Abstract
Background
The goal of this study is to report the characteristics and long‐term clinical outcomes of patients with spontaneous coronary artery dissection (SCAD) and to identify factors associated with recurrent SCAD.
Methods and Results
This is a retrospective cohort study that included patients who underwent coronary angiography for evaluation of acute myocardial infarction between 2006 and 2016. Among 26 598 patients hospitalized with a principal diagnosis of acute myocardial infarction, 208 (0.78%) were diagnosed with SCAD. Patients with SCAD were younger (49.0±11.6 versus 65.6±12.2 years) and more likely to be women (88.9% versus 31.6%). Atherosclerotic risk factors, such as hypertension, hyperlipidemia, obesity, and diabetes mellitus, were less prevalent. Median follow‐up was 4.7 years. Mortality was lower in patients with SCAD (1‐year mortality: 2.4% versus 8.8%; P<0.001). After using propensity score matching to control for differences in age, sex, and comorbidities, the difference in mortality was no longer present, suggesting that lower mortality in patients with SCAD is attributed primarily to their baseline characteristics. Recurrent SCAD occurred in 22 patients (10.6%). Multivariate Cox regression modeling showed concomitant fibromuscular dysplasia (hazard ratio, 5.1; 95% CI, 1.6–15.8; P=0.005) and migraine headaches (hazard ratio, 3.4; 95% CI, 1.4–8.4; P=0.008) to be associated with increased risk of recurrent SCAD.
Conclusions
Among patients with acute myocardial infarction, patients with SCAD have a lower risk of mortality, which is attributed primarily to their younger age, female sex, and low prevalence of atherosclerotic risk factors. Risk of recurrent SCAD persists years after the initial presentation. Patients with fibromuscular dysplasia and migraine are at higher risk for recurrent SCAD.
Keywords: acute coronary syndrome, spontaneous coronary artery dissection, women
Subject Categories: Women, Acute Coronary Syndromes, Coronary Artery Disease
Clinical Perspective
What Is New?
Patients with spontaneous coronary artery dissection (SCAD) had lower short‐ and long‐term mortality compared with other patients presenting with acute coronary syndrome; this improved survival is driven primarily by the baseline characteristics of patients with SCAD, who are younger and have fewer atherosclerotic risk factors.
SCAD recurrence continues to be observed years after initial presentation.
Fibromuscular dysplasia and migraine are associated with increased risk of recurrent SCAD.
What Are the Clinical Implications?
Patients with SCAD require long‐term follow‐up as recurrent SCAD can be observed years after initial presentation.
Screening for SCAD‐associated extracoronary arteriopathies should be considered.
Introduction
Spontaneous coronary artery dissection (SCAD) is increasingly recognized as an important cause of acute coronary syndrome (ACS) in young patients.1 SCAD is characterized by spontaneous development of a false lumen within the coronary artery wall, either from an intimal tear or through spontaneous hemorrhage arising from the vasa vasorum within the vessel wall.2, 3, 4 This results in compression of the true lumen and compromise of coronary flow. The vast majority of patients with SCAD present with ACS, with up to half of the cases being ST‐segment–elevation myocardial infarction; life‐threatening ventricular arrhythmias occur in ≈4% of cases.5
In the general population, SCAD is the cause of ACS in the minority of cases, accounting for 1% to 4% of cases.6, 7 In contrast, SCAD is an important cause of ACS in young women, accounting for almost a quarter of cases of ACS in women <50 years of age.8, 9 Remarkably, patients with SCAD tend not to have conventional risk factors for coronary artery disease.5, 10 The underlying pathophysiological mechanisms of SCAD are not well understood, although several factors, including connective tissues disease, fibromuscular dysplasia, emotional or physical stress, and pregnancy, were associated with SCAD and are believed to increase a patient's susceptibility.1, 11
The optimal management strategy is not well defined. It is unclear if secondary preventive treatments proved to be effective in patients with ACS attributable to atherosclerotic disease confer similar benefits in patients with SCAD. No randomized trials exist to guide therapy. The long‐term outcome of patients with SCAD is not well established, with published case series reporting significantly different rates of morbidity and mortality.10, 12, 13 The variation in outcomes between published series may be partly because of referral bias inherent in registry studies conducted at major tertiary referral centers.
The purpose of this study was to perform a population‐based analysis, using a community‐based cohort of patients followed by a health system in southern California, to determine the clinical characteristics, treatment strategy, and long‐term clinical outcomes of patients with SCAD and to identify clinical factors associated with recurrent SCAD.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. Institutional review board approval was obtained.
Study Population
This is a retrospective cohort study using prospectively collected data from the Kaiser Permanente Southern California (KPSC) Health System. KPSC is a regional integrated healthcare system serving >4 million members. Members enroll through the Kaiser Foundation Health Plan for comprehensive healthcare insurance that includes pharmaceutical benefits. KPSC provides care to an ethnically and socioeconomically diverse population broadly representative of the racial/ethnic groups in southern California. Comprehensive information on the medical care KPSC members receive is prospectively captured electronically through a centralized data warehouse, with electronic data sets with linked information on demographics and administrative, pharmacy, laboratory, and healthcare use data from both ambulatory visits and hospitalizations. The present study was approved by the KPSC Institutional Review Board. A waiver of informed consent was obtained because of the observational nature of the study.
Patients ≥18 years of age, admitted to the hospital with a principal diagnosis of acute myocardial infarction between January 1, 2006, and December 31, 2016, were identified using International Classification of Diseases, Ninth Revision (ICD‐9), or International Classification of Diseases, Tenth Revision (ICD‐10), codes 412.x, 414.12, I21.x, I22.x, I23.x, or I25.42. Only patients who underwent cardiac catheterization for coronary angiography were included. Each patient was only included in the study once. The date of first diagnosis of acute myocardial infarction during the study period was used as the index date. The 1‐year period before the index date was defined as the baseline window. Patients who were not KPSC members or did not have continuous 1‐year membership before the date of admission were excluded to allow adequate follow‐up data. Comorbidities at baseline were identified using ICD‐9 or ICD‐10 codes. Exposure to cardiac medications was extracted from outpatient pharmacy dispensing records. The subset of patients with coronary artery dissection was identified using the ICD‐9 or ICD‐10 codes 414.12 and I25.42. A detailed manual review of the medical records was performed by 2 physicians (R.C., M.S.L.) to confirm the diagnosis of SCAD, identify the treatment provided, and adjudicate outcomes. Patients with iatrogenic coronary artery dissection were excluded from the SCAD group. Concomitant diagnoses, including migraine and fibromuscular dysplasia, were made by patients’ treating clinicians and abstracted through manual chart review. Screening for fibromuscular dysplasia was performed at the discretion of the treating clinicians.
Outcomes
Mortality data were extracted from a mortality data file, with integrated death information derived from multiple sources, including California state death master files, Social Security Administrative death master files, hospital deaths, and insurance enrollment records.
Statistical Analysis
Descriptive statistics for categorical data were reported in absolute numbers and percentages. Continuous variables were analyzed by calculating mean values and SDs. Differences in categorical data were compared by Fisher's exact test. Differences in continuous data were compared by Student t tests. A 2‐sided P<0.05 was considered statistically significant. The propensity score for SCAD was calculated with logistic regression to estimate the probability of disease assignment on the basis of baseline variables, including age, sex, race/ethnicity, and comorbidities (history of hypertension, hyperlipidemia, obesity, diabetes mellitus, stroke, renal failure, myocardial infarction, heart failure, atrial fibrillation, hypothyroidism, and lung disease). For each patient with SCAD, a corresponding comparison patient with acute myocardial infarction was selected in a 1:1 manner on the basis of the nearest propensity score using a caliper of 0.1 with no replacement. Kaplan‐Meier curves were constructed to compare survival of patients with SCAD compared with other patients presenting with ACS. Univariate and multivariate Cox proportional hazards regression analyses were performed to identify clinical factors associated with recurrent SCAD. The multivariate Cox proportional hazard model included the following variables: age, hypertension, fibromuscular dysplasia, migraine, and exposure to β blockers, statins, and P2Y12 inhibitors (clopidogrel, prasugrel, and ticagrelor). Statistical analyses were performed using STATA 14 (StataCorp, College Station, TX).
Results
Baseline Characteristics
Between 2006 and 2016, there were 26 598 patients discharged with a principal diagnosis of acute myocardial infarction who underwent coronary angiography and met the inclusion criteria. In this group, 208 (0.78%) were diagnosed with SCAD. Compared with patients without SCAD, patients with SCAD were younger (49.0±11.6 versus 65.6±12.2 years of age) and more likely to be women (88.9% versus 31.6%) (Table 1). Patients with SCAD were less likely to have prevalent atherosclerotic risk factors, such as hypertension (30.8% versus 64.8%), hyperlipidemia (27.9% versus 62.2%), obesity (18.7% versus 21.1%), diabetes mellitus (8.2% versus 35.6%), and chronic kidney disease (4.3% versus 24.3%). They were also less likely to have significant medical comorbidities, including congestive heart failure (0.5% versus 11.4%), chronic obstructive pulmonary disease/asthma (15.4% versus 19.5%), history of stroke (1.9% versus 11.3%), history of acute myocardial infarction (6.7% versus 15.7%), or atrial fibrillation (3.4% versus 7.8%).
Table 1.
Baseline Characteristics
| Characteristics | Full Cohort | Propensity Score–Matched Cohort | ||||
|---|---|---|---|---|---|---|
| No SCAD (n=26 390) | SCAD (n=208) | P Value | No SCAD (n=208) | SCAD (n=208) | P Value | |
| Age, y | 65.6±12.2 | 49.0±11.6 | <0.001 | 49.1±12.2 | 49.0±11.6 | 0.91 |
| <35 | 204 (0.8) | 18 (8.7) | 18 (8.7) | 18 (8.7) | ||
| 35–50 | 2547 (9.7) | 93 (44.7) | 98 (47.1) | 93 (44.7) | ||
| 50–<65 | 9728 (36.9) | 78 (37.5) | 71 (34.1) | 78 (37.5) | ||
| ≥65 | 13 911 (52.7) | 19 (9.1) | 21 (10.1) | 19 (9.1) | ||
| Men | 18 051 (68.4) | 23 (11.1) | <0.001 | 21 (10.1) | 23 (11.1) | 0.87 |
| Race/ethnicity | <0.001 | 0.40 | ||||
| White | 13 848 (52.5) | 70 (33.7) | 72 (34.6) | 70 (33.7) | ||
| Black | 2715 (10.3) | 33 (15.9) | 45 (21.6) | 33 (15.9) | ||
| Hispanic | 6714 (25.4) | 86 (41.4) | 79 (38.0) | 86 (41.4) | ||
| Asian | 2198 (8.3) | 13 (6.3) | 7 (3.4) | 13 (6.3) | ||
| Other | 915 (3.5) | 6 (2.9) | 5 (2.4) | 6 (2.9) | ||
| Hypertension | 17 107 (64.8) | 64 (30.8) | <0.001 | 65 (31.3) | 64 (30.8) | 1.0 |
| Hyperlipidemia | 16 419 (62.2) | 58 (27.9) | <0.001 | 57 (27.4) | 58 (27.9) | 1.0 |
| Obesity | 5560 (21.1) | 39 (18.7) | 0.86 | 40 (19.2) | 39 (18.7) | 1.0 |
| Diabetes mellitus | 9401 (35.6) | 17 (8.2) | <0.001 | 20 (9.6) | 17 (8.2) | 0.73 |
| Prior MI | 4129 (15.7) | 14 (6.7) | <0.001 | 15 (7.2) | 14 (6.7) | 1.0 |
| Congestive heart failure | 2999 (11.4) | 1 (0.5) | <0.001 | 1 (0.5) | 1 (0.5) | 1.0 |
| Atrial fibrillation | 2061 (7.8) | 7 (3.4) | <0.001 | 7 (3.4) | 7 (3.4) | 1.0 |
| History of stroke/TIA | 2987 (11.3) | 4 (1.9) | <0.001 | 1 (0.5) | 4 (1.9) | 0.4 |
| Hypothyroidism | 2823 (10.7) | 15 (7.2) | 0.012 | 17 (8.2) | 15 (7.2) | 0.85 |
| Rheumatologic diseases | 716 (2.7) | 5 (2.4) | 0.69 | 8 (3.9) | 5 (2.4) | 0.58 |
| COPD/asthma | 5138 (19.5) | 32 (15.4) | 0.008 | 31 (14.9) | 32 (15.4) | 1.0 |
| Chronic kidney disease | 6415 (24.3) | 9 (4.3) | <0.001 | 8 (3.9) | 9 (4.3) | 1.0 |
| Depression | 3846 (14.6) | 29 (13.9) | 0.23 | 28 (13.5) | 29 (13.9) | 1.0 |
| BMI, kg/m2 | 28.5±6.6 | 29.4±6.7 | <0.001 | 30.5±6.6 | 29.4±6.7 | 0.12 |
Values are given as mean±SD or number (percentage). BMI indicates body mass index; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; SCAD, spontaneous coronary artery dissection; TIA, transient ischemic attack.
Using a propensity score matching technique, 208 patients without SCAD were identified to match the 208 patients with SCAD. The 2 groups were well matched in age, sex, ethnicity, and comorbidities (Table 1).
Clinical Presentation and Coronary Anatomy
One fifth of patients with SCAD (19.7%) presented with ST‐segment–elevation myocardial infarction (Table 2). Significant arrhythmia, including ventricular tachycardia or ventricular fibrillation, occurred in 10 patients (4.8%). The most commonly involved coronary artery was the left anterior descending artery (42.4%). Only 10.8% of SCAD cases involved >1 coronary artery territory.
Table 2.
Presentation and Angiographic Characteristics of Patients With SCAD
| Variable | Patients, No. (%) |
|---|---|
| Presentation (n=208) | |
| STEMI | 41 (19.7) |
| VT/VF | 10 (4.8) |
| Coronary artery involved (n=203)a | |
| SCAD involving >1 coronary artery | 22 (10.8) |
| Left main artery | 4 (2.0) |
| Left anterior descending artery | 86 (42.4) |
| Diagonal/septal artery | 21 (10.3) |
| Ramus artery | 4 (2.0) |
| Left circumflex artery | 13 (6.4) |
| Obtuse marginal artery | 44 (21.7) |
| Right coronary artery | 25 (12.3) |
| PDA | 19 (9.4) |
| PLV | 16 (7.9) |
PDA indicates posterior descending artery; PLV, posterior left ventricular artery; SCAD, spontaneous coronary artery dissection; STEMI, ST‐segment–elevation myocardial infarction; VF, ventricular fibrillation; VT, ventricular tachycardia.
Coronary angiogram was available for review in 203 patients.
Initial Management
Compared with other patients who presented with acute myocardial infarction, patients with SCAD were more likely to be treated conservatively (Table 3). A lower proportion of patients with SCAD underwent revascularization with coronary artery bypass grafting (4.3% versus 17.3%; P<0.001) or percutaneous coronary intervention (11.1% versus 56.0%; P<0.001). The difference in rates of percutaneous coronary artery intervention remained significant in the propensity score–matched cohort.
Table 3.
Treatment Details
| Treatments | Full Cohort | Propensity Score–Matched Cohort | ||||
|---|---|---|---|---|---|---|
| No SCAD (n=26 390) | SCAD (n=208) | P Value | No SCAD (n=208) | SCAD (n=208) | P Value | |
| Revascularization | ||||||
| CABG | 4568 (17.3) | 9 (4.3) | <0.001 | 15 (7.2) | 9 (4.3) | 0.29 |
| PCI | 14 775 (56.0) | 23 (11.1) | <0.001 | 87 (41.8) | 23 (11.1) | <0.001 |
| Medications | ||||||
| P2Y12 | 18 244 (69.2) | 146 (70.2) | 0.76 | 123 (59.1) | 146 (70.2) | 0.02 |
| Statins | 22 913 (86.5) | 168 (80.8) | 0.025 | 159 (76.4) | 168 (80.8) | 0.34 |
| β Blockers | 22 984 (87.1) | 173 (83.2) | 0.097 | 167 (80.3) | 173 (83.2) | 0.53 |
| ACEs/ARBs | 20 602 (78.1) | 119 (57.2) | <0.001 | 137 (65.9) | 119 (57.2) | 0.09 |
| Aldosterone antagonists | 1207 (4.6) | 2 (1.0) | 0.007 | 7 (3.4) | 2 (1.0) | 0.18 |
| CCBs | 5162 (19.6) | 39 (18.8) | 0.86 | 44 (21.2) | 39 (18.8) | 0.63 |
Values are given as number (percentage). ACE indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; CCB, calcium channel blocker; P2Y12, P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor); PCI, percutaneous coronary intervention; SCAD, spontaneous coronary artery dissection.
Medications used in the SCAD cohort were largely comparable to the general acute myocardial infarction cohort, with a high proportion of patients treated with P2Y12 inhibitors (clopidogrel, prasugrel, and ticagrelor), statins, and β blockers. A higher proportion of patients without SCAD were treated with angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers. However, analysis using propensity score matching showed that these differences were no longer significant after baseline characteristics were taken into consideration.
Short‐ and Long‐Term Outcomes
Follow‐up was 4.7±3.1 years. During the follow‐up period, there were 7 deaths in the SCAD cohort and 6533 deaths in the non‐SCAD cohort. Short‐term outcomes for the patients with SCAD were relatively favorable, with a 30‐day mortality rate of 1.4%, compared with 4.1% in the non‐SCAD cohort (Table 4). At 1 year, mortality was 8.8% in the non‐SCAD group and 2.4% in the SCAD group. Figure 1 shows the Kaplan‐Meier survival estimates for the patients with SCAD compared with other patients presenting with ACS. In the full cohort, patients with SCAD had lower long‐term mortality (log‐rank P<0.01). However, after matching for age, sex, ethnicity, and other baseline characteristics, no significant differences in the short‐ or long‐term mortality were observed between the 2 groups. This suggests the difference in prognosis observed between patients with and without SCAD may be primarily explained by differences in their baseline characteristics.
Table 4.
Short‐Term Mortality
| Mortality | Full Cohort | Propensity Score–Matched Cohort | ||||
|---|---|---|---|---|---|---|
| No SCAD (n=26 390) | SCAD (n=208) | P Value | No SCAD (n=208) | SCAD (n=208) | P Value | |
| On day of presentation | 139 (0.52) | 2 (0.96) | 0.3 | 0 (0) | 2 (0.96) | 0.50 |
| At 30 d | 1068 (4.1) | 3 (1.4) | 0.05 | 2 (1.0) | 3 (1.4) | 1.0 |
| At 90 d | 1437 (5.5) | 3 (1.4) | 0.008 | 2 (1.0) | 3 (1.4) | 1.0 |
| At 1 y | 2326 (8.8) | 5 (2.4) | <0.001 | 3 (1.4) | 5 (2.4) | 0.72 |
Values are given as number (percentage). SCAD indicates spontaneous coronary artery dissection.
Figure 1.
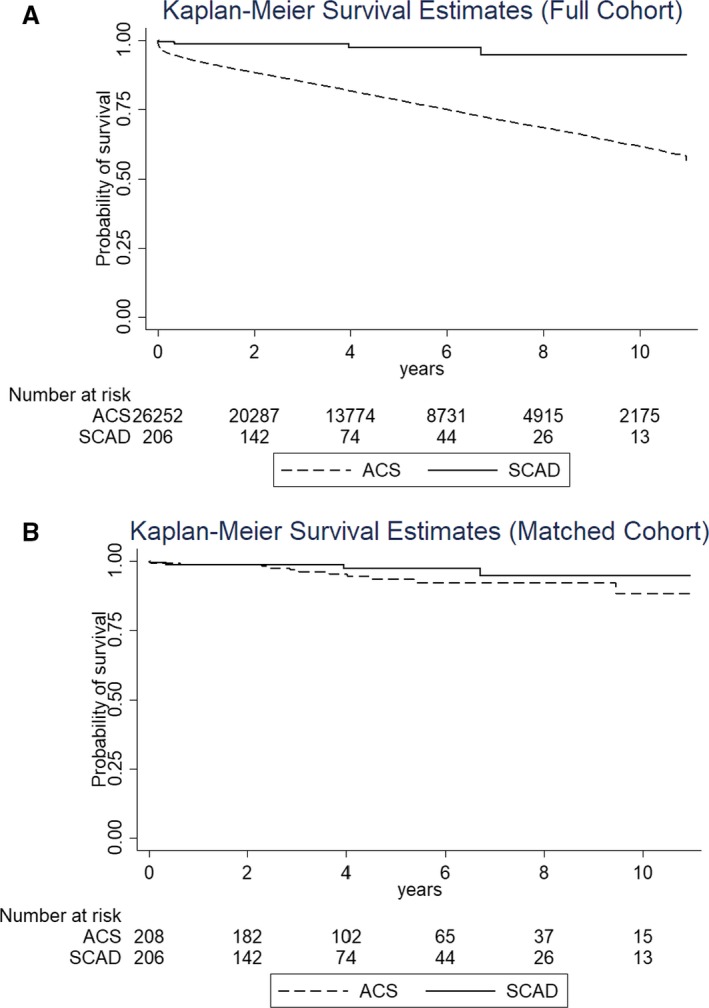
Kaplan‐Meier survival estimates for long‐term mortality of patients with spontaneous coronary artery dissection (SCAD) compared with patients with acute coronary syndrome (ACS) in the full cohort (log‐rank P<0.001; A) and in the propensity‐matched cohort (log‐rank P=0.21; B).
Screening for Fibromuscular Dysplasia
Screening for fibromuscular dysplasia was obtained at the discretion of the treating clinicians. Comprehensive screening, with imaging from the brain to the pelvis, was performed in 90 patients. Among the 90 patients who underwent comprehensive screening, 9 were diagnosed with fibromuscular dysplasia.
Factors Associated With Recurrent SCAD
Recurrent SCAD occurred in 22 patients (10.6%). Of these patients, 9 (4.3%) had recurrent SCAD within 1 year. Four patients experienced recurrent SCAD >5 years after the initial event. One patient presented with recurrent SCAD 10.5 years after. A Kaplan‐Meier event‐free survival curve is shown in Figure 2. All patients with recurrent SCAD were women. Patients with recurrent SCAD were younger and more likely to have a diagnosis of fibromuscular dysplasia or migraine (Table 5). From multivariate Cox regression analyses, fibromuscular dysplasia was associated with a 5‐fold increase in risk of recurrent SCAD (hazard ratio, 5.1; 95% CI, 1.6–15.8; P=0.005). Migraine headache was associated with a 3.4‐fold increase in risk of recurrent SCAD (hazard ratio, 3.4; 95% CI, 1.4–8.4; P=0.008). No association was observed between treatment with antiplatelet agents, β blockers, or statins and recurrent SCAD.
Figure 2.
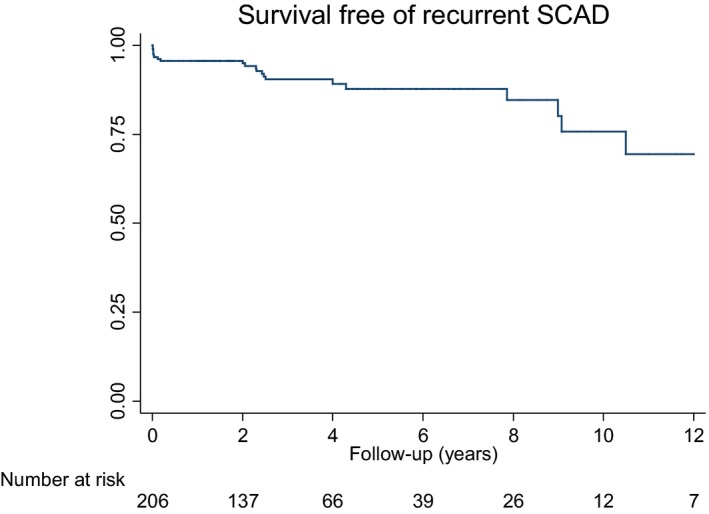
Survival free of recurrent spontaneous coronary artery dissection (SCAD).
Table 5.
Univariate and Multivariate Predictors of Recurrent SCAD
| Variable | No Recurrence (n=186)a | Recurrent SCAD (n=22)a | Univariate Predictors | Multivariate Predictorsb | ||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |||
| Age, y | 49.6±11.8 | 44.2±8.8 | 0.96 (0.92–1.00) | 0.07 | 0.95 (0.90–0.99) | 0.028 |
| Women | 163 (87.7) | 22 (100) | ··· | ··· | ··· | ··· |
| Hypertension | 58 (31.2) | 6 (27.3) | 0.97 (0.38–2.5) | 0.95 | ··· | ··· |
| Pregnancy associated | 16 (8.6) | 1 (4.5) | 0.34 (0.1–2.7) | 0.32 | ··· | ··· |
| FMD | 5 (2.7) | 4 (18.2) | 5.8 (2.0–17.5) | 0.002 | 5.1 (1.6–15.8) | 0.005 |
| Migraine | 27 (14.5) | 8 (36.4) | 3.6 (1.5–8.7) | 0.004 | 3.4 (1.4–8.4) | 0.008 |
| P2Y12 | 129 (69.4) | 17 (77.3) | 1.6 (0.6–4.5) | 0.33 | ··· | ··· |
| β Blockers | 155 (83.3) | 18 (81.8) | 1.0 (0.4–3.1) | 0.94 | ··· | ··· |
| Statins | 148 (79.6) | 20 (90.9) | 2.4 (0.6–10.4) | 0.24 | ··· | ··· |
FMD indicates fibromuscular dysplasia; P2Y12, P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor); SCAD, spontaneous coronary artery dissection.
Values are given as mean±SD or number (percentage).
Included age, hypertension, FMD, migraine, β blockers, statins, and P2Y12.
Discussion
In this large population‐based study with a racially diverse cohort, the prevalence of SCAD was 0.78% in patients presenting with ACS and undergoing coronary catheterization. Patients with SCAD were more likely to be young women. Short‐ and long‐term survival was higher in patients with SCAD. After propensity score matching, there was no difference in mortality between patients with and without SCAD, suggesting that the favorable short‐ and long‐term survival for patients with SCAD may be primarily explained by their baseline characteristics.
This cohort is one of the largest contemporary series of patients with SCAD with long longitudinal follow‐up information.11 Compared with other series, this cohort is unique in its racial and ethnic diversity. The 2 largest published SCAD series comprise >80% white patients,8, 13 whereas this cohort comprises 45% Hispanic Americans and 16% blacks. This cohort also differs from other published series in that most series include patients referred to tertiary referral centers and may be limited by referral bias and survivor bias. The patient cohort in this study was drawn from the community and comprises the entire spectrum of SCAD severity.
Despite differences in the racial/ethnic makeup, the presentation and clinical manifestation of SCAD in this cohort are comparable to other series. Ventricular arrhythmias occurred in 10 patients (4.8%), similar to the 3% to 11% previously reported rate of ventricular arrhythmias or sudden cardiac death.1, 5, 8, 12, 14 The left anterior descending artery is the artery most commonly involved (seen in 42.2% of patients), and 11% of patients had dissection involving >1 artery. These rates are remarkably similar to coronary angiographic characteristics reported in a predominantly white cohort.13
Compared with other patients presenting with ACS, a significantly higher proportion of patients with SCAD were treated conservatively. Only 4.3% of patients with SCAD were treated with coronary artery bypass grafting and 11.1% of patients were treated with percutaneous coronary intervention. The low rate of revascularization is in accordance with expert consensus that recommends conservative management as the preferred initial treatment strategy.1, 11 Observational studies reported an increased rate of coronary complications with revascularization attempts and suboptimal rates of procedural success.1, 5, 8, 12 With percutaneous intervention, one study reported a 22.2% rate of procedural‐related complications.15 With coronary artery bypass grafting, one study reported a high rate of graft failures, which was believed to be attributable to subsequent healing of the native vessel, resulting in competitive flow that led to graft occlusion.8 Case series in which patients underwent follow‐up repeated angiography found angiographic healing of the dissected coronary segments in most patients with SCAD who were managed conservatively.8, 16 The low rate of short‐term mortality in the SCAD cohort, despite a low revascularization rate, supports conservative management as a viable initial approach.
There are no randomized controlled trials comparing different medical treatment strategies for SCAD. Despite the lack of clear evidence to support the use of antiplatelet therapy in patients with SCAD, most patients with SCAD were treated with a P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor), at a rate similar to patients without SCAD who presented with ACS. It is possible that, in the absence of guidelines specifically written for the SCAD population, many physicians opted to follow the guidelines written for acute coronary syndrome, despite the distinct pathophysiological processes involved in SCAD and atherosclerotic plaque rupture.17 Similarly, the vast majority of patients with SCAD in this cohort was discharged with prescriptions for β blockers and statins. Whether β blockers and statin therapy confer benefit in SCAD is not well established. One study showed a reduction in recurrent SCAD associated with β‐blocker therapy.13 However, a theoretical risk of exacerbating vasospasm exists with β‐blocker treatment.1 With statin therapy, one study suggests an association with SCAD recurrence, whereas another showed no difference in clinical outcomes.8, 13 No association was seen between treatment with antiplatelet agents, β blockers, or statins and the risk of recurrent SCAD in our cohort. In the absence of clear evidence, use of these medications probably should be decided on the basis of the presence of other risk factors, balancing a potential benefit with their adverse effect profile.
Notwithstanding the lack of established therapies, the long‐term survival of patients with SCAD is higher compared with patients without SCAD who presented with ACS. Using propensity score matching to control for baseline characteristics, we found that the higher survival observed in patients with SCAD is attributed primarily to patients’ baseline characteristics, with age being an important contributor.
Recurrent SCAD is a major concern. In our series, recurrent SCAD continues to be an issue, even >5 years out from the initial event. One patient in our cohort experienced recurrent SCAD >10 years after the initial presentation. The persistent risk of recurrence and the lack of effective treatment likely contribute to the anxiety and depression experienced by patients with SCAD.18
Using Cox regression models to evaluate clinical predictors of recurrent SCAD, fibromuscular dysplasia and migraine headache were independently associated with a higher incidence of SCAD recurrence. Migraine headache is associated with an increased incidence of recurrent SCAD by 3.4‐fold, and fibromuscular dysplasia is associated with an increased incidence of recurrent SCAD by 5‐fold. These associations support the hypothesis that SCAD, migraine headache, and fibromuscular dysplasia may represent manifestations of systemic vascular abnormalities and an underlying propensity to injury involving different vascular beds.19 It must be emphasized that the lack of comprehensive screening is a significant limitation, and the association between fibromuscular dysplasia and migraine with recurrent SCAD is a finding that should be considered hypothesis generating. Nevertheless, our findings support expert consensus that recommends extracoronary screening with vascular imaging from the brain to the pelvis to diagnose underlying associated vascular conditions.1 The prevalence of fibromuscular dysplasia is lower in our population compared with other published series.13, 20 This may be attributable to incomplete screening (as only half of the population in this series underwent complete imaging from the brain to the pelvis). The racial and ethnic makeup of our population (with high proportions of Hispanic Americans and blacks) may also have contributed to the differences in baseline comorbidities compared with other published series.
Several limitations of this study should be acknowledged. This is a nonrandomized observational analysis and, as such, the diagnosis and management decisions were likely to be not uniform in the study population as these were at the discretion of the treating clinicians. Cases were initially identified using ICD‐9 and ICD‐10 codes, and it is possible that these codes did not capture all the SCAD cases. Underdiagnosis of SCAD is possible, especially early in the study time frame. On the other hand, among cases identified, manual chart review was performed for all cases to allow for improved case ascertainment. Because of the observational nature of the study, patients were not systematically screened for migraine and fibromuscular dysplasia; and these diagnoses, especially if in a mild form, could be missed in a subset of patients. The population studied is an insured population in the United States, with good access to health care. As such, the findings may not be generalizable to patients in the United States who lack health insurance or patients in developing countries with limited access to care.
In conclusion, among patients presenting with ACS who underwent coronary angiography, the subgroup with SCAD had a lower incidence of short‐ and long‐term mortality compared with those without SCAD. This improved survival is driven primarily by the baseline characteristics of patients with SCAD, who are younger, who are more likely to be women, and who have fewer atherosclerotic risk factors. SCAD recurrence remains a concern in long‐term follow‐up. Patients with fibromuscular dysplasia and migraine are at the highest risk for recurrent SCAD.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e012570 DOI: 10.1161/JAHA.119.012570.)
References
- 1. Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian‐Engoren C, Economy KE, Ganesh SK, Gulati R, Lindsay ME, Mieres JH, Naderi S, Shah S, Thaler DE, Tweet MS, Wood MJ; American Heart Association Council on Peripheral Vascular Disease; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Genomic and Precision Medicine; and Stroke Council . Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. 2018;137:e523–e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saw J, Mancini GB, Humphries K, Fung A, Boone R, Starovoytov A, Aymong E. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter Cardiovasc Interv. 2016;87:E54–E61. [DOI] [PubMed] [Google Scholar]
- 3. Alfonso F, Paulo M, Gonzalo N, Dutary J, Jimenez‐Quevedo P, Lennie V, Escaned J, Banuelos C, Hernandez R, Macaya C. Diagnosis of spontaneous coronary artery dissection by optical coherence tomography. J Am Coll Cardiol. 2012;59:1073–1079. [DOI] [PubMed] [Google Scholar]
- 4. Paulo M, Sandoval J, Lennie V, Dutary J, Medina M, Gonzalo N, Jimenez‐Quevedo P, Escaned J, Banuelos C, Hernandez R, Macaya C, Alfonso F. Combined use of OCT and IVUS in spontaneous coronary artery dissection. JACC Cardiovasc Imaging. 2013;6:830–832. [DOI] [PubMed] [Google Scholar]
- 5. Saw J, Aymong E, Sedlak T, Buller CE, Starovoytov A, Ricci D, Robinson S, Vuurmans T, Gao M, Humphries K, Mancini GB. Spontaneous coronary artery dissection: association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ Cardiovasc Interv. 2014;7:645–655. [DOI] [PubMed] [Google Scholar]
- 6. Nishiguchi T, Tanaka A, Ozaki Y, Taruya A, Fukuda S, Taguchi H, Iwaguro T, Ueno S, Okumoto Y, Akasaka T. Prevalence of spontaneous coronary artery dissection in patients with acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2016;5:263–270. [DOI] [PubMed] [Google Scholar]
- 7. Rashid HN, Wong DT, Wijesekera H, Gutman SJ, Shanmugam VB, Gulati R, Malaipan Y, Meredith IT, Psaltis PJ. Incidence and characterisation of spontaneous coronary artery dissection as a cause of acute coronary syndrome—a single‐centre Australian experience. Int J Cardiol. 2016;202:336–338. [DOI] [PubMed] [Google Scholar]
- 8. Tweet MS, Eleid MF, Best PJ, Lennon RJ, Lerman A, Rihal CS, Holmes DR Jr, Hayes SN, Gulati R. Spontaneous coronary artery dissection: revascularization versus conservative therapy. Circ Cardiovasc Interv. 2014;7:777–786. [DOI] [PubMed] [Google Scholar]
- 9. Saw J, Aymong E, Mancini GB, Sedlak T, Starovoytov A, Ricci D. Nonatherosclerotic coronary artery disease in young women. Can J Cardiol. 2014;30:814–819. [DOI] [PubMed] [Google Scholar]
- 10. Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS, Gulati R. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation. 2012;126:579–588. [DOI] [PubMed] [Google Scholar]
- 11. Adlam D, Alfonso F, Maas A, Vrints C; Writing Committee . European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. 2018;39:3353–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lettieri C, Zavalloni D, Rossini R, Morici N, Ettori F, Leonzi O, Latib A, Ferlini M, Trabattoni D, Colombo P, Galli M, Tarantini G, Napodano M, Piccaluga E, Passamonti E, Sganzerla P, Ielasi A, Coccato M, Martinoni A, Musumeci G, Zanini R, Castiglioni B. Management and long‐term prognosis of spontaneous coronary artery dissection. Am J Cardiol. 2015;116:66–73. [DOI] [PubMed] [Google Scholar]
- 13. Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, Mancini GBJ. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. 2017;70:1148–1158. [DOI] [PubMed] [Google Scholar]
- 14. Nakashima T, Noguchi T, Haruta S, Yamamoto Y, Oshima S, Nakao K, Taniguchi Y, Yamaguchi J, Tsuchihashi K, Seki A, Kawasaki T, Uchida T, Omura N, Kikuchi M, Kimura K, Ogawa H, Miyazaki S, Yasuda S. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: a report from the Angina Pectoris‐Myocardial Infarction Multicenter Investigators in Japan. Int J Cardiol. 2016;207:341–348. [DOI] [PubMed] [Google Scholar]
- 15. Conrotto F, D'Ascenzo F, Cerrato E, Fernandez‐Ortiz A, Gonzalo N, Macaya F, Tamburino C, Barbanti M, van Lavieren M, Piek JJ, Applegate RJ, Latib A, Spinnler MT, Marzullo R, Iannaccone M, Pavani M, Crimi G, Fattori R, Chinaglia A, Presbitero P, Varbella F, Gaita F, Escaned J. Safety and efficacy of drug eluting stents in patients with spontaneous coronary artery dissection. Int J Cardiol. 2017;238:105–109. [DOI] [PubMed] [Google Scholar]
- 16. McGrath‐Cadell L, McKenzie P, Emmanuel S, Muller DW, Graham RM, Holloway CJ. Outcomes of patients with spontaneous coronary artery dissection. Open Heart. 2016;3:e000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ; ACC/AHA Task Force Members; Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons . 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. [DOI] [PubMed] [Google Scholar]
- 18. Liang JJ, Tweet MS, Hayes SE, Gulati R, Hayes SN. Prevalence and predictors of depression and anxiety among survivors of myocardial infarction due to spontaneous coronary artery dissection. J Cardiopulm Rehabil Prev. 2014;34:138–142. [DOI] [PubMed] [Google Scholar]
- 19. Kok SN, Hayes SN, Cutrer FM, Raphael CE, Gulati R, Best PJM, Tweet MS. Prevalence and clinical factors of migraine in patients with spontaneous coronary artery dissection. J Am Heart Assoc. 2018;7:e010140 DOI: 10.1161/JAHA.118.010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prasad M, Tweet MS, Hayes SN, Leng S, Liang JJ, Eleid MF, Gulati R, Vrtiska TJ. Prevalence of extracoronary vascular abnormalities and fibromuscular dysplasia in patients with spontaneous coronary artery dissection. Am J Cardiol. 2015;115:1672–1677. [DOI] [PubMed] [Google Scholar]


