Abstract
Objective
To evaluate the long-term clinical and radiological outcome of matrix-assisted autologous chondrocyte implantation (mACI) for articular cartilage defects in the knee joint.
Design
Clinical evaluation was assessed in 21 patients with full-thickness cartilage defects, International Cartilage Repair Society (ICRS) grade IV. Clinical scoring was performed preoperatively and 12 years after transplantation using the International Knee Documentation Committee (IKDC) score, the Lysholm score, the Knee injury and Osteoarthritis Outcome Score (KOOS), and the Noyes sports activity rating scale. Morphologic evaluation of the repair tissue was assessed by magnetic resonance imaging (MRI) in 14 patients using the Kreuz-Henderson score.
Results
Clinical evaluation revealed significant improvement in the IKDC, the Lysholm, the KOOS, and the Noyes score. Morphological evaluation by MRI showed moderate to complete defect filling in 10 of 14 patients, demonstrating normal to nearly normal values in mean 74.29% of all assessed parameters. Significant correlation of the parameter cartilage signal and clinical outcome was found with the IKDC, Lysholm, and KOOS subscales ADL (activities of daily living) and QoL (quality of life).
Conclusions
The clinical and radiological outcomes 12 years after transplantation suggest the confirmation of the promising results of the mid-term follow-up. This study therefore provides first indications that the implantation of mACI might be a suitable option for long-term cartilage repair. Future controlled studies need to address the exact parameters influencing the long-term outcome of mACI.
Keywords: mACI; BioSeed-C, knee, cartilage regeneration, long-term results
Introduction
In the developing progress of articular cartilage repair, autologous chondrocyte implantation (ACI) has become a first-line treatment for large chondral defects.1 Although first-generation ACI (pACI) has been well-documented, substantial knowledge about the long-term progression of third-generation mACI is rare.
In the past decades, different types of matrices for mACI have been developed.2 In mACI based on a fibrin-polymer graft, autologous in vitro expanded chondrocytes are 3-dimensionally rearranged in a bioresorbable polymer scaffold that allows arthroscopic implantation.3 Clinical and radiological evaluation showed significant improvement in clinical outcome and joint function as well as satisfactory defect filling by magnetic resonance imaging (MRI) analysis in short-term4 and mid-term5 follow-up. Similar results were also achieved in an osteoarthritis subgroup at 4 years after transplantation.6 These clinical findings demonstrate the efficacy and reliability of the transplant for ACI, suggesting it as an effective treatment option for the regeneration of posttraumatic and/or osteoarthritic defects of the knee.
In this study, we show the long-term clinical and radiological outcome of mACI for the treatment of focal articular cartilage defects of the knee by clinical, functional, and MRI evaluation.
Materials and Methods
Patients
From December 2001 to December 2003, 79 patients were treated with mACI for their symptomatic shouldered, focal, posttraumatic, or degenerative cartilage defects in the knee with International Cartilage Repair Society (ICRS) classification IV.7 Short-term (n = 20)4 to mid-term (n = 52)5 follow-up of this patient population reporting clinical and MRI results as well as 4-year follow-up of the osteoarthritis subgroup (n = 19)6 have been published previously.
In this study, 21 patients gave consent to clinical follow-up at 12 years after transplantation and 14 patients allowed structural evaluation by MRI analysis. Clinical evaluation was carried out by comparison of the situation documented preoperatively and 12 years after surgery by clinical scores and MRI assessment.
Characteristics of the study population can be seen in Table 1 . Femoral or high tibial osteotomies were indicated with varus or valgus malalignments >5° after investigation of axial deformities by radiographs of the entire leg.
Table 1.
Characteristics of the Patient Population.
| Characteristic | 12-Year Cohort (n = 21) | Initial Cohort (n = 79) |
|---|---|---|
| Sex, n (%) | ||
| Female | 13 (61.9) | 30 (38.0) |
| Male | 8 (38.1) | 49 (62) |
| Age, y | ||
| Mean (range) | 45.35 (29-63) | 36.0 (17-64) |
| Median | 42.67 | — |
| Body mass index, kg/m2 | ||
| Mean (range) | 26.08 (18.59-37.14) | 22.33 (17.49-32.49) |
| Median | 24.54 | 22.49 |
| Follow-up rate, y | ||
| Mean (range) | 11.75 (10.17-13.08) | |
| Median | 11.67 | |
| First lesion | ||
| Defect size, cm2 | ||
| Mean (range) | 4.34 (1.5-9.6) | 4.84 (2.0-15.0) |
| Median | 4.4 | — |
| ICRS classification | IV (n = 21) | IV (n = 79) |
| Localization, n (%) | ||
| Femoral condyle | 12/21 (57.1) | 55/79 (69.6) |
| Retropatellar | 6/21 (28.6) | 17/79 (21.5) |
| Trochlea | 3/21 (14.3) | 6/79 (7.6) |
| Tibia | 0/21 (0) | 1/79 (1.3) |
| Singular lesion, n (%) | 19/21 (90.5) | 58/79 (73.4) |
| Second lesion, n (%) | 2/21 (9.5) | 21/79 (26.6) |
| Defect size, cm2 | ||
| Mean (range) | 2.0 (1-3) | 2.6 (0.5-4) |
| Median | 2.0 | — |
| ICRS classification | III-IV | III-IV |
| Localization, n (%) | ||
| Lateral femoral condyle | 1/2 (50) | 3/21 (14.3) |
| Trochlea | 1/2 (50%) | 5/21 (23.8) |
| Medial femoral condyle | 5/21 (23.8) | |
| Retropatellar | 8/21 (38.1) | |
| Concomitant surgery, n (%) | 9/21 (42.9) | 34/79 (43.0) |
| Anterior cruciate ligament reconstruction | 4/9 (44.4) | 4/34 (11.8) |
| Microfracture/drilling | 2/9 (22.2) | 5/34 (14.7) |
| Osteotomy | 3/9 (33.4) | 21/34 (61.7) |
| Medial capsular shift | 0/9 (0) | 2/34 (5.9) |
| Lateral release, n (%) | 0/9 (0%) | 2/34 (5.9) |
| Patients without concomitant surgery, n (%) | 12/21 (57.1) | 45/79 (57.0) |
| With singular defect | 12/21 (57.1) | 45/79 (57.0) |
| With secondary lesion | 0 | 0 |
| Previous surgical procedures, n (%) | 8/21 (38.1) | 55/79 (69.6) |
| Anterior cruciate ligament reconstruction | 3/8 (37.5) | 12/55 (21.8) |
| Microfracture/drilling | 2/8 (25.0) | 13/55 (23.6) |
| Shaving | 1/8 (12.5) | 9/55 (16.4) |
| Meniscectomy | 2/8 (25.0) | 20/55 (36.4) |
| Osteotomy | 1/8 (12.5) | 1/55 (1.8) |
Surgical Implantation and Follow-up Treatment
Isolation of autologous chondrocytes, cell expansion, and implantation of BioSeed-C (BioTissue Technologies GmbH, Freiburg, Germany) has been performed as described previously.4-6 In brief, approximately 250 mg of healthy cartilage tissue were harvested from a nonweightbearing zone of the knee joint by a first arthroscopic procedure and chondrocytes were isolated and in vitro expanded under GMP conditions. A polymer-based scaffold consisting of polyglycolic/polylactic acid (polyglactin, Vicryl, and polydioxanone [Ethicon GmbH, Norderstedt, Germany]) was used for 3-dimensional arrangement of autologous chondrocytes with fibrin gluing. After debridement of the defects, the autologous chondrocyte grafts were adjusted to the defect size and fixed by transosseous anchoring technique.3-6
Rehabilitation started on the first day after surgery. Patients were mobilized on crutches and subjected to continuous passive motion as described previously4-6,8 ( Table 2 ).
Table 2.
Rehabilitation Protocol.
| Weeks 1-6 | After day of operation, start of continuous passive motion on the knee machine. Mobilization and partial loading with 15% of bodyweight (i.e., sole of the foot contact). Isometric tension exercises. |
| Weeks 7-12 | Gradually increase the loading, specific strengthening exercises. Use of crutches to take weight off the knee that has been operated on. Possible ergometric training at a gentle level. Active physiotherapy. |
| From week 13 | Gradually increase the weight bearing and muscular and coordination exercises up to full weight bearing. Gentle exertion (e.g., cycling, jogging) from 6 months. More strenuous activities (e.g., tennis, football) from 12 months. |
Evaluation of Clinical and Radiological Results
Clinical and functional outcome was evaluated by the International Knee Documentation Committee (IKDC) score,9 the Lysholm score,10 the Knee injury and Ostheoarthritis Outcome (KOOS) score,11 and the Noyes sports activity rating scale.12 Visualization of the knee joint by MRI has been investigated in a 1.5-T MRI scanner (Siemens, Erlangen, Germany) and the data were evaluated using the Henderson and Kreuz scoring system ( Table 3 ).13,14 The analysis of the images was blinded and performed by a clinician and an independent musculoskeletal radiologist, who has not been involved in the initial study.
Table 3.
Magnetic Resonance Imaging Analysis of 14 Patients at 12 Years After Graft Transplantation According to Henderson Kreuz Score.a
| Rating | Defect Filling | Graft Hypertrophy | Cartilage Signal | Subchondral Edema | Effusion |
|---|---|---|---|---|---|
| 1 Normal | 8 (complete filling) | 5 (≤125%) | 7 (normal) | 4 (no edema) | 1 (no effusion) |
| 2 Mild | 0 (≥50%) | 5 (≤150%) | 3 (slight areas of hyperintensity) | 10 (mild edema) | 9 (mild effusion) |
| 3 Moderate | 2 (<50%) | 0 (≤200%) | 0 (larger areas of hyperintensity) | 0 (moderate edema) | 3 (moderate effusion) |
| 4 Abnormal | 4 (full-thickness defect) | 0 (>200%) | 4 (absent) | 0 (distinct edema) | 1 (distinct effusion) |
The number of repair sites corresponds to the number of patients.
For structural evaluation of the repair tissue, a 1.5-T MRI (Avanto, Siemens, Erlangen, Germany) with a dedicated 8-channel-knee-coil was used with following sequences: Fast spin-echo proton-density weighted (repetition time/echo time [TR/TE] 2810/31 ms) in coronal and sagittal plane, Fast spin-echo proton-density weighted with spectral fat saturation (TR/TE 3370/36 ms) in transversal, coronal and sagittal plane, Fast spin-echo T2-weighted with spectral fat-saturation (TR/TE 5880/60 ms) in sagittal plane, The spatial resolution in plane was 320 × 320 up to 512 × 512 pixels in a field-of-view of 1002 mm.
Statistical Analysis
For statistical analysis of the clinical outcome, the Kolmogorov–Smirnov method was applied for testing normal distribution of data with SigmaStat 3.5 software (Systat Software Inc, San Jose, CA, USA). Normal distributed data of IKDC and KOOS subscales Symptom and Quality of Life were analyzed by the parametric Student t test. Differences were considered significant at P < 0.05. Nonparametric data of KOOS, Lysholm, and Noyes scores were analyzed by Mann-Whitney rank sum test, considering differences significant at P < 0.05.
For analysis of correlation between clinical scores and MRI parameters, the Pearson product moment test was used, correlation was considered significant at P < 0.05.
Results
Clinical Evaluation
In 21 patients, the IKDC score showed highly significant improvement (P ≤ 0.001) at 12 years postoperatively compared with patients’ preoperative situation, increasing from a mean 46.92 ± 13.63 to 71.15 ± 16.56 ( Fig. 1 ).
Figure 1.
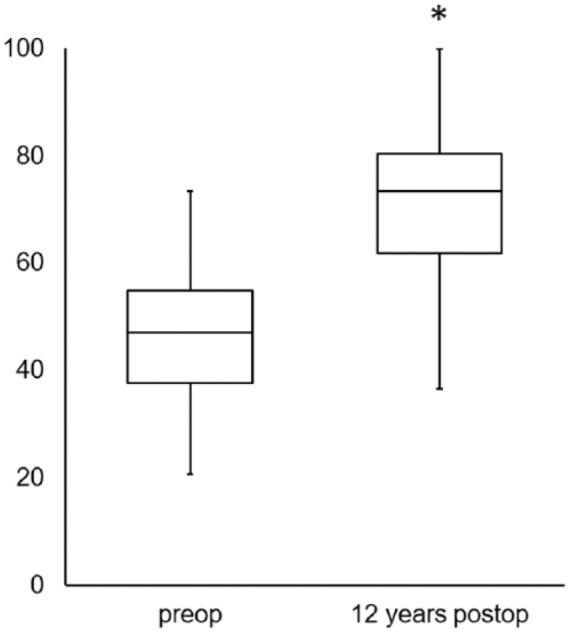
Clinical outcome assessed by the International Knee Documentation Committee (IKDC) score revealed significant improvement (*P ≤ 0.001) 12 years after transplantation compared to preoperative findings. The values are presented as the median with the end of the boxes defining the 25th and 75th percentiles and error bars defining the minimum and maximum.
Twelve years after transplantation, clinical evaluation determined by the Lysholm score in 21 patients showed an increase of median 56 to 86, therefore significant improvement (P ≤ 0.001) compared with the preoperative situation ( Fig. 2 ).
Figure 2.
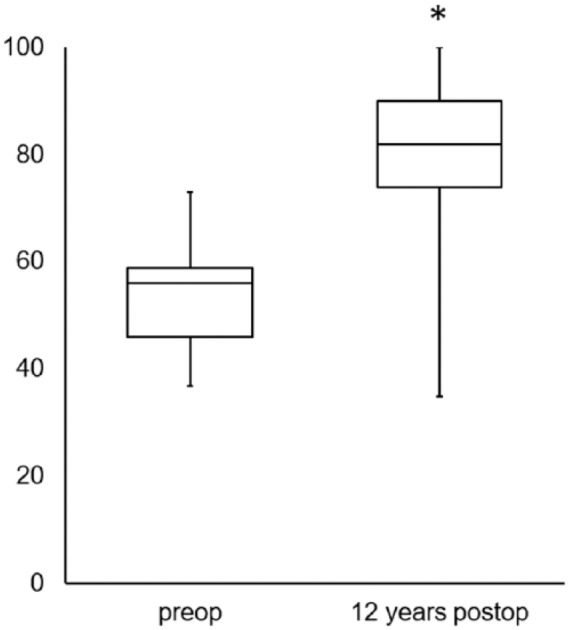
Clinical outcome assessed by the Lysholm score revealed significant improvement (*P ≤ 0.001) 12 years after transplantation compared with preoperative findings. The values are presented as the median with the end of the boxes defining the 25th and 75th percentiles and error bars defining the minimum and maximum.
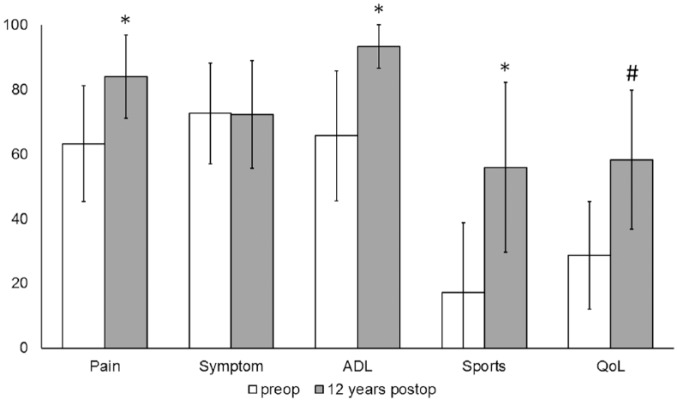
Evaluation of the clinical and functional outcome in 19 patients assessed by the KOOS score revealed significant improvement in the subscales Pain, Activities of Daily Living (ADL), Sports and Recreation (Sport/Rec), and Quality of Life (QoL) (P ≤ 0.001). Subscales increased in mean scores Pain from 63.2 ± 17.88 to 84.0 ± 12.88, ADL from 65.64 ± 20.09 to 93.0±9.47, Sports/Rec from 17.11 ± 21.62 to 56.0±26.26, and QoL from 28.71 ± 16.64 to 58.0 ± 21.45. The subscale Symptom remained almost constant compared with the preoperative situation (72.53 ± 15.48 to 72.0 ± 16.69 ( Fig. 3 ).
Figure 3.
Clinical outcome assessed by the Knee injury and Osteoarthritis Outcome Score (KOOS) score revealed significant improvement (*P ≤ 0.001) 12 years after transplantation in the subscales Pain, ADL and Sport/Rec compared to preoperative findings. Subscale QoL revealed significant improvement (#P ≤ 0.001) in long-term follow up. The KOOS values are presented as a mean value with error bars defining the standard deviation.
Further functional analysis in 21 patients determined by the Noyes score revealed a median increase from 20 to 75 indicating a significant increase in sports activities (P ≤ 0.001) ( Fig. 4 ).
Figure 4.
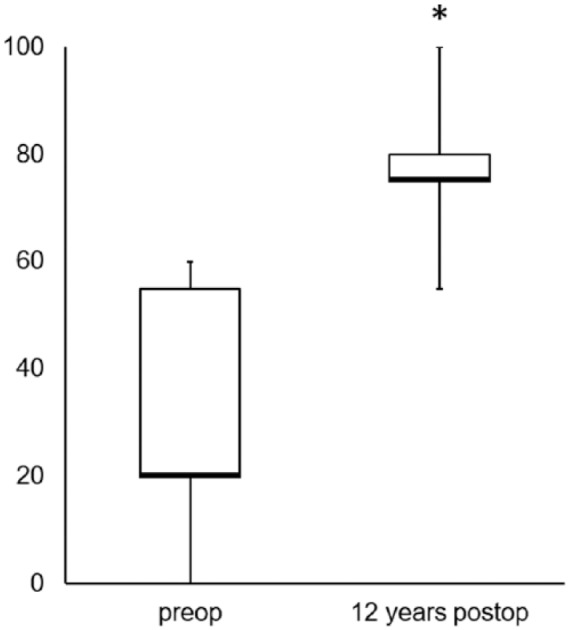
Clinical outcome assessed by the Noyes score revealed significant improvement (*P ≤ 0.001) 12 years after transplantation compared with preoperative findings. The values are presented as the median (bold line) with the end of the boxes defining the 25th and 75th percentiles and error bars defining the minimum and maximum.
Magnetic Resonance Imaging and Evaluation
After assessment of clinical and functional outcome, 14 patients gave consent to further structural evaluation by MRI ( Table 3 ). MRI data of remaining 7 patients could not be acquired due to limited compliance and participation.
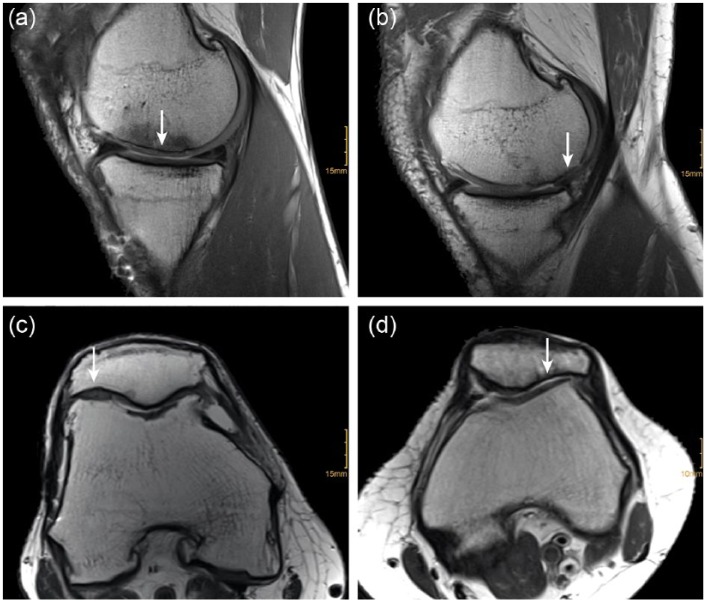
Images analyzed by the Henderson and Kreuz scoring system showed normal to abnormal defect filling 12 years after transplantation. Eight of 14 patients presented with complete filling of the defect, in 2 patients the defect was filled less than 50% ( Fig. 5 ). Four patients showed full-thickness cartilage defects and no defect filling. 10 patients showed no or mild (≤150%) hypertrophy presenting normal or nearly normal cartilage signal. In 7 patients, the defects showed normal cartilage signal whereas three patients presented with slight areas of hyperintensity. All patients showed no or only mild subchondral edema. Mild bone marrow edema was evident in 10 patients and absent in 4 patients. Ten to 13 years after transplantation, 10 patients showed no or mild effusion, whereas moderate to severe effusion was evident in 4 patients.
Figure 5.
Exemplary demonstration of morphologic magnetic resonance imaging (MRI) analysis. Sagittal T1-weighted images show complete (a) and moderate (b), axial proton density–weighted images show complete (c) and missing (d) defect filling at the repair site (arrow), located at the medial femoral condyle (a + b) and patella (c + d).
In overall MRI analysis, normal or nearly normal values were present in average 74.29% of all subcategories, whereas moderate or abnormal values could be found in mean 25.71% ( Fig. 6 ).
Figure 6.
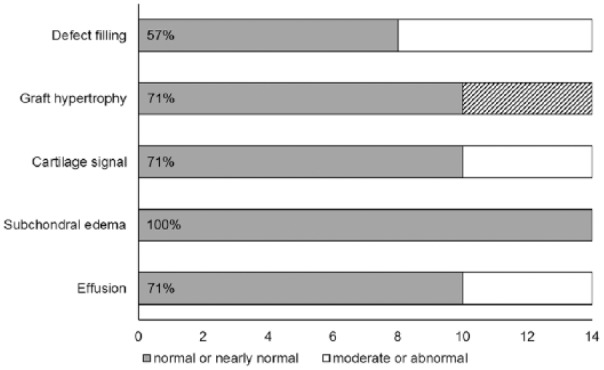
Distribution of the respective parameters assessed by magnetic resonance imaging analysis of 14 patients at 12 years after transplantation. Normal or nearly normal values could be found in mean 74.29% of all subcategories. In subcategory “graft hypertrophy,” 4 patients are not included due to missing repair tissue (shaded area).
Analysis of the correlation between MRI parameters and clinical scores revealed significant correlation between the cartilage signal and the IKDC (r = −0.544, P = 0.0441), Lysholm (r = −0.537, P = 0.0479), KOOS ADL (r = −0.596, P = 0.0244), and KOOS QoL (r = −0.546, P = 0.0434).
Discussion
In this study, we showed for the first time a 12-year clinical and radiological outcome of mACI for the treatment of focal articular cartilage defects of the knee joint. Clinical evaluation showed significant improvement in all assessed scores compared with the preoperative situation. Detailed analysis of the current data compared with the mid-term follow-up results5 showed no significant changes in all clinical scores, thus proving persistent results in patient satisfaction, function and activity. This is in line with previous findings demonstrating the maintenance of good clinical mid-term results even up to 10 to 20 years after pACI transplantation.15-17 High levels of physical activity as measured in the Noyes sports activity rating scale additionally correspond with previous findings, that physical training improves the long-term outcome after ACI treatment.18
Apparently, although clinical assessment revealed significant changes in IKDC, Lysholm, Noyes, and in the KOOS subscales Pain, ADL, Sports/Rec, and QoL, the patients estimated no change in the subscale Symptom, a subscale focusing on the subjective perception of stiffness, grinding and motion. Similar effects on the KOOS subscale Symptom in case of long-term clinical outcome of mACI have already been observed previously (see below).19
Nevertheless, further analysis of the significant improvements KOOS subscales Pain, ADL, Sport/Rec and QoL at 12 years after transplantation compared to the preoperative situation exceed a minimal perceptible clinical improvement (MPCI) that can be considered not only as statistically significant but also as clinically relevant.20
Different studies describe the long-term clinical outcome of the use of pACI reporting of significant improvement in IKDC, Lysholm, Tegner, and KOOS score,17,21-23 whereas studies investigating the long-term results of mACI are rare. Ten-year follow-up results with a hyaluronic acid–based scaffold correspond with our findings, indicating significant improvement in the IKDC, Noyes and KOOS subscales Pain and QoL. Investigation of KOOS Symptom, ADL, and Sport/Rec as well as Tegner, Brittberg, and VAS showed no significant differences compared with the preoperative situation. In addition, the authors stated a slight downward tendency of all clinical scores in long-term progression.19 Clinical follow-up at 15 years postoperatively based on a collagen membrane showed significant improvement in the Lysholm, IKDC and Tegner score.24 However, this study is limited to subjective scores and no morphological analysis by MRI is provided.
Comparison of the clinical results of the current study to mid-term follow-up suggests long-term durability of mACI in case of clinical outcome and function since no significant differences could be detected, including IKDC (P = 0.151), Lysholm (P = 0.251), KOOS Pain (P = 1.0), KOOS Symptom (P = 0.151), KOOS ADL (P = 0.41), KOOS Sport/Rec (P = 0.681), KOOS QoL (P = 0.722), and Noyes (P = 0.215) (data not shown).5,6
Regarding morphological analysis by MRI, we could show the presence of normal to nearly normal values in 74.29% according to the Henderson-Kreuz scoring system. This is in line with previous findings, demonstrating ACI as a long-term solution in more than 70% of affected patients.25 Complete defect filling could be observed in 57% of all cases. Full-thickness cartilage defects were evident in 4 cases; however, 3 of these cases were located retropatellar. After complete defect filling at 4 years after transplantation5, these patients subsequently experienced the loss of their transplant during the following years. This corresponds with previous findings, since retropatellar lesions were reported to lead to inferior results and were associated with higher rates of complications in the use of both ACI14,16,26 and microfracture.27 Since other authors state excellent results in retropatellar cartilage repair,21,28 pACI might be considered as a successful approach, however, it still remains challenging regarding the particular biomechanical environment of the localization.28 Altogether, evaluation of the long-term outcome of mACI for retropatellar defects requires further data.
In the present study, subchondral edema was evident in 71% of the patients. This is in line with other investigators reporting persistent subchondral edema in up to more than 80% in first-generation pACI21,22 and in mACI.19 Although bone marrow edema could be identified as a possible prognostic factor of clinical outcome and knee function,29 subchondral edema showed no correlation with the clinical results in this study, demonstrating that the incidence of bone marrow edema still remains controversial.30
MRI has widely been described as a noninvasive method suitable for cartilage repair tissue monitoring in short and mid-terms.31-34 However, in terms of long-term progression, deteriorating correlation of MRI parameters has been frequently observed.19,21,31,35 Poor correlation therefore might be interpreted as possible degenerative progression and/or unrelated knee injury or dysfunction.31 The trend of deteriorating MRI parameters along with stable clinical outcomes consequently challenges the potential of MRI for the long-term repair tissue monitoring.21,31 However, compared with previous findings of the long-term outcome of matrix-assisted ACI, we could still demonstrate significant correlation of the cartilage signal with the IKDC, Lysholm, and KOOS subscales ADL and QoL. This is in line with a comprehensive analysis, identifying graft hypertrophy and repair tissue signal as the critical parameters in the correlation of MRI with clinical outcome after ACI.31 Nevertheless, the current results are based on a relatively small follow-up cohort. Therefore, the role of MRI in long-term monitoring still remains unclear and requires further investigation.
Although histological analysis of BioSeed-C by second-look arthroscopy assessed in patients of the same population in short-term follow up showed complete filling, good integration, and formation of hyaline-like repair tissue,4 the lack of histological evaluation is a limitation of this study.
A further limitation is the heterogeneous composition of the study population, comprehending different concomitant surgical procedures such as femoral or high tibial osteotomy, ACL reconstruction or microfracture/drilling of secondary lesions. Although possibly influencing the final results, the different approaches can be considered as a necessary intervention to provide uniform biomechanical requirements and comparable loading of the defect area.5,36 Altogether, these patients of the current study population represent cases in daily practice.
The study population 12 years after surgery involved a reduced follow-up rate due to limited patient participation and compliance as found to be associated with patient recruitment over long-term follow-up periods.19,23,24 However, the extent of the current study population is equivalent to the previous studies of long-term matrix-assisted ACI.19,24
During the past years, different studies have been reporting successful long-term outcome of pACI as a cartilage repair procedure. Follow-up of minimum 10 years showed survivorship of the repair site in 71% of the treated patients together with improved function in 75%37 and satisfactory survival rates corresponding to significant clinical improvements could be reported up to 20 years after surgery.38
Although first-generation ACI has been that well documented, substantial knowledge about the long-term progression of matrix-assisted ACI is still missing.39 The reexamined patients of the current study presented with significant improvement in patient satisfaction, clinical outcome, and function 12 years after transplantation and the morphological evaluation by MRI revealed normal to nearly normal values in more than 70%. The consistency of the data acquired at 4 years postoperatively together with a persistent correlation between MRI parameters and clinical outcomes might suggest the long-term durability of the transplant. Together with a thorough reference to the mentioned limitations, this study provides first indications that the implantation of mACI might be a suitable option for long-term cartilage repair.
Future controlled clinical studies have to elucidate the exact conditions influencing the long-term outcome and the appropriate parameters for long-term MRI monitoring of matrix-assisted ACI for the treatment of focal articular cartilage defects of the knee joint.
Footnotes
Authors’ Note: The study was performed at the Freiburg University Medical Center.
Acknowledgment and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RHK received grant for the patients’ travel expenses from the company TransTissue Technologies GmbH, Charitéplatz 1/Virchowweg 11, 6.OG, D-10117 Berlin, Germany.
Ethical Approval: The study was approved by the ethics committee of the University of Freiburg (ID 490/12).
Informed Consent: Written informed consent was obtained from all subjects before the study.
Trial Registration: This study has been registered at the German Clinical Trials Register (Registration No. DRKS00010658).
ORCID iD: Richard Horst Kalkreuth  https://orcid.org/0000-0001-9869-9451
https://orcid.org/0000-0001-9869-9451
References
- 1. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 2. Marlovits S, Zeller P, Singer P, Resinger C, Vécsei V. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol. 2006;57(1):24-31. doi: 10.1016/j.ejrad.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 3. Erggelet C, Sittinger M, Lahm A. The arthroscopic implantation of autologous chondrocytes for the treatment of full-thickness cartilage defects of the knee joint. Arthroscopy. 2003;19(1):108-10. doi: 10.1053/jars.2003.50025. [DOI] [PubMed] [Google Scholar]
- 4. Ossendorf C, Kaps C, Kreuz PC, Burmester GR, Sittinger M, Erggelet C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther. 2007;9(2):R41. doi: 10.1186/ar2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kreuz PC, Müller S, Freymann U, Erggelet C, Niemeyer P, Kaps C, et al. Repair of focal cartilage defects with scaffold-assisted autologous chondrocyte grafts: clinical and biomechanical results 48 months after transplantation. Am J Sports Med. 2011;39(8):1697-705. doi: 10.1177/0363546511403279. [DOI] [PubMed] [Google Scholar]
- 6. Kreuz PC, Müller S, Ossendorf C, Kaps C, Erggelet C. Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: four-year clinical results. Arthritis Res Ther. 2009;11(2):R33. doi: 10.1186/ar2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):58-69. [DOI] [PubMed] [Google Scholar]
- 8. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;(391 Suppl):S362-9. [DOI] [PubMed] [Google Scholar]
- 9. Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, et al. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2001;29(5):600-13. [DOI] [PubMed] [Google Scholar]
- 10. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-4. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 11. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 12. Noyes FR, Barber SD, Mooar LA. A rationale for assessing sports activity levels and limitations in knee disorders. Clin Orthop Relat Res. 1989;(246):238-49. [PubMed] [Google Scholar]
- 13. Henderson I, Francisco R, Oakes B, Cameron J. Autologous chondrocyte implantation for treatment of focal chondral defects of the knee—a clinical, arthroscopic, MRI and histologic evaluation at 2 years. Knee. 2005;12(3):209-16. doi: 10.1016/j.knee.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 14. Kreuz PC, Steinwachs M, Erggelet C, Krause SJ, Ossendorf C, Maier D, et al. Classification of graft hypertrophy after autologous chondrocyte implantation of full-thickness chondral defects in the knee. Osteoarthritis Cartilage. 2007;15(12):1339-47. doi: 10.1016/j.joca.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 15. Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30(1):2-12. [DOI] [PubMed] [Google Scholar]
- 16. Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-34. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 17. Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117-24. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 18. Kreuz PC, Steinwachs M, Erggelet C, Lahm A, Krause S, Ossendorf C, et al. Importance of sports in cartilage regeneration after autologous chondrocyte implantation: a prospective study with a 3-year follow-up. Am J Sports Med. 2007;35(8):1261-8. doi: 10.1177/0363546507300693. [DOI] [PubMed] [Google Scholar]
- 19. Aldrian S, Zak L, Wondrasch B, Albrecht C, Stelzeneder B, Binder H, et al. Clinical and radiological long-term outcomes after matrix-induced autologous chondrocyte transplantation: a prospective follow-up at a minimum of 10 years. Am J Sports Med. 2014;42(11):2680-8. doi: 10.1177/0363546514548160. [DOI] [PubMed] [Google Scholar]
- 20. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niemeyer P, Porichis S, Steinwachs M, Erggelet C, Kreuz PC, Schmal H, et al. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am J Sports Med. 2014;42(1):150-7. doi: 10.1177/0363546513506593. [DOI] [PubMed] [Google Scholar]
- 22. Pelissier A, Boyer P, Boussetta Y, Bierry G, Van Hille W, Hamon P, et al. Satisfactory long-term MRI after autologous chondrocyte implantation at the knee. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2007-12. doi: 10.1007/s00167-013-2428-9. [DOI] [PubMed] [Google Scholar]
- 23. Rosa D, Balato G, Ciaramella G, Soscia E, Improta G, Triassi M. Long-term clinical results and MRI changes after autologous chondrocyte implantation in the knee of young and active middle aged patients. J Orthop Traumatol. 2016;17(1):55-62. doi: 10.1007/s10195-015-0383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gille J, Behrens P, Schulz AP, Oheim R, Kienast B. Matrix-associated autologous chondrocyte implantation: a clinical follow-up at 15 years. Cartilage. 2016;7(4):309-15. doi: 10.1177/1947603516638901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biant LC, Bentley G, Vijayan S, Skinner JA, Carrington RWJ. Long-term results of autologous chondrocyte implantation in the knee for chronic chondral and osteochondral defects. Am J Sports Med. 2014;42(9):2178-83. doi: 10.1177/0363546514539345. [DOI] [PubMed] [Google Scholar]
- 26. Niemeyer P, Steinwachs M, Erggelet C, Kreuz PC, Kraft N, Köstler W, et al. Autologous chondrocyte implantation for the treatment of retropatellar cartilage defects: clinical results referred to defect localisation. Arch Orthop Trauma Surg. 2008;128(11):1223-31. doi: 10.1007/s00402-007-0413-9. [DOI] [PubMed] [Google Scholar]
- 27. Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, et al. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22(11):1180-6. doi: 10.1016/j.arthro.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 28. Gomoll AH, Gillogly SD, Cole BJ, Farr J, Arnold R, Hussey K, et al. Autologous chondrocyte implantation in the patella: A multicenter experience. Am J Sports Med. 2014;42(5):1074-81. doi: 10.1177/0363546514523927. [DOI] [PubMed] [Google Scholar]
- 29. Niemeyer P, Salzmann G, Steinwachs M, Südkamp NP, Schmal H, Lenz P, et al. Presence of subchondral bone marrow edema at the time of treatment represents a negative prognostic factor for early outcome after autologous chondrocyte implantation. Arch Orthop Trauma Surg. 2010;130(8):977-83. doi: 10.1007/s00402-010-1049-8. [DOI] [PubMed] [Google Scholar]
- 30. Gomoll AH, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434-47. doi: 10.1007/s00167-010-1072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blackman AJ, Smith MV, Flanigan DC, Matava MJ, Wright RW, Brophy RH. Correlation between magnetic resonance imaging and clinical outcomes after cartilage repair surgery in the knee: a systematic review and meta-analysis. Am J Sports Med. 2013;41(6):1426-34. doi: 10.1177/0363546513485931. [DOI] [PubMed] [Google Scholar]
- 32. Choi YS, Potter HG, Chun TJ. MR imaging of cartilage repair in the knee and ankle. Radiographics. 2008;28(4):1043-59. doi: 10.1148/rg.284075111. [DOI] [PubMed] [Google Scholar]
- 33. Domayer SE, Welsch GH, Dorotka R, Mamisch TC, Marlovits S, Szomolanyi P, et al. MRI monitoring of cartilage repair in the knee: a review. Semin Musculoskelet Radiol. 2008;12(4):302-17. doi: 10.1055/s-0028-1100638. [DOI] [PubMed] [Google Scholar]
- 34. Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52(3):310-9. doi: 10.1016/j.ejrad.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 35. Moradi B, Schönit E, Nierhoff C, Hagmann S, Oberle D, Gotterbarm T, et al. First-generation autologous chondrocyte implantation in patients with cartilage defects of the knee: 7 to 14 years’ clinical and magnetic resonance imaging follow-up evaluation. Arthroscopy. 2012;28(12):1851-61. doi: 10.1016/j.arthro.2012.05.883. [DOI] [PubMed] [Google Scholar]
- 36. Bode G, Ogon P, Pestka J, Zwingmann J, Feucht M, Südkamp N, et al. Clinical outcome and return to work following single-stage combined autologous chondrocyte implantation and high tibial osteotomy. Int Orthop. 2015;39(4):689-96. doi: 10.1007/s00264-014-2547-z. [DOI] [PubMed] [Google Scholar]
- 37. Minas T, Von Keudell A, Bryant T, Gomoll AH. The John Insall Award: A minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res. 2014;472(1):41-51. doi: 10.1007/s11999-013-3146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ogura T, Mosier BA, Bryant T, Minas T. A 20-year follow-up after first-generation autologous chondrocyte implantation. Am J Sports Med. 2017;45(12):2751-61. doi: 10.1177/0363546517716631. [DOI] [PubMed] [Google Scholar]
- 39. Mundi R, Bedi A, Chow L, Crouch S, Simunovic N, Enselman ES, et al. Cartilage restoration of the knee: a systematic review and meta-analysis of level 1 studies. Am J Sports Med. 2016;44(7):1888-95. doi: 10.1177/0363546515589167. [DOI] [PubMed] [Google Scholar]