Abstract
Objectives
To determine current epidemiology and clinical characteristics of cerebrospinal fluid (CSF) shunt surgery, including revisions.
Methods
A retrospective, multicentre, registry-based study was conducted based on 10 years’ data from the UK Shunt Registry, including primary and revision shunting procedures reported between 2004 and 2013. Incidence rates of primary shunts, descriptive statistics and shunt revision rates were calculated stratified by age group, geographical region and year of operation.
Results
41 036 procedures in 26 545 patients were submitted during the study period, including 3002 infants, 4389 children and 18 668 adults. Procedures included 20 947 (51.0%) primary shunt insertions in 20 947 patients, and 20 089 (49.0%) revision procedures. Incidence rates of primary shunt insertions for infants, children and adults were 39.5, 2.4 and 3.5 shunts per 100 000 person-years, respectively. These varied by geographical subregion and year of operation. The most common underlying diagnoses were perinatal intraventricular haemorrhage (35.3%) and malformations (33.9%) in infants, tumours (40.5%) and malformations (16.3%) in children, and tumours (24.6%), post-haemorrhagic hydrocephalus (16.2%) and idiopathic normal pressure hydrocephalus (14.2%) in adults. Ninety-day revision rates were 21.9%, 18.6% and 12.8% among infants, children and adults, respectively, while first-year revision rates were 31.0%, 25.2% and 17.4%. The main reasons for revision were underdrainage and infection, but overdrainage and mechanical failure continue to pose problems.
Conclusions
Our report informs patients, carers, clinicians, providers and commissioners of healthcare, researchers and industry of the current epidemiology of shunting for CSF disorders, including the potential risks of complications and frequency of revision.
Keywords: Neurosurgery, CSF shunting, Epidemiology, Registry
Background
Disorders of the cerebrospinal fluid (CSF) circulation, often abbreviated using the term hydrocephalus, are uncommon but potentially life threatening, with annual incidence rates ranging from a few dozens to more than 300 per 100 000 population depending on geographical region, age group and aetiology.1 CSF shunt surgery uses a valved tubing system to divert CSF into other body cavities. It was introduced at the end of the 1930s and has reduced dramatically the mortality and neuronal damage caused by this disorder.2 Shunt surgery has also become a treatment option for severe cases of idiopathic intracranial hypertension (IIH).3 Despite improvements in surgical technique and shunt devices, significant complications still occur including overdrainage and underdrainage, shunt infections, and shunt mechanical failures such as obstruction, fracture and disconnection.4
Prenatal folate supplementation and improvements in in utero screening and the treatment of meningitis are among the factors that may have reduced hydrocephalus incidence in infants and children.5 6 Rising levels of obesity and an ageing population are leading to increasing incidence of IIH7 and normal pressure hydrocephalus (NPH),8 respectively.
Our understanding of CSF shunt epidemiology needs to be updated in order to inform the development of care pathways, clinical trials and technological innovation. At present, there is no recent report on the epidemiology of CSF shunt surgery based on large populations followed over time, and inclusive of all age groups and major aetiologies, hence this study in the UK and Ireland, based on data from the UK Shunt Registry (UKSR).
Methods
Study design and sample selection
This was a retrospective, multicentre, registry-based study, using 10 years’ data from the UKSR. The UKSR is a large UK–Ireland database, established in 1993, to collect data about CSF shunt and shunt-related procedures carried out in all major neurosurgical centres in these countries.6 Registration criteria include all shunt procedures performed in each centre, including new insertions and revisions (covering removal, reconnection, ligation, externalisation and reservoir/adjunct insertion). During the study period, data input in the registry consisted of a multistep method, where information was handwritten by local teams, posted to the registry and, after relevant data quality checks, entered into an electronic database (Dendrite UK). Case ascertainment was audited by comparing the number of cases reported to the UKSR against the number of reportable cases as determined from the operating theatre log books.
A continuous study period for the study was selected where all annual submissions were at least 90% of the year with the highest submission volume. Primary external ventricular drain insertions were excluded from the analyses.
Statistical analyses
Annual incidence rates of new shunt procedures were calculated per 100 000 population for each year of interest using reference populations for each relevant year as recorded in the UK Office for National Statistics and the Central Statistics Office of Ireland. Average incidence rates were calculated using the amount of person-time observed (equation 1), where n is the total number of new shunt procedures, t is the time interval under study (10 years) and N is the average annual population during that time interval. CIs for incidence rates were constructed following the approach proposed by Bégaud et al. 9
| (1) |
First-year and 90-day revision rates, defined as the number of patients who had their first revision within 1 year or within 90 days, respectively, were calculated for all patients whose primary shunt and follow-up period were within the study interval. Stratification of these rates by age group was based on patient age at primary shunting.
Demographic and clinical data were analysed using descriptive statistics. Diagnoses followed the UKSR classification system (online supplementary material 1) and were grouped into broader diagnostic groups (table 1), according to the cause of the CSF disturbance.6 Reasons for revision included underdrainage (including proximal malfunction or blockage), overdrainage, shunt infection, wound infection, fracture, migration and disconnection. Continuous variables were summarised using their mean and SD, or their median and IQR, depending on their frequency distribution. Categorical variables were summarised using counts and percentages, with 95% CI where appropriate. Histograms were used to represent the distribution of age at primary diagnosis, stratified by diagnostic group. Timelines were used to represent trends in absolute and relative numbers of shunt procedures per year. Geographical trends were analysed using the Public Health England regional classification of patient residence.
Table 1.
Shunt procedures and patient demographics, by age group, among patients with known age
| Age of patient at time of procedure | |||
| Infant (<1 year) | Children (1 to <17 years) | Adults (≥17 years) | |
| Total no of procedures, n* | 4458 | 8742 | 27 310 |
| Age (years) at primary shunt, median (IQR) | 0.2 (0.1–0.4) | 7.6 (3.2–12.4) | 55.3 (39.3–68.5) |
| Female:male ratio at primary shunt | 5:7 | 6:7 | 6:5 |
| Primary shunt insertions, n (%) | 2756 (61.8) | 2525 (28.9) | 15 263 (55.9) |
| Underlying diagnosis for primary shunt†, n (%) | |||
| Malformations‡ | 934 (33.9) | 411 (16.3) | 1125 (7.4) |
| Perinatal intraventricular haemorrhage | 973 (35.3) | 160 (6.3) | 94 (0.6) |
| Tumour (benign, malignant, unspecified) | 152 (5.5) | 1022 (40.5) | 3750 (24.6) |
| Post-haemorrhagic (AVM, SAH, unspecified) | 28 (1.0) | 48 (1.9) | 2473 (16.2) |
| Idiopathic normal pressure hydrocephalus | 0 (0) | 0 (0) | 2173 (14.2) |
| Idiopathic intracranial hypertension | 10 (0.4) | 122 (4.8) | 1160 (7.6) |
| Infection (meningitis, cerebral abscess, unspecified) | 184 (6.7) | 108 (4.3) | 472 (3.1) |
| Cyst (colloid, arachnoid, unspecified) | 84 (3.0) | 124 (4.9) | 522 (3.4) |
| Trauma | 30 (1.1) | 55 (2.2) | 525 (3.4) |
| Acquired other | 127 (4.6) | 168 (6.7) | 1242 (8.1) |
| Idiopathic other | 22 (0.8) | 26 (1.0) | 162 (1.1) |
| Unknown (diagnosis not specified) | 212 (7.7) | 279 (11.1) | 1565 (10.3) |
| First-year revision rates, n (%)§ | 772 (31.0) | 584 (25.2) | 2406 (17.4) |
| 90-day revision rates, n (%)§ | 591 (21.9) | 462 (18.6) | 1919 (12.8) |
| Reasons for primary shunt revision¶, n (%): | |||
| Underdrainage | 317 (64.9) | 199 (66.3) | 759 (57.2) |
| Shunt infection | 81 (16.5) | 35 (11.7) | 158 (11.9) |
| Disconnection | 25 (5.1) | 12 (4.0) | 93 (7.0) |
| Migration | 17 (3.4) | 6 (2.0) | 101 (7.6) |
| Overdrainage | 7 (1.4) | 24 (8.0) | 101 (7.6) |
| Fracture | 8 (1.6) | 5 (1.6) | 30 (2.2) |
| Wound infection | 3 (0.6) | 1 (0.3) | 8 (0.6) |
| More than one reason | 30 (6.1) | 18 (6.0) | 75 (5.6) |
| Primary shunt revisions that included shunt replacement with external ventricular drain | 55 (5.5) | 21 (2.6) | 106 (3.3) |
*For 526 (1.3%) procedures, in 486 (1.8%) patients, the age of the patient was unknown.
†Including only procedures of primary shunt insertion.
‡Malformations included aqueduct stenosis, Dandy-Walker, Chiari, malformations with spina bifida, unspecified congenital and other malformations.
§First-year and 90-day revision rates were calculated for all patients whose primary shunt was inserted in the study period, and who were followed up for at least 1 year and 90 days, respectively.
¶Excluding revision procedures with unknown/no reason specified. Among the 119 patients with multiple reasons for revision of primary shunt, each reason was present in different percentages, and included underdrainage (74%), disconnection (46%), migration (33%), shunt infection (18%), fracture (15%), overdrainage (11%) and wound infection (2%).
AVM, cerebral arteriovenous malformation;SAH, subarachnoid haemorrhage.
jnnp-2018-319927supp001.pdf (13.8KB, pdf)
Most analyses were carried out separately for three distinct age groups: infants, defined as children younger than 1 year; children 1 to less than 17 years old; adults 17 years or older.
Reporting rates, defined as the percentage of reported over reportable shunts, were calculated for each centre-year included in the case ascertainment audit. These rates were grouped into high (≥75%) versus low (<75%) case ascertainment for comparison. Potential impact on the results of case ascertainment, and trends in the distribution of missing data within reported cases, were explored using descriptive statistics.
Case ascertainment and missing data
Information on case ascertainment was available for 39 (85%) centres, for a median of 4 (out of 10) years per centre. Case ascertainment varied by centre-year, with average case ascertainment of primary shunt surgery of 79.5%. There was some variation in the distributions of diagnostic groups and reasons for revision, by group of high versus low case ascertainment, while those distributions were very similar in centre-years with unknown and high case ascertainment (online supplementary material 2).
jnnp-2018-319927supp002.pdf (363.1KB, pdf)
Within reported cases, age and sex were missing in 1.8% and 0.4% patients, respectively. Underlying diagnosis was missing in 10.5% patients. This percentage was lower in infants and varied depending on the centre of operation. Reasons for revision were missing in 42.4% procedures overall and in 57.9% procedures among first revisions. These percentages were lower among infants, increased over time, and there was variation between diagnostic groups and centres. Centre-years with high average case ascertainment had higher percentages of missing reason for revision. Patient residence on primary shunt was missing in 11.8% of the patients, and this percentage increased over the years and varied between centres. The centre of operation was missing in 1.3% shunt procedures (online supplementary material 3).
jnnp-2018-319927supp003.pdf (140.6KB, pdf)
Further details on missing data and case ascertainment are included in online supplementary materials 2 and 3.
Ethical considerations
The UKSR was established in 1993 as a national audit project under the auspices of the Medical Audit Committee of the East Anglian Regional Health Authority. Participating institutions submit data to the UKSR in line with local policies and approval procedures. This study did not require the use of identifiable data, and the manuscript does not contain personal information about an identifiable living individual.
Results
Sample and cohort selection
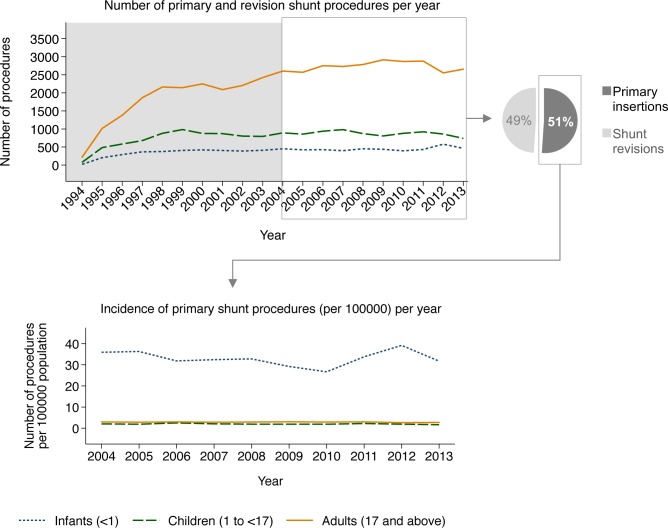
Between 1994 and 2013, 69 207 shunt procedures in 43 288 patients were submitted. Yearly submissions increased over time, until they reached a plateau (figure 1). The year with the highest number of submissions was 2011 (n=4267), and between 2004 and 2013 all years were within 90.8% and 99.8% of the 2011 submissions. Thus, this study focused on 10 years’ data from the UKSR, from 2004 to 2013.
Figure 1.
Absolute number (top left), distribution by type (primary or revision) (top right) and incidence (bottom) of yearly shunt procedures submitted to the UK Shunt Registry, by age group (infants, children and adults).
During the study period, 41 institutions participated in the UKSR. These represented all the National Health Service (NHS) neurosurgery centres and one private hospital performing shunt surgery in the UK during the study period, and one of the two neurosurgery institutions performing shunt surgery in Ireland. Of the participating centres, 9 (22%) carried out more than 50%, and 16 (39%) performed more than 75%, of all the reported procedures. Generally, centres operated on patients residing within their geographical region (at least 80% of their patients), with the exception of London and the South East, where a large percentage of their patients (about 50% in London and about 30% in the South East) resided in a different UK region (online supplementary material 4).
jnnp-2018-319927supp004.pdf (73.9KB, pdf)
Shunt surgery epidemiology
A total of 41 036 procedures in 26 545 patients were submitted during the study period, including 3002 (11.5%) infants, 4389 (16.9%) children and 18 668 (71.6%) adults. Procedures included 20 947 (51.0% [95% CI 50.6 to 51.5]) primary shunt insertions in 20 947 patients, and 20 089 (49.0% [48.5 to 49.4]) revision procedures of either those primary shunts or of shunts inserted prior to the study period (table 1).
Among patients who were followed up for at least 1 year since their primary shunt, 3783 (20.0% [19.4 to 20.5]) needed a revision within 1 year. This proportion was about 1.8 times higher in infants than in adults and about 1.4 times higher in children than in adults (table 1). The overall 90-day revision rate for primary shunt insertions was 14.6% (14.1 to 15.1) (2993 procedures, after excluding 53 patients first shunted during the last 90 days of the study period). By age group, this was about 1.7 times higher in infants than in adults and about 1.5 times higher in children than in adults (table 1). Furthermore, most (60.0% [58.6 to 61.3]) first revisions of primary shunt procedures occurred within 90 days.
Overall incidence rate of primary shunt insertions was 3.1 (3.1 to 3.2) per 100 000 person-years. Stratified by age, this rate was 32.8 (31.6 to 34.1) in infants, 2.0 (1.9 to 2.1) in children and 2.9 (2.8 to 2.9) in adults. Accounting for average case ascertainment, estimated actual incidence of primary shunt surgery was 3.8 per 100 000 person-years, overall; and 39.5, 2.4 and 3.5 for infants, children and adults, respectively. Although there were no clear time trends, there was some variation in these incidence rates by year of surgery (figure 1).
Regional variation
Among infants, some regions had more than 40 cases per 100 000 person-years, several other regions had less than 20 cases per 100 000 person-years and the remaining regions had incidence rates that were in between those figures (figure 2). Among adults and children, incidence rates also demonstrated regional variation, ranging from 0.9 to 4.6 cases per 100 000 person-years.
Figure 2.
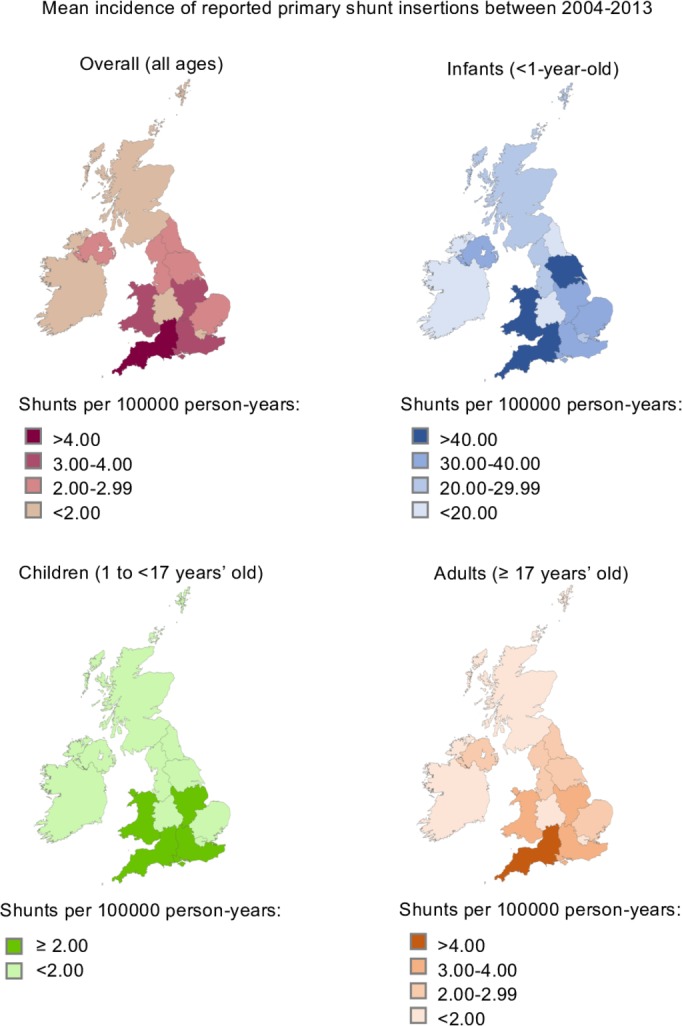
Mean incidence of reported primary shunt insertions between 2004 and 2013 (per 100 000 person-years), by region of patients’ residence and age group (infants, children and adults).
Demographics, underlying diagnosis for shunting and reasons for revision
Infants
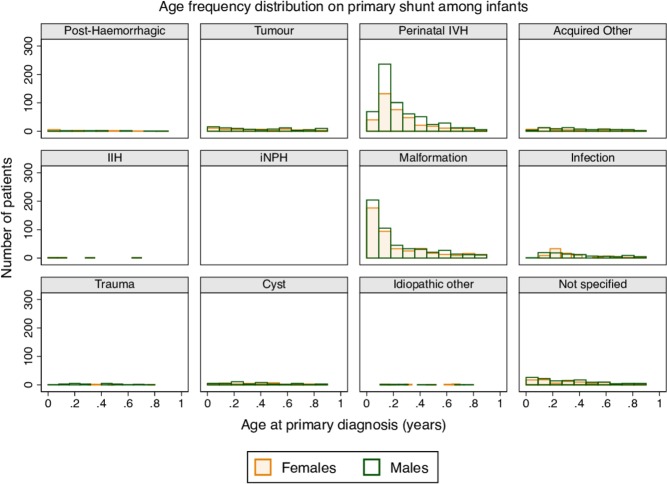
Primary shunt insertions represented 61.8% (60.4 to 63.3) of all procedures in infants with a median age of 2.4 months at first insertion, and with more males than females in most diagnostic groups (table 1). The most common underlying diagnoses were perinatal intraventricular haemorrhage (IVH) (35.3% [33.5 to 37.1]) and malformations (33.9% [32.1 to 35.7]) (table 1), and these percentages increased over time. The most common reasons for revision were underdrainage (64.9% [60.5 to 69.2]) and shunt infection (16.5% [13.4 to 20.2]) (table 1).
Age and sex distributions at primary shunt insertion differed by diagnostic group (figure 3). Most infants with malformations and IVH were operated on early, with about 90% (87.5% and 90.4%, respectively) being operated on at 6 months of age or younger. In the remaining diagnostic groups, cases were evenly spread across the entire age band. Female:male ratios ranged from 8:9 (infection) to 1:4 (trauma) (figure 3).
Figure 3.
Age frequency distribution at primary shunt insertion in infants (1 year old), by sex and underlying diagnostic group. IIH, idiopathic intracranial hypertension; iNPH, idiopathic normal pressure hydrocephalus; IVH, intraventricular haemorrhage.
Children
Primary shunt insertions represented 28.9% (27.9 to 29.8) of all procedures in children with a median age of 7.6 years at first insertion. As in infants, there were less females than males overall (table 1).
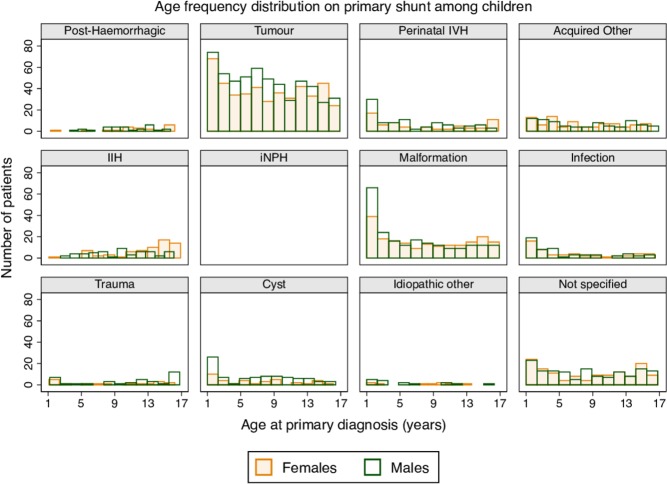
The most common diagnoses were tumours (40.5% [38.6 to 42.5]) and malformations (16.3% [14.8 to 17.7]) (table 1). Underdrainage was the most common reason for revision (66.3% [60.7 to 71.7]), followed by shunt infection (11.7% [8.2 to 15.9]) (table 1).
As with infants, age and sex distributions at primary insertion varied depending on underlying diagnosis. The number of tumour-related shunts decreased with age (figure 4), while the remaining diagnostic groups had relatively even distributions. Exceptions were children with malformations, cysts or IVH, who were diagnosed and/or operated on at earlier ages, and female children with IIH who were operated on at later ages (figure 4). There were generally less females than males, with female:male ratios ranging from 1:1 (infections and other unspecified acquired conditions) to 2:5 (cysts). The exception was children with IIH among whom there was a higher female:male ratio (5:4) (figure 4).
Figure 4.
Age frequency distribution at primary shunt insertion in children (1 to <17 years old), by sex and underlying diagnostic group. IIH, idiopathic intracranial hypertension; iNPH, idiopathic normal pressure hydrocephalus; IVH, intraventricular haemorrhage.
Adults
Primary shunt insertions represented 55.9% (55.3 to 56.5) of all procedures in adults with a median age of 55.3 years at first insertion. In contrast to the younger age groups, there were more females than males overall (table 1).
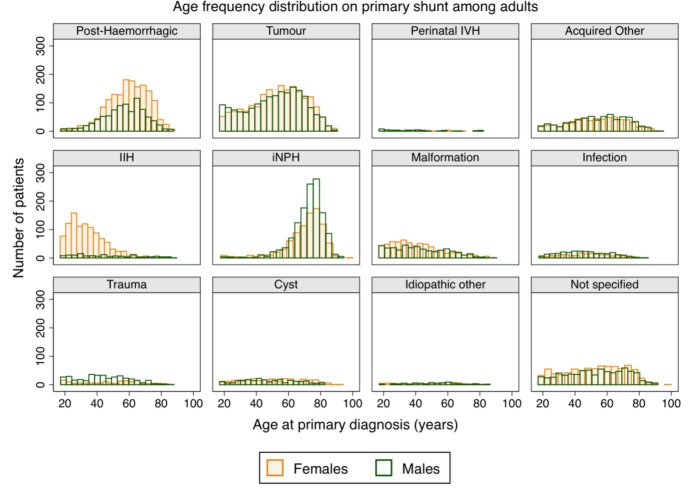
The most common diagnoses in adults were tumours (24.6% [23.9 to 25.3]) and post-haemorrhagic hydrocephalus (16.2% [15.6 to 16.8]) (table 1), with post-haemorrhagic events being mainly subarachnoid haemorrhage (93.3%) followed by arteriovenous malformation (6.0%). Over time, there was a decrease in the number of adults shunted due to haemorrhage and tumours, while there was an increase of adults shunted for idiopathic NPH (iNPH), and female adults shunted for IIH. As in the younger age groups, underdrainage was the most common reason for revision (57.3% [54.6 to 60.0]), followed by shunt infection (11.9% [10.2 to 13.8]) (table 1).
Age and sex distributions at primary shunt insertion also varied depending on underlying diagnosis among adults (figure 5). Most primary shunts were inserted during the first six decades of life, except for iNPH (most done after the age of 65). The higher number of females was particularly marked for IIH (8:1 female:male ratio) and post-haemorrhagic hydrocephalus (9:5 female:male ratio). The exception was traumatic hydrocephalus, where there were more males (2:5 female:male ratio) (figure 5).
Figure 5.
Age frequency distribution at primary shunt insertion in adults (≥17 years old), by sex and underlying diagnostic group. IIH, idiopathic intracranial hypertension; iNPH, idiopathic normal pressure hydrocephalus; IVH, intraventricular haemorrhage.
Discussion
This study represents, to our knowledge, the most comprehensive population-based epidemiological report on CSF shunt surgery to date. Our data are stratified by 3 age groups and 11 diagnostic groups, a comprehensive framework which has not been previously reported. This provides a picture of the current status of CSF shunt surgery at a national level, and establishes a benchmark both for comparisons with future studies and for quality-of-care audits. Previous reports of shunt surgery epidemiology are limited by methodology (subsample-based estimations),10–12 by time period (two to four decades old),11–13 or by being focused on specific age groups14–16 or specific diagnoses.15 17 18
Our results are based on nationally collected data from a purposively developed registry, including all the NHS neurosurgery units and one private hospital in the UK and one of the two neurosurgery centres of Ireland, and are based on a 10-year period with the highest volume of submissions. Data on case ascertainment were used to improve and contextualise our calculations. These features of our study helped us overcome the limitations of other retrospective studies based on clinical coding in patient records or discharge registries,10 11 13 and possible sampling bias in single-centre studies.12 16 18
Low reporting rates in some centre-years could have biased or limited the accuracy of some of our estimates. However, group comparisons by case ascertainment showed that centre-years with high and unknown case ascertainment had similar distributions of diagnosis and revision reasons (with a few exceptions), indicating that our results may be a valid representation of the target population.
Missing data within reported cases was another limitation, which is a common feature of retrospective analyses. We found variation by age group (it was often less common in infants) and centre of operation, as well as a slight increase in missing data over time, and therefore the risk of some bias in our estimates cannot be completely discarded. The percentage of missing reason for revision was considerably higher than in other data fields, possibly because it is not always apparent what the cause for revision is at the time of surgery. With the information at hand, it was not possible to determine whether data were missing at random, that is, not related to the value of the missing information, after controlling for available data.
Overall epidemiology of shunt surgery
We found an overall incidence rate of 3.1 primary insertions per 100 000 person-years, adjusted to 3.8 after accounting for average case ascertainment. This rate is lower than previously reported figures using subsample-based estimations, ranging from 5.5 (California, 1990–2000)11 to 6.9 (German subpopulation, 2003–2012).10 Incidence rates of shunt insertions in our study varied across different geographical regions, particularly in infants. We noted a small yearly variation in shunt surgery incidence rates, similar to other studies.10 15 17
Differing demographic characteristics of our study population as compared with others10 11 could partly explain the observed differences in shunting rates. Methodological considerations could have also affected our findings. Lower case ascertainment in certain regions or years, and higher percentage of patients with a missing address in some centres, could be behind some of the regional differences in shunting incidence. However, lower regional incidence rates did not necessarily correlate with low case ascertainment (online supplementary material 2).
Regional variations could also be influenced by some centres operating on a large volume of patients coming from different geographical regions, although this only significantly affected two UK regions (online supplementary material 4). While UK regional incidence rates were not affected by non-participation, in Ireland incidence rates were underestimated in our study, as it was not possible to correct for the population size covered by the non-participating hospital in this region.
In epidemiological terms, CSF shunts are uncommon procedures, and incidence rates of uncommon events are highly vulnerable to small changes in absolute numbers. Nonetheless, the latter can be mitigated by the inclusion of a large population over a long period, as performed in this study and demonstrated with our narrow CIs.
Therefore, considering all of the above factors, the observed variations likely represent some actual epidemiological differences for each underlying diagnosis,19 differing clinical practice in decision to shunt, and alternative treatment options,3 20 in line with previous reports.21
Shunt surgery in infants
During infancy, the incidence rate of primary insertions was 32.8 per 100 000 person-years and 39.5 after accounting for case ascertainment. These rates are within the range of published figures for the diagnosis of congenital hydrocephalus (14.0–74.7 per 100 000 in England and/or Wales, 2003–2014),22 although the latter included live births, stillbirths and termination of pregnancies, and not all cases would have required shunting. Incidence rates in our study were higher than previously reported figures for shunt surgery for congenital hydrocephalus alone (24.8 per 100 000 in Denmark 1986–1998),14 although acquired disorders represented about 20% of diagnoses in our population.
Most primary shunt interventions among infants were for malformations and perinatal IVH, a trend that increased over time, followed by tumours and infections, in line with findings of a recent review on infantile hydrocephalus.23 Our findings are also consistent with previous studies which found higher frequency of hydrocephalus in infant males than females.1 5
Shunt surgery in children
Children had the lowest incidence of primary shunt insertions as compared with the other two age groups, with 2.0 primary shunt insertions per 100 000 person-years and 2.4 after accounting for case ascertainment. We are not aware of previous reports on the incidence of shunt surgery that have distinguished children from infants. A study in California 1990–2000 reported a slightly lower proportion of both infants and children who had a shunt insertion than in our study.11
The most common diagnoses underlying the first shunt were tumours (two in five children) followed by malformations and a history of perinatal IVH in the youngest children. The incidence of tumours of the central nervous system is higher in younger children than in adolescents, although it increases with age during adulthood.24 Although malformations and perinatal IVH are present at or soon after birth, hydrocephalus may not become symptomatic and require a shunt immediately or at all.25
Shunt surgery in adults
We found an incidence rate of 2.9 primary insertions per 100 000 person-years among adults, 3.5 after accounting for case ascertainment. Tumours, post-haemorrhagic hydrocephalus, iNPH and IIH were the most common specific diagnostic groups. Incidence rate in our cohort was lower than that reported in a recent smaller study on adult shunt surgery epidemiology in Sweden of 4.7.15 A key difference between the two studies is the incidence of patients with iNPH, which was 2.2 per 100 000 person-years in the Swedish cohort versus 0.5 (14% of 3.5) per 100 000 person-years in our cohort, despite similar population pyramids in both countries.26 There was an increase in patients with iNPH in our cohort over time. Prevalence rates of iNPH have also been found to vary between countries.27 These variations probably reflect the well-known controversies over the diagnosis and management of iNPH.
The age at which shunts were inserted and effect of gender reflect the epidemiology of the underlying conditions and the population age distribution, and are in line with previous smaller studies.17 27 28 iNPH is a disease of the elderly. Eight females for every male were shunted for IIH and nine females for every five males for post-haemorrhagic hydrocephalus, while shunts for post-traumatic hydrocephalus were inserted more often in males up to their fifties. Gender differences are poorly understood but have been explained by hormonal effects3 28 and participation in different occupational and leisure activities.29
Shunt revision surgery
About half (49.0%) of the workload of surgery for CSF diversion was dedicated to the revision of primary procedures, which is similar to the USA (48% revisions between 1988 and 1991).13 By age group, infants followed by children had the highest revision rates compared with adults, which is in line with previous reports.4 11 In our study, there were about 40% more primary shunts revised for infection in infants than in adults, probably related to their higher risk for shunt infections.11 Overall 1-year and 90-day (first) revision rates were 20.0% and 14.6%, respectively, which were generally lower than those found in more historical studies.4 No reason was provided for revision in 42.4% of cases, so caution is required in the interpretation of these data.
Besides methodological, demographic and institutional/geographical differences, the lower revision rates found in our population could be related in part to a reduction in shunt infections over time. This reduction probably reflects the introduction of strict operating theatre protocols, prophylactic antibiotics and antimicrobial-impregnated catheters.6 30
Despite technological advances in shunting systems during the past decades, mechanical problems with shunt devices, compounded by ill-defined patient-related factors, are still a major reason for shunt revision. The majority of the revisions were attributed to underdrainage (57%–66%, depending on the age group) and shunt infection (12%–17%). Overdrainage (especially in adults and children), migration (especially in adults) and disconnection (in all age groups) represented up to about one-fifth of all reasons for revision, depending on the age group. This highlights the need for further improvements in both surgical technique and shunt device technology.
Lastly, patients and their families need to be counselled about the current rates of complications of shunt surgery that our study has documented. Shunt revisions pose a considerable financial burden on health services. For example, in the USA, the average costs of shunt revisions, as estimated for infections, are US$45 714 and US$56 104 per patient in adults and children, respectively.31
Conclusions
We present epidemiological findings about CSF shunt surgery based on a national registry monitored for case ascertainment and data quality. It includes 10 years’ data, all possible underlying diagnoses, and separate results for three age groups. We would encourage future epidemiology studies to report their results stratified by age groups, and by aetiology, and to make use of electronic reporting to decrease the risk of missing data.
Our report should help to inform patients, carers, clinicians, providers and commissioners of healthcare, regulators, designers of clinical trials and implant suppliers of the current epidemiology of shunting for CSF disorders.
Acknowledgments
The authors are grateful for the participation of centres across the UK and Ireland which perform shunt surgery. These centres supply our data and also help to fund the registry. We are very grateful to the Medical Devices Agency (now MHRA), the Association for Spina Bifida and Hydrocephalus (ASBAH, now SHINE) and Spina Bifida Hydrocephalus Scotland for their support. The research was supported by the Medical Audit Committee of the East Anglian Regional Health Authority and the National Institute for Health Research (NIHR) Brain Injury MedTech Co-operative based at Cambridge University Hospitals NHS Foundation Trust and University of Cambridge. We thank Elizabeth Tabone for her meticulous contributions to data entry and data quality checks. Finally, we are very grateful to Professor Gordon Murray for his invaluable critique.
Footnotes
Twitter: The first epidemiology study of CSF Shunting based on the UK Shunt Registry is now available @bit.ly/2TZ6Czn. This is a collective national effort, supported by SBNS, NIHR Brain Injury MIC, MHRA, SHINE and SBH Scotland to improve our knowledge on this life-saving procedure
Collaborators: UKSR Collaborators: David Allcutt; Fiona Arnold; Richard Ashpole; Eric Ballantyne; Cristina Bleil; Howard Brydon; Diederik Bulters; Marian Byrne; Tom Cadoux-Hudson; Claire Cairns; Kerry Cameron; Maria Cartmill; Christopher Chandler; Munchi S Choksey; Sally-Ann Collins; Kimona Colthrust; Claudia Craven; Darach Crimmins; Giles Critchley; Matthew Crocker; Simon Cudlip; Marek Czosnyka; Zofia Czosnyka; Linda D’Antona; Emma Dewhurst; Laurence Dunn; Richard Edwards; Fiona Evans; Helen Fernandes; Amy Fieldsend; Graham Flint; Matthew Garnett; Aprajay Golash; John Goodden; Lois Gourley; Jennifer Hallet; Jan Hardy; Dawn Hennigan; Katie Herbert; Liz Hinde; Damian Holliman; Peter Hutchinson; Michael Jenkinson; Ian Kamaly; Jothy Kandasamy; Nicole Keong; Sara Kewin; Andrew King; Angelos Kolias; Sarah Kvedaras; Afroditi Lalou; Paul Leach; Donald MacArthur; Conor Mallucci; John Martin; Bruce Mathew; Roy McConnell; Catherine McMahon; John McMullan; Emma Moran; Nitin Mukerji; Eva Nabbanja; Roddy O’Kane; Jody O’Connor; Gerry O’Reilly; Marios Papadopoulos; Ian Pople; Chittoor Rajaraman; Roberto Ramirez; Joana Ramos; Sheila Ross; Nicholas Ross; Thomas Santarius; Amar Saxena; Mano Shanmuganathan; Saurabh Sinha; Guirish Solanki; Roger Strachan; Dominic Thompson; Simon Thompson; John Thorne; Lewis Thorne; Kim Thurlby; Martin Tisdall; Simon Tizzard; Lorraine Todd; Ahmed Toma; Carole Turner; Shungu Ushewokunze; Raghu Vindlacheruvu; John Wadley; Laurence Watkins; Peter Whitfield; Mark Wilson; Bassel Zebian.
Contributors: JDP established the UK Shunt Registry in 1993 and has had overall responsibility up to the present time. HKR and HMS contributed to data acquisition, data management and storage and quality assurance of the UKSR. RFM, AJJ and JDP conceived the analysis plan, RFM carried out data quality checks, cleansing and analyses, and all authors contributed to data interpretation. RFM drafted the manuscript and all remaining authors critically revised it. All authors approved the final version of the manuscript for publication.
Funding: UKSR participating NHS Trusts and National Institute for Health Research.
Competing interests: The UKSR is supported by annual subscription fees from each participating institution. Support for a research fellowship program has been established by industry, who receive data on the performance and usage of their own products to facilitate their post-marketing surveillance. The UK Shunt Registry has complete independence over data collection, analysis and publication. AJJ is supported by an NIHR academic clinical lectureship award. JDP was an NIHR Senior Investigator (2009–2014) and is Honorary Clinical Director of the NIHR Brain Injury Health Technology Co-operative (2012–2017), now MedTech Co-operative (2018–2023). The registry has responsibilities to the Society of British Neurological Surgeons and the Medicines and Healthcare products Regulatory Agency.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
UKSR collaborators:
David Allcutt, Fiona Arnold, Richard Ashpole, Eric Ballantyne, Cristina Bleil, Howard Brydon, Diederik Bulters, Marian Byrne, Tom Cadoux-Hudson, Claire Cairns, Kerry Cameron, Maria Cartmill, Christopher Chandler, Munchi S Choksey, Sally-Ann Collins, Kimona Colthrust, Claudia Craven, Darach Crimmins, Giles Critchley, Matthew Crocker, Simon Cudlip, Marek Czosnyka, Zofia Czosnyka, Linda D’Antona, Emma Dewhurst, Laurence Dunn, Richard Edwards, Fiona Evans, Helen Fernandes, Amy Fieldsend, Graham Flint, Matthew Garnett, Aprajay Golash, John Goodden, Lois Gourley, Jennifer Hallet, Jan Hardy, Dawn Hennigan, Katie Herbert, Liz Hinde, Damian Holliman, Peter Hutchinson, Michael Jenkinson, Ian Kamaly, Jothy Kandasamy, Nicole Keong, Sara Kewin, Andrew King, Angelos Kolias, Sarah Kvedaras, Afroditi Lalou, Paul Leach, Donald MacArthur, Conor Mallucci, John Martin, Bruce Mathew, Roy McConnell, Catherine McMahon, John McMullan, Emma Moran, Nitin Mukerji, Eva Nabbanja, Roddy O'Kane, Jody O’Connor, Gerry O’Reilly, Marios Papadopoulos, Ian Pople, Chittoor Rajaraman, Roberto Ramirez, Joana Ramos, Sheila Ross, Nicholas Ross, Thomas Santarius, Amar Saxena, Mano Shanmuganathan, Saurabh Sinha, Guirish Solanki, Roger Strachan, Dominic Thompson, Simon Thompson, John Thorne, Lewis Thorne, Kim Thurlby, Martin Tisdall, Simon Tizzard, Lorraine Todd, Ahmed Toma, Carole Turner, Shungu Ushewokunze, Raghu Vindlacheruvu, John Wadley, Laurence Watkins, Peter Whitfield, Mark Wilson, and Bassel Zebian
References
- 1. Dewan MC, Rattani A, Mekary R, et al. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J Neurosurg 2018;37:1–15. 10.3171/2017.10.JNS17439 [DOI] [PubMed] [Google Scholar]
- 2. Notarianni C, Vannemreddy P, Caldito G, et al. Congenital hydrocephalus and ventriculoperitoneal shunts: influence of etiology and programmable shunts on revisions. J Neurosurg Pediatr 2009;61:547–52. 10.3171/2009.7.PEDS08371 [DOI] [PubMed] [Google Scholar]
- 3. Mollan SP, Ali F, Hassan-Smith G, et al. Evolving evidence in adult idiopathic intracranial hypertension: pathophysiology and management. J Neurol Neurosurg Psychiatry 2016;87:982–92. 10.1136/jnnp-2015-311302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stein SC, Guo W. Have we made progress in preventing shunt failure? A critical analysis. J Neurosurg Pediatr 2008;146:40–7. 10.3171/PED-08/01/040 [DOI] [PubMed] [Google Scholar]
- 5. Persson E-K, Hagberg G, Uvebrant P. Hydrocephalus prevalence and outcome in a population-based cohort of children born in 1989–1998. Acta Paediatr 2005;94:726–32. 10.1080/08035250510027336 [DOI] [PubMed] [Google Scholar]
- 6. Richards HK, Seeley HM, Pickard JD. Efficacy of antibiotic-impregnated shunt catheters in reducing shunt infection: data from the United Kingdom shunt registry. J Neurosurg Pediatr 2009;52:389–93. 10.3171/2009.4.PEDS09210 [DOI] [PubMed] [Google Scholar]
- 7. Markey KA, Mollan SP, Jensen RH, et al. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol 2016;15:78–91. 10.1016/S1474-4422(15)00298-7 [DOI] [PubMed] [Google Scholar]
- 8. Brean A, Eide PK. Prevalence of probable Idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand 2008;118:48–53. 10.1111/j.1600-0404.2007.00982.x [DOI] [PubMed] [Google Scholar]
- 9. Bégaud B, Martin K, Abouelfath A, et al. An easy to use method to approximate Poisson confidence limits. Eur J Epidemiol 2005;20:213–6. 10.1007/s10654-004-6517-4 [DOI] [PubMed] [Google Scholar]
- 10. Lemcke J, Stengel D, Stockhammer F, et al. Nationwide incidence of normal pressure hydrocephalus (NPH) assessed by insurance claim data in Germany. Open Neurol J 2016;10:15–24. 10.2174/1874205X01610010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu Y, Green NL, Wrensch MR, et al. Ventriculoperitoneal shunt complications in California: 1990 to 2000. Neurosurgery 2007;61:557–63. 10.1227/01.NEU.0000290903.07943.AF [DOI] [PubMed] [Google Scholar]
- 12. Hardie NA, Molgaard CA, Laws ER, et al. Incidence and effectiveness of cerebrospinal fluid shunts in Olmsted County, Minnesota, 1956–1981. Neuroepidemiology 1986;5:95–104. 10.1159/000110819 [DOI] [PubMed] [Google Scholar]
- 13. Bondurant CP, Jimenez DF. Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg 1995;23:254–9. 10.1159/000120968 [DOI] [PubMed] [Google Scholar]
- 14. Christensen JH, Hansen LK, Garne E. [Congenital hydrocephalus—prevalence and prognosis. Mortality and morbidity in a population-based study]. Ugeskr Laeger 2003;165:466–9. [PubMed] [Google Scholar]
- 15. Sundström N, Malm J, Laurell K, et al. Incidence and outcome of surgery for adult hydrocephalus patients in Sweden. Br J Neurosurg 2017;31:21–7. 10.1080/02688697.2016.1229749 [DOI] [PubMed] [Google Scholar]
- 16. Tisell M, Höglund M, Wikkelsø C. National and regional incidence of surgery for adult hydrocephalus in Sweden. Acta Neurol Scand 2005;112:72–5. 10.1111/j.1600-0404.2005.00451.x [DOI] [PubMed] [Google Scholar]
- 17. Brean A, Fredø HL, Sollid S, et al. Five-year incidence of surgery for idiopathic normal pressure hydrocephalus in Norway. Acta Neurol Scand 2009;120:314–6. 10.1111/j.1600-0404.2009.01250.x [DOI] [PubMed] [Google Scholar]
- 18. Martín-Láez R, Caballero-Arzapalo H, Valle-San Román N, et al. Incidence of idiopathic normal-pressure hydrocephalus in northern Spain. World Neurosurg 2016;87:298–310. 10.1016/j.wneu.2015.10.069 [DOI] [PubMed] [Google Scholar]
- 19. Garne E, Loane M, Addor M-C, et al. Congenital hydrocephalus – —prevalence, prenatal diagnosis and outcome of pregnancy in four European regions. Eur J Paediatr Neurol 2010;14:150–5. 10.1016/j.ejpn.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 20. Paulsen AH, Due-Tønnessen BJ, Lundar T, et al. Cerebrospinal fluid (CSF) shunting and ventriculocisternostomy (ETV) in 400 pediatric patients. Shifts in understanding, diagnostics, case-mix, and surgical management during half a century. Childs Nerv Syst 2017;33:259–68. 10.1007/s00381-016-3281-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kraemer MR, Sandoval-Garcia C, Bragg T, et al. Shunt-dependent hydrocephalus: management style among members of the American Society of Pediatric Neurosurgeons. J Neurosurg Pediatr 2017;14:216–24. 10.3171/2017.2.PEDS16265 [DOI] [PubMed] [Google Scholar]
- 22. Isaacs AM, Riva-Cambrin J, Yavin D, et al. Age-specific global epidemiology of hydrocephalus: systematic review, metanalysis and global birth surveillance. PLoS One 2018;13 10.1371/journal.pone.0204926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tully HM, Dobyns WB. Infantile hydrocephalus: a review of epidemiology, classification and causes. Eur J Med Genet 2014;57:359–68. 10.1016/j.ejmg.2014.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kieran MW, Walker D, Frappaz D, et al. Brain tumors: from childhood through adolescence into adulthood. JCO 2010;28:4783–9. 10.1200/JCO.2010.28.3481 [DOI] [PubMed] [Google Scholar]
- 25. Hamilton MG. Treatment of hydrocephalus in adults. Semin Pediatr Neurol 2009;16:34–41. 10.1016/j.spen.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 26. European Statistical System 2011 census. Available: https://ec.europa.eu/CensusHub2/query.do?step=selectHyperCube&qhc=false [Accessed 20 Mar 2018].
- 27. Martín-Láez R, Caballero-Arzapalo H, López-Menéndez Luis Ángel, et al. Epidemiology of idiopathic normal pressure hydrocephalus: a systematic review of the literature. World Neurosurg 2015;84:2002–9. 10.1016/j.wneu.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 28. Gangemi M, Cavallo LM, Di Somma A, et al. Hydrocephalus onset after microsurgical or endovascular treatment for acute subarachnoid hemorrhage. retrospective Italian multicenter study. Transl Med UniSa 2014;9. [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen R, Fiest KM, McChesney J, et al. The International incidence of traumatic brain injury: a systematic review and meta-analysis. Can J Neurol Sci 2016;43:774–85. 10.1017/cjn.2016.290 [DOI] [PubMed] [Google Scholar]
- 30. Konstantelias AA, Vardakas KZ, Polyzos KA, et al. Antimicrobial-impregnated and -coated shunt catheters for prevention of infections in patients with hydrocephalus: a systematic review and meta-analysis. J Neurosurg 2015;75:1096–112. 10.3171/2014.12.JNS14908 [DOI] [PubMed] [Google Scholar]
- 31. Parker SL, McGirt MJ, Murphy JA, et al. Cost savings associated with antibiotic-impregnated shunt catheters in the treatment of adult and pediatric hydrocephalus. World Neurosurg 2015;83:382–6. 10.1016/j.wneu.2014.06.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2018-319927supp001.pdf (13.8KB, pdf)
jnnp-2018-319927supp002.pdf (363.1KB, pdf)
jnnp-2018-319927supp003.pdf (140.6KB, pdf)
jnnp-2018-319927supp004.pdf (73.9KB, pdf)