Abstract
Objectives
Staining with toluidine blue is a well-established procedure for the histological assessment of cartilaginous- and chondrogenic-differentiated tissues. Being a cationic dye, toluidine blue staining visualizes proteoglycans in a tissue because of its high affinity for the sulfate groups in proteoglycans. It is generally accepted that metachromatic staining with toluidine blue represents cartilaginous matrix and that the degree of positive staining corresponds with the amount of proteoglycans.
Design
Articular cartilage and pellets of chondrocytes or bone marrow stromal cells were analyzed with a standardized staining procedure for toluidine blue.
Results
In the present study, we illustrate why such an assumption is invalid unless a detailed description of the procedure and/or reference to a detailed published method are provided. This is because the staining specificity and intensity depend, as we have shown, on the pH of the staining solution, the use of dehydration, and on staining time.
Conclusions
We can, therefore, suggest a well-controlled standardized protocol for toluidine blue staining, which provides an easy and simple selective staining technique for the assessment of cartilage tissue and proteoglycan development in chondrogenic differentiation. If this procedure is not used, then investigators must provide sufficient technical information concerning the staining protocol to allow an assessment of the validity of the staining results.
Keywords: cartilage tissue, toluidine blue, metachromasia
Introduction
Today there is unanimity on the use of toluidine blue staining as a standard procedure for the histological assessment of chondrogenic differentiated cells and cartilaginous tissue maturity and quality. Toluidine blue is a cationic dye that visualizes the presence of proteoglycans in a tissue because of its high affinity for the sulfate groups of proteoglycans.1 For example, if a preparation of hyaline articular cartilage is staining with toluidine blue, the coloring would be blue cell nuclei, light blue cytoplasm, and pink or purple extracellular matrix.2 Metachromasia is a phenomenon in which anionic polymerized intermolecular bonds of high charge density facilitate cationic dye-dye interactions, which result in large spectral shifts.3-5 The degree of metachromatic color depends on the anionic radicals increasing in the order carboxylate, phosphate, and sulfate and when the intermolecular bond distance is brought closer.4,5 Conversely, orthochromatic is the opposite phenomenon where no relative color change is observed when bound to tissue substances.5 We and other investigators have often stated that chondrogenesis, or the presence of cartilaginous matrix, may be demonstrated by the occurrence of metachromatic staining with toluidine blue. However, we have also emphasized that such a statement is only valid if the detailed procedure, or a reference to the relevant technical protocol, is also provided. This is because staining selectivity and intensity might depend on, for example, dye concentration, pH of the staining solution, staining time, and use of dehydration. In the present study, to justify this critical stance, we demonstrate that pH of the staining solution, the use of dehydration, and time influence the specificity and intensity of toluidine blue and the metachromatic staining of chondrogenesis in vitro and cartilaginous matrix in vivo.
Methods
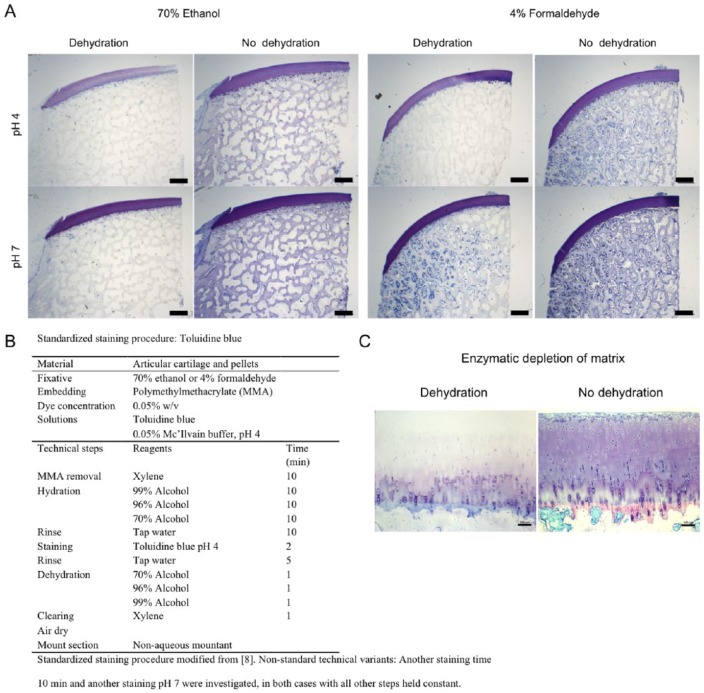
Biopsies of articular cartilage were taken from the knee of Göttingen minipigs (GM) and fixed in 70% ethanol or 4% formaldehyde.6 The GM biopsies were collected in compliance with the Danish Law on Animal Experimentation and approved by the Danish Ministry of Justice Ethical Committee (J.nr. 2012-15-2934-00301). Pellets were established by culturing human articular chondrocytes or bone marrow stromal cells (BMSCs) for 28 days in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 nM dexamethasone, 50 µg/mL L-ascorbic acid 2-phosphate, 40 µg/mL L-proline, 1 mM sodium pyruvate, 1% ITS+, and 10 ng/mL TGFβ3. Chondrocytes were isolated as previously described.7 BMSCs were isolated from bone marrow aspirate using Ficoll-Hypaque centrifugation and cultured on plastic for 14 days in DMEM with 10% FCS prior to pellet culture. Bone marrow aspirate was harvested after written consent was obtained and protocol approved by the local ethics committee under the Danish National Committee on Health Research Ethics (#M-2082134). The samples were dehydrated through the graded ethanol (70% to 96% v/v), cleared in 2-isopropanol, and xylene prior to infiltration with methyl methacrylate at 4°C. Embedding in polymethylmethacrylate (MMA) was performed with N,N-dimethyl-p-tulidine as an activator at −20°C. Sections of 7 µm were cut using a microtome (Richtert Jung polycot). Sections were stained with the standardized staining procedure for toluidine blue according to Figure 1B . Toluidine blue and tissue morphology were analyzed using a Zeiss AX10 microscope equipped with a digital color camera (Axiocam 506 color Zeiss). For enzymatic depletion of matrix, protease (Sigma-Aldrich, P6911) was used for 30 minutes at room temperature prior to the standardized staining procedure for toluidine blue.
Figure. 1.
(A) Representative images showing toluidine blue staining of the joint tissue. Panels compare effects of fixation, dehydration, and pH. Sections were stained for 2 minutes. Scale bars 1 mm. (B) Standardized staining procedure modified from Kramer and Windrum.8 Nonstandard technical variants: Another staining time 10 minutes and another staining pH 7 were investigated, in both cases with all other steps held constant. (C) Representative images showing toluidine blue staining of enzymatic depleted cartilage tissue. The panel compares effect of dehydration with no dehydration. Fixation was 70% ethanol and sections were stained at pH 4 for 2 minutes. Scale bars 100 µm.
Results
Histochemical evaluation compares the effects of dehydration, fixation, and pH on the staining of toluidine blue in knee joint tissue. In general, metachromatic color is more dominant at pH 4 compared with pH 7. However, the metachromatic staining is hidden under the orthochromatic blue color at pH 7. The influence of dehydration is visualized with metachromasia seen being increased after dehydration. In sections stained at pH 4 and then dehydrated, the articular cartilage stains strongly pink (metachromasia). There was no obvious difference in staining efficacy when using different fixatives ( Fig. 1A ). Enzymatic depletion of articular cartilage matrix ( Fig. 1C ) visualizes the differences in staining intensity of toluidine blue at pH 4. The matrix structure is depleted and there is no basis for metachromatic staining as indicated with dehydration.
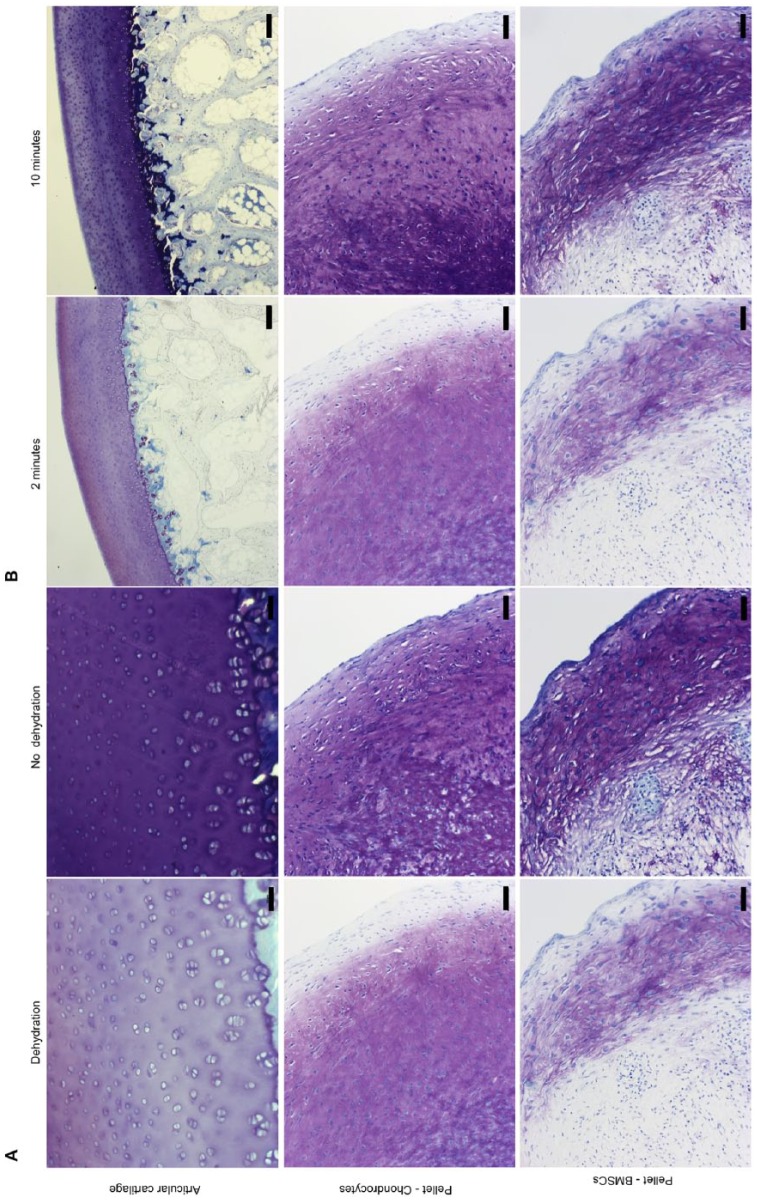
Figure 2A shows the influence of dehydration on toluidine blue staining of cartilaginous matrix in tissue sections and in chondrogenically differentiated cells in pellet culture. Again, alcoholic dehydration selectively removed orthochromatic (blue) stain, thus enhancing the visibility of metachromatically stained substrates. The alcohol-labile orthochromatic color was removed and alcohol-stable metachromatic color enhanced. Especially, the periphery of the pellet visualizes the influence of dehydration. Additionally, reducing the staining time also made the metachromatic staining of cartilaginous and differentiated tissue more obvious, as shown in Figure 2B .
Figure. 2.
Representative images showing toluidine blue staining of sections of cartilage and of pelleted cells. (A) Panels compare effects of dehydration with no dehydration. Fixation was with 70% ethanol and sections were stained at pH 4 for 2 minutes. Scale bars 50 µm. (B) Panels compare 2 staining times. Fixation was in 70% ethanol, and sections were stained at pH 4 for 2 and 10 minutes. Scale bars: 200 µm for articular cartilage and 50 µm for pellets.
Histologic evaluations using toluidine blue of the tissue and cell morphology is often used as a surrogate marker for differentiation and development. In Figure 2B , toluidine blue staining of a pellet culture of chondrocytes and BMSCs demonstrates the importance of also assessing the tissue morphology because only few cells would be categorized as chondrocytes. We observed no architectural tissue morphology identical to articular cartilage but the cells localized in the pellets were nevertheless able to synthesis and secrete proteoglycans since metachromasia were observed.
Discussion
In this short communication, we emphasize the importance of reporting the detailed procedure of the toluidine blue staining used for evaluating the presence of proteoglycans in sections of cartilaginous tissue and pellet culture of chondrocytes or BMSCs. Technical information, or references to the specific histochemical staining procedure used, is rarely provided in published work. Because of its widespread application for visualization of proteoglycans, we, therefore, found it useful to highlight technically important steps involved when using toluidine blue in order to increase the validity of the result obtained. Under the right circumstances, toluidine blue is a highly selective dye for detecting proteoglycans since the cationic dye requires a flexible anionic polymerized macromolecular structure with a high charge density in order to stain metachromatically. We have shown the importance of documenting the pH when evaluating metachromasia and found that in cases where metachromasia is overshadowed by the monomeric orthochromatic (blue color) acidic pH adjustments can reduce the orthochromatic effect and enhance the metachromatic color. This supports Singers early work concluding that: “the availability of binding sites directs function of the solution environment, notably pH.”5 By lowering the pH toluidine blue decreased the affinity for proteins and reducing backing staining.
Two fixatives were compared: formaldehyde and ethanol. There was no difference to see in the staining outcome, and both showed the same responses adjusting the pH. Metachromasia is obtained when the staining survives alcohol dehydration before mounting indicating irreversible staining.8 Sylvén and Kramer both argued that dehydrating the sections of interest in alcohol after staining was the only way to ensure the “true” metachromasia.4,8 Whereas Pearse distinguished between 2 staining techniques, the alcohol stable and labile metachromasia, but advocated wet section analysis.9 We have demonstrated the importance of dehydration and staining time through the removal of alcohol-labile staining and saturated staining. Especially, Figure 1C shows how interpretations based on staining intensity can be misleading without the presence of dehydration. There is no single standard procedure; we used graded alcohol and stained for 2 minutes, others have used acetone and different staining times.10-15 In this study, we demonstrate that 0.05% w/v toluidine blue at pH 4 for 2 minutes following dehydration differentiates glycosaminoglycans and proteoglycans by metachromasia staining of cartilage tissue and that proteoglycan development can be seen in morphologically undifferentiated tissue by use of well-controlled toluidine blue staining procedure. There is no doubt that if the staining is used under the right circumstances, not strongly orthochromatic, then this is an easy and simple differential staining technique for the assessment of chondrogenic differentiation. Therefore, we believe it is important to inform the detailed staining procedure in order to justify the result obtained.
Footnotes
Acknowledgments and Funding: We gratefully thank Bjørn Christensen and Morten Lykke Olesen for the articular cartilage biopsies (GM) and Kris Hede for the pellets. We wish to thank Anette Baatrup for laboratory assistance and the Danish Rheumatism Association for funding the PhD of Natasja Leth Joergensen.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval for this study was obtained from Danish Ministry of Justice Ethical Committee (J.nr. 2012-15-2934-00301 and the Danish National Committee on Health Research Ethics (#M-2082134).
Animal Welfare: The GM biopsies were collected in compliance with the Danish Law on Animal Experimentation.
References
- 1. Schmitz N, Laverty S, Kraus VB, Aigner T. Basic methods in histopathology of joint tissues. Osteoarthritis Cartilage. 2010;18(Suppl 3):S113-S116. doi: 10.1016/j.joca.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 2. Martin IC, Kerawala CJ, Reed M. The application of toluidine blue as a diagnostic adjunct in the detection of epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(4):444-6. doi: 10.1016/S1079-2104(98)90071-3. [DOI] [PubMed] [Google Scholar]
- 3. Sridharan G, Shankar AA. Toluidine blue: a review of its chemistry and clinical utility. J Oral Maxillofac Pathol. 2012;16(2):251-5. doi: 10.4103/0973-029X.99081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sylvén B. Metachromatic dye-substrate interactions. J Cell Sci. 1954;95:327-58. [Google Scholar]
- 5. Bergeron JA, Singer M. Metachromasy: an experimental and theoretical reevaluation. J Biophys Biochem Cytol. 1958;4(4):433-57. doi: 10.1083/jcb.4.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen BB, Foldager CB, Olesen ML, Vingtoft L, Rölfing JHD, Ringgard S, et al. Experimental articular cartilage repair in the Göttingen minipig: the influence of multiple defects per knee. J Exp Orthop. 2015;2(1):13. doi: 10.1186/s40634-015-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Joergensen NL, Le DQS, Andersen OZ, Foss M, Danielsen CC, Foldager CB, et al. Topography-guided proliferation: distinct surface microtopography increases proliferation of chondrocytes in vitro. Tissue Eng Part A. 2015;21(21-22):2757-65. doi: 10.1089/ten.tea.2014.0697. [DOI] [PubMed] [Google Scholar]
- 8. Kramer H, Windrum GM. The metachromatic staining reaction. J Histochem Cytochem. 1955;3(3):227-37. doi: 10.1177/3.3.227. [DOI] [PubMed] [Google Scholar]
- 9. Pearse AGE. A review of modern methods in histochemistry. J Clin Pathol. 1951;4(1):1-36. doi: 10.1136/jcp.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4(4):415-28. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 11. Sheehan DC, Hrapchak BB. Theory and practice of histotechnology. Maryland Heights, MO: Mosby; 1973. [Google Scholar]
- 12. Bodian MM, Lake BD. The recital approach to neuropathology. Br J Surg. 1963;50:702-14. [DOI] [PubMed] [Google Scholar]
- 13. Wolman M. Amyloid, its nature and molecular structure. Comparison of a new toluidine blue polarized light method with traditional procedures. Lab Invest. 1971;25(2):104-10. [PubMed] [Google Scholar]
- 14. Bancroft JD, Gamble M. Theory and practice of histological techniques. 5th ed. Edinburgh, Scotland: Churchill Livingstone; 2002. [Google Scholar]
- 15. An YH, Martin KL. Handbook of histology methods for bone and cartilage. Berlin, Germany: Springer Science & Business Media; 2003. [Google Scholar]