Abstract
Objective
In orthopedic joint injection, the most frequently used local anesthetics are ropivacaine, bupivacaine, and 1% or 2% lidocaine. The aim of this study was to examine effects of these various anesthetics on the viability of human chondrocytes. Our hypothesis was that all local anesthetics tested damage human chondrocytes in vitro.
Methods
Primary human chondrocytes were isolated and cultured from 6 donated human knee joints (mean age of donors 61.2 years). Local anesthetics were added to these cultures. Toxicity analysis was performed by visualization of cell structure using light microscopy. Determination of vital chondrocytes was performed by use of a Casy cell counter. Chondrocytes’ cell death was examined by fluorescence microscopy and an XTT ELISA assay.
Results
Light microscope and fluorescence microscope data revealed a defect cell structure and increased number of dead cells after addition of 1% or 2% lidocaine and bupivacaine but not ropivacaine. We were able to show an increased level of XTT activity after treatment with bupivacaine, 2% lidocaine or ropivacaine. The count of vital chondrocytes was significantly decreased after treatment with bupivacaine, 1% or 2% lidocaine, and ropivacaine.
Conclusions
The data show that treatment with local anesthetics induces cell damage of human chondrocytes in vitro. Ropivacaine seems to be a local anesthetic with the lowest toxic potential on human chondrocytes, a feature that may favor its preference for use in joint injection.
Keywords: human chondrocytes, local anesthetics, toxicity, cell damage
Introduction
Today, the injection of local anesthetics (LAs) with or without use of cortisone belongs to the standard procedures of orthopedics. LAs are used for the treatment of osteoarthritis pains or after an arthroscopic joint operation in the form of a local infiltration in order to reduce postsurgical pain.1-4 In total knee or hip arthroplasty, the use of LAs also plays an increasing role. Here they are used in the form of a local infiltration anesthesia (LIA) and show comparably good analgesic effects, for example, during the utilization of pain catheters.3,5-7 Lidocaine, ropivacaine, and bupivacaine contribute to the most often-used LAs. Nevertheless, until this day there is no uniform recommendation as to which local anesthetic for joint injection is best suited. In various in vitro and in vivo studies, a toxic effect toward human cartilage has occasionally been proven.2,8-11
Breu et al.9 examined the toxic effect of bupivacaine, ropivacaine, and mepivacaine with regard to human cartilage. It could be demonstrated that all LAs tested were cartilage-toxic, and degenerative cartilage showed higher cell impairment than intact cartilage did.9 In another study, Grishko et al.12 showed that the treatment of cartilage with lidocaine, bupivacaine, and ropivacaine leads to a cellular dysfunction of the mitochondria and thus to cell death via apoptosis. In comparison with that work, Piper and Kim13 could prove that ropivacaine is less cartilage-toxic than bupivacaine is.
The aim of the present work was to examine whether all of the LAs tested here are cell-toxic toward human cartilage, and/or whether there is in fact an LA available that is less cell-toxic. To that end, the hypothesis of the study was that all LAs used in practice these days are cell-damaging to human cartilage in vitro.
Materials and Methods
Tissue culture plastic ware was obtained from BD (Germany). Culture medium, phosphate buffered saline (PBS), trypsin, fetal calf serum (FCS), and all other reagents were obtained from Invitrogen (Germany).
Chondrocyte Isolation and Culture
Chondrocyte isolation was performed as described previously.14 Human cartilage was obtained from 6 donors who had knee osteoarthritis. Donors were included only when they had not already presented any kind of infectious signals or tumor disease. The mean age of donors was 61.17 years. Experimental protocols were approved by the local ethics committee (3769-05/13). Human cartilage was minced and digested in medium containing 1 mg/mL of pronase (Roche, Germany) for 30 minutes at 37°C. Digestion medium was discarded, and the tissue was digested with medium containing 1 mg/mL clostridial collagenase (Roche, Germany) at 37°C for 12 hours. Next, the digested solution was filtered (70 µm Nylon, BD Falcon, Germany) and centrifuged at 1,200 rpm for 10 minutes. The cell pellet was washed 3 times with PBS. Then, chondrocytes were suspended in Dulbecco’s modified Eagle medium Hams-F12 with 10% FCS, 1% penicillin/ streptomycin, and cultured at 37°C in 95% air and 5% CO2.
Chondrocytes Treatment and Detection of Cell Structure
Chondrocytes were cultured and grown on 6-well plates at a subconfluent density using 500 µL of medium, after which 500 µL of anesthetic solution were added for 30 minutes. Following anesthetic solutions were used: Lidoject 1% Hexal, 10 mg/mL (lidocaine hydrochloride 1–water 50 mg); 0.5% Bucain-Actavis 0.5% hyperbar, 5 mg/mL (bupivacaine hydrochloride 1–water 25 mg); Xylocitin-Ioc 2% mibe, 20 mg/mL (lidocaine hydrochloride 1–water 100 mg); Ropivacain-HCL B. Braun, 2 mg/mL (ropivacaine hydrochloride 1–water 21.16 mg). PBS-treated chondrocytes were used as a negative control. Triton X 100 as a known toxic reagent was used as a positive control. Immediately after incubation, the results were interpreted by using light microscopy analysis (Axiovert 25 C Light Microscope, lens 10 × 0.25, ocular 10 × 18 Zeiss, Jena, Germany). The view fields were then digitized with a digital camera (Canon EOS 500D, 15.1 megapixels).
Determination of Total Cell Numbers and Vital Cells
Isolated human chondrocytes were counted by using Casy cell counter and grown on 24-well plates at a density of 2 × 104 cells per well. After 24 hours, 500 µL of the various anesthetics were added for 30 minutes (1% lidocaine, 2% lidocaine, bupivacaine, ropivacaine). Then cells were additionally washed twice with 100 µL of 0.9% physiological sodium chloride and detached using 100µl of trypsin. The detection of living and total cell numbers was determined by the Casy cell counter and Analyser System (Schärfe-System, Reutlingen, Germany).
Detection of Necrosis Using Fluorescence Microscopy
Chondrocytes, cultured on chamber slides with a density of 2 × 104 cells/cm2, were treated with 500 µL of the various anesthetics for 30 minutes (at concentrations shown above). PBS-treated chondrocytes were used as a negative control. Triton X 100 was used as a positive control. After the washing procedure, cells were stained with fluorescein and propidium iodide (PI) (20 μL/mL) for 15 minutes. Documentation was performed immediately by fluorescence microscopy (microscope CKX 41, Olympus, Hamburg, Germany).
XTT ELISA
The XTT assay was used for the spectrophotometric quantification of cell growth and viability. This is based on the cleavage of the tetrazolium salt XTT which forms a formazan dye by metabolic active cells. This conversation only occurs in viable cells. An increase in overall XTT activity is, in direct proportion, the result of an increase in the number of living cells. Cells, grown in a 96-well tissue culture plates, were treated with the individual anesthetics’ concentrations shown above or with PBS (negative control) and Triton X 100 (positive control) for 30 minutes. After washing with PBS twice, cells were incubated with the XTT solution for 4 hours. After this incubation period, formazan solution is formed, and this is spectrophotometrically quantified using an enzyme-linked immunosorbent assay (ELISA) plate reader.
Statistical Analysis
A nonparametric Wilcoxon matched-pairs test was used as indicated in the legends. A P value of <0.05 was considered to be significant, and a P value of <0.001 was considered to be highly significant.
Results
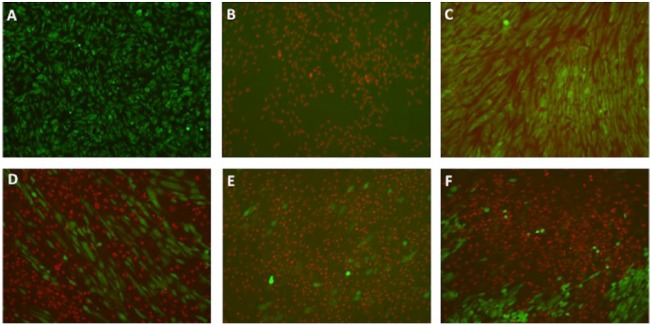
After an incubation time of 30 minutes, human chondrocytes that were treated with 1% or 2% lidocaine and bupivacaine showed an increased number of cells with defects in the cell structure. There were no defective chondrocytes when cultured with PBS or ropivacaine ( Fig. 1A and C ). Chondrocytes incubated with 1% or 2% lidocain or bupivacaine were globular, shrunken and all showed partial losses of cell contacts ( Fig. 1D and E ). Cells treated with Triton X 100 showed a totally defected cell structure ( Fig. 1B ).
Figure 1.
Light microscopy shows damage to chondrocytes after local anesthetics incubation. (A) Control chondrocytes treated with phosphate buffered saline (PBS), negative control. (B) Chondrocytes treated with Triton X 100, positive control. (C) Chondrocytes treated with ropivacaine, (D) bupivacaine, (E) 1% lidocaine, or (F) 2% lidocaine. One representative picture is shown.
The vital cell count of local anesthetic-treated chondrocytes showed a significant decrease of these: 795,322 ± 223,886 cells, that is, in comparison to negative controls having 100% vitality. Treatment with bupivacaine revealed a decrease in vital cell number to 67% (530,501 ± 301,951 cells), lidocaine 1% led to a reduction of vital cells down to 38% (303,917 ± 176,643 cells), whereas ropivacaine showed the lowest vital cell number reduction to 79% (627,045 ± 268,837 cells). Treatment with lidocaine 2% led to 43% live cells (340,102 ± 183,924 cells) and Triton X100 as positive control for toxic events left only 1% live cells (4,765 ± 1,732 cells, P < 0.05) ( Fig. 2 ). Only the addition of ropivacaine to chondrocytes showed significantly more vital cells in comparison with bupivacaine and xylocaine-treated chondrocytes, P < 0.05 ( Fig. 2 ).
Figure 2.

Vital cell counts after local anesthetics treatment in percent of control vital count. Untreated chondrocytes are shown compared with chondrocytes treated with ropivacaine, 1% or 2% lidocaine, bupivacaine or Triton X 100 as positive control. Values given as mean ± standard error of mean (SEM). Nonparametric Wilcoxon matched-pairs test. *P < 0.05, **P < 0.01.
Fluorescein and PI staining of chondrocytes treated with PBS showed vital cells (green colored) only, and no necrosis ( Fig. 3A ). Cells treated with Triton X 100 as a positive control for toxicity, inducing necrosis as well, showed PI positive cells (red colored) and no vital cells (green colored) ( Fig. 3B ). Chondrocytes treated with ropivacaine showed vital cells and only a very low number of necrotic cells. Most necrotic cells (red colored) in combination with few vital cells (green colored) ( Fig. 3C ) were detected after treatment with 1% or 2% lidocaine and bupivacaine ( Fig. 3D and E ).
Figure 3.
Detection of cell necrosis by fluorescence microscopy. Chondrocytes treated with phosphate buffered saline (PBS; negative control), Triton X 100 (positive control) or local anesthetics were stained with fluorescein and propidium iodide. Vital chondrocytes are shown as fluorescein positive (green fluorescence) and PI negative (red fluorescence). Necrotic cells are shown fluorescein PI positive (red fluorescence) and fluorescein negative (green fluorescence). (A) PBS-treated control cultures. (B) Chondrocytes treated with Triton X 100. (C-F) Chondrocytes treated with (C) ropivacaine, (D) bupivacaine, (E) 1% lidocaine, or (F) 2% lidocaine. One representative picture is shown.
XTT activity was analyzed in the supernatant of each cell culture. Compared with control chondrocytes (i.e., those treated with PBS), we noted significantly increased XTT activity even after 30 minutes of treatment with each local anesthetic, which indicated a beginning cell necrosis ( Fig. 4 ). There was low XTT activity at each time point after the treatment with 1% lidocaine in comparison to all other local anesthetics tested.
Figure 4.
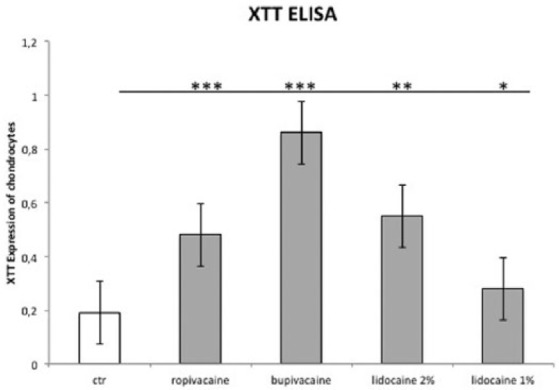
XTT assay for determination of cytotoxicity. Untreated chondrocytes are shown compared with chondrocytes treated with ropivacaine, 1 or 2% lidocaine, or bupivacaine. Values given as mean ± standard error of mean (SEM). Nonparametric Wilcoxon matched-pairs test. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The results of the present study show that all the local anesthetics tested here damage human chondrocytes in in vitro monolayer culture. In our study, when compared with bupivacaine or 1% and 2% lidocaine, ropivacaine was the anesthetic with the least toxicity toward human cartilage. According to the current literature data, all these anesthetics do show a good local analgesic effect.1-7 Over and above that, a cell-toxic effect could be also observed in several studies dedicated to cartilage ( Table 1 ).13,15-17 At present, there is to our knowledge however no uniform recommendation as to which anesthetic should definitely be used for local joint infiltration. Breu et al.9 were able to prove in an in vitro study that bupivacaine, ropivacaine, and mepivacaine are all cell-toxic in human cell cartilage. After treatment with these anesthetics, viability, apoptosis, and necrosis of the cartilage corpuscles were examined by means of flow cytometry. All anesthetics are cell-toxic with regard to cartilage. In line with that, degenerative cartilage showed higher cell impairment than intact cartilage did. In that study, not one of the three anesthetics tested could be observed to have any single advantage.9
Table 1.
In Vitro Studies of Local Anesthetics’ Toxicity against Chondrocytes.
| Study | Methods | Results |
|---|---|---|
| Dragoo et al. (2012)10 | Evaluation of chondrocytes viability after treatment of single doses of 1% lidocaine, 0.25% bupivacaine, and 0.5% ropivacaine | Treatment of 1% lidocaine resulted in a significant decrease in chondrocyte viability versus control No differences were seen after the treatment with bupivacaine or ropivacaine |
| Breu et al. (2013)9 | Cytotoxicity of 0.5% bupivacaine, 0.75% ropivacaine, and 2% mepivacaine on human chondrocytes | Bupivacaine, ropivacaine, and mepivacaine are chondrotoxic. Cellular death rates were higher in osteoarthritic cartilage compared with intact cartilage |
| Grishko et al. (2010)12 | Apoptosis and mitochondrial dysfunction in human chondrocytes after treatment with 2%, 1%, and 0.5% lidocaine, 0.5% and 0.25% bupivacaine, and 0.5% and 0.2% ropivacaine | Lidocaine, bupivacaine, and ropivacaine cause delayed mitochondrial dysfunction and apoptosis in human chondrocytes |
| Piper and Kim (2008)13 | Comparison of 0.5% ropivacaine and 0.5% bupivacaine toxicity in human chondrocytes and articular cartilage | 0.5% ropivacaine is significantly less toxic than 0.5% bupivacaine in chondrocyte culture and human articular cartilage |
| Sherman et al. (2015)11 | Toxicity of 1%, and 0.5% lidocaine, 0.25%, 0.125%, and 0.0625% bupivacaine on chondrocyte and synoviocyte viability and metabolism | 1% and 0.5% lidocaine, 0.25% and 0.125% bupivacaine were severely chondrotoxic after a single exposure Chondrocytes and synoviocytes remained viable after treatment with 0.0625% bupivacaine |
| Ickert et al. (2015)18 | Toxicity of 0.5% bupivacaine, 0.75% ropivacaine on human chondrocytes | Ropivacaine is less toxic than bupivacaine A dose- and time-dependent manner of cytotoxic effects on human chondrocytes for bupivacaine and ropivacaine could be shown |
| Jacobs et al. (2011)19 | Effect of 1%, and 2% lidocaine on viability of human cartilage cells | Lidocaine are significantly more toxic to human articular chondrocytes than a 0.9% saline |
| This study | Toxicity of 1% lidocaine, 0.5% bupivacaine, 2% xylocaine, or 0.2% ropivacaine against human chondrocytes | All tested local anesthetics induce cell damage on human chondrocytes Ropivacaine is less toxic than lidocaine, xylocaine or bupivacaine |
Dragoo et al.10 also examined in an in vitro study the cartilage-toxic effect of lidocaine, bupivacaine, and ropivacaine. After treatment for 3 hours, a 1% (w/v) lidocaine solution showed a significantly toxic effect on cartilage corpuscles. With 0.25% bupivacaine or 0.5% ropivacaine, this negative effect was not reproduced.10 According to that study, ropivacaine and bupivacaine thus seem to be less cartilage-toxic than lidocaine does.10 In addition to the study of Dragoo et al.,10 the research group of Piper and Kim13 compared ropivacaine and bupivacaine with each other with regard to the damaging influence on human cartilage. Both local anesthetics showed a negative influence on the cell vitality in comparison with the control group, which had been treated with a 0.9% saline solution. It could also be shown that bupivacaine had a significantly higher toxic potential when compared to ropivacaine.13 These observations match the results that we have obtained here.
This study is limited by the fact that it does not represent an in vivo situation. In vitro results may show higher cell toxicity than in vivo examinations based on direct chondrocyte incubation. Local anesthetics in vitro did not have to pass various barriers such as synovial membrane or cartilage matrix. An extrapolation to the process in cartilage tissue is therefore not valid. Also, we used a test on osteoarthritic cells cultured as dedifferentiated cells. Such cells may be more susceptible to toxic agents. Therefore, further studies with cells from healthy donors are needed.
Conclusions
Our study showed that ropivacaine is toxic to growing chondrocytes in vitro, but less than bupivacaine and 1% or 2% lidocaine in comparable doses. The clinical significance of our findings is still unclear.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study had the approval of the local ethics committee of the FSU Jena (3769-05/13).
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: Not applicable.
ORCID iD: Timo Zippelius  https://orcid.org/0000-0003-2166-268X
https://orcid.org/0000-0003-2166-268X
References
- 1. Lefevre N, Klouche S, de Pamphilis O, Herman S, Gerometta A, Bohu Y. Peri-articular local infiltration analgesia versus femoral nerve block for postoperative pain control following anterior cruciate ligament reconstruction: prospective, comparative, non-inferiority study. Orthop Traumatol Surg Res. 2016;102(7):873-7. [DOI] [PubMed] [Google Scholar]
- 2. Ravnihar K, Barlič A, Drobnič M. Effect of intra-articular local anesthesia on articular cartilage in the knee. Arthroscopy. 2014;30(5):607-12. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Z, Yang Q, Xin W, Zhang Y. Comparison of local infiltration analgesia and sciatic nerve block as an adjunct to femoral nerve block for pain control after total knee arthroplasty: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(19):e6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou Y, Yang TB, Wei J, Zeng C, Li H, Yang T, et al. Single-dose intra-articular ropivacaine after arthroscopic knee surgery decreases post-operative pain without increasing side effects: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2016;24(5):1651-9. [DOI] [PubMed] [Google Scholar]
- 5. Barastegui D, Robert I, Palau E, Haddad S, Reverte-Vinaixa M, Lorente L, et al. Can local infiltration analgesia increase satisfaction in postoperative short-term pain control in total knee arthroplasty? J Orthop Surg (Hong Kong). 2017;25(1):2309499017690461. [DOI] [PubMed] [Google Scholar]
- 6. Hu B, Lin T, Yan SG, Tong SL, Yu JH, Xu JJ, et al. Local infiltration analgesia versus regional blockade for postoperative analgesia in total knee arthroplasty: a meta-analysis of randomized controlled trials. Pain Physician. 2016;19(4):205-14. [PubMed] [Google Scholar]
- 7. Seangleulur A, Vanasbodeekul P, Prapaitrakool S, Worathongchai S, Anothaisintawee T, McEvoy M, et al. The efficacy of local infiltration analgesia in the early postoperative period after total knee arthroplasty: a systematic review and meta-analysis. Eur J Anaesthesiol. 2016;33(11):816-31. [DOI] [PubMed] [Google Scholar]
- 8. Baker JF, Mulhall KJ. Local anaesthetics and chondrotoxicty: What is the evidence? Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2294-301. [DOI] [PubMed] [Google Scholar]
- 9. Breu A, Rosenmeier K, Kujat R, Angele P, Zink W. The cytotoxicity of bupivacaine, ropivacaine, and mepivacaine on human chondrocytes and cartilage. Anesth Analg. 2013;117(2):514-22. [DOI] [PubMed] [Google Scholar]
- 10. Dragoo JL, Braun HJ, Kim HJ, Phan HD, Golish SR. The in vitro chondrotoxicity of single-dose local anesthetics. Am J Sports Med. 2012;40(4):794-9. [DOI] [PubMed] [Google Scholar]
- 11. Sherman SL, Khazai RS, James CH, Stoker AM, Flood DL, Cook JL. In vitro toxicity of local anesthetics and corticosteroids on chondrocyte and synoviocyte viability and metabolism. Cartilage. 2015;6(4):233-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grishko V, Xu M, Wilson G, Pearsall AW., 4th Apoptosis and mitochondrial dysfunction in human chondrocytes following exposure to lidocaine, bupivacaine, and ropivacaine. J Bone Joint Surg Am. 2010;92(3):609-18. [DOI] [PubMed] [Google Scholar]
- 13. Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90(5):986-91. [DOI] [PubMed] [Google Scholar]
- 14. Röhner E, Kolar P, Seeger JB, Arnholdt J, Thiele K, Perka C, et al. Toxicity of antiseptics against chondrocytes: what is best for the cartilage in septic joint surgery? Int Orthop. 2011;35(11):1719-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chu CR, Coyle CH, Chu CT, Szczodry M, Seshadri V, Karpie JC, et al. In vivo effects of single intra-articular injection of 0.5% bupivacaine on articular cartilage. J Bone Joint Surg Am. 2010;92(3):599-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kreuz PC, Steinwachs M, Angele P. Single-dose local anesthetics exhibit a type-, dose-, and time-dependent chondrotoxic effect on chondrocytes and cartilage: a systematic review of the current literature. Knee Surg Sports Traumatol Arthrosc. Epub 2017 Mar 13. doi: 10.1007/s00167-017-4470-5. [DOI] [PubMed] [Google Scholar]
- 17. Piper SL, Kramer JD, Kim HT, Feeley BT. Effects of local anesthetics on articular cartilage. Am J Sports Med. 2011;39(10):2245-53. [DOI] [PubMed] [Google Scholar]
- 18. Ickert I, Herten M, Vogl M, Ziskoven C, Zilkens C, Krauspe R, et al. Opioids as an alternative to amide-type local anaesthetics for intra-articular application. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2674-81. [DOI] [PubMed] [Google Scholar]
- 19. Jacobs TF, Vansintjan PS, Roels N, Herregods SS, Verbruggen G, Herregods LL, et al. The effect of lidocaine on the viability of cultivated mature human cartilage cells: an in vitro study. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1206-13. [DOI] [PubMed] [Google Scholar]