dorsal root ganglion has been found as a new target for chronic pain treatment. However, it is not clear whether the change of EGFR expression in the dorsal root ganglion contributes to neuropathic pain development. In this study, we used a chronic compression of unilateral lumbar dorsal root ganglions (CCD)-induced rat neuropathic pain model and found that CCD caused the upregulation of both phosphorylated EGFR and total EGFR expression in compressed lumbar 4/5 (L4/L5) dorsal root ganglions by western blotting and immunohistochemistry methods. Either inhibition of EGFR activation by EGFR inhibitor or knockdown of EGFR expression by EGFR small interference RNA (siRNA) relieved CCD-induced pain hypersensitivities to mechanical, thermal, and cold stimuli in rats. Moreover, EGFR knockdown reversed CCD-induced the increase of intracellular mammalian target of rapamycin (mTOR) expression as well as the activation of the satellite glial cells in the ipsilateral compressed L4/L5 dorsal root ganglions. These findings suggest that not only activated EGFR but also total EGFR contribute to CCD-induced neuropathic pain by enhancing intracellular mTOR signaling.
Keywords: EGFR, mTOR, dorsal root ganglion, neuropathic pain, chronic compression of dorsal root ganglions
Introduction
Neuropathic pain arises from injuries or diseases affecting the somatosensory nervous system at any level of the peripheral or central nervous system,1 which is characterized by intermittent burning pain, allodynia, and hyperalgesia, as well as spontaneous ongoing pain.2 Neuropathic pain is usually caused by trauma (e.g., peripheral nerve, dorsal root, dorsal root ganglion (DRG), spinal cord, or brain injury) and some disorders (e.g., multiple sclerosis, stroke, human immunodeficiency virus-induced neuropathy, and diabetes) in clinic and is best treated with a combination of multiple therapeutic approaches. However, current therapeutic approaches of neuropathic pain management although provide symptomatic but still not unsatisfactory relief. More and more work is endeavoring to explore the new potential targets or strategy for antinociception.
The epidermal growth factor receptor (EGFR) belongs to the well-studied ErbB family of receptor tyrosine kinases which regulate cellular growth, survival, proliferation, and differentiation of fibroblasts and hepatocytes.3–5 A recent study showed that the gene coding EGFR had genetic association with temporomandibular disorder, a human chronic pain syndrome.4 EGFR inhibitors, the first-line treatment for nonsmall cell lung cancer, have been reported analgesic effect in neuropathic pain patients and preclinical neuropathic pain models.6,7 Furthermore, it has been found that phosphorylated EGFR was transiently activated in affected DRGs of murine inflammatory pain or neuropathic pain models.4 EGFR inhibitors showed short analgesic effect (less than 1 h) on these animal models.4 Furthermore, it has been demonstrated that phosphoinositide 3 kinase (PI3K)/Protein kinase B (Akt)/mammalian target of rapamycin (mTOR) cellular signaling pathway is one of the pathway mediating EGFR-activated pain behaviors.4 Therefore, it seems that EGFR is a potential target for pain management.
Chronic compression of unilateral lumbar DRGs (CCD)-induced neuropathic pain model has been used to mimics low back pain and radicular pain syndromes caused by multilevel nerve root compression and intervertebral foramina stenosis in human.8 In this study, we observed the upregulation of EGFR expression in injured DRGs of neuropathic pain models and further explored the effect of EGFR upregulation in CCD-induced pain hypersensitivities. We found that unilateral CCD induced the increase of phosphorylated EGFR (p-EGFR) and total EGFR (t-EGFR) expression in ipsilateral compressed L4/L5 DRGs. Either inhibition of EGFR activation by its inhibitor or knockdown of EGFR expression by EGFR small interference RNA (siRNA) relieved CCD-induced pain hypersensitivities to mechanical, thermal, and cold stimuli. EGFR knockdown also restored the expression of total EGFR and its intracellular downstream target mTOR in compressed L4/L5 DRGs of CCD rats. Moreover, EGFR knockdown reversed the increased expression of glutamine synthetase (GS), a marker for satellite glial cells, in compressed DRGs of CCD rats.
Materials and methods
Animals
Adult male Sprague Dawley (SD) rats (200–250 g) or three- to four-week-old SD rats were kept in an environment with adequate temperature (25 ± 1°) and ventilation in a light–dark cycle of 12 h–12 h. The animals had water and food ad libitum. All the experimental protocols were approved by the Institutional Animal Ethics Committee of the Xi’an Jiaotong University Health Science Center (No. XJ20120117) and are consistent with the ethical guidelines of International Association for the Study of Pain. All the efforts were made to minimize animal suffering and to reduce the number of animals used.
Neuropathic pain models
The neuropathic pain models including spinal nerve ligation (SNL) and CCD in rats were developed based on the previous publications. For SNL surgery,9,10 lumbar 5 (L5) spinal nerve was exposed, ligated, and transected under isoflurane anesthetization. Sham group underwent the identical operation but had no ligation and transection.
For CCD surgery,11,12 the L4 and L5 intervertebral foramina were exposed and L-shaped stainless steel rods (4 mm in length and 0.6 mm in diameter) were carefully inserted into the L4 and L5 foramina to compress the DRGs. The surgical procedure in sham group was identical to that in CCD group, except that the stainless steel rods were not inserted into the intervertebral foramina.
DRG cell culture and siRNA transfection
EGFR siRNA (5′-GAUGGAGUCAGCAAGUGUATT-3′; 5′-UACACUUGCUGACUCCAUCTT-3′) and negative control (NC) siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′; 5′-ACGUGACACGUUCGGAGAATT-3′) were designed and synthesized by Genepharma (Shanghai, China). The DRGs from three- to four-week-old SD rats were harvested and cultured for examining the knockdown efficiency of EGFR siRNA. All harvested DRGs were then digested with 0.25% trypsin solution without EDTA (Beyotime Biotechnology, Shanghai, China). Following trituration and centrifugation, dissociated cells were resuspended and cultured in cold Neurobasal™-A Medium (Gibco/ThermoFisher Scientific, Waltham, MA) with 10% fetal bovine serum (JR Scientific, Woodland, CA), B-27™ Supplement (1×) (Gibco/ThermoFisher Scientific), 100 units per ml penicillin, and 100 µg per ml streptomycin (Beyotime Biotechnology) in a six-well plate precoated with 50 µg per ml poly-D-lysine (Beyotime Biotechnology). The cultured cells were incubated in an incubator with 95% O2, 5% CO2, and at 37° 7. After 24 h incubation, EGFR siRNA (250 pmol) or equivalent NC siRNA was delivered by Lipo6000 transfection reagent (Beyotime Biotechnology) into cultured cells in six well plates. Two days later, the cultured cells were harvested into radio immunoprecipitation assay (RIPA) lysis buffer for western blotting.
Intrathecal catheter implantation and drug delivery
For intrathecal drug delivery, a polyethylene (PE)-10 catheter was implanted into the subarachnoid space of the spinal cord under isoflurane anesthesia. The detailed procedure has been described in our previous publication.13 Rats showing neurologic deficits postoperatively were excluded from the study; 10 µl of siRNAs, EGFR inhibitor, or vehicles were administered intrathecally followed by flushing of 10 µl sterile normal saline.
EGFR siRNA, NC siRNA, or vehicle control (phosphate buffer saline (PBS)) was administered intrathecally from the third day after CCD surgery once daily for four days. Lipo6000 transfection reagent (Beyotime Biotechnology) was used as a delivery vehicle for siRNA to improve delivery efficiency and prevent degeneration of siRNA.14,15
Gefitinib (HY-50895, MedChem Express, Shanghai, China) or vehicle (20% DMSO) was administered intrathecally by single injection on day 7 after CCD surgery to observe the acute effect of gefitinib or once daily for seven days from the first day after CCD surgery to observe the chronic effect.
Behavior tests
Paw withdrawal thresholds (PWTs) in response to mechanical stimuli were measured with the up–down testing paradigm.16–18 Briefly, the unrestrained rat was placed in a Plexiglass chamber on an elevated mesh screen after adaptation to experimental environment. Calibrated von Frey filaments in log increments of force (0.41, 0.69, 1.20, 2.04, 3.63, 5.50, 8.51, 15.14, and 26.00 g) were applied to the plantar surface of the hind paws of the rat. The 2.04-g stimulus was applied first. If a positive response occurred, the next smaller von Frey filament was used; or not, the next larger filament was used. The test stopped when (1) a negative response to the 26.00-g filament or (2) 3 stimuli after the first positive response. The PWT was calculated with the formula provided by Dixon by converting the pattern of positive and negative responses to a 50% threshold value.17,18
Paw withdrawal latencies (PWLs) to noxious heat were measured with a Model 37370 Analgesic Meter (UGO, Italy).10,16 Rats were placed in a Plexiglas chamber on a glass plate. A radiant heat was applied by a light beam to the middle of the plantar surface of each hind paw. The light beam was turned off once the rat has a strong paw withdrawal response. The PWL was defined as the length of time between the start of the light beam and paw withdrawal response. Five values in each trial were recorded at 10-min intervals and averaged as PWL value for each paw. A cutoff time of 20 s was used to avoid tissue damage to paw.
The cold hyperalgesia was measured with a model ZH-6C cold plate (Zheng-Hua Biologic, Anhui, China). Each rat was placed in a cylindrical transparent Plexiglas chamber (height: 28 cm, diameter: 20 cm) on a round cold plate (diameter: 19 cm). The temperature of cold plate was set at 0° t. The positive response of rat indicating cold hyperalgesia exhibited as a quick and strong paw flinching. The latency of the first positive response was recorded. Each trial was repeated three times at 10-min intervals. A cutoff time of 60 s was used to avoid tissue damage of both hind paws.
RNA extraction, reverse transcription and quantitative real-time polymerase chain reaction
L4/L5 DRGs were collected and kept in RNAlater stabilization solution (Thermo Scientific). DRGs were homogenized in a cold tissue homogenizer (Shanghai Jingxin, China), and RNA was extracted using RNAeasy™ RNA extraction kit (Beyotime Biotechnology). RNA concentration was quantitated using a nanodrop spectrophotometer (Thermo Scientific); 500 ng RNA was reverse transcribed with oligo (dT) primer using the RevertAid First strand cDNA Synthesis Kit (Thermo Scientific) according to the manufacturer’s instructions. Quantitative polymerase chain reaction (qPCR) was performed on a 20 µl reaction with 20 ng of cDNA, 250 nM forward and reverse primers, and 10 µl of BeyoFast™ SYBR Green qPCR Mix (Beyotime Biotechnology) by using the Egfr primer (Forward: 5′-ACAACACCCTGGTCTGGAAG-3′; Reverse: 5′-GCCCTTCTGGTTGTTGACAT-3′) or Gapdh primer (Forward: 5′-TCGGTGTGAACGGATTTGGC-3′; Rerverse: 5′-TCCCATTCTCGGCCTTGACT-3′) in a BIO-RAD CFX96 real-time PCR system (Bio-Rad Laboratories, Hercules, CA). The cycle parameters were an initial 3-min incubation at 95° 5, followed by 40 cycles of 95° 5 for 10 s, 60° 0 for 30 s, and 72° 2 for 30 s. All data were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), an internal control. Ratios of mRNA levels in ipsilateral side to contralateral side were calculated using the △Ct method (2−△Ct).
Western blotting
The L4/L5 DRGs or spinal dorsal horns were collected after decapitation of rats under deep anesthetization by chloral hydrate (400 mg/kg). The DRGs or spinal dorsal horns were homogenized with ice-cold RIPA lysis buffer (50 mM Tris, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride, pH7.4) with protease and phosphatase inhibitor cocktail (Beyotime Biotechnology) using Shanghai Jingxin tissue homogenizer. After the crude homogenate was centrifuged at 4° t for 15 min at 1000 g, the supernatants were collected for cytosolic proteins. Protein concentration was measured using a Bradford Protein Assay Kit (Beyotime Biotechnology). The equal amount samples were heated for 5 min at 99° 9 and loaded onto a 8% separating sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis gel. The proteins were then electrophoretically transferred onto a nitrocellulose membrane followed by blocking with 3% nonfat milk in Tris-buffered saline containing 0.1% Tween-20. The first antibodies included rabbit antiphosphor-EGFR (Tyr1068, p-EGFR, 1:1000; Beyotime Biotechnology), rabbit anti-EGFR (1:1000; Beyotime Biotechnology), rabbit anti-mTOR (1:1000; Cell Signaling Technology), mouse anti-GS (1:1000; EMD Millipore, Darmstadt, Germany), or mouse anti-β-actin (1:1000; Beyotime Biotechnology) antibodies. After incubating with the first antibodies overnight, the membranes were incubated in horseradish peroxidase-conjugated antirabbit or antimouse secondary antibody (1:3000; EMD Millipore) for 2 h. The proteins on membrane were detected by western peroxide reagent and luminol/enhancer reagent (Immobilon Western Chemiluminescent HRP Substrate; EMD Millipore) and visualized using the Champchemi System with SageCapture software (Sagecreation Service for Life Science, Beijing, China). The intensity of blots was quantified by using NIH Image J software. Each blot from the targeted protein was normalized to the corresponding β-actin. The average value from the control groups was set as 100% after normalization. The relative levels of the targeted protein from time points or the treated groups were determined by dividing the normalized values from these groups by the average value of the control groups.
Immunofluorescence
The rats were deeply anesthetized with chloral hydrate (400 mg/kg) and perfused transcardially through the left cardiac ventricle with 100 ml of perfusion buffer, followed by 250 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4, at room temperature for 15 min. Subsequently, the L4/L5 DRGs was removed and postfixed for 24 h at 4° t and then dehydrated with 30% sucrose in 0.1 M PB for 48 h. DRGs were embedded in optimum cutting temperature medium (Tissue Tek OCT; Sakura) and were cut at 20 µm using a Leica CM3050 S cryostat. DRG sections were placed directly on gelatin-covered slides. After rinsing with PBS for 10 min, slides with DRG sections were incubated with 10% normal goat serum for 1 h. For single labeling of EGFR, DRG sections were incubated overnight at 4° t with rabbit anti-EGFR (1:100, Beyotime Biotechnology) antibody. The sections were then incubated in goat antirabbit antibody conjugated to Alexa Fluor 488 (1:200, Abcam) for 2 h at room temperature. The primary antiserum was omitted for control DRG sections. The slides were coverslipped with SouthernBiotech Fluoromount-G (SouthernBiotech, Birmingham, AL). Pictures were captured by an Olympus BX53 fluorescence microscope. For colabeling of EGFR with DRG cell markers, DRG sections were incubated with rabbit anti-EGFR antibody (1:100; Beyotime Biotechnology) and mouse antineurofilament-200 (NF200, 1:500; Sigma), mouse anticalcitonin gene-related peptide (CGRP, 1:50; Abcam), or mouse anti-GS (1:500; EMD Millipore) overnight at 4° t, followed by goat antirabbit antibody conjugated to Alexa Fluor 488 (1:200; Abcam) and goat antimouse antibody conjugated to Cy3 (1:200; Abcam) for 2 h at room temperature. Cy3-conjugated-Avidin (1:200, Abcam) was used as the second antibody for double labeling of EGFR with biotinylated-isolectin B4 (IB4, 1:100, Sigma). The slides were coverslipped with SouthernBiotech Fluoromount-G (SouthernBiotech) or antifade mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (Beyotime Biotechnology). All colabeled pictures were viewed and captured using Nikon C2 confocal microscope (Nikon, Tokyo, Japan). All the pictures were quantified by NIH Image J Software.
Statistical analysis
The animals were distributed into various treatment groups randomly. The number of rats is more than six in each group in behavior experiment and more than three in western blotting or immunofluorescence experiments. All of the results were given as means ± standard error of the mean. The data were statistically analyzed with SigmaPlot 12.5 software (San Jose, CA) by two-tailed, paired Student’s t test for two group comparison, one-way analysis of variance (ANOVA) for more than two-group comparison or two-way repeated measure (RM) ANOVA for results from behavior tests. When ANOVA showed a significant difference, post hoc Tukey method was used to perform pairwise comparisons between means. P < 0.05 was considered significant.
Results
Increased EGFR expression in neuropathic pain models
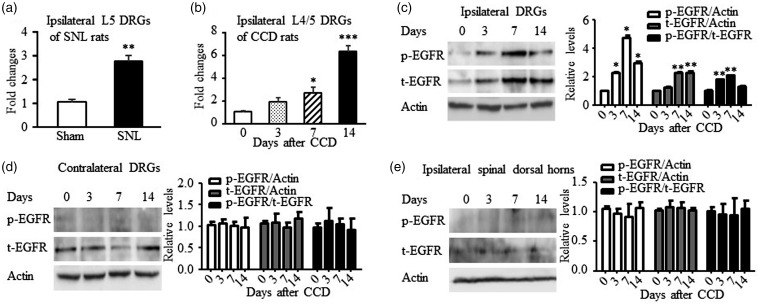
Previous RNA-seq analysis had shown a significant increase of EGFR mRNA levels in injured DRGs of SNL-induced neuropathic pain mice.19 Using quantitative real time-PCR, we confirmed the increase of EGFR mRNA level in injured DRGs in SNL rats on day 7 after SNL surgery. EGFR mRNA level in SNL rats is 2.60-folds of sham rats (P < 0.01, Figure 1(a)).
Figure 1.
Expression changes of EGFR mRNA and protein in the DRG and spinal dorsal horn in neuropathic pain models. (a) EGFR mRNA increased in the ipsilateral L5 DRG on day 7 after SNL. N = 5 rats per group. **P < 0.01 versus the corresponding sham group by two-tailed unpaired Student’s t test. (b) EGFR mRNA increased in the ipsilateral L4/L5 DRG on days 3, 7, and 14 after CCD. N = 3 or 4 rats/time point. One-way ANOVA (expression vs. time points) followed by post hoc Tukey test, *P < 0.05, ***P < 0.001 versus the corresponding naive rats (day 0). (c) Phosphor-EGFR (p-EGFR) and total EGFR (t-EGFR) increased in the ipsilateral L4/L5 DRGs after CCD surgery. N = 3 or 4 rats/time point. One-way ANOVA (expression vs. time points) followed by post hoc Tukey test, *P < 0.05, ***P < 0.001 versus the corresponding naive rats (day 0). (d and e) No significant changes in p-EGFR and t-EGFR were seen in the contralateral L4/L5 DRGs (d) and the ipsilateral L4/L5 spinal dorsal horns (e) at all observed time points. N = 3 or 4 rats/time point. One-way ANOVA (expression vs. time points) followed by post hoc Tukey test. CCD: chronic compression of DRG; DRG: dorsal root ganglion; EGFR: epidermal growth factor receptor; SNL: spinal nerve ligation.
CCD induces pain hypersensitivities in rats by causing DRG injury via compressing nerve root and DRG,8,12 which is more close to clinical neuropathic pain conditions. Therefore, we further examined EGFR expression using CCD-induced neuropathic pain model. As expected, CCD increased EGFR mRNA and protein levels in compressed DRGs. The mRNA levels of EGFR is 1.88, 2.92, and 5.97-folds of control group (0 day) on days 3, 7 and 14, respectively (F3,13 = 29.10, P < 0.001, Figure 1(b)). The significant changes were observed only on days 7 and 14 by a one-way ANOVA followed by post hoc Tukey method. However, a significant increase on day 3 was also observed if using a two-tailed, paired Student’s t test. Consistently, EGFR protein levels increased by 2.27 and 2.32-folds on days 7 and 14, respectively (F3,15 = 23.15, P < 0.001, Figure 1(c)). EGFR total protein did not change significantly on day 3 after CCD by western blotting. By normalizing to β-actin, the activated p-EGFR significantly increased by 2.30, 4.79, and 3.00-folds on days 3, 7, and 14 (F3,11 = 5.37, P < 0.05, Figure 1(c)), while the ratio of p-EGFR to total EGFR (t-EGFR) increased 1.81 and 2.11-folds on days 3 and 7 (F3,11 = 45.77, P < 0.001, Figure 1(c)). The p-EGFR/t-EGFR ratio did not significantly change on day 14 after surgery. These results indicate that CCD not only causes the activation of EGFR but also leads to the upregulation of EGFR mRNA and protein expression in injured DRGs. The increased p-EGFR is partially due to the increase of total EGFR protein level. Unilateral CCD did not affect the expression of p-EGFR and t-EGFR in contralateral L4/L5 DRGs (P > 0.05, Figure 1(d)) and ipsilateral L4/L5 spinal dorsal horns (P > 0.05, Figure 1(e)) at all observed time points. In comparison, sham surgery did not cause any change on EGFR mRNA and protein level (data not shown).
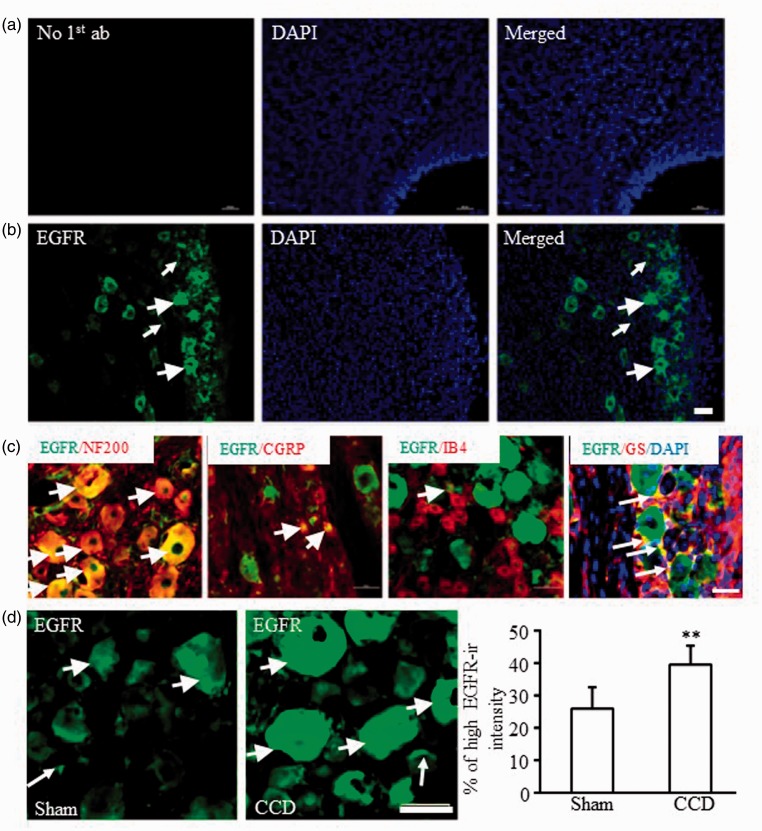
We further observed EGFR expression in DRG using immunofluorescence method. We first examined the specificity of EGFR antibody by omission of primary antibody (Figure 2(a)). No positive signals were detected only except DAPI showed the nuclei staining in control DRG slices (Figure 2(a)). Significant EGFR immunoreactivity (EGFR-ir) was observed in both neuronal and nonneuronal cells as shown in Figure 2(b). Dr. Martin et al. has reported that EGFR distributed averagely in DRG neurons by counting neuron numbers with EGFR-ir at different sizes.4 However, this classification is not accurate to distinct the expression of EGFR in different types of DRG neurons. In DRG, medium/large neurons with myelinated A fibers was usually labeled by NF200, small DRG peptidergic neurons by CGRP, and small nonpeptidergic DRG neurons by IB4.20–22 By colocalization of EGFR with NF200, CGRP, or IB4, we found that about 80.1% of EGFR-positive neurons were colocalized with NF200, 14.9% with CGRP, and only 5.5% with IB4 (Figure 2(c)). We also observed EGFR expression in satellite glial cells by colocalization of EGFR with GS, a marker for satellite glial cells (Figure 2(c)). The cellular nuclei were shown by DAPI to better recognize the structure of satellite glial cells. To be consistent with previous report,4 we analyzed the neurons with high EGFR-ir intensity. Around 25.86 (±6.55) % of DRG neurons showed high EGFR-ir intensity in sham group. The high EGFR-ir intensity neurons increased by 1.53-folds in CCD group compared to sham group (P < 0.05, Figure 2(d)).
Figure 2.
Distribution of EGFR protein in the lumbar DRGs of rats. (a) No EGFR signals were detected in the control DRG slices without EGFR antibody incubation. (b) EGFR was expressed in the cytosol of neurons and nonneuronal cells around cellular nuclei (labeled by DAPI) in the DRG. (c) EGFR was colocalized with NF200, CGRP, IB4, or glutamine synthetase. (d) CCD increased the numbers of DRG neurons with high EGFR-ir intensity in the ipsilateral L4/L5 DRGs. N = 5 rats/group. **P < 0.01 versus the corresponding sham group by two-tailed unpaired Student’s t test. Short arrows: DRG neurons with high EGFR-ir intensity; Long arrows: glial cells with EGFR-ir. Scale bars: 50 µm. CCD: chronic compression of dorsal root ganglion; CGRP: calcitonin gene-related peptide; DAPI: 4′,6-diamidino-2-phenylindole; EGFR: epidermal growth factor receptor; EGFR-ir: EGFR immunoreactivity; IB4: isolectin B4; NF200, neurofilament-200.
Inhibition of EGFR activation by EGFR inhibitor attenuates CCD-induced pain hypersensitivities
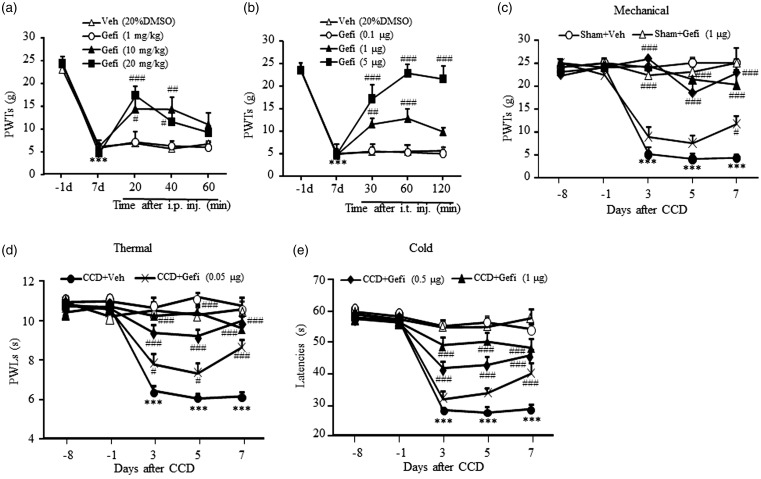
It has been reported that EGFR inhibitors produced complete and dose-dependent reversal of allodynia for 40 min in chronic constriction injury of sciatic nerve or spared nerve injury-induced-neuropathic pain models.4 We further examined the effect of gefitinib on CCD-induced mechanical pain hypersensitivity. Ipsilateral PWTs to von Frey stimuli decreased significantly on day 7 post-CCD compared to baseline (***P < 0.001, vs. −1 day, Figure 3(a) and (b)). Intraperitoneal injection (i.p.) of gefitinib dose dependently increased PWTs on the ipsilateral side at 20 min and 40 min after drug injection compared to vehicle treatment (Figure 3(a), F12,129 = 3.98, #P < 0.05; ##P < 0.01, ###P < 0.001, vs. Veh). Intrathecal injection (i.t.) of gefitinib dose dependently increased PWTs at 30 min and 60 min on the ipsilateral side compared to vehicle treatment (Figure 3(b), F12,159 = 13.20, ##P < 0.01, ###P < 0.001, vs. Veh). Moreover, the analgesic effect of i.t. injection of 5 µg gefitinib lasted for at least 2 h (Figure 3(b), ###P < 0.001, vs. Veh).
Figure 3.
Effect of EGFR inhibitor gefitinib on CCD-induced nociceptive hypersensitivities. Single injection of gefitinib intraperitoneally (i.p., a) or intrathecally (i.t., b) dose dependently reversed the decrease in PWTs to mechanical stimulation. All rats were performed CCD surgery. N = 7 or 8 rats/group. Two-way RM ANOVA (effect vs. group × time interaction) followed by post hoc Tukey test, ***P < 0.001 versus baseline value before CCD surgery. #P < 0.05, ##P < 0.01, ###P < 0.001, versus the corresponding time point in the vehicle (Veh, 20% DMSO) group or versus the value before drug administration. I.t. injection of gefitinib (once daily for 7 days) dose dependently blocked the decrease of PWTs to mechanical stimulation (c), PWLs to thermal stimulation (d), or positive response latencies to cold stimulation (e) on the ipsilateral side of CCD rats. The first injection starts at 30 min before CCD surgery. N = 6 rats/group. Two-way RM ANOVA (effect vs. group × time interaction) followed by post hoc Tukey test, ***P < 0.001 versus the corresponding time point in the sham + Veh group. #P < 0.05, ###P < 0.001 versus the corresponding time point in the CCD + Veh group. CCD: chronic compression of dorsal root ganglion; i.p.: intraperitoneal; i.t.: intrathecal; PWL: paw withdrawal latency; PWT: paw withdrawal threshold.
To determine the analgesic action of repeated administration of EGFR inhibitors on CCD-induced pain hypersensitivities, we intrathecally injected gefitinib at different doses (0.05, 0.5, 1 µg) starting on day 1 after CCD surgery once daily for seven days and observed paw withdrawal responses to mechanical, thermal, and cold stimuli in rats. Increased pain hypersensitivities to mechanical, thermal, or cold stimuli in CCD rats were attenuated dose dependently by repeated injection of gefitinib (Figure 3(c) to (e)). Compared with vehicle treatment, gefitinib at the doses of 1 µg and 0.5 µg completely or partially reversed the decrease of PWTs to mechanical stimulation, PWLs to thermal or positive response latencies to cold stimulation on the ipsilateral side from days 3 to 7 post-CCD (Figure 3(c): F20,179 = 9.37, Figure 3(d): F20,179 = 11.64, Figure 3(e): F20,179 = 15.80, ***P < 0.001, vs. Sham + Veh, ###P < 0.001, vs. CCD + Veh). Gefitinib at 0.05 µg did not significantly affect CCD-induced pain hypersensitivities during initial five days while finally lead to pain relief on day 7 post-CCD (#P < 0.05, ###P < 0.001, vs. CCD + Veh, Figure 3(c) to (e)). Gefitinib at the dose of 1 µg did not affect basal paw withdrawal responses in sham rats (P > 0.05, Figure 3(c) to (e)). However, it should be noted that repeated administration of the higher dose of gefitinib (5 µg or 10 µg) caused the decrease of PWTs and PWLs in both sham and CCD rats (data not shown).
EGFR knockdown by EGFR siRNA attenuates CCD-induced pain hypersensitivities
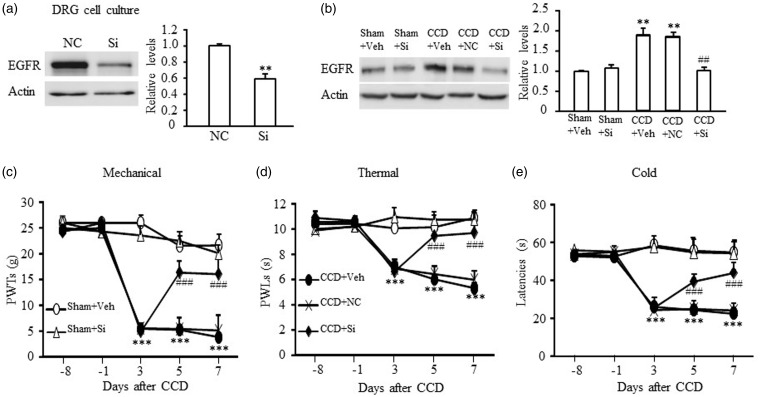
In order to determine the role of total EGFR in the development of CCD-induced pain hypersensitivities, we tested the effect of i.t. injection of EGFR siRNA on CCD-induced pain hypersensitivities. We first examined the knockdown efficiency of EGFR siRNA by using in vitro DRG cell culture (Figure 4(a)). Transfection of EGFR siRNA (250 pmol) significantly reduced total EGFR expression compared to NC siRNA (P < 0.01, Figure 4(a)). In in vivo experiment, we intrathecally injected siRNAs once daily for four days starting on day 3 post-CCD and found that EGFR siRNA (10 µM/10 µl), but not NC siRNA (10 µM/10 µl), diminished the CCD-induced increase in the level of EGFR protein in the ipsilateral L4/L5 DRG (F4,14 = 12.51, **P < 0.01 vs. Sham + Veh, ##P < 0.01, vs. CCD + Veh; Figure 4(b)). No significant changes in the basal level of EGFR protein were seen from the EGFR siRNA plus sham surgery group (Figure 4(b)), which may due to the low basal level of EGFR expression. Three days after surgery, CCD induced the significant decrease in PWTs to mechanical stimulation, PWLs to thermal or positive response latencies to cold stimulation in all groups with CCD operation (***P < 0.001, vs. Sham + Veh, Figure 4(c) to (e)). EGFR siRNA dramatically blocked CCD-induced increase of EGFR expression and thereby increased PWTs to von Frey filaments stimulation, PWLs to thermal and positive response latencies to cold stimulation on the ipsilateral side on days 5 and 7 after CCD surgery (Figure 4(c): F16,149 = 10.98, Figure 4(d): F16,149 = 19.30, Figure 4(e): F16,149 = 19.48, ###P < 0.001, vs. CCD + Veh or CCD + NC). NC siRNA had no effect on CCD-induced mechanical allodynia, thermal, or cold hyperalgesia (P > 0.05, Figure 4(c) to (e)). Basal mechanical, thermal, or cold responses on the ipsilateral sides of sham rats were not affected by i.t. injection of either siRNA (Figure 4(c) to (e)).
Figure 4.
Effect of EGFR siRNA (Si) on CCD-induced nociceptive hypersensitivities. (a) The expression level of EGFR protein was markedly reduced by treating with EGFR siRNA (250 nM) in in vitro DRG cell culture. N = 3 repeats (six wells from three rats) per treatment. *P < 0.01 versus NC siRNA by two-tailed unpaired Student’s t test. (b) Intrathecal injection of EGFR siRNA (10 µM in 10 µl) blocked the increase of EGFR induced by CCD and did not affect the basal expression of EGFR in sham group. The first injection starts on day 3 post-CCD and once daily for four days. Ipsilateral L4/L5 DRGs were harvested on day 7 after surgery. N = 3 rats/time point. One-way ANOVA (effect vs. the treated groups) followed by post hoc Tukey test, **P < 0.01, ***P < 0.001 versus the sham + Veh group. ##P < 0.01 or ###P < 0.001 versus the CCD + Veh group. Intrathecal injection of EGFR siRNA reversed the decrease of PWTs to mechanical stimulation (c), PWLs to thermal stimulation (d), or positive response latencies to cold stimulation (e) on the ipsilateral side in CCD rats. N = 6 rats/group. Two-way RM ANOVA (effect vs. group × time interaction) followed by post hoc Tukey test. ***P < 0.001 versus the corresponding time point in the sham + Veh group. ###P < 0.001 versus the corresponding time point in the CCD + Veh or the CCD + NC group. CCD: chronic compression of DRG; DRG: dorsal root ganglion; EGFR: epidermal growth factor receptor; NC: negative control; PWL: paw withdrawal latency; PWT: paw withdrawal threshold.
mTOR signaling pathway mediates the effect of EGFR
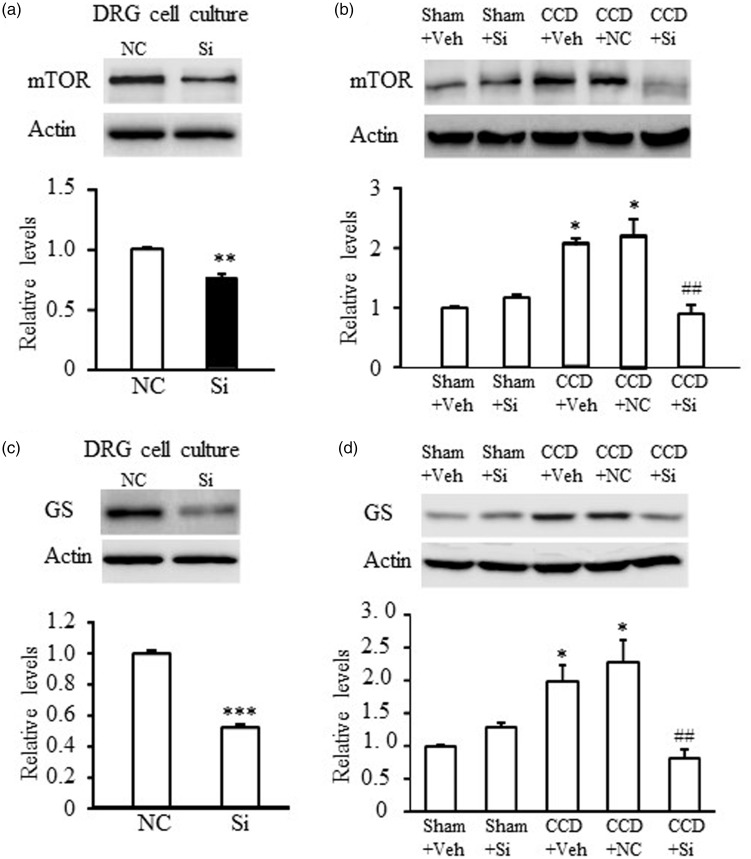
It has been demonstrated that activation of EGFR enhanced nociception through a mechanism involving mTOR signaling pathway. We further confirmed that mTOR was a downstream effector of EGFR by examining the effect of EGFR knockdown on mTOR expression. In in vitro DRG cell culture, transfection of EGFR siRNA markedly reduced mTOR expression compared to transfection with NC siRNA (P < 0.01 for mTOR; Figure 5(a)). In in vivo experiments, repeated i.t. injection of EGFR siRNA for four days reversed CCD-induced the upregulation of mTOR expression (F4,14 = 9.09, *P < 0.05 vs. Sham + Veh, ##P < 0.01, vs. CCD + Veh; Figure 5(b)). EGFR siRNA did not alter the basal level of mTOR in the ipsilateral L4/L5 DRG of sham rats (Figure 5(b)). These results suggested that mTOR might mediate the pro-nociceptive effect of EGFR under the chronic condition of neuropathic pain.
Figure 5.
Intracellular signaling molecule mTOR mediated nociceptive effect of EGFR. The expression level of mTOR (a) or GS (c) was markedly reduced by treating with EGFR siRNA (Si, 250 nM) in in vitro DRG cell culture. N = 3 repeats (six wells from three rats) per treatment. **P < 0.01, ***P < 0.001 versus NC siRNA treatment by two-tailed unpaired Student’s t test. Intrathecal injection of EGFR siRNA (10 µM in 10 µl vehicle solution) blocked the increase of mTOR (b) or GS (d) induced by CCD and did not affect their basal expressions in sham group. The first injection was administered on day 3 post-CCD and once daily for four days. Ipsilateral L4/L5 DRGs were harvested on day 7 after surgery. N = 3 rats/time point. One-way ANOVA (effect vs. the treated groups) followed by post hoc Tukey test, *P < 0.05 versus the sham + Veh group. ##P < 0.01 versus the CCD + Veh group. CCD: chronic compression of DRG; DRG: dorsal root ganglion; GS: glutamine synthetase; mTOR: mammalian target of rapamycin; NC: negative control.
As EGFR was expressed in DRG nonneuronal cells, we further tested if EGFR knockdown affected satellite glial cells activation. In in vitro DRG cell culture, which included DRG neuron and nonneuronal cells, EGFR siRNA largely reduced the expression of GS, a marker for satellite glial cells (P < 0.05, Figure 5(c)). Consistently, EGFR knockdown by i.t. injection of EGFR siRNA reversed the increase of GS expression in compressed DRGs of CCD rats (F4,14 = 8.32, *P < 0.05 vs. Sham + Veh, ##P < 0.01, vs. CCD + Veh; Figure 5(d)). These results suggested glial EGFR might also contribute to neuropathic pain development.
Discussion
It has been reported that activated EGFR contributes to neuropathic pain via mTOR and other signaling pathway.4,7 In this study, we found that not only activated EGFR but also total EGFR involved in peripheral mechanism of neuropathic pain. EGFR are not only expressed in DRG neurons but also in satellite glial cells in DRG. Most of EGFR positive neurons colocalized with NF200-positive large myelinated DRG neurons, a few with CGRP-positive DRG neurons, and very few with IB4-positive DRG neurons. Both p-EGFR and t-EGFR increased in injured DRGs in CCD-induced neuropathic pain rats. Either EGFR inhibition by its inhibitor gefitinib or EGFR knockdown by EGFR siRNA gradually relieved pain hypersensitivities. EGFR knockdown reversed the increase of EGFR, as well as mTOR expression, induced by CCD. Moreover, CCD also caused the activation of DRG satellite glial cells which can be deactivated by EGFR knockdown. These findings suggest that not only activated EGFR but also total EGFR contribute to the development of neuropathic pain.
CCD mechanically deformed DRG so that mimics low back pain and radicular pain syndromes caused by multilevel nerve root compression and intervertebral foramina stenosis radiculopathies and tumors in human.8 Several studies have shown that unilateral CCD surgery caused pain hypersensitivities not only on the ipsilateral side but also on the contralateral side.11,12,23 However, not all of animals exhibited pain hypersensitivities on the contralateral side in our preliminary experiments. Moreover, we did not find any significant change of p-EGFR and t-EGFR in contralateral DRGs. We therefore focused on the ipsilateral side to study involvement of EGFR in CCD-induced pain hypersensitivities. The increase of EGFR mRNA expression in injured DRGs of neuropathic pain mice has been screened based on a RNA-seq analysis for DRGs from SNL mice and sham mice.19 Our current study further confirmed the upregulation of both EGFR mRNA and protein in injured DRGs of CCD-induced neuropathic pain model.
Previous studies have demonstrated the analgesic effect of EGFR inhibitor possibly by blocking EGFR activation.4,7 Our study also showed that EGFR inhibitor gefitinib by i.p. injection or i.t. injection reversed CCD-induced mechanical allodynia dose dependenly. Moreover, i.t. injection of gefitinib prolonged analgesic time on CCD-induced mechanical allodynia. It suggested that i.t. administration may be more effective for pain relief. Furthermore, repeated i.t. injection of gefitinib or EGFR siRNA produced the accumulating analgesic effect on CCD-induced pain hypersensitivities. The effect of EGFR siRNA on pain behaviors and EGFR expression indicates a key role of total EGFR in the development of neuropathic pain. As CCD did not cause any change of EGFR in spinal cord, it is not likely that EGFR in spinal cord contributes to the analgesic effect of EGFR inhibitor or EGFR siRNA.
The downstream effects of EGFR were mediated by a number of important signaling pathways, including mitogen-activated protein kinase (MAPK) and PI3K/AKT/mTOR.4,24 It has been demonstrated that epiregulin, one of the EGFR ligands, activated EGFR and then potentiated pain behaviors through enhanced PI3K/AKT/mTOR signaling but not extracellular signal-regulated kinase (ERK) signaling.4 Our results showed that DRG EGFR knockdown not only reversed the increase of EGFR expression but also reversed the increase of mTOR expression in CCD rats. Therefore, mTOR, the downstream of EGFR, may also mediate the chronic effect of EGFR on nociceptive behaviors in CCD rats.
Not only mTOR signaling pathway, EGFR also affects opioid receptors,25 β‑adrenergic receptors,26 cannabinoid type 1, and transient receptor potential vanilloid 1 (TRPV1) receptors,27 which are important for pain processing. A recent study has reported that the upregulation of epiregulin in the blood activated EGFRs on DRG neurons to induce hypersensitivity through transactivation of TRPV1 and the mTOR signaling pathway, which increases MMP-9 translation.4 In addition, DRG satellite glial cells have been demonstrated an important role in the development and maintenance of neuropathic pain.20,28 Consistent with previous studies,4,29,30 EGFR was not only expressed in DRG neurons but also in satellite glial cells, which were activated in compressed DRGs by CCD surgery. EGFR knockdown deactivated satellite glial cells in compressed DRGs of CCD rats. The deactivated satellite glial cells may due to the knockdown effect of EGFR siRNA on EGFR in satellite glial cells. As activated glial cells release pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor-α, leading to further activation of glia and act as a secondary stimulus,31,32 the decreased EGFR expression level in satellite cells may reduce the release proinflammatory cytokines and then relieve CCD-induced pain hypersensitivities.
In conclusions, we demonstrate that not only activated EGFR but also total EGFR expression contribute to neuropathic pain development by regulating mTOR signaling. Both EGFR inhibition and EGFR knockdown are potential ways for neuropathic pain treatment. However, the expression of EGFR in both DRG neurons and glial cells make the antinociceptive effect of EGFR inhibitors and EGFR siRNA complicated.
Author Contributions
L. Liang conceived of the project and designed experiments, analyzed the data, and wrote the manuscript. F.-Q. Huo and S. Jia provided advices on the project. S. Wang, S. Liu, W. Liu, and B. V. P. Nagendra did behavior tests. L. Xu, X. Zhu, and L. Tian performed western blotting and immunofluorescence experiments. Y. Chen harvested tissues. Y. Wang did CCD surgery. S. Wang, S. Liu, and L. Xu equally contributed to this work.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors thank for the support from the National Natural Sciences Foundation of China (81701112, 31871065 to L. Liang; 81870886 to F.-Q. Huo) and the China Postdoctoral Science Foundation Grant (2018M633527) to L. Liang.
References
- 1.Jones RC, III, Lawson E, Backonja M. Managing neuropathic pain. Med Clin North Am 2016; 100: 151–167. [DOI] [PubMed] [Google Scholar]
- 2.Woolf CJ. Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology 2007; 106: 864–867. [DOI] [PubMed] [Google Scholar]
- 3.Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol 2008; 128: 1365–1374. Jun [DOI] [PubMed] [Google Scholar]
- 4.Martin LJ, Smith SB, Khoutorsky A, Magnussen CA, Samoshkin A, Sorge RE, Cho C, Yosefpour N, Sivaselvachandran S, Tohyama S, Cole T, Khuong TM, Mir E, Gibson DG, Wieskopf JS, Sotocinal SG, Austin JS, Meloto CB, Gitt JH, Gkogkas C, Sonenberg N, Greenspan JD, Fillingim RB, Ohrbach R, Slade GD, Knott C, Dubner R, Nackley AG, Ribeiro-da-Silva A, Neely GG, Maixner W, Zaykin DV, Mogil JS, Diatchenko L. Epiregulin and EGFR interactions are involved in pain processing. J Clin Invest 2017; 127: 3353–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Citri A, Yarden Y. EGF-ERBB s: towards the systems level. Nat Rev Mol Cell Biol 2006; 7: 505–516. [DOI] [PubMed] [Google Scholar]
- 6.Kersten C, Cameron MG. Cetuximab alleviates neuropathic pain despite tumour progression. BMJ Case Rep 2012; 2012: 1–6. doi: 10.1136/bcr.12.2011.5374 [DOI] [PMC free article] [PubMed]
- 7.Kersten C, Cameron MG, Laird B, Mjaland S. Epidermal growth factor receptor-inhibition (EGFR-I) in the treatment of neuropathic pain. Br J Anaesth 2015; 115: 761–767. [DOI] [PubMed] [Google Scholar]
- 8.Briggs CA, Chandraraj S. Variations in the lumbosacral ligament and associated changes in the lumbosacral region resulting in compression of the fifth dorsal root ganglion and spinal nerve. Clin Anat 1995; 8: 339–346. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Liang L, Miao X, Wu S, Cao J, Tao B, Mao Q, Mo K, Xiong M, Lutz B M, Bekker A, Tao Y-X. Contribution of the suppressor of variegation 3-9 homolog 1 in dorsal root ganglia and spinal cord dorsal horn to nerve injury-induced nociceptive hypersensitivity. Anesthesiology 2016; 125: 765–778. Oct [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao JY, Liang L, Gu X, Li Z, Wu S, Sun L, Atianjoh FE, Feng J, Mo K, Jia S, Lutz BM, Bekker A, Nestler EJ, Tao YX. DNA methyltransferase DNMT3a contributes to neuropathic pain by repressing Kcna2 in primary afferent neurons. Nat Commun 2017; 8: 14712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu SJ, Xing JL. An experimental model for chronic compression of dorsal root ganglion produced by intervertebral foramen stenosis in the rat. Pain 1998; 77: 15–23. Jul [DOI] [PubMed] [Google Scholar]
- 12.Xie Y-B, Zhao H, Wang Y, Song K, Zhang M, Meng F-C, Yang Y-J, He Y-S, Kuang F, You S-W, You H-J, Xu H. Bilateral neuropathy of primary sensory neurons by the chronic compression of multiple unilateral DRGs. Neural Plast 2016; 2016: 2130901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang L, Fan L, Tao B, Yaster M, Tao YX. Protein kinase B/Akt is required for complete Freund’s adjuvant-induced upregulation of Nav1.7 and Nav1.8 in primary sensory neurons. J Pain 2013; 14: 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Mao Y, Liang L, Wu S, Yuan J, Mo K, Cai W, Mao Q, Cao J, Bekker A, Zhang W, Tao YX. The transcription factor C/EBPbeta in the dorsal root ganglion contributes to peripheral nerve trauma-induced nociceptive hypersensitivity. Sci Signal 2017; 10: eaam5345. [DOI] [PMC free article] [PubMed]
- 15.Otsuka S, Adamson C, Sankar V, Gibbs K M, Kane-Goldsmith N, Ayer J, Babiarz J, Kalinski H, Ashush H, Alpert E, Lahav R, Feinstein E, Grumet M. Delayed intrathecal delivery of RhoA siRNA to the contused spinal cord inhibits allodynia, preserves white matter, and increases serotonergic fiber growth. J Neurotrauma 2011; 28: 1063–1076. [DOI] [PubMed] [Google Scholar]
- 16.Li Z, Gu X, Sun L, Wu S, Liang L, Cao J, Lutz B M, Bekker A, Zhang W, Tao Y-X. Dorsal root ganglion myeloid zinc finger protein 1 contributes to neuropathic pain after peripheral nerve trauma. Pain 2015; 156: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980; 20: 441–462. [DOI] [PubMed] [Google Scholar]
- 18.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. Jul [DOI] [PubMed] [Google Scholar]
- 19.Wu S, Marie Lutz B, Miao X, Liang L, Mo K, Chang YJ, Du P, Soteropoulos P, Tian B, Kaufman AG, Bekker A, Hu Y, Tao YX. Dorsal root ganglion transcriptome analysis following peripheral nerve injury in mice. Mol Pain 2016; 12: 1744806916629048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang L, Wang Z, Lu N, Yang J, Zhang Y, Zhao Z. Involvement of nerve injury and activation of peripheral glial cells in tetanic sciatic stimulation-induced persistent pain in rats. J Neurosci Res 2010; 88: 2899–2910. [DOI] [PubMed] [Google Scholar]
- 21.Luo H, Cheng J, Han JS, Wan Y. Change of vanilloid receptor 1 expression in dorsal root ganglion and spinal dorsal horn during inflammatory nociception induced by complete Freund’s adjuvant in rats. Neuroreport 2004; 15: 655–658. 22 [DOI] [PubMed] [Google Scholar]
- 22.Yu L, Yang F, Luo H, Liu FY, Han JS, Xing GG, Wan Y. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund’s adjuvant. Mol Pain 2008; 4: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatashita S, Sekiguchi M, Kobayashi H, Konno S, Kikuchi S. Contralateral neuropathic pain and neuropathology in dorsal root ganglion and spinal cord following hemilateral nerve injury in rats. Spine (Phila Pa 1976) 2008; 33: 1344–1351. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Zheng L, Zhang J, Dai B, Wang N, Chen Y, He L. Antitumor activity of taspine by modulating the EGFR signaling pathway of Erk1/2 and Akt in vitro and in vivo. Planta Med 2011; 77: 1774–1781. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Long H, Wu Z, Jiang X, Ma L. EGF transregulates opioid receptors through EGFR-mediated GRK2 phosphorylation and activation. Mol Biol Cell 2008; 19: 2973–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Wu WKK, Yu L, Li ZJ, Sung JJY, Zhang ST, Cho CH. Epidermal growth factor-induced esophageal cancer cell proliferation requires transactivation of beta-adrenoceptors. J Pharmacol Exp Ther 2008; 326: 69–75. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Wang Z, Capo-Aponte JE, Zhang F, Pan Z, Reinach PS. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp Eye Res 2010; 91: 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F-Y, Sun Y-N, Wang F-T, Li Q, Su L, Zhao Z-F, Meng X-L, Zhao H, Wu X, Sun Q, Xing G-G, Wan Y. Activation of satellite glial cells in lumbar dorsal root ganglia contributes to neuropathic pain after spinal nerve ligation. Brain Res 2012; 1427: 65–77. [DOI] [PubMed] [Google Scholar]
- 29.Huerta JJ, Diaz-Trelles R, Naves FJ, Llamosas MM, Del Valle ME, Vega JA. Epidermal growth factor receptor in adult human dorsal root ganglia. Anat Embryol 1996; 194: 253–257. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed Z, Read ML, Berry M, Logan A. Satellite glia not DRG neurons constitutively activate EGFR but EGFR inactivation is not correlated with axon regeneration. Neurobiol Dis 2010; 39: 292–300. [DOI] [PubMed] [Google Scholar]
- 31.Koltzenburg M, Bennett DL, Shelton DL, McMahon SB. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur J Neurosci 1999; 11: 1698–1704. [DOI] [PubMed] [Google Scholar]
- 32.Cheng C-F, Cheng J-K, Chen C-Y, Lien C-C, Chu D, Wang S-Y, Tsaur M-L. Mirror-image pain is mediated by nerve growth factor produced from tumor necrosis factor alpha-activated satellite glia after peripheral nerve injury. Pain 2014; 155: 906–920. [DOI] [PubMed] [Google Scholar]