Abstract
Objective
Limitations of matrix-assisted autologous chondrocyte implantation to regenerate functional hyaline cartilage demand a better understanding of the underlying cellular/molecular processes. Thus, the regenerative capacity of a clinically approved hydrogel collagen type I implant was tested in a standardized bovine cartilage punch model.
Methods
Cartilage rings (outer diameter 6 mm; inner defect diameter 2 mm) were prepared from the bovine trochlear groove. Collagen implants (± bovine chondrocytes) were placed inside the cartilage rings and cultured up to 12 weeks. Cartilage-implant constructs were analyzed by histology (hematoxylin/eosin; safranin O), immunohistology (aggrecan, collagens 1 and 2), and for protein content, RNA expression, and implant push-out force.
Results
Cartilage-implant constructs revealed vital morphology, preserved matrix integrity throughout culture, progressive, but slight proteoglycan loss from the “host” cartilage or its surface and decreasing proteoglycan release into the culture supernatant. In contrast, collagen 2 and 1 content of cartilage and cartilage-implant interface was approximately constant over time. Cell-free and cell-loaded implants showed (1) cell migration onto/into the implant, (2) progressive deposition of aggrecan and constant levels of collagens 1 and 2, (3) progressively increased mRNA levels for aggrecan and collagen 2, and (4) significantly augmented push-out forces over time. Cell-loaded implants displayed a significantly earlier and more long-lasting deposition of aggrecan, as well as tendentially higher push-out forces.
Conclusion
Preserved tissue integrity and progressively increasing cartilage differentiation and push-out forces for up to 12 weeks of cultivation suggest initial cartilage regeneration and lateral bonding of the implant in this in vitro model for cartilage replacement materials.
Keywords: bovine cartilage punch model, collagen type I hydrogel, matrix-associated cartilage transplantation (MACT), (immuno)histology, implant push-out force
Introduction
Partial- and full-thickness traumatic or degenerative cartilage lesions of the knee are common and lead to progressive deterioration of the cartilage and finally osteoarthritis. Efficient and reliable methods for successful cartilage repair, including autologous chondrocyte implantation (ACI), microfracture, and osteoarticular transfer/mosaicplasty, are thus highly sought and have shown some promise in initial cartilage regeneration.1,2
These methods, however, mostly do not stop the progression of cartilage degeneration and usually result in an unpredictable outcome concerning (1) defect coverage, (2) fibrous or hyaline features of the repair tissue, and (3) considerably weaker mechanical properties and higher permeability of the repair tissue compared with native cartilage.3,4 Novel tissue engineering techniques, for example, second-generation ACI techniques such as matrix-assisted chondrocyte implantation (MACI), have led to an improvement of traditional methods and have recently entered clinical practice, usually aiming at providing stable and long-lasting attachment of the regenerated cartilage to the defect area.5-8 These techniques show the advantage of a decreased operation time, limited surgical trauma, and lack of complications associated with the use of periosteum in first-generation ACI (e.g., graft overgrowth) and have employed materials such as collagen membranes (type I/III collagen membrane).9-11
One commercial MACI product (type I collagen-based CaReS) has been extensively applied in Europe (and “off-label” in the United States) and is currently used with some clinical success.12 CaReS, a 3-dimensional hydrogel, is first seeded with in vitro–enriched autologous chondrocytes and then introduced into the debrided cartilage defect. Studies in the large animal model Goettinger minipig have recently shown that even a cell-free collagen type I hydrogel yields high-quality cartilage repair tissue comparable to that obtained after implantation of the cell-seeded implant. This suggests that cells migrating into the defect site from the surrounding cartilage may participate in the regeneration of cartilage.13-16 However, to our knowledge, there are presently no in vitro studies addressing the cellular and molecular mechanisms of cartilage regeneration in cartilage-implant constructs containing this cell-based collagen implant.
The main aim of the study was thus to analyze the behavior of this collagen implant in an in vitro model and to assess whether the results reflect its clinical performance for the therapy of cartilage defects. The following hypotheses were tested: (1) the experimental in vitro model is suitable for pre-testing of implants intended for the clinical regeneration of cartilage defects, (2) the model allows the description of the cellular and molecular processes underlying cartilage regeneration in vitro, (3) the cell-free and/or cell-loaded collagen implant supports the regeneration of the cartilage defect (via cell migration and tissue formation) in the in vitro model by its physicochemical and molecular structure.
For this purpose, a novel cartilage implant model was developed consisting of (1) “host” cartilage cylinders resected from bovine femoral condyles using standardized punches and (2) inserted matrix implants. The cylinders were filled with the cell-free or cell-loaded collagen I scaffold, cultured for periods of up to 12 weeks, and then subjected to (1) (immuno)histological staining (hematoxylin/eosin, safranin O; aggrecan, collagens 1 and 2); (2) protein assays (dimethyleneblue-binding [DMB]-test for tissue proteoglycan analysis; enzyme-linked immunosorbent assay [ELISA] for aggrecan, collagens 1 and 2); (3) transcriptional analysis (expression of aggrecan, collagen 1, and collagen 2); and (4) measurement of implant push-out force.
Methods
Preparation/Culture of Cartilage Rings with Cell-Free or Cell-Loaded Collagen Implants
Bovine cartilage was obtained from the knee joint of German Holstein Friesian Cattle (age 24 months).17 Collagen implants (Amedrix GmbH, Esslingen; Germany; cell-free or cell-seeded with bovine chondrocytes; ± cells; cell isolation and seeding according to internal guidelines of the producer) were placed into the inner defect of the cartilage rings ( Fig. 1 ). The constructs were then embedded in an agarose cylinder in 48-well plates ( Fig. 1A-E ) and cultured for 0, 4, 8, 10, and 12 weeks at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s culture medium (DMEM; containing 5% fetal calf serum [FCS], 1% gentamycin, and 0.1% ITS (insulin-transferrin-selenium)–culture supplement [final concentrations: 5 μg/mL insulin and transferrin, 5 ng/mL selenic acid; BD Biosciences, Heidelberg, Germany]). Media were changed 3 times a week.
Figure 1.
Scheme of the in vitro model. For embedding of the cartilage-implant constructs, hot liquid agarose (2%) was added into the wells of a 48-well plate (A). Cylindrical pockets of a defined size (6 mm) were created by inserting a metal-pin plate into the hot agarose until it gelated (B, C). The central defects of the cartilage rings (diameter 2 mm) were filled with the collagen implant (cell-free/cell-loaded; diameter 6 mm) using forceps (C1) and, after embedding the resulting constructs into the agarose (D), culture medium was added (E). After in vitro culture, cartilage-implant constructs were subjected to histological characterization. Also, gene expression of chondrocytes isolated from the “host” cartilage, cells on the cartilage surface, and the collagen implant was analyzed (F). At the protein level, the amount of cartilage components released into the supernatant, as well as the remaining content in “host” cartilage rings and the cells located on the cartilage surface was quantified.
In each experimental series, 120 technical replicates of cartilage rings each were obtained from one animal each for both cell-free and cell-loaded collagen implants (n = 5 and 6 experimental series, respectively) and subsequently analyzed histologically (n = 4), biochemically (n = 10; n = 5 each for real time polymerase chain reaction [RT-PCR] and protein extraction), and biomechanically (n = 10; total of 24 samples for each of the 5 time points; see below). Supernatants were pooled over 1 week and stored at −20°C for further ELISA analysis.
Viability Assay
Cartilage rings were obtained at weekly intervals from in vitro culture, minced using a scalpel and a pair of scissors and initially digested with pronase E (Merck, Darmstadt, Germany; 1 mg/mL) in serum-free DMEM for 1 hour at 37°C, 5% CO2, and 95% humidity under constant stirring. After three washes in phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4 × 2 H2O, 2 mM KH2PO4; pH 7.4), they were incubated for 17 hours at 37°C in DMEM containing 5% FCS (Invitrogen, Darmstadt, Germany) and collagenase P (0.1 mg/mL; Roche, Mannheim, Germany). Chondrocytes were then centrifuged (1500 rpm; Eppendorf mini spin; Eppendorf, Hamburg, Germany) for 10 minutes, washed 3 times with PBS containing 5% FCS, and counted.
A total of 1 × 104 cells were seeded in culture slides (Becton Dickinson–Discovery Labware Products, Bedford, MA, USA) and cultivated for 1 day. Viability was assessed using fluorescein diacetate/propidium iodide life/dead staining.18 Three images each were taken in 3 chambers of the culture slides using a fluorescence microscope Axiovert200M, an AxioCam MRm camera, and the AxioVision Rel4.8 program (all Zeiss, Oberkochen, Germany). Viable and dead cells were counted in all images using the cellprofiler software (www.cellprofiler.org)19 and viability was expressed as the mean percentage of viable cells.
Histology and Immunohistology
Paraffin sections of the cartilage-implant constructs were cut into 6-µm sections and stained with hematoxylin and eosin (H&E) and safranin O (to assess the proteoglycan content).
For aggrecan immunhistology, sections were treated with chondroitinase ABC (0.25 U/mL; Sigma-Aldrich; 37°C, 90 minutes), blocked with hydrogen peroxide and 10% goat serum/Tris-biuffered saline (TBS), and incubated overnight at 4°C with the primary antibody (0.1 µg/mL; clone: MA85A95; GenTex, Irvine, CA, USA).
For collagen 1 and 2 staining, the epitopes were first demasked by incubation with proteinase K (code S3004; diluted 1:50; Dako, Hamburg, Germany) at room temperature (RT) for 15 minutes. The sections were then subjected to blocking of the endogenous peroxidase activity with 0.5% hydrogen peroxide in methanol for 10 minutes, blocking with 25% normal bovine serum albumin (BSA)/TBS for 30 minutes (both at RT), and overnight incubation at 4°C with a primary antibody to bovine collagen 1 (2 µL/mL; polyclonal rabbit IgG; Acris, Herford, Germany) or collagen 2 (10 µg/mL; polyclonal rabbit IgG; Acris, Herford, Germany).
This was followed by incubation for 1 hour at RT with a secondary antibody (anti-mouse or anti-rabbit) coupled to horseradish peroxidase (HRP; for collagen 1 and 2 antibodies) or alkaline phosphatase (ALP; for aggrecan) and visualization of the HRP with diaminobenzidine (DAB) and the ALP with Fast Red (both Sigma Aldrich). Sections were then counterstained with hematoxylin and mounted with Aquatex (Merck, Darmstadt, Germany) for light microscopy.
(Isotype-matched) control immunoglobulins consistently yielded negative results.
Score for Cell Migration
Cell migration onto/into the collagen implants was evaluated by 3 independent observers using a scoring system, which consisted of 4 levels (0 = implant without cells, 1 = single adherent cells, 2 = several adherent cells, 3 = cell-layer on the implant).17 Data were expressed as the means ± standard error of the mean of the values of the three individual observers.
Histology/Immunohistology Scores for Safranin O, Collagen 1, Collagen 2, and Aggrecan
The intensity of the red color in the safranin O staining or the positive signal in the immunostaining for aggrecan, collagen 2, and collagen 1 were semiquantitatively evaluated using a scoring system with 4 levels (0 = no staining; 1 = weak staining; 2 = moderate staining; 3 = strong staining).17 Scoring was performed by 3 independent observers, data were expressed as the means ± standard error of the mean of the values of the 3 individual observers.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
For the isolation of RNA from (1) cells located in the matrix of the “host” cartilage, (2) cells located on the cartilage surface (see below), and (3) cells migrated onto and into the collagen implants (± cells; Fig. 1F ), fine pointed forceps were used to first remove the cartilage-implant constructs from the agarose wells and then carefully separate the collagen implants (± cells) from the surrounding cartilage ring. A total of ten collagen implants were pooled and stored in a tube with 300 µL lysis buffer at −80°C for subsequent RNA isolation.
The empty cartilage rings were then treated for 1 minute in a tube with 300 µL lysis buffer under continuous shaking to obtain the RNA from the cells located on the surface of the cartilage rings.17,20 Subsequently, the rings were removed from the buffer and both components were separately stored at −80°C.
The cartilage rings were disintegrated with a pair of scissors in 800 µL TriZol (Life Technologies, Carlsbad, CA, USA), incubation at RT for 15 minutes, and centrifugation at 12,000 rpm for 3 minutes.21 Total RNA from these samples, the lysed cell fractions (cells on cartilage surface) or the collagen implants was then isolated using the Quiagen Kit (RNeasy mini kit; Qiagen, Hilden, Germany; including a DNase digestion).
The RNA eluate (12 µL each) was primed with oligo(d)T for 10 minutes at 72°C and reverse transcribed for one hour at 42°C using superscript II (Invitrogen). To quantify aggrecan, collagens 1 and 2, and the housekeeping gene aldolase, qRT-PCR (i-cycler PCR system; BioRad, Munich, Germany) was performed using the primers listed in Table 1 and PCR products from bovine chondrocytes as standards. Gene expression in all samples was normalized to the relative expression level of aldolase. Product specificity was confirmed by melting curve analysis and initial cycle sequencing of the PCR products.
Table 1.
Primers, Product Length, and Specific Amplification Conditions for Real Time Polymerase Chain Reaction.a
| Gene | Upstream Primer (5′-3′) | Downstream Primer (3′-5′) | Product Length | Annealing Temperature (°C) | Melting Temperature (°C) |
|---|---|---|---|---|---|
| Aggrecan | CAGAGTTCAGTGGGACAGCA | AGACACCCAGCTCTCCTGAA | 193 | 60 | 84 |
| Collagen 2 | CATCTGGTTTGGAGAAACCATC | GCCCAGTTCAGGTCTCTTAG | 600 | 61 | 83 |
| Collagen 1 | AGCCAGCAGATCGAGAACAT | ACACAGGTCTCACCGGTTTC | 185 | 60 | 86 |
| Aldolase | CACCGGATTGTGGCTCCGGG | CGCCCCCGATGCAGGGATTC | 170 | 58 | 88 |
General amplification protocol (40 cycles): initial denaturation for 90 seconds at 95°C; denaturation for 20 seconds at 94°C, specific primer annealing temperature (see above) for 20 seconds, amplification at 72°C for 30 seconds, additional heating step at 84°C; denaturation for 1 minute at 95°C; cooling to 60°C (holding for 10 seconds).
Protein Extraction
To determine the relative protein amounts of aggrecan, collagen 2, and collagen 1, protein was extracted from (1) the “host” cartilage (after removal of the collagen implants) and (2) the cells located on the cartilage surface (see above). Protein from the “host” cartilage was isolated by disintegration in 1000 µL of 4 M GuHCL with a pair of scissors and incubation for 48 hours at 4°C under rotation. Protein from the cells located on the cartilage surface was isolated using the acetone precipitate of the lysis buffer according to the supplier’s instruction of the RNeasy mini kit (Qiagen).
Quantification of Glycosaminoglycans
Glycosaminoglycans released from the cartilage-implant constructs into the supernatant during culture, as well as the remaining content in the cartilage rings and the cells located on the cartilage surface, were quantified using the DMB assay.22,23 Supernatants were analyzed by pooling the supernatants of the respective week and group. Briefly, 25 µL of pooled supernatant or extracted/precipitated proteins, respectively, were applied to microtiter plates with or without dilution in 0.05 M sodium acetate buffer (pH 6.8). After addition of 200 µL DMB reagent (containing 1.9-dimethyleneblue (21 µg/mL), 55 nM formic acid; pH 6.8]), absorption was read at 525 nm. A dilution series of a chondroitin-4 sulfate standard (Sigma-Aldrich) was used for the generation of a standard curve and the calculation of the results.
Enzyme-Linked Immunosorbent Assay (ELISA)
Aggrecan, collagen 2, and collagen 1 concentrations in the supernatant during culture, as well as the remaining content in the cartilage rings and the cells located on the cartilage surface, were quantified using ELISA kits. Supernatants of the cartilage-implant constructs (± cells) after 0, 4, 8, 10, and 12 weeks of cultivation were analyzed by pooling the supernatants of the respective week and group. Aggrecan, collagen 2, and collagen 1 concentrations were then measured according to the protocols of commercially available ELISA kits (Chondrex, Redmond, WA, USA; BlueGene, Shanghai, China). Absorption at 490 nm was measured using a Fluostar Optima Reader (BMG Labtech GmbH).
Biomechanical Testing
Biomechanical testing (n = 10 for each time point) was performed using a static universal test system (Zwicki 1120, Zwicki/Roelli, Ulm, Germany). The maximal force required to push out the implant from the cartilage rings (Fmax(insert)) was determined using a cylindrical indenter (diameter 1.8 mm; i.e., 0.2 mm less than the diameter of the central defect). To address remaining friction forces between indenter and empty “host” cartilage ring, a second identical test was then performed and the resulting force (Fmax(empty)) was subtracted from the initial push-out force [Fmax(insert) − Fmax(empty) = ΔFmax(res)] and used for further analysis. Values were given in Newtons and, in order to allow a comparison with published studies, in kPa after dividing the values in Newtons by the lateral surface area of the implant cylinder (0.8163 mm2).
Statistical Analysis
Results were expressed as means ± standard error of the mean. Statistical analysis was performed using the Mann-Whitney U test and the software SPSS 22.0 (P ≤ 0.05).
Results
Cell-Free Collagen Implants
Morphological Characteristics, Viability, and Cell Migration
In the case of cell-free cartilage-implant constructs, lateral contact of the collagen insert to the cylindrical defect was maintained throughout tissue culture for 12 weeks ( Fig. 2 ). Despite relatively long in vitro culture periods (up to 12 weeks), resident cartilage cells showed vital morphology without signs of alterations and positive nuclear staining, thus pointing to suitable culture conditions ( Fig. 2 ). The matrix integrity of the cartilage seemed to be largely unaffected during the whole culture period ( Fig. 2 ), although cartilage zones located close to the edge of the defect were characterized by the appearance of proliferation-induced cell clusters as a possible reaction to the initial mechanical tissue disruption (starting at 4 weeks; Fig. 2 ; see hash). In addition, late time points showed empty chondrocyte lacunae as a possible sign of chondrocyte emigration from the “host” cartilage ring ( Fig. 2 ; see arrow).
Figure 2.
Hematoxylin and eosin staining of the cartilage-implant constructs (cell-free or cell-loaded implants) after placement into the inner defect of the cartilage rings and subsequent culture for 0, 4, 8, 10, or 12 weeks. Morphological characteristics of the cells in cartilage, implant and at the cartilage-implant interface; # proliferation-induced cell clusters; → empty chondrocyte lacunae; * cellular multilayer.
The high viability of the chondrocytes in the cartilage rings was confirmed by fluorescein diacetate/propidium iodide live-dead staining of chondrocytes enzymatically isolated from the cartilage at weekly intervals, resulting in viability rates of >94% throughout the entire culture period ( Fig. 3 ).
Figure 3.
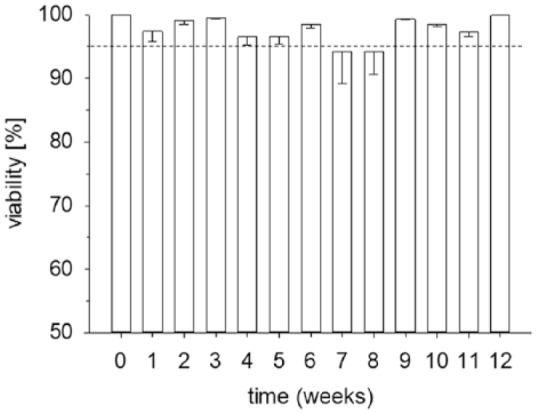
Viability of the chondrocytes in the cartilage ring throughout culture. Chondrocytes were enzymatically isolated from the cartilage at weekly intervals and cultivated for 1 day. Viability was assessed using fluorescein diacetate/propidium iodide staining. Data are expressed as means ± standard error of the mean (SEM); the dashed line indicates a viability of 95%.
Cartilage-implant constructs showed progressive formation of a cellular multilayer on the surface of the cartilage rings throughout cultivation ( Fig. 2 ; see asterisks), as well as initial migration of chondrocytes onto and into the initially cell-free collagen type 1 implants starting at 4 weeks of in vitro culture ( Figs. 2 and 4 ). There was a significant increase of the cell colonization from 0 weeks to all other time points and from 4 weeks to 8 and 12 weeks ( Fig. 4 ).
Figure 4.
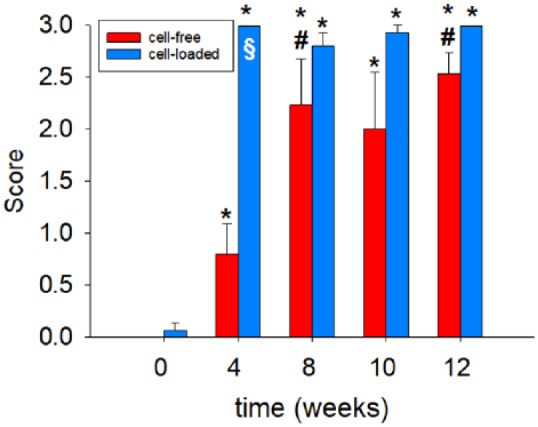
Semiquantitative scoring of cell migration onto/into the collagen implants after culture for 0, 4, 8, 10, or 12 weeks (cell-free; cell-loaded). Degree of migration: 0 = implant without cells, 1 = single adherent cells, 2 = several adherent cells, 3 = cell layer on implant; values are shown as means ± standard error of the mean (SEM); symbols indicate P ≤ 0.05 versus *0 weeks and #4 weeks; §versus cell-free.
Both the cells on/in the collagen implants and on the cartilage surface showed mixed fibroblastic and chondrocytic features ( Fig. 2 ; see asterisk for cell-loaded implants at 12 weeks) and positively stained for safranin O, aggrecan, and collagen 2, but only very weakly or negative for collagen 1 ( Fig. 5 ).
Figure 5.
(Immuno)staining of the collagen implants (cell-free) after placement into the inner defect of the cartilage rings and subsequent culture for 0, 4, 8, 10, or 12 weeks. Safranin O staining and immunostaining for aggrecan, collagen 2, and collagen 1 (for quantification see Fig. 6 ); staining with (isotype-matched) control immunoglobulins consistently yielded negative results.
Content of Proteoglycans, Aggrecan, Collagen 2, and Collagen 1
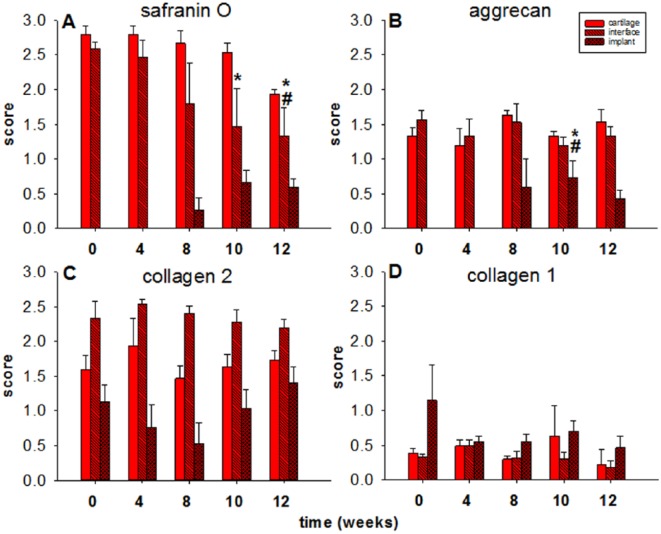
The finding of a preserved matrix integrity in the surrounding cartilage ring was further supported by a very limited, nonsignificant decrease of the safranin O staining intensity over time (from a semiquantitative score of 2.8 for the freshly isolated cartilage to a score of 1.9 at 12 weeks; Figs. 5 and 6A ); this suggested a minimal loss of proteoglycan during 12 weeks of in vitro culture.
Figure 6.
Semiquantitative analysis of “host” cartilage, interface, and collagen implants (cell-free). Score: 0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining; values are shown as means ± standard error of the mean (SEM); symbols indicate P ≤ 0.05 versus *0 weeks, #4 weeks.
At the cartilage-implant interface there was also a loss of safranin O staining (from 2.6 to 1.3), which reached statistical significance for the 10- and 12-week values in comparison with the 0-week and/or 4-week values ( Figs. 5 and 6A ). In the implant, staining was first noticed at 8 weeks and then reached a plateau at 10 and 12 weeks ( Figs. 5 and 6A ), indicating proteoglycan deposition into the implant.
As for the safranin O staining, a largely constant immunostaining for aggrecan was observed in the surrounding cartilage ring and at its interface with the implant throughout the 12-week culture period (constant scores between 1.2 and 1.7 over time; Figs. 5 and 6B ), again suggesting a minimal loss of proteoglycan during in vitro culture.
Also, staining for aggrecan in the implant was first noticed at 8 weeks and then reached a plateau at 10 weeks (P ≤ 0.05 vs. 0 and 4 weeks) and 12 weeks ( Figs. 5 and 6B ), confirming proteoglycan deposition into the implant.
As in the case of aggrecan, largely constant immunostaining for collagen 2 was observed throughout the 12-week culture period in surrounding cartilage ring (scores between 1.5 and 1.9), cartilage-implant interface (scores between 2.2 and 2.5), and implant (scores between 0.5 and 1.4), without any significant differences among the different time points ( Figs. 5 and 6C ), again supporting the concept of a preserved matrix integrity.
Very little immunostaining for collagen 1 (except for the implant 0-week value, scores mostly between 0.2 and 0.7) was observed in surrounding cartilage ring, interface, and implant, without any significant differences among the different time points ( Figs. 5 and 6D ).
DMB Assay (Proteoglycan Content; Tissue Extracts and Culture Supernatant)
As observed by safranin O staining and aggrecan immunostaining, there was a limited, nonsignificant decrease of the glycosaminoglycan (GAG) content in the surrounding cartilage ring over time (from 4911 µg/mL for the freshly isolated cartilage to 3627 µg/mL at 12 weeks, with an intermediate peak of 5785 µg/mL at 4 weeks; Supplementary Figure 1A, available in the online version of the article).
The chondrocytes that migrated onto the surface of the cartilage ring generally showed an approximately 10-fold lower GAG content, with a significant decrease over time (from 537 µg/mL for freshly isolated cartilage to 378 µg/mL at 12 weeks, with an intermediate peak of 689 µg/mL at 8 weeks; P ≤ 0.05 vs. 4 and 8 weeks for the 10-week time point, P ≤ 0.05 vs. 4 weeks for the 12-week time point; Supplementary Figure 1A).
Also, in the culture supernatant, there was a limited, nonsignificant decrease of the GAG content over time (from 193 µg/mL at 4 weeks to 146 µg/mL at 12 weeks; Supplementary Figure 1A). This was confirmed by a limited, nonsignificant decrease of the aggrecan release into the supernatant over time (ELISA; from 17 ng/mL at 4 weeks to 10 ng/mL at 12 weeks; scores not shown).
ELISA (Collagens 2 and 1; tissue Extracts and Culture Supernatant)
A significant decrease of the collagen 2 content was observed in the surrounding cartilage ring over time (from 1091 ng/mL at 0 weeks to 296 ng/mL at 12 weeks, P ≤ 0.05 vs. 0 weeks for the 8-week time point; Supplementary Figure 1C). The chondrocytes migrated onto the surface of the cartilage ring showed an approximately 4-fold higher collagen 2 content, with a nonsignificant decrease over time (from 3757 ng/mL at 0 weeks to 2644 ng/mL at 12 weeks; Supplementary Figure 1C).
There was also a limited, nonsignificant decrease of the collagen 2 release into the supernatant over time (from 1381 ng/mL at 4 weeks to 269 ng/mL at 12 weeks; Supplementary Figure 1C).
In contrast to the collagen 2 release, there was a limited, but significant increase of the collagen 1 release into the supernatant over time (from 185 ng/mL at 4 weeks to a peak of 422 ng/mL at 8 weeks and a subsequent plateau; P ≤ 0.05 vs. 4 weeks for the 8-week time point; data not shown).
Gene Expression for Aggrecan, Collagen 2, and Collagen 1 (RT-PCR)
Aggrecan expression showed a slight increase over time in cartilage ring (maximum 4-fold increase at 12 weeks), cartilage surface cells (maximum 7-fold; 12 weeks), and, interestingly, in the implant (maximum 3-fold; 10 weeks; Fig. 7A ).
Figure 7.
Real-time polymerase chain reaction analysis for aggrecan, collagen 1, and collagen 2 (cell-free implants). mRNA expression for aggrecan (A), collagen 2 (B), collagen 1 (C), aggrecan/collagen 1 ratio (D), and collagen 2/collagen 1 ratio (E) was determined prior to and after 4, 8, 10, and 12 weeks of in vitro culture; relative gene expression of the cells located in the “host” cartilage matrix (cartilage), on the cartilage surface (cartilage surface), and on/in the collagen implant (implant); values are expressed as means ± standard error of the mean (SEM); symbols indicate P ≤ 0.05 *versus 0 weeks; #versus 4 weeks; xversus 8 weeks; +versus 10 weeks.
Whereas collagen 2 expression in the cartilage ring was constant or even significantly decreased (P ≤ 0.05 for 4, 10, and 12 weeks vs. 0 weeks), collagen 2 expression in surface cells and implant rose from baseline values to a transient peak at 8 weeks of 13-fold and 9-fold, respectively, and thereafter decreased again ( Fig. 7B ).
Whereas collagen 1 expression in cartilage ring and surface cells rose to a transient peak at 8 weeks of 61-fold and 30-fold, respectively, and thereafter decreased again (surface cells P ≤ 0.05 for 12 vs. 4 weeks), collagen 1 expression in the implant was constant or even decreased over time ( Fig. 7C ).
The above changes were reflected in a constant aggrecan/collagen 1 ratio in the cartilage ring, and a transient peak at 10 weeks for surface cells (37-fold) and implant (13-fold; Fig. 7D ).
As for the aggrecan/collagen 1 ratio, there was a marginally decreased collagen 2/collagen 1 ratio in the cartilage ring (P ≤ 0.05 for 8, 10, and 12 weeks vs. 4 weeks), and a transient peak at 8 or 10 weeks for surface cells (7-fold) and implant (13-fold; Fig. 7E ).
Cell-Loaded Collagen Implants
Morphological Characteristics and Cell Migration
The findings in cell-loaded cartilage-implant constructs were generally comparable to those in cell-free cartilage-implant constructs, with lateral contact of the implant to the cylindrical defect, vital morphology of the resident cartilage for up to 12 weeks cells, and largely preserved matrix integrity ( Fig. 2 ).
As in the case of cell-free constructs, cell-loaded cartilage-implant constructs also showed progressive formation of a cellular multilayer on the surface of the cartilage rings throughout cultivation (in part with chondrocytic features; Fig. 2 ; see asterisk at 12 weeks). However, a substantial presence/migration of chondrocytes onto and into the initially cell-free collagen type 1 implants occurred already after 4 weeks of in vitro culture ( Figs. 2 and 4 ). There was again a significant increase of the cell colonization from 0 weeks to all other time points ( Fig. 4 ).
Interestingly, cell-loaded collagen implants showed a significantly faster cell colonization than initially cell-free collagen implants (P ≤ 0.05 at 4 weeks; Fig. 4 ).
Content of Proteoglycans, Aggrecan, Collagen 2, and Collagen 1
Also, in this group, there was a very limited, nonsignificant decrease of the safranin O staining intensity over time in the cartilage ring (from a semiquantitative score of 2.4 for the freshly isolated cartilage to a score of 1.5 at 12 weeks; Figs. 8 and 9A ).
Figure 8.
(Immuno)staining of the collagen implants (cell-loaded) after placement into the inner defect of the cartilage rings and subsequent culture for 0, 4, 8, 10, or 12 weeks. Safranin O staining and immunostaining for aggrecan, collagen 2, and collagen 1 (for quantification see Fig. 9 ); staining with (isotype-matched) control immunoglobulins consistently yielded negative results.
Figure 9.
Semiquantitative analysis of “host” cartilage, interface, and collagen implants (cell-loaded). Score: 0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining; values are shown as means ± standard error of the mean (SEM); symbols indicate P ≤ 0.05 versus *0 weeks, #4 weeks, +10 weeks; §versus cell-free.
At the interface, there was also a loss of safranin O staining (from 2.3 to 0.9), which reached statistical significance for the 8, 10, and 12-week values in comparison to the 0-week values ( Figs. 8 and 9A ).
In the implant, significantly elevated staining was first noticed at 4 weeks and then reached a plateau thereafter ( Figs. 8 and 9A ; P ≤ 0.05 vs. 0 weeks for 4, 8, 10, and 12 weeks).
As for the safranin O staining, a largely constant immunostaining for aggrecan was observed in surrounding cartilage ring and interface throughout the 12-week culture period (constant scores between 1.1 and 1.9 over time; Figs. 8 and 9B ).
Also, substantial staining for aggrecan in the implant was first noticed at 4 weeks and reached a plateau thereafter ( Figs. 8 and 9A ; P ≤ 0.05 vs. 0 weeks for 4, 8, or 12 weeks). Notably, at 4 and 12 weeks the score for cell-loaded implants was significantly higher than that in cell-free implants (compare Figs. 6B and 9B ).
Also, for collagen 2, largely constant immunostaining was observed throughout the 12-week culture period in surrounding cartilage ring (scores between 1.9 and 2.2), interface (between 1.6 and 2.4), and implant (scores between 0.7 and 1.4), without any significant differences among the different time points ( Figs. 8 and 9C ).
Very little immunostaining for collagen 1 (except for one 0-week value, scores mostly between 0.2 and 0.8) was observed in surrounding cartilage ring, interface, and implant, for the latter with significantly lower values versus 0 weeks at 4, 8, 10, and 12 weeks ( Figs. 8 and 9D ).
DMB Assay (Proteoglycan Content; Tissue Extracts and Culture Supernatant)
In the cell-loaded cartilage-implant constructs, there was a limited, nonsignificant decrease of the GAG content in the surrounding cartilage ring over time with a peak at 4 weeks of in vitro culture (from 5485 µg/mL for the freshly isolated cartilage to 4820 µg/mL at 12 weeks; intermediate peak of 6373 µg/mL at 4 weeks; Supplementary Figure 1B).
Interestingly, the GAG content in the surrounding cartilage ring of cell-loaded constructs was significantly higher than that of cell-free constructs at all time points (Supplementary Figure 1A and B).
The chondrocytes migrated onto the surface of the cartilage ring again showed an approximately 10-fold lower GAG content, with a significant decrease over time (from 449 µg/mL for the freshly isolated cartilage to 405 µg/mL at 12 weeks; intermediate peak of 516 µg/mL at 4 weeks; P ≤ 0.05 vs. 8 weeks for the 10-week time point; Supplementary Figure 1B). There was only a marginally lower GAG content in cell-loaded versus cell-free cartilage-implant constructs (compare Supplementary Figure 1A and B).
In the supernatant, there was a limited, decrease of the GAG release over time (from 190 µg/mL at 4 weeks to 154 µg/mL at 12 weeks; P ≤ 0.05 vs. 4 weeks for the 8- and 10-week time points), as confirmed by a limited, significant decrease of the aggrecan release into the supernatant over time (ELISA; from 24 ng/mL at 4 weeks to 10 ng/mL at 12 weeks; P ≤ 0.05 vs. 10 weeks for the 12-week time point; data not shown).
ELISA (Collagens 2 and 1; Tissue Extracts and Culture Supernatant)
As observed for the cell-free cartilage-implant constructs, there was a limited, nonsignificant decrease of the collagen 2 content in the surrounding cartilage ring up to 8 weeks of in vitro culture with a slight increase thereafter (from 1580 ng/mL at 0 weeks to 523 ng/mL at 12 weeks; Supplementary Figure 1D). The chondrocytes migrated onto the surface of the cartilage ring showed an approximately 3-fold higher collagen 2 release than the cartilage ring itself, with a nonsignificant, slight decrease over time (from 3234 ng/mL at 0 weeks to 2611 ng/mL at 12 weeks; Supplementary Figure 1D). Also, there was a limited, nonsignificant decrease of the collagen 2 release into the supernatant up to 10 weeks of culture with a slight increase thereafter (from 2115 ng/mL at 4 weeks to 854 ng/mL at 12 weeks; Supplementary Figure 1D).
In contrast to the collagen 2 release, there was nonsignificant increase of the collagen 1 release into the supernatant over time (from 270 ng/mL at 4 weeks to a peak of 429 ng/mL at 10 weeks and a subsequent plateau; data not shown).
Gene Expression for Aggrecan, Collagen 2, and Collagen 1 (RT-PCR)
Whereas aggrecan expression in cartilage ring and implant rose to a transient peak at 8 weeks of 10-fold and 6-fold, respectively, and thereafter decreased again, aggrecan expression in the surface cells was constant over time ( Fig. 10A ).
Figure 10.
Real time polymerase chain reaction analysis for aggrecan, collagen 1, and collagen 2 (cell-loaded implants). mRNA expression for aggrecan (A), collagen 2 (B), collagen 1 (C), aggrecan/collagen 1 ratio (D) and collagen 2/collagen 1 ratio (E) was determined prior to and after 4, 8, 10, and 12 weeks of in vitro culture; relative gene expression of the cells located in the “host” cartilage matrix (cartilage), on the cartilage surface (cartilage surface), and on/in the collagen implant (implant); values are expressed as means ± standard error of the mean (SEM); symbols indicate P ≤ 0.05 versus #4 weeks; §versus cell-free.
The collagen 2 expression in cartilage ring and surface cells was constant over time. In contrast, the collagen 2 expression in the implant rose to a transient peak at 8 weeks of 7-fold and thereafter decreased again ( Fig. 10B ).
Collagen 1 expression reached a 10-week nonsignificant peak in cartilage ring (10-fold in comparison to 0 weeks) and surface cells (30-fold), and a 12-week nonsignificant peak in the implant (11-fold; Fig. 10C ).
The aggrecan/collagen 1 ratio in the cartilage ring showed an increase over time with a 12-week, 32-fold peak in comparison with 0 weeks. In contrast, the aggrecan/collagen 1 ratio of the surface cells and the implant was mostly constant over time ( Fig. 10D ).
The collagen 2/collagen 1 ratio in cartilage ring, surface cells, and implant only marginally changed over time, without any significant differences among the different time points ( Fig. 10E ).
Push-Out Forces of the Cultivated Cartilage-Implant Constructs (Biomechanical Testing)
Strikingly, the push-out force for cell-free implants showed a progressive increase during culture (from 0.002 ± 0.000 N or 2.560 ± 0.680 kPa at 0 weeks to a peak of 0.061 ± 0.056 N or 75.858 ± 68.780 kPa at 8 weeks and a subsequent plateau; P ≤ 0.05 vs 0 weeks for 4, 8, 10, and 12 weeks; Fig. 11 ).
Figure 11.

Biomechanical push-out testing of the cartilage-implant constructs (cell-free or cell-loaded implants). Values are expressed as means ± standard error of the mean (SEM); the symbols indicate P ≤ 0.05 *versus 0 weeks; #versus 4 weeks.
This was also the case for cell-loaded implants, which showed a progressive increase during culture from 0.012 ± 0.007 N (15.026 ± 9.095 kPa) at 0 weeks to a peak of 0.126 ± 0.031 N (154.344 ± 38.032 kPa) at 12 weeks (P ≤ 0.05 vs. 4 weeks for 8, 10, and 12 weeks; Fig. 11 ). At any time point, the values for cell-loaded implants were numerically higher than those for cell-free implants ( Fig. 11 ).
Discussion
The aim of the present study was to test the long-term performance (12 weeks) of a collagen type 1 hydrogel in a standardized in vitro bovine cartilage punch model. The main findings of the study were that (1) cartilage-implant constructs revealed vital morphology, preserved matrix integrity, progressive, but only slight proteoglycan loss in the “host” cartilage ring, and decreasing proteoglycan release into the culture supernatant and (2) cell-free or cell-loaded collagen implants both showed substantial cell immigration/colonization, progressively increased levels of aggrecan (mRNA/protein) and collagen 2 (mRNA increasing/protein constant), and significantly augmented push-out forces of the implants from the “host” cartilage ring over time. Compared with cell-free collagen implants, interestingly, cell-loaded implants displayed significantly earlier cell immigration/colonization, earlier and longer lasting deposition of aggrecan, and tendentially higher push-out forces.
The results of the present study are thus in good agreement with the known biocompatibility of the already clinically approved CaReS implant, in either cell-free or cell-loaded form, as a scaffold material in general and its good performance as an implant material for cartilage repair in vitro and in vivo.16,24-27 In addition, the study provides in vitro data concerning the dynamic molecular processes underlying cartilage regeneration and may be applicable for systematic in vitro screening of future cartilage replacement materials.
As previously published, our present in vitro cartilage regeneration model is highly suitable for the testing of chondral implants with proven or expected clinical potential,17,20,28-34 with easily available, ethically unproblematic, and reproducible tissue supply and the possibility of long-term in vitro culture for up to 12 weeks without major cartilage degeneration. Modified in vitro systems, including subchondral bone (thus addressing the contribution of bone-derived secreted factors or progenitor cells) may provide a more complex understanding of the performance of the cartilage-bone unit in cartilage regeneration than cartilage only-based models. However, such systems may present with technical challenges such as the complete removal of the cartilage layer without damage to the subchondral bone lamella (unpublished data35,36).
On the other hand, cartilage zones located close to the edge of the defect contained proliferation-induced cell clusters as a possible reaction to the initial mechanical tissue disruption and showed empty chondrocyte lacunae. While these changes may indicate some degree of cartilage degeneration in the “host” cartilage,37 they also likely suggest an attempt to repair damaged cartilage and seed the cartilage implant.38
Long-term stability of the “host” cartilage ring over time was indicated by (1) limited histological signs of cartilage degeneration and chondrocyte viability rates of >94% throughout culture, (2) limited loss of proteoglycan and stable protein content of collagen 2 (and very small amounts of collagen 1), (3) largely constant or even increasing mRNA expression for aggrecan and/or collagen 2, and (4) progressively increased anchoring of the implant in the sense of “lateral bonding.” This shows that the present system allows long-term in vitro culture, which may yield meaningful results with an at least partial transferability to the in vivo situation.
Both cell-free and cell-loaded cartilage-implant constructs showed substantial colonization of the implant, which was paralleled by signs of chondrocyte emigration especially from the superficial regions of the “host” cartilage ring. This confirms the high cyto- or biocompatibility of the type 1 collagen–based implants in the present in vitro system, as previously reported in experimental16,39,40 and clinical in vivo studies.12,25-27 Interestingly, cell loading of the collagen implants appeared to accelerate and/or amplify the process of cartilage regeneration in the implant, as indicated by significantly faster cell colonization. These results argue for an advantage of cell-loaded versus cell-free collagen implants, in agreement with earlier studies on cell-seeded or cell-containing implants based on collagen or other cartilage replacement materials.41-44 Alternatively, in vivo microfracturing of the subchondral bone plate below the cartilage implant can be used to favor cell immigration into initially cell-free implants and optimize the conditions for successful cartilage repair.39,40,45
Cell-free and cell-loaded collagen implants both showed progressive deposition of the matrix molecule aggrecan and a stable protein content of collagen 2 throughout culture, again demonstrating the cytocompatibility of the implant material and the active production of cartilage-specific matrix molecules by vital chondrocytes.46 This was further underlined by the progressively increasing mRNA levels for aggrecan and collagen 2 in the implant.
In addition, the locally produced, cartilage-specific matrix molecules appeared to be successfully retained in the collagen implants, as indicated by a progressive decrease of the aggrecan and collagen 2 release into the culture supernatant over time. These results are in good agreement with in vitro data obtained with other clinically applied cartilage implants.41,43,47 As discussed above in the context of cell immigration, cell loading of the collagen implants accelerated and/or amplified cartilage regeneration, as emphasized by a significantly earlier and more long-lasting appearance/presence of aggrecan, again supporting an advantage of cell-loaded cartilage implants.There were no apparent signs of chondrocyte de-differentiation in either cell-free or cell-loaded collagen implants over time, since (1) cartilage-specific aggrecan protein content increased to a plateau at approximately 8 weeks and thereafter, (2) collagen 2 (and collagen 1) protein content was stable over time, and (3) the mRNA ratios for aggrecan/collagen 1 and collagen 2/collagen 1 were either constant or considerably increased throughout in vitro culture. The present in vitro model of cartilage repair may thus provide the local conditions to support the phenotypic stability of the chondrocytes in both “host” cartilage and implant.
The favorable mRNA ratios for aggrecan/collagen 1 and collagen 2/collagen 1 in the implant in the present study (an indication for a phenotypic stabilization of the chondrocytes) are in clear contrast to the results obtained upon culture of human osteoarthritic or nonosteoarthritic chondrocytes in the same collagen implant, but without the surrounding cartilage ring.48-51 This strongly indicates that the present “in situ organ” culture system generates a local milieu favoring the long-term preservation of the differentiated chondrocyte phenotype, cartilage matrix production, and lateral bonding, a process likely involving reciprocal effects of the cartilage ring and the implant on each other (unpublished data). These results are in good agreement with previous reports on culture systems applying such a surrounding cartilage ring,28,31,33,34 but are addressed for the first time on a molecular level in the current study.
Both cell-free and cell-loaded collagen implants showed significantly increased push-out forces from the “host” cartilage ring over time, with tendentially higher values for cell-loaded implants. Thus, well maintained cell vitality, substantial cell migration, and sustained local matrix production appear to have functional consequences in the sense of augmented lateral bonding of the implant to the surrounding “host” cartilage. Lateral bonding at the cartilage-implant interface and the resulting push-out forces are likely influenced by various factors, including (1) the character of the tissue formed at the interface,52 (2) sprouting of extracellular matrix components in the interfacial zone, and (3) the presence or absence of cell death at the interface.53
The increase of the push-out forces during the 12-week culture for cell-free or cell-loaded collagen implants (from 2.560 to 54.259 kPa or from 15.026 to 154.344 kPa, respectively) led to final values, which are comparable to those previously reported for poly(glycolic acid)-construct tissues after 5 weeks of culture31 or for cell-loaded agarose hydrogels.28 This shows that collagen implants, in either cell-free or cell-loaded form, may be well suitable as a scaffold material in general and as an implant material for cartilage repair in vivo.12,25-27
The method applied in the present study to measure the push-out force of the collagen implants for the first time took into consideration the blank value obtained by a second push-through of the indenter after the initial push-out of the implant. This approach may help to obtain reproducible results by addressing technical artifacts due to factors such as differences between the defect sizes in individual samples or variations of the implant hydrogel diameter due to variable water content.28,31,33
Conclusion
In the present in vitro bovine cartilage punch model, largely preserved tissue integrity, limited proteoglycan release, as well as progressively increasing cartilage differentiation and push-out forces (for up to 12 weeks of cultivation) suggest initial cartilage regeneration and imply lateral bonding of the current collagen implant to the surrounding cartilage. On the basis of significantly faster cell colonization and a significantly earlier and more long-lasting appearance/presence of aggrecan, cell-loaded implants may show an advantage over cell-free cartilage implants. The present in vitro cartilage regeneration model thus proved well suitable to demonstrate the regenerative capacity of this clinically approved hydrogel collagen type 1 implant and to decipher some of the underlying molecular processes.
Supplementary Material
Footnotes
Acknowledgments and Funding: The authors are grateful to Cordula Müller and Ulrike Körner for expert technical assistance, as well as to Maren Siedentop, Daniela Warnecke, and Fabian Holzner for expert biomechanical testing of implant push-out forces. We gratefully acknowledge the partial financial support of the Bundesministerium für Bildung und Forschung (BMBF), grant references 13N12601 and 0315577C.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: T.Graeve is a member of the Amedrix GmbH.
Supplemental Material: The supplementary material for this article is available online.
ORCID iD: Peter Foehr  https://orcid.org/0000-0003-1042-546X
https://orcid.org/0000-0003-1042-546X
References
- 1. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432-63. [DOI] [PubMed] [Google Scholar]
- 2. Musumeci G, Castrogiovanni P, Leonardi R, Trovato FM, Szychlinska MA, Di Giunta A, et al. New perspectives for articular cartilage repair treatment through tissue engineering: a contemporary review. World J Orthop. 2014;5(2):80-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dell’Accio F, De Bari C, El Tawil NM, Barone F, Mitsiadis TA, O’Dowd J, et al. Activation of WNT and BMP signaling in adult human articular cartilage following mechanical injury. Arthritis Res Ther. 2006;8(5):R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ye K, Di Bella C, Myers DE, Choong PF. The osteochondral dilemma: review of current management and future trends. ANZ J Surg. 2014;84(4):211-7. [DOI] [PubMed] [Google Scholar]
- 5. Kon E, Verdonk P, Condello V, Delcogliano M, Dhollander A, Filardo G, et al. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am J Sports Md. 2009;37(Suppl 1):156S-66S. [DOI] [PubMed] [Google Scholar]
- 6. Dewan AK, Gibson MA, Elisseeff JH, Trice ME. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. BioMed Res Int. 2014;2014:272481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bachmann G, Basad E, Lommel D, Steinmeyer J. MRI in the follow-up of matrix-supported autologous chondrocyte transplantation (MACI) and microfracture [in German]. Radiologe. 2004;44(8):773-82. [DOI] [PubMed] [Google Scholar]
- 8. Gobbi A, Chaurasia S, Karnatzikos G, Nakamura N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: a nonrandomized prospective trial. Cartilage.2015;6(2):82-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bartlett SPKW, Skinner JA, Carrington RWJ, Briggs TWR, Bentley G. Collagen-covered versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a comparison of tourniquet times. Eur J Orthop Surg Traumatol. 2006;16(4):315-7. [Google Scholar]
- 10. Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy. 2007;23(4):381-7. [DOI] [PubMed] [Google Scholar]
- 11. Gillogly SD, Wheeler KS. Autologous chondrocyte implantation with collagen membrane. Sports Med Arthrosc Rev. 2015;23(3):118-24. [DOI] [PubMed] [Google Scholar]
- 12. Schneider U, Rackwitz L, Andereya S, Siebenlist S, Fensky F, Reichert J, et al. A prospective multicenter study on the outcome of type I collagen hydrogel-based autologous chondrocyte implantation (CaReS) for the repair of articular cartilage defects in the knee. Am J Sports Med. 2011;39(12):2558-65. [DOI] [PubMed] [Google Scholar]
- 13. Andereya S, Maus U, Gavenis K, Müller-Rath R, Miltner O, Mumme T, et al. First clinical experiences with a novel 3D-collagen gel (CaReS) for the treatment of focal cartilage defects in the knee [in German]. Z Orthop Ihre Grenzgeb. 2006;144(3):272-80. [DOI] [PubMed] [Google Scholar]
- 14. Maus U, Schneider U, Gravius S, Müller-Rath R, Mumme T, Miltner O, et al. Clinical results after three years use of matrix-associated ACT for the treatment of osteochondral defects of the knee [in German]. Z Orthop Unfall. 2008;146(1):31-7. [DOI] [PubMed] [Google Scholar]
- 15. Welsch GH, Mamisch TC, Zak L, Blanke M, Olk A, Marlovits S, et al. Evaluation of cartilage repair tissue after matrix-associated autologous chondrocyte transplantation using a hyaluronic-based or a collagen-based scaffold with morphological MOCART scoring and biochemical T2 mapping: preliminary results. Am J Sports Med. 2010;38(5):934-42. [DOI] [PubMed] [Google Scholar]
- 16. Schneider U, Schmidt-Rohlfing B, Gavenis K, Maus U, Mueller-Rath R, Andereya S. A comparative study of 3 different cartilage repair techniques. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2145-52. [DOI] [PubMed] [Google Scholar]
- 17. Pretzel D, Linss S, Ahrem H, Endres M, Kaps C, Klemm D, et al. A novel in vitro bovine cartilage punch model for assessing the regeneration of focal cartilage defects with biocompatible bacterial nanocellulose. Arthritis Res Ther. 2013;15(3):R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kunisch E, Maenz S, Knoblich M, Ploeger F, Jandt KD, Bossert J, et al. Short-time pre-washing of brushite-forming calcium phosphate cement improves its in vitro cytocompatibility. Tissue Cell. 2017;49(6):697-710. [DOI] [PubMed] [Google Scholar]
- 19. Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7(10):R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pretzel D, Pohlers D, Weinert S, Kinne RW. In vitro model for the analysis of synovial fibroblast-mediated degradation of intact cartilage. Arthritis Res Ther. 2009;11(1):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruettger A, Neumann S, Wiederanders B, Huber R. Comparison of different methods for preparation and characterization of total RNA from cartilage samples to uncover osteoarthritis in vivo. BMC Res Notes. 2010;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173-7. [DOI] [PubMed] [Google Scholar]
- 23. Chandrasekhar S, Esterman MA, Hoffman HA. Microdetermination of proteoglycans and glycosaminoglycans in the presence of guanidine hydrochloride. Anal Biochem. 1987;161(1):103-8. [DOI] [PubMed] [Google Scholar]
- 24. Petri M, Broese M, Simon A, Liodakis E, Ettinger M, Guenther D, et al. CaReS (MACT) versus microfracture in treating symptomatic patellofemoral cartilage defects: a retrospective matched-pair analysis. J Orthop Sci. 2013;18(1):38-44. [DOI] [PubMed] [Google Scholar]
- 25. Efe T, Theisen C, Fuchs-Winkelmann S, Stein T, Getgood A, Rominger MB, et al. Cell-free collagen type I matrix for repair of cartilage defects-clinical and magnetic resonance imaging results. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):1915-22. [DOI] [PubMed] [Google Scholar]
- 26. Schuettler KF, Struewer J, Rominger MB, Rexin P, Efe T. Repair of a chondral defect using a cell free scaffold in a young patient—a case report of successful scaffold transformation and colonisation. BMC Surg. 2013;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schuttler KF, Schenker H, Theisen C, Schofer MD, Getgood A, Roessler PP, et al. Use of cell-free collagen type I matrix implants for the treatment of small cartilage defects in the knee: clinical and magnetic resonance imaging evaluation. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1270-6. [DOI] [PubMed] [Google Scholar]
- 28. Vinardell T, Thorpe SD, Buckley CT, Kelly DJ. Chondrogenesis and integration of mesenchymal stem cells within an in vitro cartilage defect repair model. Ann Biomed Eng. 2009;37(12):2556-65. [DOI] [PubMed] [Google Scholar]
- 29. Dunzel A, Rudiger T, Pretzel D, Kopsch V, Endres M, Kaps C, et al. The bovine cartilage punch model: a tool for the in vitro analysis of biomaterials and cartilage regeneration [in German]. Orthopade. 2013;42(4):254-61. [DOI] [PubMed] [Google Scholar]
- 30. Enders JT, Otto TJ, Peters HC, Wu J, Hardouin S, Moed BR, et al. A model for studying human articular cartilage integration in vitro. J Biomed Mater Res A. 2010;94(2):509-14. [DOI] [PubMed] [Google Scholar]
- 31. Hunter CJ, Levenston ME. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Eng. 2004;10(5-6):736-46. [DOI] [PubMed] [Google Scholar]
- 32. Madry H, Zurakowski D, Trippel SB. Overexpression of human insulin-like growth factor-I promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001;8(19):1443-9. [DOI] [PubMed] [Google Scholar]
- 33. Obradovic B, Martin I, Padera RF, Treppo S, Freed LE, Vunjak-Novakovic G. Integration of engineered cartilage. J Orthop Res. 2001;19(6):1089-97. [DOI] [PubMed] [Google Scholar]
- 34. Secretan C, Bagnall KM, Jomha NM. Effects of introducing cultured human chondrocytes into a human articular cartilage explant model. Cell Tissue Res. 2010;339(2):421-7. [DOI] [PubMed] [Google Scholar]
- 35. Mika J, Clanton TO, Ambrose CG, Kinne RW. Surgical preparation for articular cartilage regeneration in the osteoarthritic knee joint. Cartilage. 2017;8(4):365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mika J, Clanton TO, Pretzel D, Schneider G, Ambrose CG, Kinne RW. Surgical preparation for articular cartilage regeneration without penetration of the subchondral bone plate: in vitro and in vivo studies in humans and sheep. Am J Sports Med. 2011;39(3):624-31. [DOI] [PubMed] [Google Scholar]
- 37. Lotz MK, Otsuki S, Grogan SP, Sah R, Terkeltaub R, D’Lima D. Cartilage cell clusters. Arthritis Rheum. 2010;62(8):2206-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morales TI. Chondrocyte moves: clever strategies? Osteoarthritis Cartilage. 2007;15(8):861-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gavenis K, Heussen N, Hofman M, Andereya S, Schneider U, Schmidt-Rohlfing B. Cell-free repair of small cartilage defects in the Goettinger minipig: the effects of BMP-7 continuously released by poly(lactic-co-glycolid acid) microspheres. J Biomater Appl. 2014;28(7):1008-15. [DOI] [PubMed] [Google Scholar]
- 40. Gavenis K, Schneider U, Maus U, Mumme T, Muller-Rath R, Schmidt-Rohlfing B, et al. Cell-free repair of small cartilage defects in the Goettinger minipig: which defect size is possible? Knee Surg Sports Traumatol Arthrosc. 2012;20(11):2307-14. [DOI] [PubMed] [Google Scholar]
- 41. Endres M, Neumann K, Zhou B, Freymann U, Pretzel D, Stoffel M, et al. An ovine in vitro model for chondrocyte-based scaffold-assisted cartilage grafts. J Orthop Surg Res. 2012;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patrascu JM, Kruger JP, Boss HG, Ketzmar AK, Freymann U, Sittinger M, et al. Polyglycolic acid-hyaluronan scaffolds loaded with bone marrow-derived mesenchymal stem cells show chondrogenic differentiation in vitro and cartilage repair in the rabbit model. J Biomed Mater Res B Appl Biomater. 2013;101(7):1310-20. [DOI] [PubMed] [Google Scholar]
- 43. Bartz C, Meixner M, Giesemann P, Roel G, Bulwin GC, Smink JJ. An ex vivo human cartilage repair model to evaluate the potency of a cartilage cell transplant. J Transl Med. 2016;14(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zscharnack M, Krause C, Aust G, Thümmler C, Peinemann F, Keller T, et al. Preclinical good laboratory practice-compliant safety study to evaluate biodistribution and tumorigenicity of a cartilage advanced therapy medicinal product (ATMP). J Transl Med. 2015;13:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Erggelet C, Endres M, Neumann K, Morawietz L, Ringe J, Haberstroh K, et al. Formation of cartilage repair tissue in articular cartilage defects pretreated with microfracture and covered with cell-free polymer-based implants. J Orthop Res. 2009;27(10):1353-60. [DOI] [PubMed] [Google Scholar]
- 46. Gavenis K, Schmidt-Rohlfing B, Mueller-Rath R, Andereya S, Schneider U. In vitro comparison of six different matrix systems for the cultivation of human chondrocytes. In Vitro Cellular Dev Biol Anim. 2006;42(5-6):159-67. [DOI] [PubMed] [Google Scholar]
- 47. Endres M, Neumann K, Schroder SE, Vetterlein S, Morawietz L, Ringe J, et al. Human polymer-based cartilage grafts for the regeneration of articular cartilage defects. Tissue Cell. 2007;39(5):293-301. [DOI] [PubMed] [Google Scholar]
- 48. Halbwirth F, Niculescu-Morzsa E, Zwickl H, Bauer C, Nehrer S. Mechanostimulation changes the catabolic phenotype of human dedifferentiated osteoarthritic chondrocytes. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):104-11. [DOI] [PubMed] [Google Scholar]
- 49. Jeyakumar V, Halbwirth F, Niculescu-Morzsa E, Bauer C, Zwickl H, Kern D, et al. Chondrogenic gene expression differences between chondrocytes from osteoarthritic and non-OA trauma joints in a 3D collagen type I hydrogel. Cartilage. 2017;8(2):191-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zwickl H, Niculescu-Morzsa E, Halbwirth F, Bauer C, Jeyakumar V, Reutterer A, et al. Correlation analysis of SOX9, -5, and -6 as well as COL2A1 and aggrecan gene expression of collagen I implant-derived and osteoarthritic chondrocytes. Cartilage. 2016;7(2):185-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zwickl H, Niculescu-Morzsa E, Nehrer S. Investigation of collagen transplants seeded with human autologous chondrocytes at the time of transplantation. Cartilage. 2010;1(3):194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moretti M, Wendt D, Schaefer D, Jakob M, Hunziker EB, Heberer M, et al. Structural characterization and reliable biomechanical assessment of integrative cartilage repair. J Biomech. 2005;38(9):1846-54. [DOI] [PubMed] [Google Scholar]
- 53. Theodoropoulos JS, De Croos JN, Park SS, Pilliar R, Kandel RA. Integration of tissue-engineered cartilage with host cartilage: an in vitro model. Clin Orthop Relat Res. 2011;469(10):2785-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.