Abstract
Background:
The objective of this study was to investigate a case of a permanently (suprapubic) catheterized woman with neurogenic bladder dysfunction. The patient had suffered from recurrent catheter-associated urinary tract infections (CAUTIs) since 2009, despite several prevention approaches and attempts. In 2013, the patient’s catheter was substituted for the BIP Foley Catheter, coated with a noble metal alloy (NMA) of gold, silver, and palladium.
Method:
This is a retrospective–prospective clinical case study covering 4 years history for the control catheters and up to 2.5 years for the anti-infective BIP Foley Catheter. CAUTI incidences, symptoms, antibiotics, catheter performance, and comfort were evaluated. Levels of proinflammatory cytokines were measured pre- and post-substitution to BIP Foley Catheter in urine of the case and of four other permanently catheterized patients. In addition, the levels of noble metals were assessed in urine of the case subject during use of the BIP Foley Catheter.
Results:
While using control catheters, the patient experienced symptomatic CAUTIs requiring antibiotics almost every month for 4 years. After 1 month with the BIP Foley Catheter, the symptoms disappeared, and the patient remained free of symptomatic CAUTIs and antibiotic treatment for the following 2.5 years, despite bacteriuria. The patient was satisfied with the comfort during insertion, use, and removal of the BIP Foley Catheter. Urinary levels of the proinflammatory cytokines interleukin (IL)-6, IL-8, and tumor necrosis factor alpha (TNFα) decreased towards normal levels post catheter type substitution. Traces of noble metals detected in a few urine samples were ⩽4% of the permitted daily exposure. The levels of the noble metals gold, silver, and palladium remaining on the BIP Foley Catheter after use were the same as for unused catheters.
Conclusion:
Long-term use of a NMA-coated catheter was associated with cessation of frequent CAUTIs. The catheter was experienced as comfortable and inflammatory markers were reduced with time. The coating was stable, with no significant metal release into urine and is therefore safe for patient use.
Keywords: CAUTI, coating, cystitis, noble metal alloy, urinary catheters, urinary tract infection
Introduction
Urinary tract infection (UTI) is the most common hospital-acquired infection (HAI) worldwide and accounts for 30–40% of all healthcare-associated infections. HAI is a leading cause of morbidity and mortality in patients seeking medical care. In addition to the suffering, HAI (including UTI) can lead to high antibiotic consumption with subsequent development of microbial resistance.1 In fact, 70% of the bacteria that cause HAI are resistant to at least one relevant antibiotic2 and the multiresistance is a global threat to human health. Medical devices account for over 50% of all HAI cases, and 75% of UTIs are associated with a urinary catheter.3,4
A catheter-associated urinary tract infection (CAUTI) is almost always preceded by bacteriuria, which develops with a frequency of 3–6% per day in catheterized patients.5 Bacteriuria can be asymptomatic and may persist as such for extended periods. Yet, in many cases bacteriuria eventually results in local or systemic symptoms, such as urgency, dysuria, abdominal pain, and fever. Moreover, microbes from the urinary tract can spread and cause sepsis, septic shock, and multi-organ failure, as reported for 2%, 0.3%, and 1.7% of cases, respectively, with hospital-acquired UTIs.6
As the risk of infection increases with duration of catheterization, CAUTI is a large health burden for patients with neurogenic bladder dysfunction who use indwelling catheters chronically. These patients suffer from potentially life-threatening voiding dysfunction owing to a brain, spinal cord, or other nerve condition, caused by, for example, stroke, spinal cord injury (SCI), multiple sclerosis, or birth defects.7 CAUTI remains one of the most prevalent morbidities for these patients, estimated to be 2.5 episodes/patient/year8 and often leads to hospitalization and reduced quality of life.9 The effect of antibiotic treatments of CAUTI is gradually declining owing to the rapid development of resistant strains.10
To reduce the CAUTI rate among patients with neurogenic bladder dysfunction, different preventive measures are available. One example is a system in which the drainage from bladder to the collection bag is closed, preventing microbes from entering the urethra.11,12 Another measure is suprapubic catheterization, which is selected for long-term comfort and the association with a lower incidence of bacteriuria, symptomatic infections, and urethral injury and strictures.13–15
Prophylactic antibiotic treatments have also been implemented for patients with neurogenic bladder dysfunction, but have been shown ineffective and associated with the development of resistant strains of microbes.16
Several urinary catheters have been specially designed to reduce the risk of bacteriuria and CAUTI. These include catheters coated with antiseptic and antimicrobial compounds, such as silver ions, antibiotics (nitrofurazone, no longer on the market), and noble metal alloy (NMA).17–19 The catheters coated with silver ions have been proven antimicrobial in vitro,20 but to the best of our knowledge, no clinical evidence for these catheters exist.
In contrast, catheters with a NMA coating, that is, BIP Foley Catheters (referred to as ‘BIP Foley’ throughout this case report) containing gold, silver, and palladium, have been studied extensively, and demonstrate an anti-infective effect on CAUTI for catheterizations of 3 days or more.21–26 The reduction of infection rates in these studies is dependent on patient group characteristics, hospital or region, catheterization duration, and the CAUTI definition.
The clinical evidence of long-term use (more than 30 days) of these catheters is limited. This additional case report about a 65–70-year-old woman with permanent catheter due to neurogenic bladder disorder is therefore of high value for understanding the long-term clinical and microbiological effects of catheters.
In addition, the urinary levels of proinflammatory markers interleukin (IL)-6, IL-8, and tumor necrosis factor alpha (TNFα) were analyzed, all involved in systemic and local inflammation.27 They have multiple roles in the inflammation, such as recruitment (IL-8) and activation (IL-6) of various immune cells.28 Their increased levels are associated with the signs and symptoms of inflammation, such as fever (IL-6, IL-8, and TNFα),29 muscle contraction (IL-6),30 heat flashes, swelling, redness (TNFα), and pain (TNFα and IL-6),28 lowering quality of life for the patient.
Some data for these markers in UTI already exist and show that urinary IL-6 and IL-8 are elevated during febrile UTI and decrease in response to antibiotic therapy.27 This case report contributes to the understanding of how these markers are affected by the presence of urinary catheters and the NMA-coating in permanently catheterized patients.
This case report includes a thorough clinical, microbiological and immunological follow up of the patient for a long period and can serve as a baseline for other cases/clinical studies with similar patients. It also serves as clinical evidence for the NMA-coating durability and nonreleasing mechanism of action.
Subject and methods
Patients
The case patient was a woman who had a neurogenic bladder dysfunction due to subarachnoid hemorrhage in 1994. Since 2006, she was permanently catheterized with an indwelling catheter via the suprapubic route. Soon thereafter, she experienced frequent symptomatic CAUTI requiring antibiotic treatment. She had no history of previous bladder stones. During a long period, a number of urotherapeutic interventions were introduced as attempts to reduce the recurrent CAUTIs (Table 1). Four additional SCI patients were included for cytokine analyses (see under “Cytokine assay”).
Table 1.
Interventions/attempts to reduce CAUTI.
| Intervention | Effect |
|---|---|
| Prophylactic: Hiprex31 | No improvement |
| Prophylactic: antibiotics32 | No improvement |
| Increased intake of water15 | No improvement |
| Rinsing bladder with sodium chloride 9 mg/ml daily. Mechanical cleaning.15 | No improvement |
| A review of hygiene procedures15 | No improvement |
| Rinsing bladder with chlorhexidine (0.2 mg/ml) twice a week for a month | No improvement |
| Bladder instillation with Uro-Tainer Suby G and dose escalation for Solution R twice a week in order to acidify patient ratios in the bladder, thereby reducing the bacterial burden | Less smelly urine 2 days after instillation, less urgency, and less leakage. Patient did however stop treatment due to continued leakage through the urethra and erythema as a reaction to the citric acid. |
| Change to silver releasing catheter (Dover Silver) 16, Nelaton 40 cm20 | Some effect. Patient became free from CAUTI for a short period. During this period, she had less smelly urine, less urgency, and less leakage through the urethra, but the symptoms reoccurred after approximately 3 months. |
| Instillation of chondroitin sulphate 20 ml 1/week as an intravesical GAG layer replenishment33 | No improvement |
| Cranberry juice34 | No improvement |
| Local estrogen, Vagifem 10 µg, 2 times/week32 | No improvement |
Material (Foley catheters)
Since 2009, the patient used Folycil, silicone, 14 or 16 Fr (Coloplast Ltd, UK), which during 2011 was changed to Dover Silver, 16 Fr (Covidien, USA), a silicone catheter coated with phosphate silver ion technology incorporated in a hydrogel coating. In April 2013, the patient switched to the NMA (gold, silver, and palladium) and hydrogel coated BIP Foley Catheter (Bactiguard AB, Sweden). The patient used one BIP Foley Catheter-Silicone 16 Fr, and then BIP Foley Catheters made of latex, 16 Fr, because this catheter type is softer and more comfortable.
Collected data
The data were collected in a retrospective–prospective way. Historical/baseline clinical data were obtained for the years 2009 up until early 2013, when the patient started to exchange catheters at the urology department of the Central Hospital, Karlstad, Sweden. Each monthly visit, serving as an evaluation point, included catheter exchange and microbiology testing, all according to standard clinical praxis.
The obtained parameters were CAUTI rate (incidence/month), clinical catheter performance and safety (adverse events). For the prospective period with BIP Foley, noble metals in urine were measured to determine the safety margin for the patient and risk for creation of selective pressure for microbial resistance. The remaining noble metals at the surface were analyzed on used catheters removed after each 1 month period and compared with the metals on unused catheters from the same production lot/batch. The urine samples for inflammatory marker analysis were collected monthly; once before and 14 times after the switch to BIP Foleys. All prospective clinical and microbiological data were collected in a case report form (paper CRF) by the patient’s urotherapist. The retrospective baseline information was retrieved from the patients’ medical records.
Definition of CAUTI
Signs and symptoms of CAUTI were based on Swedish guidelines for UTIs (STRAMA; Drug Therapeutic Committee and the Health and Medical Care Administration of the Stockholm County Council, Sweden), and modified for a patient with neurogenic bladder disorders. Only symptomatic CAUTI was recorded and was defined as bacteriuria together with at least one symptom (fever >38°C, malaise, fatigue, pyuria, coloration of urine, smelly urine, pain in urinary bladder, urine leakage, urgency, cloudy urine, bacteremia) with no other recognized cause. As pain in bladder, urine leakage, and urgency may be caused by the neurogenic dysfunction, only over-habitual levels of these symptoms were considered as related to CAUTI.
Urine sample collection
Urine samples for microbiology were collected some days before each planned catheter exchange visit and during unplanned visits owing to CAUTI symptoms of the patient. Urine samples for inflammatory markers and metal analysis were collected via the catheter before the catheter change. The samples were centrifuged for 15 min at 200g at room temperature (RT) and the supernatants were then stored at −20°C. As controls, urine samples from 12 healthy anonymous individuals (25–60 years old, 1:1 ratio female and male) were collected and analyzed.
Catheter collection
Catheters were collected after use during each planned patient visit and analyzed for noble metals after ethylene oxide (ETO) sterilization.
Microbiological testing
Microbial analyses of urine were conducted at the Clinical Microbiology laboratory at the Central Hospital in Karlstad, according to clinical praxis.
Cytokine assays
Levels of IL-6, IL-8, and TNF-α were measured in all urine samples using electro-chemiluminescence immunoassay plates from a Multi Array® (MSD, Rockville MD, USA), using a sector imaging S2400 instrument (MSD).
Cytokine results for the same cytokines (IL-6, IL-8, and TNF-α) in urine of four permanently catheterized patients from another study recently performed at Rehab Station Stockholm (RSS) in Sweden, have been added as supportive data for the conclusion of this case study. Control catheters from Biocath (Bard, Covington, GA, USA) or Rüsch (Teleflex Medical, Morrisville, NC, USA) were used during baseline, and then switched to BIP Foleys, used during two visits/catheter exchanges covering approximately 4 months in total (M1–M4). The urine samples were collected and prepared as described above at baseline and at the two visits after switch to BIP Foley. The cytokine profiles were analyzed with Biochip Array technology (Randox Biochip, Crumlin, UK).
Metal analysis
The levels of coating metals (i.e. gold, silver, and palladium) present on catheters and in urine samples were analyzed by ALS Scandinavia, Lulea, Sweden, using inductively coupled plasma sector field mass spectrometry (ICP-SFMS) in accordance with EN ISO 17294-1. ALS is accredited by SWEDAC with an acknowledgement of competence, comparable with ISO 9000 certification in the industry.
ALS Scandinavia method VK-038 was used to analyze noble metals on the catheter surface. Extraction of metals from a small piece was performed in HNO3(sp), HF(sp) and H2O2, heated in a microwave, and stabilized with HCl before dilution with milliQ water. The extraction solutions and urine samples were analyzed using ICP-SFMS.
Statistical analysis
In the Karlstad case, statistical differences of levels of inflammatory markers was calculated with Mann–Whitney. Three initial visits month 0 (M0) to month 2 (M2) were excluded due to antibiotic use, which strongly influences the level of cytokines, because the interest of this study is in evaluating the effect of a coated catheter and not antibiotics. For confirmatory calculation of the relative reduction of cytokines in the Rehab study, Student’s t test (one-sided) was used. Statistical significance is found if p ⩽ 0.05.
Permissions
Both the Karlstad case study and the Rehab study were approved by the Central ethical committee in Uppsala (Dnr: 2015/041) and in Stockholm (Dnr: 2011/1838-31/1), respectively. Informed consent was collected from all participants in both studies.
Results
Clinical data historical baseline
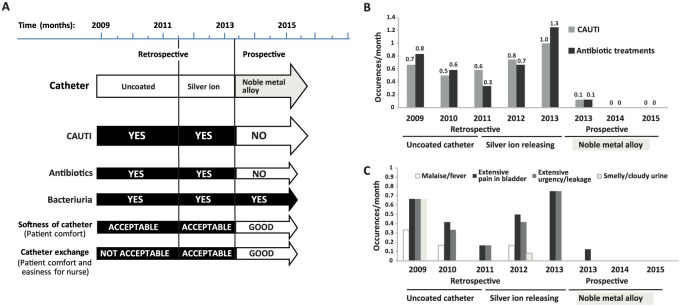
During the historical baseline period, the patient experienced frequent symptomatic CAUTIs (0.5–1 CAUTIs/month), requiring antibiotics (0.3–1 antibiotic treatments/month); see Figure 1.
Figure 1.
Overall clinical outcome during the historical baseline (with uncoated and silver ion catheters) and after introduction of noble metal alloy coated BIP Foley. (A) Schematic presentation of catheter-associated urinary tract infection (CAUTI) incidence, antibiotic use, bacteriuria, and comfort parameters for the patient and study nurse (softness and easiness of catheter exchange). (B) Average CAUTI/month and antibiotic treatments/month for the period 2009–2015. (C) Average occurrence of different CAUTI symptoms/month for the period 2009–2015.
During the antibiotic treatments, the symptoms temporarily disappeared but reoccurred as soon as the treatment was completed. Attempts to reduce CAUTIs were made by introducing various interventions and prophylactic antibiotic therapy (Table 1), most often without any beneficial effects for the patient. The introduction of Urotainer and the silver-ion-releasing catheter were the only interventions that lead to a reduction of the CAUTI symptoms (Table 1). However, the Urotainer treatment was stopped owing to side effects and the silver-releasing catheter was only effective temporarily.
The symptoms of neurogenic tense bladder, for example, discomfort due to hardness of the catheter (experienced as some pain) and painful catheter exchanges worsened during the CAUTI episodes when the pain and smooth muscle tonus/contractions further increased in the bladder.
Clinical data after switch to BIP Foley catheter
The patient was free of both symptomatic CAUTIs and antibiotic treatments from the second month with BIP Foley and through the entire period of 2.5 years (Figure 1) of use. Moreover, the patient was satisfied with the comfort and softness of the product, and both the urotherapist and the patient were pleased with the ease of catheter insertion and removal. No catheter malfunctions and no adverse events were observed during the study period of 2.5 years using BIP Foley.
Microbiology
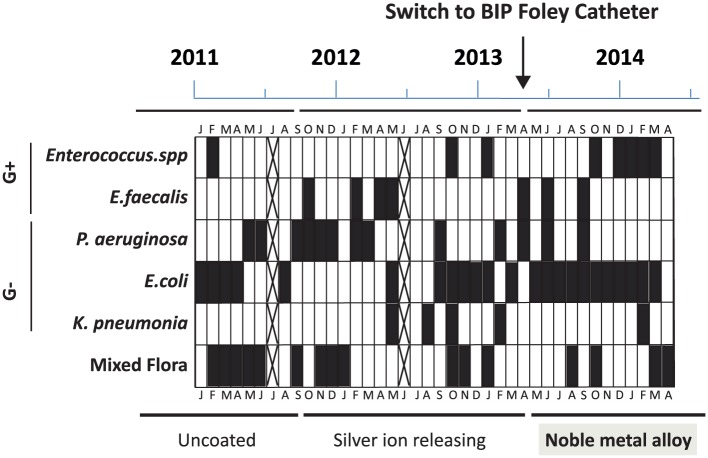
The microbiology data were obtained monthly (except for 2 months marked with crosses in Figure 2) during both the historical baseline (from 2011) and after switch to BIP Foley (from 2013). No correlation between febrile CAUTI and any bacterial specific specie(s) was found. Pseudomonas aeruginosa and Enterococcus faecalis were frequently detected in urine at baseline and occasionally during the first 5 months after switch to BIP Foley but not after that (Figure 2). Escherichia coli and Enterococcus spp. were the most dominant at the end of the evaluation period.
Figure 2.
Microbial etiology of infections and bacteriuria (black fields = detected microbes and crossed fields = no data). No correlations between any specific species and febrile catheter-associated urinary tract infection (CAUTI) were found, but P. aeruginosa and E. faecalis were not detected in the later phase of the study, when the patient had been free from infection for approximately half a year.
Inflammatory markers
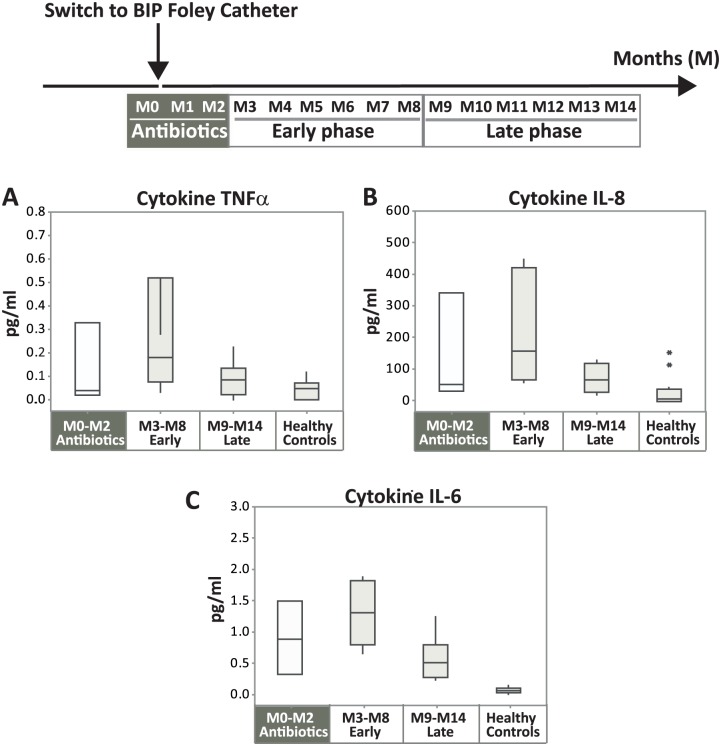
Levels of cytokines were assessed in urine to monitor inflammatory processes in the urinary tract. Urine samples were collected monthly during the study period, 1 month prior and 14 months after exchange to BIP Foley M0–M14), in which the levels of proinflammatory cytokines IL-6, IL-8, and TNFα were measured. Box plots in Figure 3 demonstrate medians and distributions of the subperiods M0–M2 (antibiotic phase), M3–M8 (early phase), and M9–M14 (late phase). M0–M2 was grouped separately because antibiotic treatment is known to reduce the cytokine levels.27
Figure 3.
Levels of proinflammatory cytokines. Box plots of cytokine levels in urine TNFα (A), IL-8 (B), and IL-6 (C) showing median, the first/third quartiles and outliers (*). The bars to the left (M0–M2, antibiotic phase) are distinguished with a gray background, because the patient was treated with antibiotics during this period. To illustrate the time effects of the use of BIP Foley on the inflammatory processes, the remaining antibiotic-free study period (M3–M14) was split into two equally long periods: early phase M3–M8 (n = 6) and late phase M9–M14 (n = 6), respectively. As reference values, healthy controls were included as a fourth category (n = 12). Median values are for early phase versus late phase: TNFα, 0.18 versus 0.08 pg/ml (A); IL-8, 162 versus 71 pg/ml (B); and IL-6, 1.3 versus 0.5 pg/ml (C). p values: M3–M8 versus M9–M14, 0.1 (A), 0.1 (B), and 0.01 (C); M3–M4 versus HC, 0.02 (A), 0.006 (B), and 0.0009 (C); M3-M4 versus HC, 0.3 (A), 0.04 (B), and 0.0009 (C).
The result was very similar for all three cytokines tested (Figure 3); the levels were low during the first 3 months (M0–M2) owing to antibiotics, increased during the following 6 months (M3–M8, early phase) indicating ongoing inflammatory processes post-infection period, and decreased during the late phase (M9–M14) coming closer to normal levels. IL-6 was lowered by 62% (statistically significant, p = 0.01), IL-8 by 56% (p = 0.1) and TNFα by 56% (p = 0.1).
Supportive cytokine data from the Rehab study
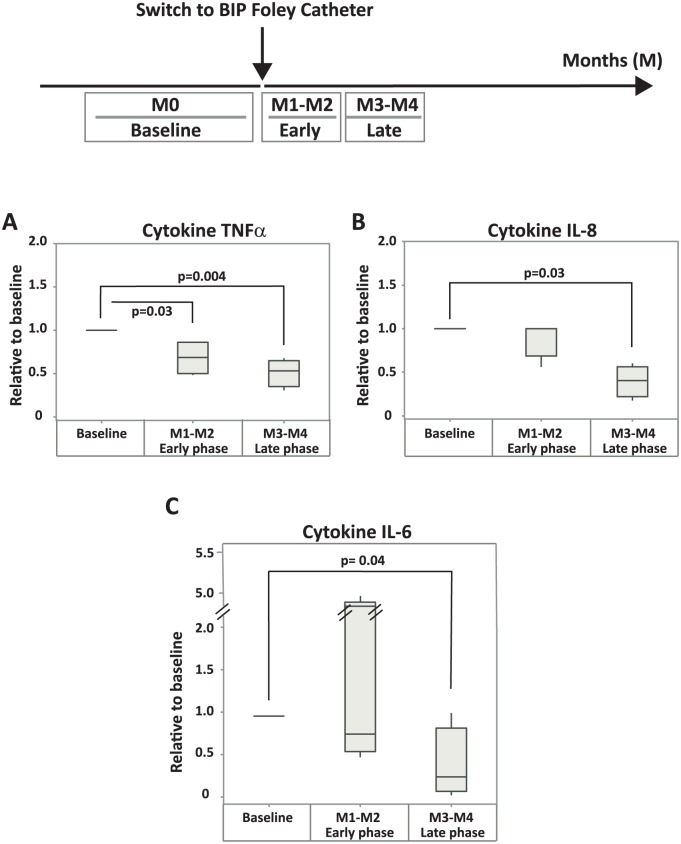
Supportive data, demonstrating declining inflammatory marker levels after switch to BIP Foley, was registered in a study with four patients at Rehab Station in Stockholm. More details of the study design can be found in the method section, under inflammatory markers. No patients in this study suffered from symptomatic CAUTI or were treated with antibiotics, but all of them had asymptomatic bacteriuria during the whole study period. The levels of the inflammatory markers in this study are presented relative to the baseline level from initiation visit (Figure 4).
Figure 4.
Supporting data: relative levels of proinflammatory cytokines in urine of patients in the Rehab study. Box plots of the cytokines TNFα (A), IL-8 (B), and IL-6 (C) showing median, and the first/third quartile. TNFα was significantly reduced already after 2 months of BIP Foley use (early phase of this study, M1–M2), whereas the decrease of IL-6 and IL-8 were significant after approximately 4 months (late phase, M3–M4) of BIP Foley use. Median values relative to baseline are after 2 and 4 months: TNFα, 0.7 and 0.5 (A); IL-8, 1.0 and 0.4 (B); IL-6, 0.8 and 0.3 (C), respectively. All statistically significant p values (⩽0.05) are shown in the figure.
The levels of all cytokines (IL-6, IL-8, and TNFα) decreased after switch to BIP Foley in a similar way as presented in Figure 3, that is, already after 2 months (M1–M2, early phase of this study) and continued to decrease after an additional 2 months (M3–M4, late phase of this study). The reduction towards baseline after 4 months was approximately 50% (p = 0.004) for TNFα, 60% (p = 0.03) for IL-8, and 70% (p = 0.04) for IL-6.
Coating/noble metal data
To assess noble metal durability of the BIP Foley coating after 1 month of use, metal levels of used and unused catheters were analyzed and compared. In total, four used and four unused catheters of the same batches were analyzed. In Figure 5(A), the mean [µg/cm2; standard error of mean (SEM) as bars] of remaining metals, gold, silver, and palladium are presented. No differences of any of the metal levels, between used and unused catheters, were found.
Figure 5.
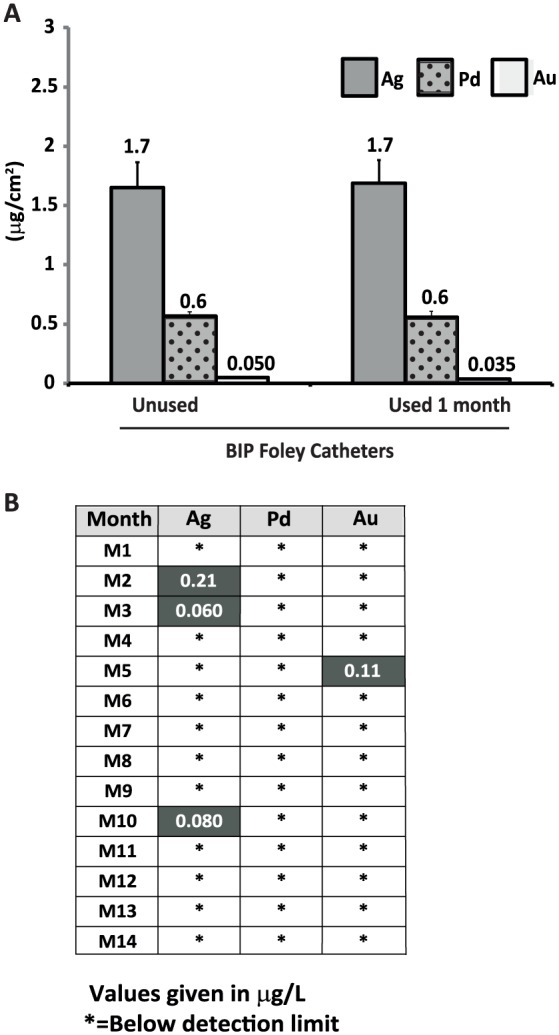
Metal content of unused and clinically used BIP Foley Catheters (A) and metal levels in urine samples (B). (A) Metal levels of four unused and four used catheters: silver, mean of 1.7 µg/cm2 (for both unused and used), palladium, mean of 0.6 µg/cm2 (for both unused and used); and gold, mean of 0.05 µg/cm2 (unused) versus 0.035 µg/cm2 (used), p = 0.06. (B) Metal levels in 14 urine samples, collected during BIP Foley use. No samples contained detectable levels of palladium, three and one samples contained very low levels of silver and gold, respectively. Detection levels (silver 0.05 µg/l, palladium 0.05 µg/l, and gold 0.1 µg/l).
The level of silver on the silver ion catheter that was used prior to BIP Foley was also assessed with ICP-SFMS (n = 3). The results revealed a mean of 5.9 µg/cm2 silver (SEM = 0.72), which is significantly higher than unused BIP Foley Catheters (mean of 1.7 µg/cm2, p = 0.011).
Urine samples collected at each visit for 14 months (M1–M14, main part of the study), were analyzed for metal levels (Figure 5(B)). Silver was detected only in three urine samples (M2, M3, and M10), at very low levels of 0.06–0.2 µg/l. Palladium was not detected in any of the analyzed samples, and gold was only detected in one sample (M5), also at a very low level (0.11 µg/l).
Discussion
Clinical data
The case of a female patient with permanent suprapubic catheter has been presented in this report. The patient had used indwelling catheters for several years and suffered from recurrent symptomatic CAUTIs, requiring frequent antibiotic treatments. Her infections became increasingly difficult to treat with time as the pathogenic microbial strains developed resistance. The patient tested BIP Foley and already after 1 month she became free from these symptomatic infections and thereby the antibiotic treatments during the entire follow-up period of more than 2 years. In addition to becoming free from CAUTI, the patient was also pleased with the softness and the exchange comfort of the catheter and her quality of life was greatly improved.
The results support the notion that the NMA coating on BIP Foleys may prevent new infections from occurring, and thereby improve the condition and quality of life for catheterized patients with recurring infections in the urinary tract. This is further supported by two previous studies, by Chung et al.22 and Estores et al.,35 evaluating the effect of NMA-coated catheters in long-term catheterized patients.22,35 The anti-infective effect of this catheter type was found to be significant in both those studies. The study by Estores et al.35 is also a case report of a neurogenic bladder dysfunction patient who became free from recurrent CAUTI after switch to the catheter with the NMA.
Microbiological data
Even if the symptomatic infections in the patient disappeared after the switch to BIP Foley, the bacteriuria remained. This is expected since the bacteria (especially E. coli) may not only colonize the catheter, but also the bladder lining,36 which may not be affected by the anti-infective material on the catheter.
Possibly, the NMA coating reduced the microbial colonization on the catheter surface or modified the microbial etiology of the bacteria causing the bacteriuria and infections, which is crucial for the involvement of the immune system and thereby type and severity of symptoms. We found in this case study that P. aeruginosa was frequently detected during baseline and occasionally during the first months with BIP Foley, but not during the following 7 months of the study. E. faecalis, also frequent during baseline, was replaced by Enterococcus spp. in general. These findings are similar to the previous case study,35 where the bacteria (P. aeruginosa and E. faecalis) were isolated from the patient’s urine during baseline, but not at the end of the study, after using NMA-coated catheter for approximately half a year. P. aeruginosa is a Gram-negative bacterium, which often has natural resistance to antibiotics and is associated with serious illness, especially in immune compromised patients. It is capable of extensive colonization and produces biofilms and toxins. E. faecalis is a Gram-positive bacterium, which can cause life-threatening nosocomial infections, and where its naturally high levels of antibiotic resistance contribute to high pathogenicity.37 These properties support the suggestion that these bacteria could be the causative agents of the symptomatic CAUTIs occurring during baseline.
E. coli, isolated during the whole evaluation period, is a Gram negative bacterium, most often harmless, but some strains may be uropathogenic. E. coli can benefit its host by colonizing the bladder and preventing pathogenic bacteria from doing so. The E. coli strain present at the end of this study was probably not pathogenic since it was not causing symptomatic CAUTIs. Interestingly, similar result was also found in the study by Estores et al.,35 where this species was the only detected one at the end of the study when the patient was free from infection and used NMA-coated catheters. Certain strains of E. coli have even been developed as treatment of symptomatic UTIs.38
Inflammatory markers
All tested proinflammatory markers (IL-6, IL-8, and TNFα) showed a similar increased trend after the antibiotic phase (M0–M2) and decreasing trend between the early (M3–M8) and late phase (M9–M14) with NMA-coated catheter.
When the subject was treated with antibiotics, the cytokine levels were suppressed. This is expected, since downregulation of cytokines is a known effect of antibiotics.27 The levels of all cytokines during the early phase, the first antibiotic-free 6-month period after switch to BIP Foley, were significantly higher than the levels in urine of healthy, noncatheterized subjects. This finding indicates that the patient, despite being free from symptoms, still had some ongoing inflammation in the urinary bladder, that may be sustained by, for example, abundant growth of bacteria39 or by remaining tissue stress/irritation, epithelial regeneration, tissue healing, and cell death,28 that may have been caused by the previous infections. This remaining inflammation is hypothesized to be reduced by the long-term use of BIP Foley.
The levels of all inflammatory markers were reduced towards normal levels in the late phase of the study (M9–M14). By then, the patient had been free from infection for 8–12 months. In line with these data, a significant reduction of cytokines in the latter phase of the Rehab study (after approximately 4 months, M3–M4) was also observed. This faster decrease is possible because these patients were not suffering from symptomatic CAUTIs.
Taken together, these results suggest that not only infections, but also inflammation decrease with time when using NMA-coated catheter. This emphasizes the importance of studying the long-term effects of the NMA-coating on infection, inflammation, and the immune system. It also highlights that it may take some time for patients to experience improvement regarding some symptoms after introduction of such a catheter.
Coating metals: minimal/no toxic release and no selective pressure for microbial resistance
The coating metals gold, silver, and palladium were not released in detectable amounts from the catheter surface to the patient’s urine in the majority of tested samples. In three urine samples silver was detected at extremely low levels of 0.06–0.2 µg/l. These levels were compared with the permitted daily exposure (PDE) limit for chronic use of silver in drugs.40 The established PDE for silver is 10 µg/day. Assuming a normal urine production of 2 l/day, the maximum concentration would be 10 µg/day divided by 2 l/day = 5 µg/l. The maximum value in sample M2 was 0.2 µg/l, which corresponds to 4% (0.2 µg/l divided by 5 µg/l) of the PDE.
This silver concentration also corresponds to 0.003% of the lowest minimal inhibition concentration (MIC), which is 8–32 mg/l among the Enterobacteriaceae family41,42 and to 0.1% of the minimum selective concentration (MSC; i.e. 250 µg/l)43 (personal communication, Professor Dan Andersson and Lisa Albrecht, MD, Department of Medical Biochemistry and Microbiology, Uppsala University, Sweden) for creating silver resistance in microbial species. Hence, NMA-coated Foleys are safe for the patient from a silver exposure perspective and create no selection pressure for microbial resistance.
Palladium was not detected in any of the urine samples, and gold was detected in only one sample (M5), also at very low levels (0.1 µg/l), corresponding to 0.2% of the PDE limit for chronic use40 (calculated as for silver and using the PDE of gold, which is 100 µg/day).
Hence, NMA-coated catheters are also safe for the patient from the palladium and gold exposure perspectives.
Durability of the coating on the catheters
In addition to the low levels of coating metals in urine, our findings reveal the same levels of coating metals on the used catheters compared with unused catheters. The findings confirm the conclusion from the clinical urine analysis: a stable coating for at least 1 month of use, the nonreleasing mechanism of action of the Bactiguard coating, and long-term durability of its action.
The coating durability entails also that it is environmentally friendly, because no metals will end up in the sewer with the patient’s urine. Further, when the catheters are disposed as waste which is burned, the miniscule amounts of metals will end up in ash which is deposited in closed systems and will not reach the storm water.44,45
Conclusion
In conclusion, we have demonstrated a case where a permanently (suprapubic) catheterized female patient presenting with recurring CAUTI was successfully treated by the substitution of her traditional type catheter for a BIP Foley Catheter with a NMA coating. The result was cessation of CAUTI symptoms for more than 2 years, better comfort, and less pain during catheter exchanges.
In addition to the clinically beneficial effects on her symptomatic infections, it was found that the NMA coating suppressed inflammatory markers in urine, indicating a gradual and parallel recovery from inflammation. Finally, our results show that the NMA is safe from a patient perspective in regard to exposure to coating metals, even in the case of chronic use. In addition, it does not create any selective pressure for microbial resistance or environmental risks.
This combination of outcomes led to an overall improvement in the quality of life for this patient. Our study suggests that long-term use of NMA-coated catheters, for example, BIP Foley Catheters, may prevent new CAUTI events from developing and indicates that usage can safely improve the condition of patients already suffering from recurrent CAUTIs.
Acknowledgments
We would like to thank Linda Bergström and Kristine Bylund for proofreading the manuscript and Erika Söderberg for laboratory assistance. Javier Sanchez was the principal investigator of the study. Javier Sanchez and Dorota Johansson contributed equally.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: None for BM, PG, ÅS, and LL. Sponsor representatives are YK-L, DJ, and JS. The sites obtained unrestricted grant support for materials and other expenses from Bactiguard.
ORCID iD: Ylva Kai-Larsen  https://orcid.org/0000-0003-2375-3163
https://orcid.org/0000-0003-2375-3163
Contributor Information
Birgitta Magnusson, Section of Urology, Central hospital Karlstad, Sweden.
Ylva Kai-Larsen, Bactiguard AB, Alfred Nobels Allé 150, 146 48 Tullinge, Stockholm, Sweden.
Petter Granlund, Section of Urology, Central hospital Karlstad, Sweden.
Åke Seiger, Rehab Station Stockholm, Spinalis, Stockholm, Sweden Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden.
Lena Lindbo, Rehab Station Stockholm, Spinalis, Stockholm, Sweden.
Javier Sanchez, Bactiguard AB, Tullinge, Stockholm, Sweden Danderyd Hospital, Stockholm, Sweden.
Dorota Johansson, Bactiguard AB, Tullinge, Stockholm, Sweden.
References
- 1. WHO/CDS/CSR/EPH/2002.12. Prevention of hospital-acquired infections: a practical guide. 2nd ed. Geneva, Switzerland: World Health Organization; http://appswhoint/medicinedocs/documents/s16355e/s16355epdf. [Google Scholar]
- 2. World health organization (WHO),http://www.who.int/about/en/ (2018, accessed 12 December 2018).
- 3. Centers for Disease Control and Prevention. Catheter-Associated Urinary Tract Infections (CAUTI), https://www.cdc.gov/hai/ca_uti/uti.html (2015, accessed 12 December 2018) [Google Scholar]
- 4. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control 2014; 3: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schumm K, Lam TB. Types of urethral catheters for management of short-term voiding problems in hospitalised adults. Cochrane Database Syst Rev 2008: CD004013. [DOI] [PubMed] [Google Scholar]
- 6. Bouza E, San Juan R, Munoz P, et al. ; Co-operative Group of the European Study Group on Nosocomial I. A European perspective on nosocomial urinary tract infections II. Report on incidence, clinical characteristics and outcome (ESGNI-004 study). European Study Group on Nosocomial Infection. Clin Microbiol Infect 2001; 7: 532–542. [DOI] [PubMed] [Google Scholar]
- 7. Ginsberg D. The epidemiology and pathophysiology of neurogenic bladder. Am J Manag Care 2013; 19: s191–s196. [PubMed] [Google Scholar]
- 8. Siroky MB. Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med 2002; 113(Suppl. 1A): 67S–79S. [DOI] [PubMed] [Google Scholar]
- 9. Jahromi MS, Mure A, Gomez CS. UTIs in patients with neurogenic bladder. Curr Urol Rep 2014; 15: 433. [DOI] [PubMed] [Google Scholar]
- 10. Shepherd AK, Pottinger PS. Management of urinary tract infections in the era of increasing antimicrobial resistance. Med Clin North Am 2013; 97: 737–757, xii. [DOI] [PubMed] [Google Scholar]
- 11. Vigil HR, Hickling DR. Urinary tract infection in the neurogenic bladder. Transl Androl Urol 2016; 5: 72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loveday HP, Wilson JA, Pratt RJ, et al. Epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2014; 86(Suppl. 1): S1–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for prevention of catheter-associated urinary tract infections (2009), https://www.cdc.gov/hicpac/pdf/CAUTI/CAUTIguideline2009final.pdf (Updated 2017, accessed 12 December 2018). [DOI] [PubMed]
- 14. Kidd EA, Stewart F, Kassis NC, et al. Urethral (indwelling or intermittent) or suprapubic routes for short-term catheterisation in hospitalised adults. Cochrane Database Syst Rev 2015: CD004203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Geng V, Farrell J, Gea-Sánchez M, et al. Guidelines for indwelling catheterization in adults. European Association of Urology Nurses; 2012. [Google Scholar]
- 16. Morton SC, Shekelle PG, Adams JL, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil 2002; 83: 129–138. [DOI] [PubMed] [Google Scholar]
- 17. Siddiq DM, Darouiche RO. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urol 2012; 9: 305–314. [DOI] [PubMed] [Google Scholar]
- 18. Singha P, Locklin J, Handa H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater 2017; 50: 20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhu Z, Wang Z, Li S, et al. Antimicrobial strategies for urinary catheters. J Biomed Mater Res A 2019; 107: 445–467. [DOI] [PubMed] [Google Scholar]
- 20. Pariente JL, Bordenave L, Jacob F, et al. Cytotoxicity assessment of latex urinary catheters on cultured human urothelial cells. Eur Urol 2000; 38: 640–643. [DOI] [PubMed] [Google Scholar]
- 21. Aljohi AA, Hassan HE, Gupta RK. The efficacy of noble metal alloy urinary catheters in reducing catheter-associated urinary tract infection. Urol Ann 2016; 8: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chung PH, Wong CW, Lai CK, et al. A prospective interventional study to examine the effect of a silver alloy and hydrogel-coated catheter on the incidence of catheter-associated urinary tract infection. Hong Kong Med J 2017; 23: 239–245. [DOI] [PubMed] [Google Scholar]
- 23. Hidalgo Fabrellas I, Rebollo Pavon M, Planas Canals M, et al. Incidence of urinary tract infections after cardiac surgery: comparative study accordind to catheterization device. Enferm Intensiva 2015; 26: 54–62. [DOI] [PubMed] [Google Scholar]
- 24. Lederer JW, Jarvis WR, Thomas L, et al. Multicenter cohort study to assess the impact of a silver-alloy and hydrogel-coated urinary catheter on symptomatic catheter-associated urinary tract infections. J Wound Ostomy Continence Nurs 2014; 41: 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rupp ME, Fitzgerald T, Marion N, et al. Effect of silver-coated urinary catheters: efficacy, cost-effectiveness, and antimicrobial resistance. Am J Infect Control 2004; 32: 445–450. [DOI] [PubMed] [Google Scholar]
- 26. Stenzelius K, Persson S, Olsson UB, et al. Noble metal alloy-coated latex versus silicone Foley catheter in short-term catheterization: a randomized controlled study. Scand J Urol Nephrol 2011; 45: 258–264. [DOI] [PubMed] [Google Scholar]
- 27. Jantausch BA, O’Donnell R, Wiedermann BL. Urinary interleukin-6 and interleukin-8 in children with urinary tract infection. Pediatr Nephrol 2000; 15: 236–240. [DOI] [PubMed] [Google Scholar]
- 28. Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin 2007; 45: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Netea MG, Kullberg BJ, Van der Meer JWM. Circulating cytokines as mediators of fever. Clin Infect Dis 2000; 31: S178–S184. [DOI] [PubMed] [Google Scholar]
- 30. Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev 2005; 33: 114–119. [DOI] [PubMed] [Google Scholar]
- 31. Hickling DR, Nitti VW. Management of recurrent urinary tract infections in healthy adult women. Rev Urol 2013; 15: 41–48. [PMC free article] [PubMed] [Google Scholar]
- 32. Epp A, Larochelle A; SOGC Urogynaecology Committee, SOGC Family Physicians Advisory Committee. Recurrent urinary tract infection. SOGC Clinical Practice Guideline No. 250, November 2010. J Obstet Gynaecol Can 2010; 32: 1082–1090. J Obstet Gynaecol Can 2011; 33: 14. [DOI] [PubMed] [Google Scholar]
- 33. Nordling J, van Ophoven A. Intravesical glycosaminoglycan replenishment with chondroitin sulphate in chronic forms of cystitis. A multi-national, multi-centre, prospective observational clinical trial. Arzneimittelforschung 2008; 58: 328–335. [DOI] [PubMed] [Google Scholar]
- 34. Vasileiou I, Katsargyris A, Theocharis S, et al. Current clinical status on the preventive effects of cranberry consumption against urinary tract infections. Nutr Res 2013; 33: 595–607. [DOI] [PubMed] [Google Scholar]
- 35. Estores IM, Olsen D, Gomez-Marin O. Silver hydrogel urinary catheters: evaluation of safety and efficacy in single patient with chronic spinal cord injury. J Rehabil Res Dev 2008; 45: 135–139. [DOI] [PubMed] [Google Scholar]
- 36. Sakarya S, Ertem GT, Oncu S, et al. Escherichia coli bind to urinary bladder epithelium through nonspecific sialic acid mediated adherence. FEMS Immunol Med Microbiol 2003; 39: 45–50. [DOI] [PubMed] [Google Scholar]
- 37. Qiao LD, Chen S, Yang Y, et al. Characteristics of urinary tract infection pathogens and their in vitro susceptibility to antimicrobial agents in China: data from a multicenter study. BMJ Open 2013; 3: e004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Darouiche RO, Green BG, Donovan WH, et al. Multicenter randomized controlled trial of bacterial interference for prevention of urinary tract infection in patients with neurogenic bladder. Urology 2011; 78: 341–346. [DOI] [PubMed] [Google Scholar]
- 39. Czaja CA, Scholes D, Hooton TM, et al. Population-based epidemiologic analysis of acute pyelonephritis. Clin Infect Dis 2007; 45: 273–280. [DOI] [PubMed] [Google Scholar]
- 40. European Medicine Agency. ICH guideline Q3D on elemental impurities. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/01/WC500180284.pdf Rf (accessed 25 July 2016).
- 41. Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. J Hosp Infect 2005; 60: 1–7. [DOI] [PubMed] [Google Scholar]
- 42. Sütterlin S. Aspects of bacterial resistance to silver. PhD Thesis, Uppsala University, Uppsala, Sweden, 2015. [Google Scholar]
- 43. Andersson D. Evolution of antibiotic resistance: the importance of low levels of antimicrobial agents. Department of Medical Biochemistry and Microbiology (IMBIM), Uppsala University, Sweden, 2016. [Google Scholar]
- 44. European Environment Agency C. European Environment Agency (2013) Managing municipal solid waste—a review of achievements in 32 European countries, 2017. http://www.eea.europa.eu/publications/managing-municipal-solid-waste
- 45. Sahoo PK, Kim K, Powell MA, et al. Recovery of metals and other beneficial products from coal fly ash: a sustainable approach for fly ash management. Int J Coal Sci Technol 2016; 3: 267–283. [Google Scholar]