Abstract
Objectives
The presence of proinflammatory low-density granulocytes (LDG) has been demonstrated in autoimmune and infectious diseases. Recently, regulatory neutrophilic polymorphonuclear myeloid-derived suppressor cells (PMN-MDSC) were identified in systemic lupus erythematosus (SLE). Because LDG and PMN-MDSC share a similar phenotype with contrasting functional effects, we explored these cells in a cohort of patients with SLE.
Methods
LDG and normal-density granulocytes (NDG) were isolated from fresh blood of healthy donors (HD) and patients with SLE. Associations between LDG and clinical manifestations were analysed. Multicolor flow cytometry and confocal imaging were performed to immunophenotype the cells. The ability of LDG and NDG to suppress T cell function and induce cytokine production was quantified.
Results
LDG prevalence was elevated in SLE versus HD, associated with the interferon (IFN) 21-gene signature and disease activity. Also, the LDG-to-lymphocyte ratio associated better with SLE disease activity index than neutrophil-to-lymphocyte ratio. SLE LDG exhibited significantly heightened surface expression of various activation markers and also of lectin-like oxidised low-density lipoprotein receptor-1, previously described to be associated with PMN-MDSC. Supernatants from SLE LDG did not restrict HD CD4+ T cell proliferation in an arginase-dependent manner, suggesting LDG are not immunosuppressive. SLE LDG supernatants induced proinflammatory cytokine production (IFN gamma, tumour necrosis factor alpha and lymphotoxin alpha) from CD4+ T cells.
Conclusions
Based on our results, SLE LDG display an activated phenotype, exert proinflammatory effects on T cells and do not exhibit MDSC function. These results support the concept that LDG represent a distinct proinflammatory subset in SLE with pathogenic potential, at least in part, through their ability to activate type 1 helper responses.
Keywords: T cells, systemic lupus erythematosus, autoimmune diseases
Key messages.
Low-density neutrophil populations may either exhibit proinflammatory (low-density granulocytes, LDG) or suppressive (polymorphonuclear myeloid-derived suppressor cells) properties. Both populations have been reported in systemic lupus erythematosus (SLE).
SLE LDG demonstrate an activated phenotype, do not suppress T cells and promote Th1 responses.
Activated SLE LDG also express lectin-like oxidised low-density lipoprotein receptor-1.
SLE normal-density granulocytes, but not LDG, may suppress T cells in an arginase-dependent manner.
Introduction
A low-density neutrophil population was identified in the peripheral blood mononuclear cell population of patients with systemic lupus erythematosus (SLE) in 1986.1 Since then, these cells have been reported in other autoimmune, cancer and infectious diseases with either proinflammatory (low-density granulocytes, LDG) or suppressive effects (neutrophilic polymorphonuclear myeloid-derived suppressor cells, PMN-MDSC).2–6 The proinflammatory nature of LDG in SLE was demonstrated by their ability to secrete tumour necrosis factor alpha (TNF-α), interferon gamma (IFN)-γ and type I IFN, cytokines frequently implicated in disease pathogenesis.3 LDG are also potent producers of neutrophil extracellular traps (NETs), which drive type I IFN production by plasmacytoid dendritic cells (pDCs) and directly contribute to endothelial cell dysfunction and vascular damage.7–10 Patients with SLE with increased circulating LDG numbers also demonstrate heightened prevalence of skin involvement, vasculitis, arterial inflammation and coronary plaque.3 11
In contrast, PMN-MDSC were described as immunoregulatory due to their ability to suppress T cell proliferation in infectious, autoimmune, cancer and metabolic diseases.12–16 The immunosuppressive mechanisms mediated by PMN-MDSC include surface expression of various checkpoint inhibitors (programmed death-ligand 1 [PD-L1], programmed death-ligand 2 [PD-L2] and CD73), along with release of enzymatic or chemical mediators (arginase-1 [Arg1] and nitric oxide synthase).17 In a lupus nephritis cohort, SLE PMN-MDSC mediated suppression in an Arg1-dependent manner, similar to what has been described in multiple cancer settings.14 The multifaceted roles of these low-density neutrophil subsets across a range of diverse indications highlight their importance in human disease.18 19
Despite contrasting functions, LDG and PMN-MDSC have been reported in SLE with strong association to SLE disease activity index (SLEDAI).3 14 Notably, both subsets utilise overlapping neutrophil surface markers for identification, including CD11b+, CD33+, CD15+ (or CD66b) and human leucocyte antigen-DR isotype (HLA-DR−), after excluding other lineage (LIN) markers (CD3, CD19, CD20 and CD56).19 20 While these subsets are thought of as immature neutrophils with reduced density, most identifying markers are also shared by the mature, terminally differentiated normal-density granulocytes (NDG) counterparts. In view of the shared similarities, a comprehensive and comparative immunophenotyping of activation and regulatory markers on the low-density and normal-density counterparts has yet to be performed in the context of linking these cells to SLE disease activity and understanding their relationship to the type I IFN axis and other components of this disease. Additionally, it remains unclear in SLE how the low-density and normal-density counterparts compare functionally in their ability to exert effects on T cells. As a result, there is ambiguity surrounding the identity, nature and role of these low-density subsets in the autoimmune space, particularly SLE.21 22
To address these knowledge gaps, we performed immunophenotypic, morphological and functional characterisation of this neutrophil subset and compared them with autologous NDG in a well-characterised SLE cohort consisting of both clinically inactive and active patients. We hypothesised that this larger cohort with representation across the disease spectrum would offer enhanced understanding of the functional and phenotypic features of these cells. Using this approach, we evaluated whether lupus LDG (i) associate with disease activity and, more specifically, the type I IFN pathway, (ii) manifest any of the PMN-MDSC regulatory effects via previously described mechanisms and (iii) differ from matching NDG on these various phenotypic and functional aspects. As most of the published literature in the SLE field refers to this abnormal neutrophil subset as “LDG,” we will henceforth use the same terminology.
Methods
Methods are provided in the online supplementary information.
annrheumdis-2018-214620supp001.docx (4.1MB, docx)
Results
Overview of SLE cohort
The demographic and clinical information for healthy donors (HD) and patients with SLE is summarised in table 1. There was no significant difference in either sex or median age. The median SLEDAI score was 2 with range of 0–13.
Table 1.
Demographics and clinical characteristics of HD and patients with lupus examined in this study
| HD* | SLE‡ | |
| Sex (M/F), n | M=7, F=73 | M=6, F=88 |
| Age, years (median, range) | 45 (35–65)† | 40 (15–73) |
| SLEDAI (median, range) | – | 2 (0–13) |
| C3, mg/dL (median, range) | – | 95.5 (44.8–184.9) |
| C4, mg/dL (median, range) | – | 16.7 (2.2–43.3) |
| ESR, mm/hour (median, range) | – | 23 (2–100) |
| CRP, mg/L (median, range) | – | 1.7 (0.15–31.4) |
| Auto-antibodies (% positive) | ||
| Anti-dsDNA | – | 70 |
| ANA | – | 95 |
| LAC | – | 28 |
| ENA | – | 85 |
| Medications (%)§ | ||
| Oral corticosteroids | – | 21 |
| Hydroxychloroquine | – | 83 |
| Azathioprine | – | 76 |
| Cyclophosphamide | – | 19 |
| Mycophenolate mofetil | – | 18 |
| Methotrexate | – | 24 |
| Biologics (belimumab/rituxan) | – | 5 |
*Human whole blood was collected from HD from the MedImmune Blood Donor programme. †Age of the HD was provided by decade. ‡SLE blood samples were collected from the National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health. Clinical parameters for patients with SLE were obtained at the time of visit by routine laboratory test. §Current medications that patients were taking.
ANA, anti-nuclear antibodies;C3, complement 3;C4, complement 4;CRP, C-reactive protein;dsDNA, double-stranded DNA; ENA, extractable nuclear antigens;ESR, erythrocyte sedimentation rate;F, female;HD, healthy donors;LAC, lupus anticoagulant;M, male;SLE, systemic lupus erythematosus;SLEDAI, SLE disease activity index.
Increased LDG in SLE associates with IFN gene signature and disease severity
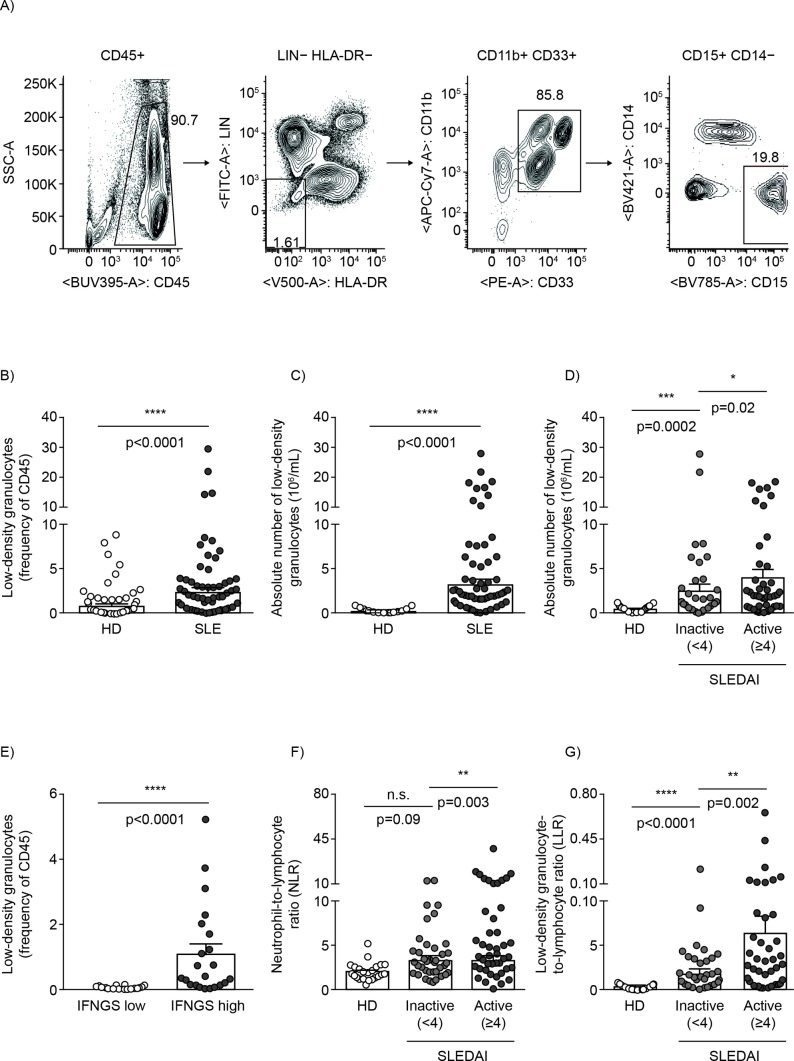
Previous studies demonstrated the increased presence of LDG in patients with SLE.1 3 14 We examined those findings by evaluating the prevalence of these cells in our cohort of HD and patients with SLE. Surface markers, previously described in both autoimmune and cancer studies, required for their identification were included.19 20 LDG, purified by density gradient centrifugation, were identified as LIN−(CD3/CD19/CD20/CD56), HLA-DR−, CD11b+, CD33+, CD15+ (figure 1A). LDG prevalence was significantly increased in patients with SLE by 2.9-fold (figure 1B; HD mean±SEM=0.81%±0.16; SLE mean±SEM=2.37%±0.45) and absolute count by 11.5-fold (figure 1C; HD mean±SEM=0.28±0.05; SLE mean±SEM=3.22±0.53). Additionally, the number of LDG demonstrated significant positive association with SLEDAI score (figure 1D). In line with the strong association with SLEDAI, LDG were observed to be 1.6-fold higher in active versus inactive patients (inactive SLE mean±SEM=2.48±0.71; active SLE mean±SEM=4.05±0.79), suggesting their close relationship with disease activity. To understand whether the observed close association with SLEDAI was due to specific clinical manifestations, LDG numbers were assessed for association with various clinical parameters and significantly associated with increased DNA binding (p=0.0165) and low complement levels (p=0.0187) (online supplementary table S1). The observed increase in LDG numbers in SLE versus HD and its association with SLEDAI validate previous observations and demonstrate efficacy of the cohort for the purposes of our study.
Figure 1.
Increased prevalence of LDG in SLE associates with disease severity and IFNGS. The frequency of circulating LDG was analysed and correlated with disease severity. (A) Flow cytometry gating strategy for identifying LDG in human PBMC. Prevalence of LDG in HD and patients with SLE (healthy n=80; SLE n=94) as (B) frequency of CD45 (HD mean±SEM=0.81±0.16; SLE mean±SEM=2.37±0.45; p<0.0001) and (C) absolute numbers (HD mean±SEM=0.28±0.05×106/mL; SLE mean±SEM=3.22±0.53×106/mL; p<0.0001). (D) Association between number of LDG with healthy as well as inactive and active patients with SLE (healthy n=80; inactive n=50; active n=44) based on SLEDAI (inactive patients mean±SEM=2.48±0.71×106/mL; active patients with SLE mean±SEM=4.05±0.79×106/mL; p=0.02]. (E) Association between the IFNGS (low n=20; high n=22) and prevalence of LDG in patients with SLE with low (mean±SEM=0.04±0.01) and high (mean±SEM=1.10±0.30; p<0.0001) IFNGS. The relationship between (F) neutrophil:lymphocyte ratio and (G) LDG:lymphocyte ratio with SLEDAI. Individual symbol represents one donor and the mean±SEM is shown. HD, healthy donors; HLA-DR, human leucocyte antigen-DR isotype; IFNGS, interferon 21-gene signature; LDG, low-density granulocytes; LIN, lineage; PBMC, peripheral blood mononuclear cells; SLE, systemic lupus erythematosus; SLEDAI, SLE disease activity index; SSC, side scatter.
The type I IFN pathway plays an important role in disease pathogenesis and exacerbation in SLE and other rheumatic diseases.23–25 LDG that undergo enhanced NET formation lead to externalisation of modified extracellular DNA.26 27 Material released from NETs stimulates pDCs to release IFN-α and myeloid cells to release type I IFNs.7 28 29 While LDG can lead to the production of type I IFNs in vitro, the in vivo association between LDG and type I IFN pathway remains unknown. To address this, we evaluated the relationship between LDG and type I IFN-regulated genes.30 The LDG frequency was significantly increased in patients with high versus low type I IFN gene signature (IFNGS; figure 1E; low mean±SEM=0.04%±0.0; high mean±SEM=1.10%±0.30; p<0.0001). This demonstrates a strong association between the presence of LDG with both clinical activity and activation of the type I IFN pathway.
While the IFNGS is a strong predictor of type I IFN–driven inflammation, the total neutrophil-to-lymphocyte ratio (NLR) is another commonly used indicator of inflammation that encompasses many pathways besides type I IFN.31 32 Given the significantly increased number of LDG in SLE, we compared NLR and evaluated utility of LDG-to-lymphocyte ratio (LLR) as a new and improved predictor of inflammation. In our cohort, while NLR demonstrated a significant difference between active and inactive patients with SLE (p=0.003), it did not significantly distinguish between inactive patients with SLE and HD (p=0.09) (figure 1F). In contrast, LLR offered better resolution and separation between the same inactive patients with SLE and HD (p<0.0001) (figure 1G). This result suggests that the LLR may be a more sensitive indicator of immune dysregulation than NLR. It would be interesting to further validate the utility of LLR over NLR in other disease areas.
SLE LDG exhibit an activated immunophenotype
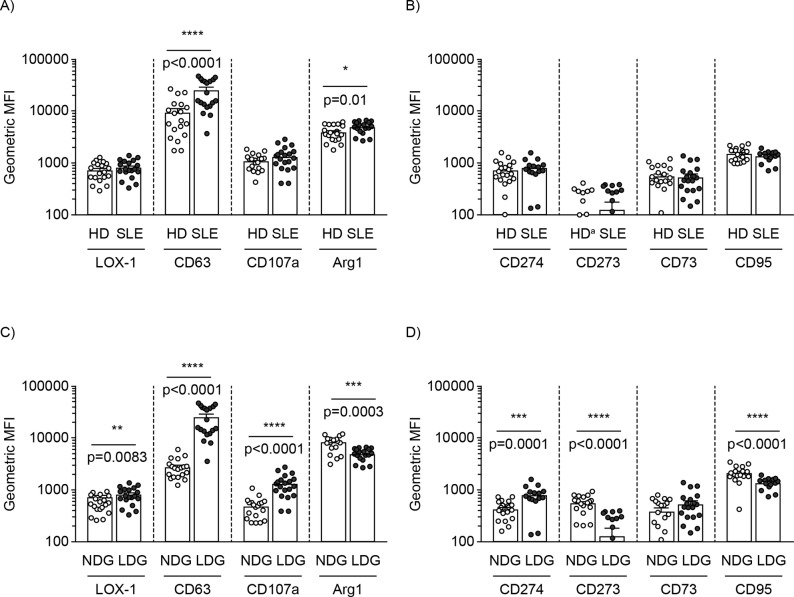
The increased prevalence of LDG across the disease spectrum validated the utility of our cohort for further analysis. Although LDG and PMN-MDSC share a similar immunophenotype, they demonstrate differences in the expression levels of various activation and regulatory markers based on their contrasting functions.19 Regulatory markers associated with the suppressive activity of PMN-MDSC include Arg1, CD73, CD274 (PD-L1) and CD273 (PD-L2),13 15 whereas degranulation markers associated with the proinflammatory role of neutrophils include CD63 (lysosome-associated membrane glycoprotein (LAMP)-3) and CD107a (LAMP-1).33 The expression of lectin-like oxidised low-density lipoprotein (oxLDL) receptor-1 (LOX-1) was demonstrated to be uniquely expressed by PMN-MDSC in cancer; however, its ability to distinguish MDSC from LDG in SLE remains unexplored.34 It is unknown how the expression levels of these markers compare between LDG and NDG. To address these questions, immunophenotyping was performed on both LDG and matching NDG from HD and patients with SLE. We first examined the immunophenotypic profile of LDG between HD and patients with SLE (figure 2A–B). As LDG share similar phenotypic markers with NDG, we then compared the expression profile of the two cell types in SLE (figure 2C–D) and HD (online supplementary figure S1).
Figure 2.
LDG exhibit activated phenotype in SLE. Both the NDG and LDG from HD and SLE were phenotyped for various activation and regulatory markers (healthy n=20; SLE n=20). Phenotyping of (A) activation and (B) regulatory markers on LDG in HD versus SLE. Phenotyping of (C) activation and (D) regulatory markers on NDG versus LDG from patients with SLE. Individual symbol represents one donor and the mean geometric MFI±SEM is shown. aBar representing geometric MFI value too low to be observed. Arg1, arginase-1; HD, healthy donors; LDG, low-density granulocytes; LOX-1, lectin-like oxidised low-density lipoprotein receptor-1; MFI, mean fluorescence intensity; NDG, normal-density granulocytes; SLE, systemic lupus erythematosus.
With the exception of intracellular Arg1 and CD63, no significant difference was observed for other markers between LDG from HD and SLE (figure 2A–B). However, when SLE LDG were compared with autologous NDG, significant differences were observed (figure 2C–D). Specifically, LDG expressed significantly higher (LOX-1, CD63, CD107a and CD274), reduced (intracellular Arg1, CD273 and CD95) or no difference (CD73) in expression levels of markers examined (figure 2C–D and HD in online supplementary figure S1). Apart from CD274, none of the other checkpoint inhibitors (CD273 and CD73) were significantly elevated on LDG versus NDG (figure 2D). Notably, the significantly higher expression of degranulation markers (CD63 and CD107a) and reduced intracellular Arg1 on SLE LDG versus NDG suggest their increased activation status (figure 2C). Of particular interest was the significantly higher surface expression of LOX-1 and CD63 on SLE LDG versus NDG.
These results confirm and expand the notion that SLE LDG and NDG differ immunophenotypically. Based on the higher surface expression of the degranulation markers, SLE LDG display an activated immunophenotype with concomitant expression of LOX-1.
SLE LDG express LOX-1 and demonstrate different morphology compared with NDG
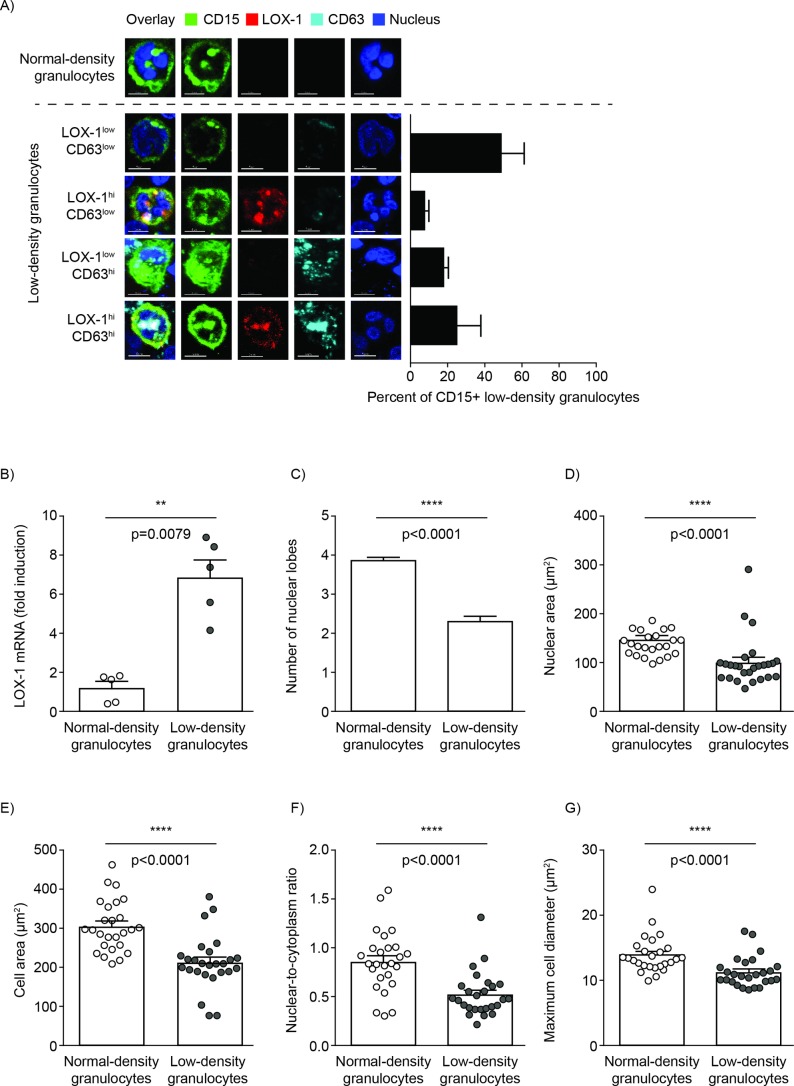
The increased expression of both LOX-1 and CD63 on LDG in SLE had not been previously described. While LOX-1 was recently suggested to be uniquely expressed by suppressive PMN-MDSC, CD63 is a granulocyte activation marker.33 34 LDG and autologous NDG from patients with SLE were analysed by confocal imaging to examine for differences in LOX-1/CD63 staining and morphology. While NDG, identified by characteristic multilobed nuclei, demonstrated dim LOX-1/CD63 staining, LDG, with more heterogeneous nuclear morphology including banded nuclei, were observed to encompass four subpopulations based on variable levels of LOX-1 and CD63 expression (figure 3A). The LOX-1 expression on LDG was validated at the messenger level (figure 3B). These data suggest that SLE LDG express LOX-1, and it was co-expressed with CD63 at variable levels.
Figure 3.
SLE LDG express LOX-1 and demonstrate different morphology compared with autologous NDG. (A) Representative confocal images of autologous SLE NDG and LDG and relative percentage of LDG subgroups. The LOX-1hi/CD63hi population was further characterised for morphology (data representative of five patients with SLE). (B) LOX-1 mRNA expression levels in SLE NDG and LDG (data representative of five patients with SLE). (C–G) Quantitative comparative assessment of various morphological parameters between SLE NDG and LOX-1hi CD63hi LDG population, (C) number of nuclear lobes, (D) nuclear area (NDG mean±SEM=160±7.4 µm2; LDG mean±SEM=101±3.4 µm2; p<0.0001), (E) cell area (NDG mean±SEM=315±14.6 µm2; LDG mean±SEM=245±9 µm2; p<0.0001), (F) nuclear-to-cytoplasm ratio (NDG mean±SEM=0.89±0.07; LDG mean±SEM=0.6±0.03; p<0.0001) and (G) cell diameter (NDG mean±SEM=14±0.72 µm; LDG mean±SEM=11.7±0.41 µm; p<0.0001). Data pooled from five patients with SLE with individual symbol representing one cell and the mean±SEM is shown. Scale bar per image is 10 µm. LDG, low-density granulocytes; LOX-1, lectin-like oxidised low-density lipoprotein receptor-1; NDG, normal-density granulocytes; SLE, systemic lupus erythematosus.
The LOX-1hi/CD63hi co-expressing population (figure 3A) was selected for further quantitative morphological assessment. Quantitatively, LDG had fewer nuclear lobes compared with autologous NDG (figure 3C; LDG mean±SEM=2.3±0.09; NDG mean±SEM=3.8±0.06). Compared with their NDG counterparts, LDG demonstrated significantly reduced nuclear area, cell area, cell diameter as well as lower nuclear-to-cytoplasm ratio (figure 3D–G). Thus, LDG represent distinct morphology compared with NDG based on the various nuclear and cellular parameters examined.
SLE NDG, but not LDG, restrict CD4+ T cell proliferation in arginase-dependent manner
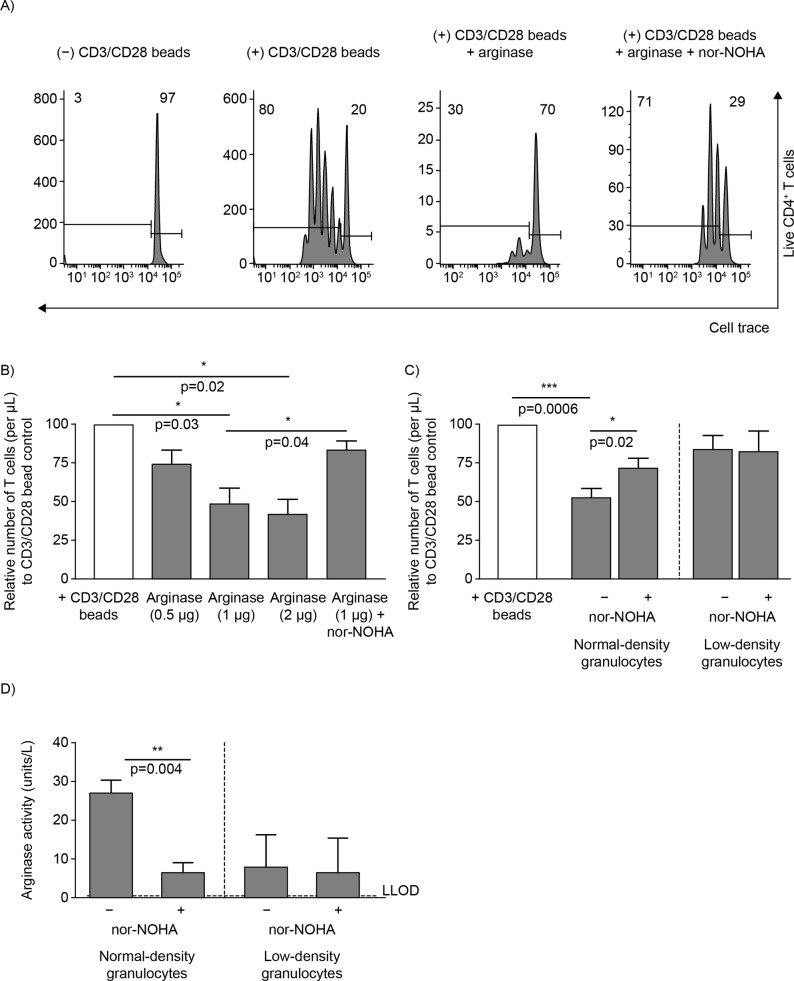
Because SLE LDG express LOX-1, which was recently suggested to be uniquely expressed by suppressive PMN-MDSC, we evaluated the cells for their suppressive capacity.34 LDG were assessed for their ability to suppress CD4+ T cell proliferation under both contact-independent and contact-dependent settings. For contact-independent suppression, the arginase mechanism, shown previously to be used by PMN-MDSC and activated neutrophils, was evaluated.14 35 NDG and LDG isolated from patients with SLE were treated overnight either in the absence or presence of arginase inhibitor nor-NOHA. The supernatant from such treated cells was then added to freshly isolated healthy naïve CD4+ T cells that were activated with anti-CD3/CD28 beads. As a positive control, the exogenously added arginase was observed to inhibit T cell proliferation in a dose-dependent manner and this was reversed by its inhibitor nor-NOHA (figure 4A–B). While SLE NDG significantly reduced the proliferative capacity of activated control CD4+ T cells compared with bead control (p=0.0006), an effect reversed by nor-NOHA (p=0.02), SLE LDG did not restrict T cell proliferation (figure 4C). Furthermore, neither NDG nor LDG isolated from HD demonstrated any suppressive effect on T cells (online supplementary figure S2B). Even in a contact-dependent setting, the SLE LDG did not inhibit T cell proliferation (online supplementary figure S2C). These results were not affected by osmotic stress due to red blood cell lysis treatment (online supplementary figure S3).
Figure 4.
SLE LDG do not restrict CD4+ T cell proliferation. Both NDG and LDG from patients with SLE were evaluated for their ability to restrict T cell proliferation in an arginase-dependent assay. The relative number of HD proliferating CD4+ T cells was calculated after 72 hours, when co-cultured with supernatant from either SLE NDG or LDG that was cultured overnight in the absence or presence of arginase inhibitor, nor-NOHA. (A) Representative plots and (B) relative number of T cells (normalised to CD3/CD28 bead controls) for different control conditions from three independent experiments. (C) Relative number of T cells (normalised to CD3/CD28 bead controls) when cultured with either NDG or LDG supernatant that was cultured overnight in the absence or presence of nor-NOHA (pooled data from six patients with SLE in three independent experiments). (D) Quantification of bioactive arginase present in the supernatant of the NDG and LDG test conditions (pooled data from six patients with SLE and the mean±SEM is shown). One unit of arginase is the amount of enzyme that will convert 1.0 µmole of L-arginine to ornithine and urea per minute at pH 9.5 and 37°C. HD, healthy donors; LDG, low-density granulocytes; NDG, normal-density granulocytes; NOHA, Nω-hydroxy-nor-arginine; SLE, systemic lupus erythematosus.
In line with the increased suppression capacity of lupus NDG in suppressing T cell proliferation by a contact-independent mechanism, these cells were found to release 3.3-fold higher bioactive arginase than LDG (figure 4D; NDG mean±SEM=27.27±3.2 enzyme units; LDG mean±SEM=8.17±3.3 enzyme units; p=0.008). The SLE NDG arginase synthesis was dampened 3.9-fold after nor-NOHA treatment (NDG − nor-NOHA mean±SEM=27.27±3.2 enzyme units; NDG + nor-NOHA mean±SEM=6.87±2.3 enzyme units). LDG did not show significant difference in amount of arginase synthesised in the absence or presence of nor-NOHA. This also corroborates our observation that SLE NDG have 1.8-fold higher intracellular Arg1 geometric mean fluorescence intensity than LDG (figure 2C; NDG mean±SEM=4996±269; LDG mean±SEM=2817±285). Additionally, no significant difference in levels of released bioactive arginase was observed between HD NDG and autologous LDG (online supplementary figure S2D). These results suggest that SLE LDG do not inhibit T cell proliferation under both contact-independent and contact-dependent settings. Additionally, the SLE NDG-driven suppression of T cell proliferation in contact-independent setting is primarily mediated by intracellular arginase that is spontaneously released by the cells in its bioactive form.
SLE LDG induce proinflammatory T cell cytokine profile
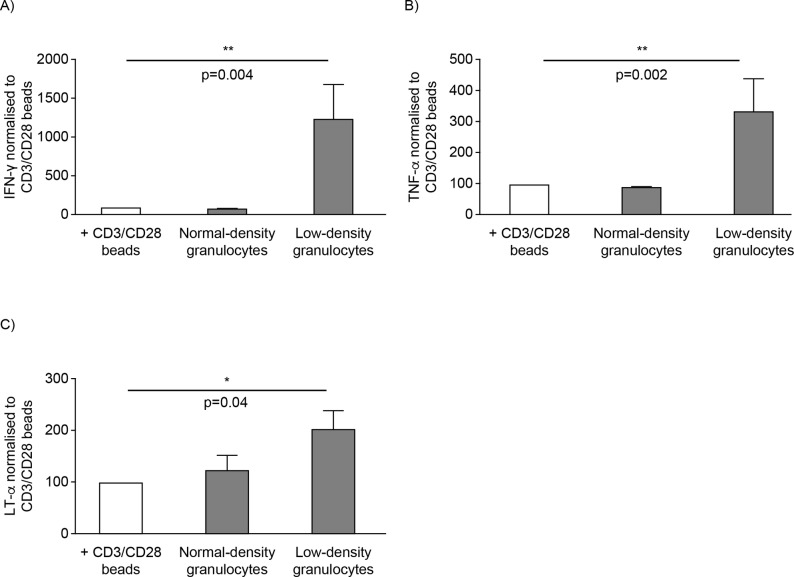
Both LDG and PMN-MDSC have been demonstrated to drive their respective functions and downstream effects on other cells via the production of key cytokines including IFN-γ and interleukin 10.3 17 Given the significant differences between NDG and LDG in their immunophenotypic profile and abilities to suppress T cell proliferation, we examined the effect of these cell types on T cell cytokine production. The supernatant from cell cultures was analysed for different cytokines by Meso Scale Discovery multiplex assay. Only SLE LDG were able to induce significantly higher production of Th1 proinflammatory cytokines IFN-γ, TNF-α and lymphotoxin alpha than bead controls (figure 5). These cytokines were not detected in supernatants from LDG or NDG (data not shown). Other cytokines and chemokines examined were either not significantly different or not detected (online supplementary table S2). Overall, functional and phenotypic analyses of these cells support that lupus LDG represent a proinflammatory subset that can activate adaptive immune responses.
Figure 5.
SLE LDG induce proinflammatory T cell cytokine profile. Normalised quantification of (A) IFN-γ, (B) TNF-α and (C) LT-α from the proliferating T cell cultures that were cultured with supernatants derived from either NDG or LDG of patients with SLE (pooled data from six patients with SLE and the mean±SEM is shown). IFN-γ, interferon gamma; LDG, low-density granulocytes; LT-α, lymphotoxin alpha; NDG, normal-density granulocytes; SLE, systemic lupus erythematosus; TNF-α, tumour necrosis factor alpha.
Discussion
The field of neutrophil biology has evolved significantly over the last decade, and some breakthroughs have focused around the role of low-density neutrophil subsets in autoimmunity, infectious diseases and cancer.15 36 LDG and PMN-MDSC exert contrasting functional effects on T cells, with LDG found to be stimulatory and PMN-MDSC suppressive. In the absence of distinguishing markers, functional assays are further needed in order to characterise these subsets as proinflammatory or suppressive. Parallel analysis of low-density and normal-density neutrophils lends important context for interpreting immunophenotypic and functional studies. While previous studies in SLE have examined these low-density neutrophil subsets, conflicting reports exist regarding their function and role in disease.3 14 21 22 Although the immunophenotypic identification strategy utilised by Wu et al is similar to the approach used in our study (CD11b+CD33+HLA-DR−), cells were designated as PMN-MDSC by these investigators because of their observed suppressive functions.14 In our hands, these cells exhibited proinflammatory functions. The cohort studied by Wu et al exhibited high disease activity, and the majority of patients had lupus nephritis, which could underlie the observed differences in function. Furthermore, in our hands, the cell sorting technique used by Wu et al may significantly modify the functional characteristics of granulocytes and myeloid cells (unpublished observations).
In multiple cancers, LOX-1 was demonstrated to be expressed at high levels on suppressive PMN-MDSC as compared with their normal-density counterparts and was thus suggested as a marker for PMN-MDSC.34 Here, we demonstrate for the first time the heightened surface expression of LOX-1 on proinflammatory LDG in an autoimmune disease. LOX-1 is a class E scavenger receptor for oxLDL, and in inflammatory diseases such as SLE, elevated oxLDL can induce granulocytic activation and degranulation.37–44 In cancer, LOX-1 has been associated with suppressive activity but is not required for regulatory function.34 LOX-1 expression can be induced by endoplasmic reticulum stress, a common feature of both cancer and autoimmunity.34 45 For these reasons, in autoimmune diseases, LOX-1 should not be used to assess whether a neutrophil is immunosuppressive or proinflammatory.
Indeed, while SLE LDG express LOX-1, they did not display any significant ability to suppress T cells in either contact-independent or contact-dependent assays. In contrast, here we demonstrated suppression mediated by transferred supernatants from overnight cultures of only lupus NDG. We observed that spontaneously released bioactive Arg1 from SLE NDG was 5-fold higher than HD NDG. Metabolism of extracellular arginine via Arg1 liberated from PMN-MDSC or neutrophil azurophil granules is a key mechanism by which these cells are thought to exert their suppressive effect on T cells.46–48 Consistent with the possible involvement of this mechanism in disease, elevated levels of Arg1 have been reported in the serum of patients with autoimmunity, cancer and infectious diseases.49–51 The enhanced suppressive ability of SLE NDG may also be due to presence of activated neutrophils in such patients that have increased neutrophil siderophore lipocalin-2 (LCN2/NGAL), which can effectively bind and remove iron.52 Sequestration of iron, a key T cell nutrient, from the microenvironment negatively impacts T cell proliferation.53 The ability to release significantly more bioactive arginase coupled with heightened presence of LCN2 allows NDG from patients with SLE to be better suppressors of T cell proliferation than HD NDG. Our data suggest that in SLE, the Arg1-dependent suppression is primarily mediated by NDG and not LDG. While NDG supernatant did not affect CD4 T cell cytokine production, LDG supernatant promoted proinflammatory Th1 cytokine response, further validating their role as drivers of inflammation. Such Th1 cytokine-producing cells have been detected in abundance in the kidneys of patients with lupus nephritis and also correlated with histological disease activity.54–56
In SLE, LDG have been shown to be highly susceptible to form NETs, a process that can lead to immune dysregulation and activation of pDC and myeloid type I IFN axis and induction of IFNGS.7 30 We demonstrated for the first time direct positive correlation between LDG and IFNGS, suggesting a close link between the presence of these cells and activation of type I IFN pathway. As such, future studies should address whether similar correlations are observed in other inflammatory diseases where the type I IFN pathway is considered to play important pathogenic roles. Further understanding the crosstalk between aberrant neutrophil subsets and the type I IFN pathway in SLE may provide the identification of additional therapeutic targets in this disease.
In conclusion, we demonstrate that SLE LDG represent a pathogenic subset associated with type I IFN activation and with the development of non-suppressive T cell activation in this disease. The observed proinflammatory role of LDG validates their putative pathogenic role in SLE.
Acknowledgments
The data were presented at the Neutrophil 2018 conference (http://theneutrophil.com). This study was supported in part by the intramural research program at NIAMS/NIH AR041199 and by MedImmune, a member of the AstraZeneca Group. Currently, DS is an employee of Eli Lilly and Company (Indianapolis, IN, USA), YLL is an employee of University of Texas MD Anderson Cancer Center (Houston, TX, USA), RMS is an employee of Novartis (Basel, Switzerland), MAS is an employee of Bristol-Myers Squibb Company (New York City, NY, USA) and KAC is an employee of the Allen Institute for Immunology (Seattle, WA, USA; kerry.casey@alleninstitute.org). Editorial support was provided by JK Associates Inc., a member of the Fishawack Group of Companies. This support was funded by MedImmune.
Footnotes
Handling editor: Josef S Smolen
Contributors: SR, RMS, MAS, RK, MJK and KAC conceived and designed the study. SR, DS, RNH, YLL, PM, CKS, ZM and SH performed the experiments and collected the data. SR, DS, RNH, YLL, PM, CKS and SH analysed and interpreted the data. All authors were involved in development, review and approval of the manuscript.
Competing interests: SR, RNH and RK are employees at MedImmune. DS, MAS and KAC were employees at MedImmune during the time work was performed on this study. Other authors have nothing to declare.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum 1986;29:1334–42. 10.1002/art.1780291105 [DOI] [PubMed] [Google Scholar]
- 2. Bennett L, Palucka AK, Arce E, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med 2003;197:711–23. 10.1084/jem.20021553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Denny MF, Yalavarthi S, Zhao W, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol 2010;184:3284–97. 10.4049/jimmunol.0902199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sagiv JY, Michaeli J, Assi S, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 2015;10:562–73. 10.1016/j.celrep.2014.12.039 [DOI] [PubMed] [Google Scholar]
- 5. Cloke T, Munder M, Taylor G, et al. Characterization of a novel population of low-density granulocytes associated with disease severity in HIV-1 infection. PLoS One 2012;7:e48939 10.1371/journal.pone.0048939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rocha BC, Marques PE, Leoratti FMdeS, et al. Type I interferon transcriptional signature in neutrophils and low-density granulocytes are associated with tissue damage in malaria. Cell Rep 2015;13:2829–41. 10.1016/j.celrep.2015.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villanueva E, Yalavarthi S, Berthier CC, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011;187:538–52. 10.4049/jimmunol.1100450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carmona-Rivera C, Zhao W, Yalavarthi S, et al. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 2015;74:1417–24. 10.1136/annrheumdis-2013-204837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol 2016;12:402–13. 10.1038/nrneph.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barrera-Vargas A, Gómez-Martín D, Carmona-Rivera C, et al. Differential ubiquitination in nets regulates macrophage responses in systemic lupus erythematosus. Ann Rheum Dis 2018;77:944–50. 10.1136/annrheumdis-2017-212617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carlucci PM, Purmalek MM, Dey AK, et al. Neutrophil subsets and their gene signature associate with vascular inflammation and coronary atherosclerosis in lupus. JCI Insight 2018;3 10.1172/jci.insight.99276. [Epub ahead of print: 19 Apr 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bowers NL, Helton ES, Huijbregts RPH, et al. Immune suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway. PLoS Pathog 2014;10:e1003993 10.1371/journal.ppat.1003993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao LY, Chung J-S, Teshima T, et al. Myeloid-derived suppressor cells in psoriasis are an expanded population exhibiting diverse T-Cell-Suppressor mechanisms. J Invest Dermatol 2016;136:1801–10. 10.1016/j.jid.2016.02.816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu H, Zhen Y, Ma Z, et al. Arginase-1–dependent promotion of T H 17 differentiation and disease progression by MDSCs in systemic lupus erythematosus. Sci Transl Med 2016;8 10.1126/scitranslmed.aae0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol 2018;19:108–19. 10.1038/s41590-017-0022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ostrand-Rosenberg S. Myeloid derived-suppressor cells: their role in cancer and obesity. Curr Opin Immunol 2018;51:68–75. 10.1016/j.coi.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solito S, Marigo I, Pinton L, et al. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 2014;1319:47–65. 10.1111/nyas.12469 [DOI] [PubMed] [Google Scholar]
- 18. Scapini P, Marini O, Tecchio C, et al. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev 2016;273:48–60. 10.1111/imr.12448 [DOI] [PubMed] [Google Scholar]
- 19. Jablonska J, Granot Z. Neutrophil, quo vadis? J Leukoc Biol 2017;102:685–8. 10.1189/jlb.3MR0117-015R [DOI] [PubMed] [Google Scholar]
- 20. Bronte V, Brandau S, Chen S-H, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016;7 10.1038/ncomms12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cripps JG, Gorham JD. MDSC in autoimmunity. Int Immunopharmacol 2011;11:789–93. 10.1016/j.intimp.2011.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crook KR, Liu P. Role of myeloid-derived suppressor cells in autoimmune disease. World J Immunol 2014;4:26–33. 10.5411/wji.v4.i1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hooks JJ, Moutsopoulos HM, Geis SA, et al. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med 1979;301:5–8. 10.1056/NEJM197907053010102 [DOI] [PubMed] [Google Scholar]
- 24. Ytterberg SR, Schnitzer TJ. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum 1982;25:401–6. 10.1002/art.1780250407 [DOI] [PubMed] [Google Scholar]
- 25. Higgs BW, Liu Z, White B, et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann Rheum Dis 2011;70:2029–36. 10.1136/ard.2011.150326 [DOI] [PubMed] [Google Scholar]
- 26. Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol 2012;189:2689–95. 10.4049/jimmunol.1201719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lood C, Blanco LP, Purmalek MM, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 2016;22:146–53. 10.1038/nm.4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garcia-Romo GS, Caielli S, Vega B, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011;3 10.1126/scitranslmed.3001201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lande R, Ganguly D, Facchinetti V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011;3 10.1126/scitranslmed.3001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yao Y, Higgs BW, Morehouse C, et al. Development of potential pharmacodynamic and diagnostic markers for Anti-IFN-α monoclonal antibody trials in systemic lupus erythematosus. Hum Genomics Proteomics 2009;1 10.4061/2009/374312. [Epub ahead of print: 17 Nov 2009]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li L, Xia Y, Chen C, et al. Neutrophil-lymphocyte ratio in systemic lupus erythematosus disease: a retrospective study. Int J Clin Exp Med 2015;8:11026–31. [PMC free article] [PubMed] [Google Scholar]
- 32. Forget P, Khalifa C, Defour J-P, et al. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes 2017;10 10.1186/s13104-016-2335-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuijpers TW, Tool AT, van der Schoot CE, et al. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood 1991;78:1105–11. [PubMed] [Google Scholar]
- 34. Condamine T, Dominguez GA, Youn J-I, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol 2016;1:aaf8943–aaf43. 10.1126/sciimmunol.aaf8943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hock BD, Taylor KG, Cross NB, et al. Effect of activated human polymorphonuclear leucocytes on T lymphocyte proliferation and viability. Immunology 2012;137:249–58. 10.1111/imm.12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kaplan MJ. Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol 2011;7:691–9. 10.1038/nrrheum.2011.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997;386:73–7. 10.1038/386073a0 [DOI] [PubMed] [Google Scholar]
- 38. Taye A, El-Sheikh AAK. Lectin-like oxidized low-density lipoprotein receptor 1 pathways. Eur J Clin Invest 2013;43:740–5. 10.1111/eci.12092 [DOI] [PubMed] [Google Scholar]
- 39. Al-Banna N, Lehmann C. Oxidized LDL and LOX-1 in experimental sepsis. Mediators Inflamm 2013;2013:1–6. 10.1155/2013/761789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Obermayer G, Afonyushkin T, Binder CJ. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J Thromb Haemost 2018;16:418–28. 10.1111/jth.13925 [DOI] [PubMed] [Google Scholar]
- 41. Hayem G, Nicaise-Roland P, Palazzo E, et al. Anti-oxidized low-density-lipoprotein (oxLDL) antibodies in systemic lupus erythematosus with and without antiphospholipid syndrome. Lupus 2001;10:346–51. 10.1191/096120301667475689 [DOI] [PubMed] [Google Scholar]
- 42. Lopez LR, Salazar-Paramo M, Palafox-Sanchez C, et al. Oxidized low-density lipoprotein and beta2-glycoprotein I in patients with systemic lupus erythematosus and increased carotid intima-media thickness: implications in autoimmune-mediated atherosclerosis. Lupus 2006;15:80–6. 10.1191/0961203306lu2267oa [DOI] [PubMed] [Google Scholar]
- 43. Maeba R, Maruyama A, Tarutani O, et al. Oxidized low-density lipoprotein induces the production of superoxide by neutrophils. FEBS Lett 1995;377:309–12. 10.1016/0014-5793(95)01336-9 [DOI] [PubMed] [Google Scholar]
- 44. Sedgwick JB, Hwang YS, Gerbyshak HA, et al. Oxidized low-density lipoprotein activates migration and degranulation of human granulocytes. Am J Respir Cell Mol Biol 2003;29:702–9. 10.1165/rcmb.2002-0257OC [DOI] [PubMed] [Google Scholar]
- 45. Morito D, Nagata K. ER stress proteins in autoimmune and inflammatory diseases. Front Immunol 2012;3 10.3389/fimmu.2012.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Munder M, Mollinedo F, Calafat J, et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood 2005;105:2549–56. 10.1182/blood-2004-07-2521 [DOI] [PubMed] [Google Scholar]
- 47. Munder M, Schneider H, Luckner C, et al. Suppression of T-cell functions by human granulocyte arginase. Blood 2006;108:1627–34. 10.1182/blood-2006-11-010389 [DOI] [PubMed] [Google Scholar]
- 48. Pillay J, Tak T, Kamp VM, et al. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci 2013;70:3813–27. 10.1007/s00018-013-1286-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang LW, Chang KL, Chen CJ, et al. Arginase levels are increased in patients with rheumatoid arthritis. Kaohsiung J Med Sci 2001;17:358–63. [PubMed] [Google Scholar]
- 50. Polat MF, Taysi S, Polat S, et al. Elevated serum arginase activity levels in patients with breast cancer. Surg Today 2003;33:655–61. 10.1007/s00595-002-2563-2 [DOI] [PubMed] [Google Scholar]
- 51. Cloke TE, Garvey L, Choi B-S, et al. Increased level of arginase activity correlates with disease severity in HIV-seropositive patients. J Infect Dis 2010;202:374–85. 10.1086/653736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li YN, Hu FL, Dai YJ, et al. Serum anti-lipocalin 2 IgG is a novel biomarker in the diagnosis of systemic lupus erythematosus. Lupus 2014;23:868–75. 10.1177/0961203314530484 [DOI] [PubMed] [Google Scholar]
- 53. Le NTV, Richardson DR. Iron chelators with high antiproliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: a link between iron metabolism and proliferation. Blood 2004;104:2967–75. 10.1182/blood-2004-05-1866 [DOI] [PubMed] [Google Scholar]
- 54. Uhm W-S, Na K, Song G-W, et al. Cytokine balance in kidney tissue from lupus nephritis patients. Rheumatology 2003;42:935–8. 10.1093/rheumatology/keg255 [DOI] [PubMed] [Google Scholar]
- 55. Masutani K, Akahoshi M, Tsuruya K, et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum 2001;44:2097–106. [DOI] [PubMed] [Google Scholar]
- 56. Kikawada E, Lenda DM, Kelley VR. IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol 2003;170:3915–25. 10.4049/jimmunol.170.7.3915 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
annrheumdis-2018-214620supp001.docx (4.1MB, docx)