Abstract
Age-related hearing loss has been associated with varied auditory cortex morphology in human neuroimaging studies. These findings have suggested that peripheral auditory system declines cause changes in brain morphology but could also be due to latent variables that affect the auditory periphery and brain. The current longitudinal study was designed to evaluate these explanations for pure-tone threshold and brain morphology associations. Thirty adults (mean age at Time 1 = 64.12 ± 10.32 years) were studied at two time points (average duration between visits = 2.62 ± 0.81 years). Small- to medium-effect size associations were observed between high-frequency pure-tone thresholds and auditory cortex gray matter volume at each time point. Although there were significant longitudinal changes in low- and high-frequency hearing measures and brain morphology, those longitudinal changes were not significantly correlated across participants. High-frequency hearing measures at Time 1 were significantly related to more lateral ventricle expansion, such that participants with higher measures exhibited larger increases in ventricle size. This ventricle effect was statistically independent of high-frequency hearing associations with auditory cortex morphology. Together, these results indicate that there are at least two mechanisms for associations between age-related hearing loss and brain morphology. Potential explanations for a direct hearing loss effect on brain morphology, as well as latent variables that likely affect both the inner ear and brain, are discussed.
Keywords: presbyacusis, pure-tone thresholds, morphometry, auditory cortex, lateral ventricle
Introduction
Nearly everyone aged 70 years and older experiences hearing loss, particularly for high-frequency sounds (Brant & Fozard, 1990; Echt, Smith, Burridge, & Spiro, 2010; Lee, Matthews, Dubno, & Mills, 2005; F. R. Lin, Thorpe, Gordon-Salant, & Ferrucci, 2011; Wiley, Chappell, Carmichael, Nondahl, & Cruickshanks, 2008). This high-frequency hearing loss can result from age-related declines in the endocochlear potential that diminishes the function of the cochlea as an amplifier and a lifetime accumulation of noise and ototoxic exposures that can damage hair cells and affect their function (Dubno, Eckert, Lee, Matthews, & Schmiedt, 2013; Schmiedt, 2010; Schuknecht, 1974; Vaden, Matthews, Eckert, & Dubno, 2017). These declines in the auditory periphery appear to affect structure and function throughout the central auditory system (Golub, 2017; Kujawa & Liberman, 2015; Lang et al., 2010; Ouda, Profant, & Syka, 2015).
An association between individual differences in pure-tone thresholds and auditory cortex morphology was first reported in a tinnitus study where Heschl’s gyrus volume was lower in middle-aged participants with elevated thresholds (Schneider et al., 2009). Similar small- to medium-effect sizes have been observed when voxel-based and gross volumetric measures of auditory cortex were used to study middle-aged to older adults with varied severity of hearing loss (Eckert, Cute, Vaden, Kuchinsky, & Dubno, 2012; Husain et al., 2011; Peelle, Troiani, Grossman, & Wingfield, 2011; Ren et al., 2018; Rigters et al., 2017; although see Alfandari et al., 2018; Boyen, Langers, de Kleine, & van Dijk, 2013). While average pure-tone threshold measures have typically been used to examine this association (Husain et al., 2011; Peelle et al., 2011; Ren et al., 2018), measures of high-frequency hearing loss have been more strongly associated with auditory cortex morphology than low-frequency hearing loss in two studies (Eckert et al., 2012; Schneider et al., 2009), whereas a measure of low-frequency hearing loss was more strongly associated with auditory cortex gray matter in another study with a large sample size (Rigters et al., 2017). These findings have been thought to reflect auditory cortex atrophy because increased cerebrospinal fluid (CSF) was also observed in some of these studies (Eckert et al., 2012; Qian, Chang, Moonis, & Lalwani, 2017).
There are two general explanations for why variation in age-related hearing loss has been associated with variation in auditory cortex morphology (Peelle & Wingfield, 2016). It is possible that increases in pure-tone thresholds cause a decline in brain morphology. Findings from animal model studies of noise-induced hearing loss suggest at least a modest causal effect of hearing loss on neurons and supporting tissue in auditory cortex (Groschel, Gotze, Ernst, & Basta, 2010; Nguyen, Khaleel, & Razak, 2017; Saljo et al., 2002; Su et al., 2017). These observations are consistent with evidence that people with sudden and unilateral hearing loss demonstrate lower gray matter volume in auditory cortex (Fan et al., 2015; Wang et al., 2016; M. Yang et al., 2014). In addition, hearing sensitivity and auditory cortex morphology may decline together in older adults because of a common mechanism affecting both the auditory periphery and the central nervous system (CNS). It is also possible that there is a general effect of aging in cross-sectional samples which increases the likelihood of observing an association between two variables that change with age, although chronological age has been controlled statistically in some auditory cortex morphology studies (Eckert et al., 2012; Rigters et al., 2017; Schneider et al., 2009).
Longitudinal studies are needed to delineate indirect effects from causal relationships between hearing thresholds and auditory cortex morphology in human participants. There has been one longitudinal study that provides modest support for the causal explanation. Change in gray matter volume over ∼6.4 years was more pronounced in older adults with elevated pure-tone thresholds measured at the beginning of the study (F. R. Lin et al., 2014). However, the extent to which changes in pure-tone thresholds track with changes in auditory cortex morphology remains unclear. Here, we examined the extent to which (a) cross-sectional associations between pure-tone thresholds and auditory cortex morphology were observed consistently across two longitudinal time points, (b) these associations were specific to auditory cortex and not to other nonauditory brain regions, and (c) pure-tone threshold changes across two longitudinal time points tracked with changes in brain morphology. That is, the overarching goal of this study was to evaluate the longitudinal support for the hypothesis that hearing loss causes changes in auditory cortex. The absence of significant longitudinal effects in the context of cross-sectional effects would provide stronger support for the alternative hypothesis that associations between hearing loss and auditory cortex morphology are mediated by a latent variable or variables.
Materials and Methods
Participants
Thirty adults (17 females) provided informed consent to participate in this longitudinal study that was approved by the Medical University of South Carolina Institutional Review Board. At the first visit (Time 1), participants had a mean age of 64.12 years (range: 43.65–84.70). At the second visit (Time 2), the same participants had a mean age of 66.74 years (range: 46.55–86.53). The mean duration between visits was 2.62 years (range: 1.26–4.97). Inclusion criteria included English as the native language (two reported fluency in a second language) and a Mini-Mental Status Examination score of at least 27 at each visit (Table 1). The exclusion criteria included a history of head trauma, seizures, conductive hearing loss or otologic disease, self-reported CNS disorders, and contraindications for safe magnetic resonance imaging scanning. This sample is a subset of 49 participants who were included in a cross-sectional study that demonstrated significant associations between pure-tone thresholds and auditory cortex morphology (Eckert et al., 2012). We recruited all previous participants who were available to examine longitudinal hearing threshold and auditory cortex associations. Requests for data used in this study can be made to the corresponding author and accessed with a data use agreement and institutional approvals.
Table 1.
Descriptive Statistics (Mean and Standard Deviation) for the Low-Frequency and High-Frequency Hearing, MMSE, and Total GM Volume Measures.
| Low-frequency hearing | High-frequency hearing | MMSE | Total GM volume | |
|---|---|---|---|---|
| Time 1 | −0.24 (0.52) | −0.55 (0.90) | 29.33 (0.76) | 667.97 (77.61) |
| Time 2 | −0.08 (0.68) | −0.43 (0.89) | 29.07 (0.98) | 658.56 (79.37) |
| Longitudinal change | * | ** | ns | ** |
Note. Longitudinal change in each measure was estimated with paired-sample t tests. MMSE = Mini-Mental Status Examination; GM = gray matter; ns = not statistically significant.
p < .05. **p < .005.
A brief hearing history update was obtained through self-report. Three of the participants reported owning a hearing aid and thus there were too few cases to examine how a hearing aid could mediate the anatomical effects described later. In addition, 13 of the participants reported some form of tinnitus, but this unstandardized self-report measure was not related to any of the auditory cortex gray matter volume measures (results not shown).
Audiologic Assessment
Otoscopy was performed to ensure that excessive cerumen did not limit the validity of audiometric assessment. Pure-tone thresholds were measured with TDH-39 headphones at conventional frequencies (250, 500, 1000, 2000, 3000, 4000, 6000, and 8000 Hz) using a Madsen OB922 clinical audiometer that was calibrated to American National Standards Institute (2010) standards. Bone-conduction testing (500, 1000, 2000, 3000, and 4000 Hz) was performed to ensure that elevated thresholds were not due to conductive hearing loss.
To replicate previous associations between high-frequency hearing thresholds and gray matter morphology (Eckert et al., 2012), a low-frequency hearing measure and a high-frequency hearing measure were calculated based on a factor analysis of pure-tone thresholds from 852 older adults (1,796 ears; mean age = 69.92 years, standard deviation = 7.24 years; 55.6% females) who participated in a study on presbyacusis and did not have conductive hearing loss or otologic disease (Dubno et al., 2008; Lee et al., 2005). This factor analysis demonstrated that two statistically unique low- and high-frequency components could explain 87% of the variance across conventional frequencies (Eckert et al., 2012). These low- and high-frequency hearing measures were calculated for this study using the following approach. (a) Each participant’s pure-tone threshold was standardized to the mean and standard deviation of the corresponding pure-tone threshold from the 852 older adults (columns 2 and 3 in Table 1 from Eckert et al., 2012). For example, a participant with a 40-dB HL threshold at 3000 Hz would have a standardized score for that threshold equal to 0.060 [x = (40 − 38.63)/22.81]. (b) The standardized score for each threshold (250 to 8000 Hz) was then multiplied by the corresponding low- or high-frequency component coefficient from the factor analysis (columns 4 and 5 in Table 1 from Eckert et al., 2012). For example, the same participant from above would have a weighted low-frequency score equal to −.004 [x = 0.060 × −0.065] and a weighted high-frequency score equal to 0.015 [x = 0.060 × 0.253] for the threshold at 3000 Hz. (c) These weighted values would then summed across frequencies (250 to 8000 Hz) for each component to create the low- and high-frequency hearing measures. Finally, R Project code to produce the low- and high-frequency measures with new audiogram data has been included in the Supplemental Materials to facilitate replication of this approach.
The low- and high-frequency measures were averaged across ears because of the strong positive correlation between ears for each measure, for example, Time 1 left and right ear high-frequency measure: r(29) = .90. Table 1 presents descriptive statistics for these standardized measures, which were scaled such that lower values indicate lower (better) thresholds (e.g., a high-frequency hearing value of −1 corresponds to ∼10-dB HL threshold at 3000 Hz, while a value of 1 corresponds to ∼60-dB HL threshold at 3000 Hz). To further elucidate how the high-frequency measure, for example, related to individual thresholds, results are presented later to demonstrate the relation between change in the high-frequency measure and change in the 3000-Hz threshold.
Image Acquisition and Preprocessing
T1-weighted images were acquired at Time 1 and Time 2 with a Siemens 3T Trio and 32 channel head coil using the following parameters: 160 slices with a 256 × 256 matrix, repetition time = 8.13 ms, echo time = 3.7 ms, flip angle = 8°, slice thickness = 1 mm, and no slice gap. These data were collected at the MUSC Center for Biomedical Imaging where standard quality control approaches were used to track and correct for any changes in scanner function over the course of this study (i.e., typical hardware failures such as a receiver malfunction in the phased-array coil). The procedures described later were used to limit potential influences of scanner drift or image quality over time as well as to replicate the cross-sectional methods used in Eckert et al. (2012).
Cross-sectional image processing
The T1-weighted images collected at Time 1 and Time 2 were rigidly aligned into anterior commissure-posterior commissure orientation and denoised (Coupe et al., 2010). Each image was bias-field corrected and segmented using the CAT12 Toolbox (Gaser & Dahnke, 2016). The segmented gray matter and white matter images were spatially normalized into a study-specific coordinate space using diffeomorphic image registration (DARTEL; Ashburner, 2007). These normalized tissue-class images were modulated to adjust the segmented gray matter probabilities for volumetric displacement that occurred during normalization. The modulated gray matter images were then smoothed with a Gaussian smoothing kernel (full-width at half maximum (FWHM) = 8 mm) to ensure that the data were normally distributed and to limit false-positive results. Jacobian determinant images were also created to examine the extent to which gray matter results could be explained by volumetric displacement relative to the study-specific template (Eckert et al., 2006). Gaussian smoothed (FWHM = 8 mm) gray matter density images were also produced to determine the extent to which the gray matter effects were present without the modulation step. Unmodulated gray matter effects were further confirmed using cortical thickness data that were collected using the CAT12 Toolbox and smoothed with a kernel (FWHM = 15 mm) that is appropriate for cortical thickness data (Spalthoff, Gaser, & Nenadic, 2018).
Longitudinal image processing
SPM12 pairwise longitudinal registration was used to estimate changes in brain morphology. This image processing step produced a T1-weighted image in the average space between the two T1-weighted images for each participant and limits the potential bias of using a single time point as the target space (Reuter, Schmansky, Rosas, & Fischl, 2012). This image processing step also produced a Jacobian determinant image that reflects the amount of warping to align the two images, which was adjusted for the duration between longitudinal time points to provide a rate of volumetric change for each voxel. The average T1-weighted image for each participant was then segmented so that the images in average space could be normalized to study-specific DARTEL template that was created for the cross-sectional image processing. The normalization parameters from this step were then used to warp the Jacobian determinant images representing the rate of longitudinal volumetric changes into the study-specific coordinate space for group-level statistical analyses. Again, the Jacobian image for the longitudinal analysis represented the rate of change for that individual, which differs from the cross-sectional analysis where the Jacobian image represents the extent of image warping to fit to the study-specific template.
Cytoarchitectonic ROI and Total Gray Matter Volume
The Anatomy toolbox cytoarchitectonic maps (Auditory Te 1.1, 1.0, 1.2, and Visual hOc1 or V1; Eickhoff et al., 2005) were used to define the cortical regions of interest (ROI) for this study, as in Eckert et al. (2012). The V1 ROI served as a control region to determine the sensory system specificity of associations with the pure-tone threshold variables. The Anatomy Toolbox was used to create ROI that were restricted to auditory and visual cortex in Montreal Neurological Institute space. The ROI were then normalized into study-specific space by warping the SPM gray matter tissue prior to the DARTEL gray matter template and applying the warping parameters to the ROI using the SPM12 Normalize function. The average gray matter volumes and Jacobian determinants from within the Te and V1 regions from Time 1 and Time 2 were obtained using MarsBar (Brett, Anton, Valabregue, & Poline, 2002). The gray matter ROI were residualized for total gray matter volume using SPSS to control for the global effect of total gray matter volume on the ROI gray matter measures, as in Eckert et al. (2012). The total gray matter volume measure was obtained by summing the probability values across each participant’s native space segmented image (Table 1). These ROI were also used to obtain the average Jacobian determinant values in the Te and V1 regions that characterized longitudinal morphologic change.
Statistics
Cross-sectional analyses
Pearson correlations were used to examine associations between the low- and high-frequency hearing measures with the auditory cortex measures at Time 1 and Time 2. These ROI analyses, in particular, were performed without multiple comparison correction because pure-tone thresholds have exhibited small- to medium-effect associations with auditory cortex morphology in previous studies (Eckert et al., 2012; Husain et al., 2011; Peelle et al., 2011; Ren et al., 2018; Rigters et al., 2017) and because we had a priori predictions that pure-tone thresholds would be associated with auditory cortex gray matter volume. Multiple regression was then used to determine the specificity of significant gray matter associations after controlling for the average Jacobian determinant from within each ROI and to examine the influence of age.
Cortical thickness analyses were performed with the CAT12 Toolbox to examine the extent to which cortical thickness was associated with the hearing measures. There were no significant effects outside of auditory cortex after correction for multiple comparison with family-wise error (p < .05) using threshold-free cluster enhancement (TFCE Toolbox v.154). The effect sizes for the cortical thickness results are shown later across the brain so that the modest magnitude of auditory cortex effects can be interpreted in the context of effects across the cortex.
Longitudinal analyses
Paired t tests were performed to determine the extent to which the low- and high-frequency threshold measures increased from baseline (Time 1), and brain morphology changed from Time 1 to Time 2. Pearson correlations were then used to determine the extent to which longitudinal changes in hearing and gray matter measures were significantly related. Threshold free cluster enhancement was used to control for multiple comparisons when examining Jacobian (implicit masking across brain voxels) and cortical thickness associations with low- and high-frequency hearing measures. Significant results were subsequently interrogated using partial correlation to determine the extent to which the effect sizes were diminished after controlling for individual differences in age, duration between visits, and sex. We also determined the extent to which these variables related to the magnitude of change in the low- and high-frequency hearing measures.
Post hoc analyses were performed to clarify a significant association between the Time 1 high-frequency hearing measure and longitudinal expansion of the lateral ventricle. First, partial correlation was performed to determine the extent to which this association could be explained by participant sex and age. Second, multiple regression was performed to determine the extent of unique variance in the high-frequency hearing measure that was related to the auditory cortex gray matter volume and longitudinal expansion of the lateral ventricle.
Results
Cross-Sectional Associations Between Cortical Morphology and Hearing
Lower gray matter volume in left primary auditory cortex was significantly associated with elevated high-frequency hearing measures at Time 1 and Time 2 (Table 2 and Figure 1), after controlling for total gray matter volume. The left Te 1.0 associations were not significant after controlling for age at either time point. However, the left Te 1.2 associations at Time 1 and Time 2 remained significant after controlling for age (p < .05). There were no significant associations between the low-frequency hearing measure and the gray matter ROI (Supplemental Materials). There were no significant associations between the hearing measures and gray matter volume in the visual cortex control region (Table 2).
Table 2.
Pearson Correlation Coefficients for the Associations Between Low- and High-Frequency Hearing Measures and the Cytoarchitectonic ROI Measures at Time 1 and Time 2.
| Left hemisphere |
Right hemisphere |
|||||||
|---|---|---|---|---|---|---|---|---|
| Hearing threshold measures | Te 1.1 | Te 1.0 | Te 1.2 | V1 | Te 1.1 | Te 1.0 | Te 1.2 | V1 |
| Low Time 1 | −0.13 | −0.17 | −0.13 | 0.18 | −0.09 | −0.28 | 0.08 | 0.13 |
| Low Time 2 | −0.21 | −0.34 | −0.21 | 0.15 | −0.10 | −0.31 | −0.16 | 0.19 |
| High Time 1 | −0.26 | −0.36* | −0.52** | 0.15 | −0.25 | −0.40* | −0.12 | 0.10 |
| High Time 2 | −0.38* | −0.43* | −0.51** | 0.07 | −0.38* | −0.41* | −0.14 | 0.02 |
Note. Low- and high-frequency hearing measures at Time 1 and Time 2 were correlated with their respective average modulated gray matter volume from within each ROI.
p < .05, after controlling for age in a multiple regression. *p < .05, uncorrected. **p < .01, uncorrected.
Figure 1.
The high-frequency hearing measure was higher in subjects with lower GM volume in the left Te 1.0 (top) and left Te 1.2 (bottom) at Time 1 and Time 2. The brain rendering insets show the left and right hemisphere Te ROI and arrows indicate from where the data shown in the scatterplot were collected. GM = gray matter.
There were no significant Pearson correlations between the average Jacobian determinant within each Te ROI and the high- or low-frequency hearing measures (all ps > .05). However, elevated high-frequency hearing measures at Time 1 and Time 2 were significantly associated with lower unmodulated gray matter density data, for example, Time 1 Te 1.0: r(29) = −.53, p = .002. Coupled with similar cortical thickness results demonstrating small- to medium-effect sizes (Figure 2), these findings indicate that the auditory cortex gray matter associations with high-frequency hearing were largely due to cortical thickness rather than the gross volumetric displacement information. Thus, there was evidence of lower auditory cortex gray matter in participants with more high-frequency hearing loss at each time point and for different gray matter measurement methods.
Figure 2.
Small- to medium-effect size associations were observed between cortical thickness and the high-frequency hearing measure, including within auditory cortex, at Time 1 and Time 2. Color scale represents Cohen’s d effect sizes (thresholded using a Z = 1.70, p < .05 uncorrected threshold and shown on the standard Freesurfer brain surface in MNI template space). Small to medium effects in the lower calcarine bank observed for the Time 1 and Time 2 data sets are not shown.
Longitudinal Associations Between Hearing and Cortical Morphology
Longitudinal changes in pure-tone thresholds
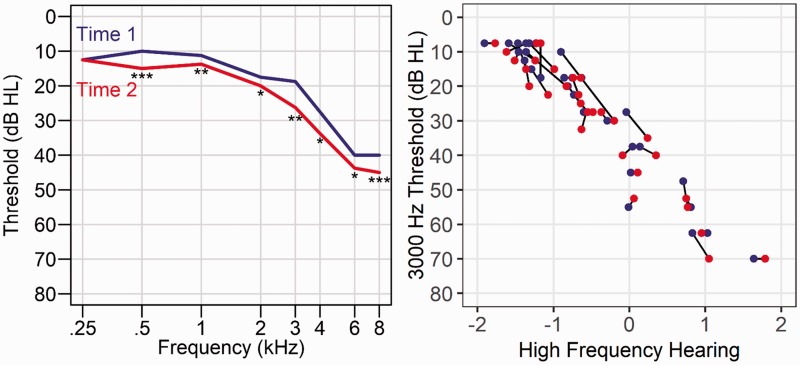
There were significant longitudinal increases in low- and high-frequency hearing measures (Table 1). Figure 3 shows these effects for each threshold and individual participant changes for the 3000-Hz threshold that contributes to the high-frequency hearing measure. The change in low- and in high-frequency hearing was not significantly correlated with age at Time 1, low frequency: r(29) = −.01, p = .97; high frequency: r(29) = .04, p = .84, or with the duration between visits, low frequency: r(29) = .16, p = .39; high frequency: r(29) = .05, p = .79.
Figure 3.
Longitudinal changes in pure-tone thresholds. Left: The median for each pure-tone threshold is presented for Time 1 (blue line) and Time 2 (red line) audiograms across the N = 30 sample. Threshold increases were observed across frequencies, except for the 250 Hz (*p < .05, **p < .01, ***p < .001). Right: Change in the 3000-Hz threshold is presented as a representative pure-tone threshold change and to show the association between the high-frequency hearing estimate and a pure-tone threshold at Time 1, blue circles: r(29) = .96, and Time 2, red circles: r(29) = .95. The black lines connect each subject’s values from Time 1 and Time 2 to show the relatively consistent threshold increases over time across participants.
Longitudinal changes in brain morphology
Figure 4 shows the significant expansion of the lateral ventricles and contraction of brain tissue from Time 1 to Time 2 across participants. The contraction of tissue included Heschl’s gyrus bilaterally, but these effects were not specific to auditory cortex and included regions bounding the major sulci (e.g., frontal operculum bounding the Sylvian fissure or primary visual cortex bounding the calcarine fissure) and subcortical structures. The pulvinar (MNI coordinate: 12 × −28 × 0) and dorsomedial nucleus (MNI coordinate: 6 × −14 × 2) of the thalamus, in particular, were regions that exhibited the most pronounced contraction with age.
Figure 4.
Volumetric changes from Time 1 to Time 2 and hearing measure associations. Jacobian determinant estimates of the rate of volumetric change demonstrated significant contraction of subcortical white matter and tissue surrounding major sulci (e.g., Sylvian fissure), with an expansion of the lateral ventricles (threshold free cluster enhancement-family-wise error p < .05; red: contraction; blue/violet: expansion; top row: left hemisphere). Relatively poorer high-frequency hearing at Time 1 predicted a faster rate of lateral ventricle volume expansion (threshold free cluster enhancement-family-wise error p < .05, violet). No significant low-frequency hearing effects were observed after multiple comparison correction.
Although significant longitudinal changes were observed for the low- and high-frequency hearing measures and brain morphology, there were no significant associations between the change in low- or high-frequency measures and change in brain morphology (gray matter ROI, cortical thickness, or Jacobian data; all ps > .05). However, participants who exhibited more high-frequency hearing loss at Time 1 also exhibited more pronounced changes brain morphology. Specifically, participants with elevated high-frequency hearing measures at Time 1 exhibited a faster rate of lateral ventricle volume expansion (Figure 4, violet cluster). A post hoc control analysis revealed that high-frequency hearing was significantly associated with the average lateral ventricle expansion (Figure 4, violet cluster) after controlling for age at Time 1, duration between visits, and sex, partial r(25) = .66, p = .0002; without controlling for these variables r(29) = .69, p = .00002, despite higher high-frequency measures at Time 1 for males compared with females, r(29) = .50, p = .005. A gif file is presented in Supplemental Materials to demonstrate the change in ventricle volume for a male participant with elevated high-frequency thresholds at both time points (shown on the left) compared with a male participant who demonstrated less change in ventricle volume and lower high-frequency thresholds at both time points (shown on the right).
Distinct High-Frequency Hearing Associations with Brain Morphology
In summary, high-frequency hearing was significantly related to auditory cortex gray matter volume at Time 1 and Time 2. In addition, the Time 1 high-frequency hearing measure was significantly related to the longitudinal change in ventricle size. These results raised the post hoc question as to whether the same variance in high-frequency hearing explained both sets of brain morphology results. A multiple regression was performed to determine the extent to which the Time 1 left Te 1.0 gray matter volume and the change in ventricle volume predicted unique variance in the Time 1 high-frequency hearing measure. Time 1 gray matter volume in the Te 1.0 ROI and rate of change in lateral ventricle expansion explained unique variance in the high-frequency hearing measure (Multiple R = .75: Te 1.0 gray matter volume: standard β = − .29, p = .033; lateral ventricle change Jacobian: standard β = .66, p = .00002), suggesting that at least two different mechanisms explain the association between Time 1 high-frequency hearing measures and change in brain morphology.
Discussion
Consistently modest associations between auditory cortex gray matter volume and high-frequency hearing measures were observed at two different time points. However, there was not strong longitudinal evidence that increasing hearing loss from Time 1 to Time 2 caused changes in brain morphology. The hearing and brain morphology variables exhibited significant change over time, but the changes in hearing changes did not track with changes in brain morphology. There are at least two explanations for these results. First, declines in auditory cortex gray matter volume due to high frequency hearing loss may occur over a longer time period and with more than the ∼2 dB of loss observed over the ∼2.5 years of this study. Second, there could be multiple common cause mechanisms that increase the risk of hearing loss and declines in brain morphology. Together, these explanations could explain why there were two unique associations between high-frequency hearing measures and brain morphology in this study.
Cross-Sectional Findings
The small but significant associations between individual variation in high-frequency hearing measures and auditory cortex gray matter volume at two time points are consistent with the findings from previous cross-sectional studies (Eckert et al., 2012; Schneider et al., 2009), and more generally with evidence that older adults with elevated pure-tone thresholds have relatively lower gray matter volume in auditory cortex (Husain et al., 2011; Peelle et al., 2011; Ren et al., 2018; Rigters et al., 2017; Schneider et al., 2009). These findings were observed across studies involving different sample sizes, age distributions, and extent of hearing loss. The associations at Time 1 and Time 2 highlight the stability of these small to medium effects.
Previous studies have included a voxel-based morphometry approach that can be sensitive to a variety of morphologic features that include cortical thickness, atrophy of surrounding structures and expansion of the Sylvian fissure space in studies of aging, and sulcal/gyral variability. This study included cortical thickness and gross volumetric approaches to examine the first two potential influences. The small- to medium-effect sizes between high-frequency hearing measures and gray matter density (unmodulated) and cortical thickness in auditory cortex indicate that the effects observed in this study were due to variation in gray matter within auditory cortex rather than surrounding morphologic features, particularly in the absence of significant deformation-based or Jacobian associations with high-frequency hearing measures. It is possible that the auditory cortex effects were underestimated because of sulcal/gyral variation. For example, the presence of a sulcus intermedius at the crown of Heschl’s gyrus is variable across people and variable in medial to lateral position where this sulcus separates the gyrus into two gyri (i.e., common-stem Heschl’s gyri; Leonard, Puranik, Kuldau, & Lombardino, 1998). Visual inspection of Heschl’s gyrus sulcal/gyral features did not suggest that the hearing measure effects were due to or were obscured because of a common-stem Heschl’s gyrus.
The hearing measure associations with cortical morphology were not specific to auditory cortex. Figure 2 shows cortical thickness associations with high-frequency hearing across the cortex. And while the visual cortex ROI did not exhibit significant associations with the hearing measures, there were associations with cortical thickness in left calcarine cortex as well as bilateral dorsal cingulate and bilateral pars opercularis in the Time 1 and Time 2 data. Some of the cortical thickness effects in this study were not consistent across time points (e.g., somatosensory cortex) and thus should be viewed with caution given the relatively small effect sizes and post hoc nature. The value of the relatively more consistent results is in showing that small to modest associations between hearing and brain morphology can be observed across the brain and may not reflect specific declines in the auditory system due to hearing loss.
Longitudinal Changes
The change in brain morphology was not significantly related to changes in the low- and high-frequency hearing measures. There was strong evidence that the change in lateral ventricle expansion was most pronounced in participants with higher high-frequency hearing measures at Time 1. This result is generally consistent with another longitudinal study showing an association between elevated hearing thresholds and more pronounced change in brain morphology, particularly for the temporal lobe (F. R. Lin et al., 2014). There were changes in perisylvian and cingulate morphology that exhibited associations with the Time 1 high-frequency threshold measure, but these were small effect sizes (not shown) and were not significant after correcting for multiple comparisons. Differences in the results from these studies could reflect differences in image analyses, statistical methods, duration of the longitudinal study, pure-tone threshold variables, and the relatively smaller sample size of this study.
Despite the differences between this study and the F. R. Lin et al. (2014) study, it is remarkable that in both studies it was Time 1 high-frequency hearing measures that predicted change in brain morphology. We did consider factors that could explain this result, but variables such as participant sex, duration between visits, and Time 1 age did not appear to mediate the association. It is also remarkable that the Time 1 auditory cortex gray matter volume and change in ventricle volume were uniquely related to variation in the high-frequency measure. Again, this suggests that there were different mechanisms underlying the relationships between (a) hearing measures and auditory cortex morphology and (b) hearing measures and change in ventricular volume.
Potential Explanations for Hearing and Brain Morphology Associations
Direct effects of hearing loss
Cross-sectional associations between hearing thresholds and auditory cortex morphology have been interpreted in the context of peripheral declines that produce damage throughout the auditory system (Eckert et al., 2012). This interpretation is consistent with evidence from people with sudden and unilateral hearing loss who exhibit atypical cortical morphology (Fan et al., 2015; Y. Lin et al., 2008; Wang et al., 2016; M. Yang et al., 2014) as well as evidence from animal studies demonstrating that noise exposure affecting the peripheral auditory system can alter central auditory system structure (Nguyen et al., 2017; Saljo et al., 2002; Su et al., 2017). In addition, some of this noise exposure could occur in development based on evidence that postnatal noise exposure can produce long-term dendritic morphology changes throughout the mouse auditory system (Ouda, Burianova, Balogova, Lu, & Syka, 2016).
There were no longitudinal effects in this study to support a causal effect of hearing loss on brain morphology, which could be due to a limitation in the design of this study. There was a broad range of age in this study (Time 1 age range: 43.65–84.70 years) and a relatively short and varied longitudinal time frame (range: 1.26–4.97 years). Thus, the cross-sectional variance in age could obscure modest changes in cortical morphology that might occur with changes in pure-tone thresholds. This interpretation appears to be supported by evidence that the high-frequency hearing and left Te 1.0 gray matter volume association was no longer significant after controlling for age differences. This may also indicate that larger hearing measure changes are necessary to detect small changes in auditory cortex gray matter volume. The cross-sectional range of the pure-tone thresholds (e.g., right ear, 3000 Hz range: 0–65 dB HL) was much broader than the longitudinal range of threshold changes (e.g., right ear, 3000 Hz range: −10 to 25 dB). Longer term longitudinal studies and the inclusion of older adults within a limited age range may be necessary to address the premise that peripheral auditory system declines produce changes in brain morphology. In addition, the selection of participants who are older (Lee et al., 2005) and have specific presbyacusis phenotypes (Vaden et al., 2017) who are expected to demonstrate more pronounced increases in hearing loss may increase sensitivity to hearing and brain morphology associations.
Common cause effects of systemic age-related changes
There are multiple common cause explanations for why hearing thresholds covary with brain morphology measures, particularly in relation to the mechanisms of sensorineural hearing loss in older adults. For example, vascular disease could affect both the inner ear and the brain (Friedland, Cederberg, & Tarima, 2009; Gates, Cobb, D’Agostino, & Wolf, 1993; Makishima, 1978). Indeed, the earliest explanations for presbyacusis included systemic atherosclerosis as a primary mechanism (Alexander, 1902; Von Fieandt & Saxen, 1937). This explanation implies that there are inner ear and brain structure and function declines because of common declines in vascular support.
The lateral ventricle findings may indicate a related but different vascular mechanism. Age-related changes in venous system drainage (Chung et al., 2010; Raz, Daugherty, Sethi, Arshad, & Haacke, 2017), particularly for veins surrounding the lateral ventricles (Satow et al., 2017), can be influenced by age-related changes in arterial pulsation and compliance that disrupts CSF regulation (Bateman, 2000; Bateman, Levi, Schofield, Wang, & Lovett, 2008). This potential venous flow explanation for changes in lateral ventricle volume seems speculative but may be possible given that the change in ventricle volume did not appear to reflect a spatial accommodation of lost tissue, change in total gray matter volume × change in ventricle volume: r(29) = −.05, ns. Given that the pressure balance between inner ear fluid and the cochlear artery influences vascular function in a way that is similar to the pressure balance between CSF and cerebrovasculature (Nakashima et al., 2003), one prediction stemming from the lateral ventricle finding is that venous outflow from the cochlea is disrupted, thereby increasing cochlear pressure (Nakashima & Ito, 1981). Perhaps increased scala media pressure has an additive effect on stria vascularis declines (Schmiedt, 2010; Schuknecht, 1974) in elevating pure-tone thresholds.
Diet may also provide an explanation for hearing threshold and brain morphology associations. Multiple studies have implicated nutrition in the prevalence of hearing loss (Gopinath, Flood, McMahon, et al., 2010; Gopinath et al., 2011; Gopinath, Flood, Rochtchina, McMahon, & Mitchell, 2010; Gopinath et al., 2014), including susceptibility to noise-induced high-frequency hearing loss (Spankovich & Le Prell, 2014). More specifically, vitamin D deficiency has been related to hearing loss in humans (Ikeda, Kobayashi, Itoh, Kusakari, & Takasaka, 1989; Kang, Choi, Kim, & Choi, 2014) and animal models (Carpinelli, Wise, & Burt, 2011; Zou et al., 2008). In addition, vitamin D has been consistently related to lateral ventricle volume (Annweiler, Annweiler, Montero-Odasso, Bartha, & Beauchet, 2014). While some participants in this study reported taking a multivitamin and vitamin D as part of our medication questions to screen for CNS disorders, there did not appear to be a strong relation between this self-report information and the lateral ventricle change or high-frequency hearing measure. Moreover, there have been inconsistent findings between vitamin intake and hearing measures (C. H. Yang, Schrepfer, & Schacht, 2015). Careful long-term study of nutrition, including the use of serum measures, is likely necessary to evaluate this potential common cause explanation.
The potential mechanisms of decline listed earlier are certainly not exhaustive, including the high likelihood that multiple genetic mutations increase susceptibility for presbyacusis (Lewis et al., 2018) and brain decline. Some hearing loss-related genes such as SYNJ2 (Manji et al., 2011) have been related to CNS disorders like Alzheimer’s disease (Gasparoni et al., 2018), which occurs with a greater frequency in people with hearing loss (F. R. Lin, Metter, et al., 2011; Patrone, Eriksson, & Lindholm, 2014). While longitudinal volume changes in the inferior lateral ventricle have been associated with SYNJ2 in Alzheimer’s patients (Koran, Hohman, Meda, & Thornton-Wells, 2014), this may be an unlikely mechanism for the lateral ventral changes observed in this study because we screened for mild cognitive impairment. The specific mechanism(s) underlying the findings from this study are not clear, but it may be useful in the design of future studies to consider mechanisms that produce peripheral nervous system effects that can precede age-related declines in the CNS, given the Time 1 high-frequency hearing measure association with lateral ventricle volume expansion. It is also worth considering the likelihood of risk-taking behaviors that contribute to hearing loss (Warner-Czyz & Cain, 2016). That is, some brain morphology and hearing associations may reflect the influence of brain morphology on decision-making that increases the risk of hearing loss.
Limitations
While the auditory cortex cross-sectional results replicate previous findings, as described earlier, the results of this study should be interpreted with respect to the wide range of participant age, limited longitudinal duration, and the modest sample size that could have limited sensitivity to small effects and limits inference about small effects sizes that were not replicated across time points. Some of these small and inconsistent effects are presented earlier (e.g., Figure 3) to provide context about the magnitude of auditory cortex effects relative to associations with varied high-frequency measures in other brain regions. In addition, the Time 1 high-frequency hearing measure effects should be considered in the context of uncertainty for how long participants had elevated thresholds as we had no objective or subjective report of when participants first experienced hearing loss. For example, it may be that noise exposure(s) and an ototoxic event(s) contributed to the elevated thresholds and brain morphology decline at one point in time rather than reflecting more gradual age-related changes in thresholds and brain morphology. Or, it may be that cortical declines are observable only after an extended period of elevated thresholds.
Conclusion
Middle-aged to older adults exhibited small but consistent associations between elevated high-frequency hearing measures and gray matter volume in putative primary auditory cortex regions. However, longitudinal changes in hearing measures and auditory cortex morphology did not change together across participants. There were longitudinal changes in lateral ventricle volume that were larger for participants with more elevated high-frequency hearing measures at Time 1. This finding supports the provocative idea that hearing loss may be a marker of and target of prevention for future changes in brain structure and function. This longitudinal lateral ventricle result was not due to the participants who largely contributed to the association between high-frequency hearing and auditory cortex morphology. Thus, there appeared to be at least two mechanisms of high-frequency hearing elevation that differentially map to brain morphology measures.
Supplemental Material
Supplemental Material for Age-Related Hearing Loss Associations With Changes in Brain Morphology by Mark A. Eckert, Kenneth I Vaden.Jr.Judy R. Dubno in Trends in Hearing
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported (in part) by the National Institutes of Health/National Institute on Deafness and Other Communication Disorders (P50 DC 000422) and MUSC Center for Biomedical Imaging. In addition, this publication was supported, in part, by the National Center for Advancing Translational Sciences of the National Institutes of Health under Grant Number UL1 TR001450. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program (C06 RR 014516) from the National Institutes of Health/National Center for Research Resources.
Supplemental material
Supplemental material is available for this article online.
References
- Alexander G. (1902) Zur pathologischen histologie des ohrlabyrinthes mit besonderer berücksichtigung des corti'schen organes. Archiv für Ohrenheilkunde 56(1–2): 1–23. [Google Scholar]
- Alfandari D., Vriend C., Heslenfeld D. J., Versfeld N. J., Kramer S. E., Zekveld A. A. (2018) Brain volume differences associated with hearing impairment in adults. Trends in Hearing 22: 2331216518763689 doi:10.1177/2331216518763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American National Standards Institute (2010) Specification for audiometers (ANSI S3.6-2010), New York, NY: Author. [Google Scholar]
- Annweiler C., Annweiler T., Montero-Odasso M., Bartha R., Beauchet O. (2014) Vitamin D and brain volumetric changes: Systematic review and meta-analysis. Maturitas 78(1): 30–39. doi:10.1016/j.maturitas.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Ashburner J. (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38(1): 95–113. doi:10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bateman G. A. (2000) Vascular compliance in normal pressure hydrocephalus. American Journal of Neuroradiology 21(9): 1574–1585. [PMC free article] [PubMed] [Google Scholar]
- Bateman G. A., Levi C. R., Schofield P., Wang Y., Lovett E. C. (2008) The venous manifestations of pulse wave encephalopathy: Windkessel dysfunction in normal aging and senile dementia. Neuroradiology 50(6): 491–497. doi:10.1007/s00234-008-0374-x. [DOI] [PubMed] [Google Scholar]
- Boyen K., Langers D. R., de Kleine E., van Dijk P. (2013) Gray matter in the brain: Differences associated with tinnitus and hearing loss. Hearing Research 295: 67–78. doi:10.1016/j.heares.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Brant L. J., Fozard J. L. (1990) Age changes in pure-tone hearing thresholds in a longitudinal study of normal human aging. The Journal of the Acoustical Society of America 88(2): 813–820. [DOI] [PubMed] [Google Scholar]
- Brett, M., Anton, J. L., Valabregue, R., & Poline, J. B. (2002). Region of interest analysis using an SPM toolbox. Paper presented at the International Conference on Functional Mapping of the Human Brain, Sendai, Japan.
- Carpinelli M. R., Wise A. K., Burt R. A. (2011) Vitamin D-deficient diet rescues hearing loss in Klotho mice. Hearing Research 275(1-2): 105–109. doi:10.1016/j.heares.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Chung C. P., Lin Y. J., Chao A. C., Lin S. J., Chen Y. Y., Wang Y. J., Hu H. H. (2010) Jugular venous hemodynamic changes with aging. Ultrasound in Medicine & Biology 36(11): 1776–1782. doi:10.1016/j.ultrasmedbio.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Coupe P., Manjon J. V., Gedamu E., Arnold D., Robles M., Collins D. L. (2010) Robust Rician noise estimation for MR images. Medical Image Analysis 14(4): 483–493. doi:10.1016/j.media.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Dubno J. R., Eckert M. A., Lee F. S., Matthews L. J., Schmiedt R. A. (2013) Classifying human audiometric phenotypes of age-related hearing loss from animal models. Journal of the Association for Research in Otolaryngology 14(5): 687–701. doi:10.1007/s10162-013-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubno J. R., Lee F. S., Matthews L. J., Ahlstrom J. B., Horwitz A. R., Mills J. H. (2008) Longitudinal changes in speech recognition in older persons. The Journal of the Acoustical Society of America 123(1): 462–475. doi:10.1121/1.2817362. [DOI] [PubMed] [Google Scholar]
- Echt K. V., Smith S. L., Burridge A. B., Spiro A., III (2010) Longitudinal changes in hearing sensitivity among men: The Veterans Affairs Normative Aging Study. The Journal of the Acoustical Society of America 128(4): 1992–2002. doi:10.1121/1.3466878. [DOI] [PubMed] [Google Scholar]
- Eckert M. A., Cute S. L., Vaden K. I., Jr., Kuchinsky S. E., Dubno J. R. (2012) Auditory cortex signs of age-related hearing loss. Journal of the Association for Research in Otolaryngology 13(5): 703–713. doi:10.1007/s10162-012-0332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M. A., Tenforde A., Galaburda A. M., Bellugi U., Korenberg J. R., Mills D., Reiss A. L. (2006) To modulate or not to modulate: Differing results in uniquely shaped Williams syndrome brains. Neuroimage 32(3): 1001–1007. doi:10.1016/j.neuroimage.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Eickhoff S. B., Stephan K. E., Mohlberg H., Grefkes C., Fink G. R., Amunts K., Zilles K. (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25(4): 1325–1335. doi:10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fan W., Zhang W., Li J., Zhao X., Mella G., Lei P., Xu H. (2015) Altered contralateral auditory cortical morphology in unilateral sudden sensorineural hearing loss. Otolology & Neurotology 36(10): 1622–1627. doi:10.1097/MAO.0000000000000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland D. R., Cederberg C., Tarima S. (2009) Audiometric pattern as a predictor of cardiovascular status: Development of a model for assessment of risk. Laryngoscope 119(3): 473–486. doi:10.1002/lary.20130. [DOI] [PubMed] [Google Scholar]
- Gaser C., Dahnke R. (2016) CAT-A computational anatomy toolbox for the analysis of structural MRI data. Human Brain Mapping 2016: 336–348. [Google Scholar]
- Gasparoni G., Bultmann S., Lutsik P., Kraus T. F. J., Sordon S., Vlcek J., Walter J. (2018) DNA methylation analysis on purified neurons and glia dissects age and Alzheimer’s disease-specific changes in the human cortex. Epigenetics & Chromatin 11(1): 41 doi:10.1186/s13072-018-0211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates G. A., Cobb J. L., D’Agostino R. B., Wolf P. A. (1993) The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Archives of Otolaryngology—Head and Neck Surgery 119(2): 156–161. [DOI] [PubMed] [Google Scholar]
- Golub J. S. (2017) Brain changes associated with age-related hearing loss. Current Opinion in Otolaryngology & Head and Neck Surgery 25(5): 347–352. doi:10.1097/MOO.0000000000000387. [DOI] [PubMed] [Google Scholar]
- Gopinath B., Flood V. M., McMahon C. M., Burlutsky G., Brand-Miller J., Mitchell P. (2010) Dietary glycemic load is a predictor of age-related hearing loss in older adults. Journal of Nutrition 140(12): 2207–2212. doi:10.3945/jn.110.128462. [DOI] [PubMed] [Google Scholar]
- Gopinath B., Flood V. M., McMahon C. M., Burlutsky G., Spankovich C., Hood L. J., Mitchell P. (2011) Dietary antioxidant intake is associated with the prevalence but not incidence of age-related hearing loss. The Journal of Nutrition Health & Aging 15(10): 896–900. [DOI] [PubMed] [Google Scholar]
- Gopinath B., Flood V. M., Rochtchina E., McMahon C. M., Mitchell P. (2010) Consumption of omega-3 fatty acids and fish and risk of age-related hearing loss. The American Journal of Clinical Nutrition 92(2): 416–421. doi:10.3945/ajcn.2010.29370. [DOI] [PubMed] [Google Scholar]
- Gopinath B., Schneider J., Flood V. M., McMahon C. M., Burlutsky G., Leeder S. R., Mitchell P. (2014) Association between diet quality with concurrent vision and hearing impairment in older adults. The Journal of Nutrition Health & Aging 18(3): 251–256. doi:10.1007/s12603-013-0408-x. [DOI] [PubMed] [Google Scholar]
- Groschel M., Gotze R., Ernst A., Basta D. (2010) Differential impact of temporary and permanent noise-induced hearing loss on neuronal cell density in the mouse central auditory pathway. Journal of Neurotrauma 27(8): 1499–1507. doi:10.1089/neu.2009.1246. [DOI] [PubMed] [Google Scholar]
- Husain F. T., Medina R. E., Davis C. W., Szymko-Bennett Y., Simonyan K., Pajor N. M., Horwitz B. (2011) Neuroanatomical changes due to hearing loss and chronic tinnitus: A combined VBM and DTI study. Brain Research 1369: 74–88. doi:10.1016/j.brainres.2010.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Kobayashi T., Itoh Z., Kusakari J., Takasaka T. (1989) Evaluation of vitamin D metabolism in patients with bilateral sensorineural hearing loss. American Journal of Otolaryngology 10(1): 11–13. [PubMed] [Google Scholar]
- Kang J. W., Choi H. S., Kim K., Choi J. Y. (2014) Dietary vitamin intake correlates with hearing thresholds in the older population: The Korean National Health and Nutrition Examination Survey. The American Journal of Clinical Nutrition 99(6): 1407–1413. doi:10.3945/ajcn.113.072793. [DOI] [PubMed] [Google Scholar]
- Koran M. E., Hohman T. J., Meda S. A., Thornton-Wells T. A. (2014) Genetic interactions within inositol-related pathways are associated with longitudinal changes in ventricle size. Journal of Alzheimer’s Disease 38(1): 145–154. doi:10.3233/JAD-130989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa S. G., Liberman M. C. (2015) Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hearing Research 330(Pt B): 191–199. doi:10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H., Jyothi V., Smythe N. M., Dubno J. R., Schulte B. A., Schmiedt R. A. (2010) Chronic reduction of endocochlear potential reduces auditory nerve activity: Further confirmation of an animal model of metabolic presbyacusis. Journal of the Association for Research in Otolaryngology 11(3): 419–434. doi:10.1007/s10162-010-0214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F. S., Matthews L. J., Dubno J. R., Mills J. H. (2005) Longitudinal study of pure-tone thresholds in older persons. Ear & Hearing 26(1): 1–11. [DOI] [PubMed] [Google Scholar]
- Leonard C. M., Puranik C., Kuldau J. M., Lombardino L. J. (1998) Normal variation in the frequency and location of human auditory cortex landmarks. Heschl’s gyrus: Where is it? Cerebral Cortex 8(5): 397–406. [DOI] [PubMed] [Google Scholar]
- Lewis M. A., Nolan L. S., Cadge B. A., Matthews L. J., Schulte B. A., Dubno J. R., Steel K. P., Dawson S. J. (2018) Whole exome sequencing in adult-onset hearing loss reveals a high load of predicted pathogenic variants in known deafness-associated genes and identifies new candidate gens. BMC Medical Genomics 11(1): 77 doi:10.1186/s12920-018-0395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. R., Ferrucci L., An Y., Goh J. O., Doshi J., Metter E. J., Resnick S. M. (2014) Association of hearing impairment with brain volume changes in older adults. Neuroimage 90: 84–92. doi:10.1016/j.neuroimage.2013.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. R., Metter E. J., O’Brien R. J., Resnick S. M., Zonderman A. B., Ferrucci L. (2011) Hearing loss and incident dementia. Archives of Neurology 68(2): 214–220. doi:10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. R., Thorpe R., Gordon-Salant S., Ferrucci L. (2011) Hearing loss prevalence and risk factors among older adults in the United States. The Journals of Gerontology Series A Biological Sciences and Medical Sciences 66(5): 582–590. doi:10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Wang J., Wu C., Wai Y., Yu J., Ng S. (2008) Diffusion tensor imaging of the auditory pathway in sensorineural hearing loss: Changes in radial diffusivity and diffusion anisotropy. Journal of Magnetic Resonance Imaging 28(3): 598–603. doi:10.1002/jmri.21464. [DOI] [PubMed] [Google Scholar]
- Makishima K. (1978) Arteriolar sclerosis as a cause of presbycusis. Otolaryngology 86(2): ORL322–ORL326. [DOI] [PubMed] [Google Scholar]
- Manji S. S., Williams L. H., Miller K. A., Ooms L. M., Bahlo M., Mitchell C. A., Dahl H. H. (2011) A mutation in synaptojanin 2 causes progressive hearing loss in the ENU-mutagenised mouse strain Mozart. PLoS One 6(3): e17607 doi:10.1371/journal.pone.0017607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T., Ito A. (1981) Effect of increased perilymphatic pressure on endocochlear potential. Annals of Otology, Rhinology & Laryngology 90(3 Pt 1): 264–266. doi:10.1177/000348948109000314. [DOI] [PubMed] [Google Scholar]
- Nakashima T., Naganawa S., Sone M., Tominaga M., Hayashi H., Yamamoto H., Nuttall A. L. (2003) Disorders of cochlear blood flow. Brain Research Reviews 43(1): 17–28. [DOI] [PubMed] [Google Scholar]
- Nguyen A., Khaleel H. M., Razak K. A. (2017) Effects of noise-induced hearing loss on parvalbumin and perineuronal net expression in the mouse primary auditory cortex. Hearing Research 350: 82–90. doi:10.1016/j.heares.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Ouda L., Burianova J., Balogova Z., Lu H. P., Syka J. (2016) Structural changes in the adult rat auditory system induced by brief postnatal noise exposure. Brain Structure & Function 221(1): 617–629. doi:10.1007/s00429-014-0929-z. [DOI] [PubMed] [Google Scholar]
- Ouda L., Profant O., Syka J. (2015) Age-related changes in the central auditory system. Cell & Tissue Research 361(1): 337–358. doi:10.1007/s00441-014-2107-2. [DOI] [PubMed] [Google Scholar]
- Patrone C., Eriksson O., Lindholm D. (2014) Diabetes drugs and neurological disorders: New views and therapeutic possibilities. The Lancet Diabetes & Endocrinology 2(3): 256–262. doi:10.1016/S2213-8587(13)70125-6. [DOI] [PubMed] [Google Scholar]
- Peelle J. E., Troiani V., Grossman M., Wingfield A. (2011) Hearing loss in older adults affects neural systems supporting speech comprehension. Journal of Neuroscience 31(35): 12638–12643. doi:10.1523/JNEUROSCI.2559-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle J. E., Wingfield A. (2016) The neural consequences of age-related hearing loss. Trends in Neuroscience 39(7): 486–497. doi:10.1016/j.tins.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z. J., Chang P. D., Moonis G., Lalwani A. K. (2017) A novel method of quantifying brain atrophy associated with age-related hearing loss. Neuroimage: Clinical 16: 205–209. doi:10.1016/j.nicl.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N., Daugherty A. M., Sethi S. K., Arshad M., Haacke E. M. (2017) Age differences in arterial and venous extra-cerebral blood flow in healthy adults: Contributions of vascular risk factors and genetic variants. Brain Structure & Function 222(6): 2641–2653. doi:10.1007/s00429-016-1362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F., Ma W., Li M., Sun H., Xin Q., Zong W., Zhao B. (2018) Gray matter atrophy is associated with cognitive impairment in patients with presbycusis: A comprehensive morphometric study. Frontiers of Neuroscience 12: 744 doi:10.3389/fnins.2018.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N. J., Rosas H. D., Fischl B. (2012) Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61(4): 1402–1418. doi:10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigters S. C., Bos D., Metselaar M., Roshchupkin G. V., Baatenburg de Jong R. J., Ikram M. A., Goedegebure A. (2017) Hearing impairment is associated with smaller brain volume in aging. Frontiers of Aging Neuroscience 9: 2 doi:10.3389/fnagi.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saljo A., Bao F., Jingshan S., Hamberger A., Hansson H. A., Haglid K. G. (2002) Exposure to short-lasting impulse noise causes neuronal c-Jun expression and induction of apoptosis in the adult rat brain. Journal of Neurotrauma 19(8): 985–991. doi:10.1089/089771502320317131. [DOI] [PubMed] [Google Scholar]
- Satow T., Aso T., Nishida S., Komuro T., Ueno T., Oishi N., Fukuyama H. (2017) Alteration of venous drainage route in idiopathic normal pressure hydrocephalus and normal aging. Frontiers of Aging Neuroscience 9: 387 doi:10.3389/fnagi.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt, R. A. (2010). The physiology of cochlear presbycusis. In Fay, Richard R. and Popper Arthur N. (Eds.), The aging auditory system (pp. 9–38). New York, NY: Springer.
- Schneider P., Andermann M., Wengenroth M., Goebel R., Flor H., Rupp A., Diesch E. (2009) Reduced volume of Heschl’s gyrus in tinnitus. Neuroimage 45(3): 927–939. doi:10.1016/j.neuroimage.2008.12.045. [DOI] [PubMed] [Google Scholar]
- Schuknecht H. F. (1974) Presbyacusis, Cambridge, England: Harvard University Press. [Google Scholar]
- Spalthoff R., Gaser C., Nenadic I. (2018) Altered gyrification in schizophrenia and its relation to other morphometric markers. Schizophrenia Research 202: 195–202. doi:10.1016/j.schres.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Spankovich C., Le Prell C. G. (2014) Associations between dietary quality, noise, and hearing: Data from the National Health and Nutrition Examination Survey, 1999–2002. International Journal of Audiology 53(11): 796–809. doi:10.3109/14992027.2014.921340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y. T., Meng X. X., Zhang X., Guo Y. B., Zhang H. J., Cheng Y. P., Bao J. X. (2017) Doxepin mitigates noise-induced neuronal damage in primary auditory cortex of mice via suppression of acid sphingomyelinase/ceramide pathway. Anatomical Record (Hoboken) 300(12): 2220–2232. doi:10.1002/ar.23677. [DOI] [PubMed] [Google Scholar]
- Vaden K. I., Jr., Matthews L. J., Eckert M. A., Dubno J. R. (2017) Longitudinal changes in audiometric phenotypes of age-related hearing loss. Journal of the Association for Research in Otolaryngology 18(2): 371–385. doi:10.1007/s10162-016-0596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Fieandt H., Saxen A. (1937) Pathologie und klinik der altersschwerhorlgkeit. Acta Otolaryngology 23: 1–85. [Google Scholar]
- Wang X., Xu P., Li P., Wang Z., Zhao F., Gao Z., Liu P. (2016) Alterations in gray matter volume due to unilateral hearing loss. Scientific Reports 6: 25811 doi:10.1038/srep25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Czyz A. D., Cain S. (2016) Age and gender differences in children and adolescents’ attitudes toward noise. International Journal of Audiology 55(2): 83–92. [DOI] [PubMed] [Google Scholar]
- Wiley T. L., Chappell R., Carmichael L., Nondahl D. M., Cruickshanks K. J. (2008) Changes in hearing thresholds over 10 years in older adults. Journal of the American Academy of Audiology 19(4): 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. H., Schrepfer T., Schacht J. (2015) Age-related hearing impairment and the triad of acquired hearing loss. Frontiers of Cellular Neuroscience 9: 276 doi:10.3389/fncel.2015.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Chen H. J., Liu B., Huang Z. C., Feng Y., Li J., Teng G. J. (2014) Brain structural and functional alterations in patients with unilateral hearing loss. Hearing Research 316: 37–43. doi:10.1016/j.heares.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Zou J., Minasyan A., Keisala T., Zhang Y., Wang J. H., Lou Y. R., Tuohimaa P. (2008) Progressive hearing loss in mice with a mutated vitamin D receptor gene. Audiology & Neurootology 13(4): 219–230. doi:10.1159/000115431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Age-Related Hearing Loss Associations With Changes in Brain Morphology by Mark A. Eckert, Kenneth I Vaden.Jr.Judy R. Dubno in Trends in Hearing