Abstract
Background
In shunt‐dependent, single‐ventricle patients, mortality remains high in the interstage period between discharge after neonatal surgery and stage 2 operation. We sought to evaluate the impact of our infant single‐ventricle management and monitoring program (ISVMP) on interstage mortality and stage 2 outcomes.
Methods and Results
This retrospective single‐center cohort study compared patients enrolled in ISVMP at hospital discharge with historical controls. The relationship of ISVMP to interstage mortality was determined with a bivariate probit model for the joint modeling of both groups, using an instrumental variables approach. We included 166 ISVMP participants (December 1, 2010, to June 30, 2015) and 168 controls (January 1, 2007, to November 30, 2010). The groups did not differ by anatomy, gender, race, or genetic syndrome. Mortality was lower in the ISVMP group (5.4%) versus controls (13%). An ISVMP infant compared with a historical control had an average 29% lower predicted probability of interstage death (adjusted probability: −0.29; 95% CI, −0.52 to −0.057; P=0.015). On stratified analysis, mortality was lower in the hypoplastic left heart syndrome subgroup undergoing Norwood operation (4/84 [4.8%] versus 12/90 [14%], P=0.03) but not in those with initial palliation of shunt only (P=0.90). ISVMP participants were younger at the time of the stage 2 operation (138 versus 160 days, P<0.001), with no difference in postoperative mortality or length of stay.
Conclusions
In this single‐center study, we report significantly lower interstage mortality for participants with hypoplastic left heart syndrome enrolled in ISVMP. Younger age at stage 2 operation was not associated with postoperative mortality or longer length of stay.
Keywords: hypoplastic left heart syndrome, interstage mortality, interstage period, single‐ventricle congenital heart disease
Subject Categories: Quality and Outcomes
Clinical Perspective
What Is New?
In this large single‐center study, implementation of an infant single‐ventricle management and monitoring program was associated with a significant reduction in interstage mortality in infants with hypoplastic left heart syndrome undergoing the Norwood operation but not in those undergoing initial neonatal palliation of shunt only.
It is worth noting that a reduction in interstage mortality was achieved by standardization of care for this patient population without the creation of a dedicated single ventricle clinic.
What Are the Clinical Implications?
Further studies are needed to identify specific components of the monitoring program that have the biggest impact on interstage survival.
Introduction
Infants with hypoplastic left heart syndrome (HLHS) and other shunt‐dependent, single‐ventricle (SV) heart defects are tenuous in the interstage period between discharge after neonatal surgery and stage 2 operation (or superior cavopulmonary connection). Despite advances in prenatal diagnosis and operative and postoperative care, interstage mortality in patients with HLHS has been reported to be ≈10%.1, 2, 3, 4 In an attempt to reduce mortality during this vulnerable period in this patient population, the concept of a home monitoring program (HMP) was first introduced by Ghanayem et al, with successful reduction in interstage mortality at a single center.5, 6 With implementation of the National Pediatric Cardiology Quality Improvement Collaborative (NPCQIC), this concept of intensive home monitoring of infants with HLHS has been adopted by a majority of centers in the United States as standard of care.7 In addition, many HMPs now include all infants with shunt‐dependent SV heart defects. The infant SV management and monitoring program (ISVMP) was implemented in our center in December 2010 with the goal of standardizing care and improving the survival of patients with shunt‐dependent SV defects in the interstage period. The primary objective of this study was to evaluate the impact of ISVMP on interstage survival in infants with shunt‐dependent SV defects. In addition, we describe postoperative outcomes after stage 2 operation and 1‐year transplant‐free survival.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patient Population
The study protocol was approved by the Institutional Review Board for the Protection of Human Subjects at the Children's Hospital of Philadelphia (CHOP). We conducted a retrospective cohort study of patients with shunt‐dependent SV defects who were enrolled in the ISVMP from December 1, 2010, to June 30, 2015. Informed consent was waived for this study. For the historical control group, we searched our institution's surgical database for all neonates with shunt‐dependent SV defects who were discharged after neonatal palliation between January 1, 2007, and November 30, 2010 (last date before the introduction of the ISVMP). All infants with shunt‐dependent SV defects were eligible for enrollment in the ISVMP. For this analysis, patients who received an aortopulmonary shunt in anticipation of a biventricular repair and those who remained inpatient during the interstage period or who died during the neonatal hospitalization were excluded (Figure 1).
Figure 1.
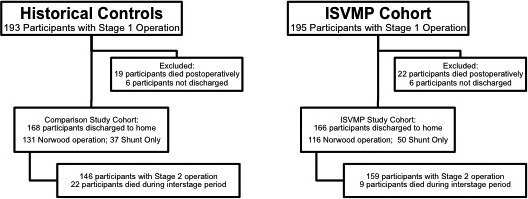
Flow diagram for patient enrollment and outcomes. Flowchart to describe total neonates undergoing surgery, including which participants were included in analysis, and outcomes. ISVMP indicates infant single‐ventricle management and monitoring program.
Home Monitoring Program
As part of the ISVMP, all patients were discharged home with a pulse oximeter and weighing scale. The components of the program included (1) standardization of the neonatal discharge criteria and process; (2) standardization of parental education before neonatal discharge; (3) daily home monitoring by parents, including daily oxygen saturation measurement, daily weight measurement, and feeding log; (4) weekly home nursing visits documenting oxygen saturation, weight, mode of feeding, and formula type and amount; (5) weekly phone calls to parents by a dedicated ISVMP nurse practitioner; (6) involvement of a registered dietician; (7) biweekly pediatrician visits; (8) biweekly (after Norwood) or monthly (other shunt‐dependent patients) cardiology visits with focused echocardiograms; (9) weekly review of patients by a dedicated ISVMP team; and (10) scheduling of standard stage 2 operation at 4 to 5 months of age. Even though we had a dedicated ISVMP physician team that included 5 cardiologists, we did not have a dedicated SV clinic. Communication and comanagement was ongoing throughout the interstage period among the ISVMP team members, particularly the dedicated nurse practitioner and the primary cardiologists.
Data Collection
Medical records were reviewed to abstract demographic and relevant surgical and medical variables from the neonatal and stage 2 hospitalizations. The interstage period started on the date of discharge from neonatal admission and ended on the date of stage 2 or death. Any admission in the interstage period was characterized as an interstage readmission. Duration of interstage readmission (in days) and events during interstage readmission, such as unplanned cardiac catheterization and surgeries other than stage 2, were recorded. Unplanned cardiac catheterization was defined as a cardiac catheterization that was not scheduled as routine before the stage 2 operation, based on review of records. Concurrent surgery at the time of stage 2 operation included pulmonary arterioplasty, atrioventricular valvuloplasty, and atrial septectomy. Study covariates pertinent to the neonatal admission included gender, presence of genetic syndrome, Norwood operation, shunt type (Blalock–Taussig or right‐ventricle‐to‐pulmonary‐artery shunt), cardiopulmonary bypass times, length of mechanical ventilation, need for cardiopulmonary resuscitation or extracorporeal membrane oxygenation, and length of hospital stay. Covariates pertinent to neonatal admission hospital discharge included weight‐for‐age Z score (WAZ), number of medications, use of digoxin, and distance of primary residence to CHOP. Echocardiographic variables during neonatal admission were obtained by review of reports.
Statistical Analysis
Demographic and clinical characteristics for the ISVMP and control groups were summarized using mean±SD or median (interquartile range [IQR]) for continuous variables and frequency (percentage) for categorical variables. For continuous variables, means or medians were compared using Student t or Wilcoxon–Mann–Whitney U tests, as appropriate. Categorical variables were compared using the χ2 or Fisher exact test.
Because inclusion in the ISVMP or control group was not randomized in this observational study, we sought to account for the presence of unmeasured factors that could be associated with exposure (ISVMP versus control) and outcome and thus be potential confounders of that association. We used a simultaneous equation approach analogous to instrumental variable (IV) analysis to account for measured and unmeasured factors that might differ between ISVMP and control patients.8, 9 This approach relies on the availability of an IV that fulfills the following characteristics: (1) It is strongly correlated with ISVMP enrollment, (2) it is independent of unmeasured confounders, and (3) it has no direct effect on outcome except through its effect on program. The IV serves as a surrogate for randomization used to extract variation in the ISVMP that is free of confounding to allow us to estimate the relationship between the ISVMP and the outcome.10 Extrapolating from other treatment effectiveness studies, we used distance to CHOP as the IV because distance to receive treatment is often associated with socioeconomic status and other unmeasured characteristics that need to be controlled.9, 11 Distance to CHOP was strongly associated with enrollment in the ISVMP but was not associated with mortality except through its association with ISVMP and thus met the IV selection criteria.
To estimate the effect of the ISVMP on interstage mortality, we conducted simultaneous‐equations bivariate probit models because we needed 2 nonlinear models for the dichotomous measures: program (ISVMP and controls) and outcome (mortality). We used probit models for our ISVMP and outcome equations and covariates associated with the ISVMP and/or outcome This model assumed a bivariate normal distribution for the error terms in the 2 equations. For ease of interpretation, we did not report the raw probit coefficient but instead report the adjusted probability of the outcome for the effect of the program (with a 95% CI). Because the ISVMP comprised several components and they were implemented for patient care concurrently, single aspects of the program were not examined individually. To examine whether the effect of the ISVMP on interstage mortality differs according to shunt type in the Norwood patients, we also ran an interaction model with a program‐by‐shunt‐type interaction and report the P value from that model.
Analysis of Impact of Age at Stage 2 Operation on Survival
The potential effect on outcome of younger age at stage 2 in the ISVMP group was examined using the Aalen additive hazards model, which was selected instead of the proportional hazards model, given that the occurrence of the stage 2 operation was not a rare event.12 Time was measured from discharge from neonatal surgery to either date of admission for stage 2 operation (event) or date of death/end of follow‐up period (censoring). A 2‐staged least squares approach was used in which the predicted values of the program on the IV were used as a covariate in the second‐stage regression of the multivariable additive hazards model.13 The estimated cumulative time‐varying effects of first‐stage fitted values of the IV were obtained and presented graphically.
All statistical analyses used 2‐sided tests and were performed using α=0.05 for the threshold of statistical significance. SAS software (v9.4; SAS Institute) and STATA v15 (StataCorp) were used, and the additive hazards model was computed using standard software.
Results
Patient Characteristics and Neonatal Hospitalization
There were 166 participants in the ISVMP group and 168 historical controls. Overall, 28 cases and 27 controls were excluded because they either died (22 cases and 19 controls) or were not discharged from the initial neonatal hospitalization (6 cases and 8 controls). Baseline characteristics were comparable between groups except that, compared with controls, ISVMP participants had greater gestational age (38.5±1.4 versus 38.1±1.7 weeks, P=0.04), were more likely to have a prenatal diagnosis (97% versus 90%, P=0.007), and were older at neonatal surgery (4 days [IQR: 3–6] versus 4 days [IQR 2–6], P=0.01; Tables 1 and 2). The groups were comparable in terms of percentage of participants undergoing the Norwood operation; however, a greater proportion of these participants received a right‐ventricle‐to‐pulmonary‐artery shunt compared with a Blalock–Taussig shunt in the ISVMP (36% and 64%, respectively, in ISVMP versus 21% and 79%, respectively, in the control group; P=0.006; Figure 2). Duration of cardiopulmonary bypass was shorter in the ISVMP group compared with controls (76 versus 80 minutes, P=0.003). At the time of hospital discharge, the groups were comparable but a higher proportion of participants in the ISVMP group had at least moderate atrioventricular valve regurgitation on echocardiography (Table 3).
Table 1.
Baseline Characteristics
| ISVMP (n=166) | Historical (n=168) | P Value | |
|---|---|---|---|
| Male sex | 92 (55) | 109 (65) | 0.1 |
| Birth weight, kg | 3.2±0.5 | 3.2±0.5 | 0.9 |
| Birth WAZ | −0.48±0.96 | −0.52±0.95 | 0.72 |
| Gestational age, wk | 38.5±1.4 | 38.1±1.7 | 0.04 |
| Prematurity (<37 wk) | 13 (8) | 20 (12) | 0.21 |
| Prenatal diagnosis | 155 (97) | 149 (90) | 0.007 |
| Presence of genetic syndrome | 6 (3.6) | 6 (3.6) | 1 |
| Presence of heterotaxy syndrome | 18 (11) | 10 (6) | 0.11 |
| Pulmonary vein abnormalities | 20 (12) | 10 (6) | 0.06 |
| Anatomy | 0.76 | ||
| HLHS | 84 (51) | 90 (54) | |
| HLHS with aortic atresia | 30 | 26 | |
| Tricuspid atresia | 12 | 10 | |
| Ebstein anomaly | 3 | 1 | |
| Atrioventricular canal defect | 20 | 15 | |
| Double‐outlet right ventricle | 13 | 15 | |
| PA/IVS | 14 | 11 | |
| Other | 20 | 26 |
Counts are expressed as n (%), and continuous variables are expressed as mean±SD. Means were compared using Student t or Wilcoxon–Mann–Whitney U tests for continuous variables, as appropriate. Categorical variables were compared using the χ2 or Fisher exact test. HLHS indicates hypoplastic left heart syndrome; ISVMP, infant single‐ventricle management and monitoring program; PA/IVS, pulmonary atresia with intact atrial septum; WAZ, weight‐for‐age Z score.
Table 2.
Hospital Characteristics at Neonatal Operation
| ISVMP (n=166) | Historical (n=168) | P Value | |
|---|---|---|---|
| Age at first operation, d | 4 (3–6) | 4 (2–6) | 0.01 |
| Norwood operation, n | 116 | 131 | 0.13 |
| BT shunt | 74 (64) | 104 (79) | 0.006 |
| RV‐PA shunt | 42 (36) | 27 (21) | |
| BT shunt only | 50 | 37 | 0.28 |
| Cardiopulmonary bypass time, min | 76 (31–90) | 80 (72–106) | 0.003 |
| Mechanical ventilation, d | 1 (1–3) | 2 (2–4) | 0.1 |
| CPR | 10 (6.3) | 16 (10) | 0.2 |
| ECMO | 6 (3.8) | 9 (5.4) | 0.5 |
| Arrhythmias | 49 (31) | 59 (36) | 0.29 |
| Length of hospital stay, d | 22 (16–35) | 24 (17–38) | 0.6 |
Counts are expressed as n (%), continuous variables are expressed as median (interquartile range). Medians were compared using Student t or Wilcoxon–Mann–Whitney U tests for continuous variables, as appropriate. Categorical variables were compared using the χ2 or Fisher exact test. BT indicates Blalock–Taussig shunt; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; ISVMP, infant single‐ventricle management and monitoring program; RV‐PA, right ventricle to pulmonary artery.
Figure 2.
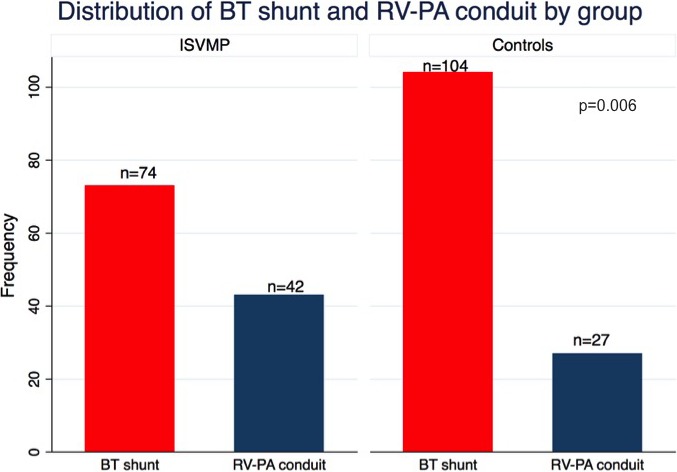
Distribution of shunt type between groups. Distribution of either BT shunt or RV‐PA shunt between the ISVMP cohort and historical controls. BT indicates Blalock–Taussig shunt; ISVMP, infant single‐ventricle management and monitoring program; RV‐PA, right ventricle to pulmonary artery.
Table 3.
Hospital Characteristics at Discharge
| ISVMP (n=166) | Historical (n=168) | P Value | |
|---|---|---|---|
| Weight at discharge, kg | 3.4 (3.1–3.8) | 3.4 (3.1–3.7) | 0.4 |
| WAZ | −1.3 (−2.0 to −0.7) | −1.4 (−2.2 to −0.8) | 0.19 |
| Prescribed digoxin | 42 (27) | 46 (29) | 0.74 |
| Ventricular functiona | 0.3 | ||
| Normal | 81 (50) | 68 (41) | |
| Mildly diminished | 66 (40) | 78 (47) | |
| Moderately diminished | 16 (10) | 18 (11) | |
| Severely diminished | 0 (0) | 1 (1) | |
| Atrioventricular valve regurgitationa | 0.01 | ||
| None or trivial | 98 (60) | 126 (77) | |
| Mild | 55 (34) | 35 (21) | |
| Moderate | 8 (5) | 3 (2) | |
| Severe | 1 (1) | 0 (0) |
Counts are expressed as n (%), continuous variables are expressed as median (interquartile range). Medians were compared using Student t or Wilcoxon–Mann–Whitney U tests for continuous variables, as appropriate. Categorical variables were compared using the χ2 or Fisher exact test. ISVMP indicates infant single‐ventricle management and monitoring program; WAZ, weight‐for‐age Z score.
On echocardiogram.
Interstage Mortality
An ISVMP participant compared with a historical control had 29% lower predicted probability of death in the interstage period using the bivariate probit model with the IV approach (adjusted probability: −0.29; 95% CI, −0.52 to −0.057; P=0.015). Results from the Aalen additive hazards model suggest that this difference in mortality does not appear to be related solely to younger age at stage 2 in the ISVMP group. Age at the stage 1 operation was included in the model. In addition, the lower probability of mortality in the ISVMP group was independent of gestational age (adjusted probability: −0.26; 95% CI, −0.5 to −0.026; P=0.03). The overall interstage mortality was lower for ISVMP participants compared with controls (5.4% versus 13%, P=0.02), and similar rates were found in the subgroup of participants with HLHS (4.8% versus 14%, P=0.03; Table 4). The effect of the ISVMP on interstage mortality did not vary by type of shunt placed during the Norwood operation (P=0.2). No relationship was observed between the ISVMP and interstage mortality in the shunt‐only group (adjusted probability: −0.055; 95% CI, −0.56 to 0.45; P=0.83). In the shunt‐only group (n=87), the interstage mortality in the ISVMP group was 10% (5/50) compared with 10.8% (4/37) among the controls (P=0.90). A majority of the shunt‐only deaths occurred in participants with pulmonary atresia with intact ventricular septum (3 each in the ISVMP and control groups).
Table 4.
Interstage and Stage 2 Operation Characteristics
| ISVMP (n=166) | Historical (n=168) | P Value | |
|---|---|---|---|
| Overall interstage mortality | 9 (5.4) | 22 (13) | 0.02 |
| Interstage mortality for HLHS subset | 4 (4.8) | 12 (14) | 0.03 |
| Interstage mortality for the shunt‐only subset | 5 (3) | 4 (2.4) | 0.90 |
| Age at stage 2 operation, d | 138 (126–158) | 160 (138–188) | <0.001 |
| Weight (kg), when measured | 6.1 (5.5–6.8) | 6.1 (4.1–6.7) | 0.97 |
| WAZ | −0.9 (−1.6 to −0.1) | −1.4 (−2 to −0.7) | <0.001 |
| At least 1 interstage admission | 104 (63) | 78 (46) | 0.002 |
| Interstage admissions per participant | 1 (0–2) | 0 (0–1) | <0.001 |
| LOS for interstage admission, d | 3 (2–7) | 4 (2–10) | 0.02 |
| Interstage unplanned catheterization | 40 (24) | 43 (26) | 0.66 |
| Stage 2 operation | 0.09 | ||
| Glenn operation | 123 (79) | 101 (69) | |
| Hemi–Fontan operation | 29 (18) | 42 (29) | |
| Kawashima operation | 4 (3) | 3 (2) | |
| Concurrent cardiac surgery at stage 2 | 95 (48) | 65 (33) | 0.002 |
| Need for ECMO | 3 (2) | 2 (1) | 0.5 |
| Repeat operation during stage 2 admission | 1 (0.7) | 2 (1.4) | 0.6 |
| Catheterization during stage 2 admission | 21 (13) | 8 (5.5) | 0.01 |
| Catheterization with intervention | 13 (8) | 5 (3) | 0.07 |
| LOS for stage 2 operation, d | 7 (6–13) | 7 (4–15) | 0.4 |
| Mortality during stage 2 admission | 4 (3) | 3 (2) | 0.5 |
| 1‐Year mortality after stage 2 | 1 (3) | 4 (5) | 0.7 |
Counts are expressed as n (%), and continuous variables are expressed as median (interquartile range). Medians were compared using Student t or Wilcoxon–Mann–Whitney U tests for continuous variables, as appropriate. Categorical variables were compared using the χ2 or Fisher exact test. The instrumental variable approach was only used to calculate the impact of ISVMP on interstage mortality. ECMO indicates extracorporeal membrane oxygenation; HLHS, hypoplastic left heart syndrome; ISVMP, infant single‐ventricle management and monitoring program; LOS, length of hospital stay; WAZ, weight‐for‐age Z score.
Unplanned Catheterizations and Readmissions in the Interstage Period
The proportion of participants undergoing unplanned catheterizations during the interstage period was comparable between groups (26% of ISVMP versus 24% of control, P=0.66). The ISVMP group had a greater percentage of participants with at least 1 interstage readmission compared with the historical control group (63% versus 46%, P=0.002) and had a greater number of interstage readmissions per participant compared with controls (1 [IQR: 0–2] versus 0 [IQR: 0–1], P<0.001; Table 4). Although the ISVMP group had more readmissions, they were shorter in duration compared with those of the control group (median: 3 days [IQR: 2–7] versus 4 days [IQR: 2–10]; P=0.02). A majority of these readmissions were observational, requiring no to minimal intervention.
Stage 2 Characteristics
Participants in the ISVMP were younger at the time of the stage 2 operation compared with the historical controls (138 versus 160 days, P<0.001), had higher median WAZ (−0.9 versus −1.4, P<0.001), and were more likely to have concurrent surgery at stage 2 (48% versus 33%, P=0.002), with pulmonary arterioplasty being the most common additional procedure (Table 4; Figure 3). During the stage 2 admission, the ISVMP group had more postoperative catheterizations in the ISVMP group (13% versus 5.5%, P=0.01), with a trend towards more interventions (13/157 versus 5/146 in controls, P=0.07). Despite this, there was no difference in length of stay or mortality after stage 2 between groups.
Figure 3.
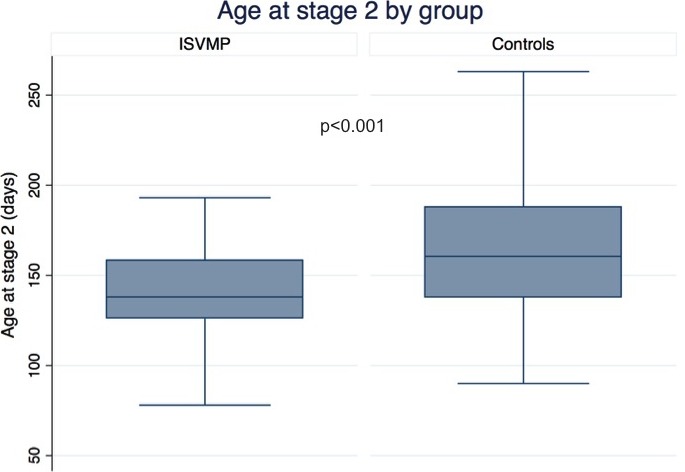
Age at stage 2 operation by group. Box plot of age at the stage 2 operation in days, comparing the ISVMP cohort and historical controls. ISVMP indicates infant single‐ventricle management and monitoring program.
Discussion
In this study, we investigated the impact of an ISVMP in a high‐volume center on interstage and stage 2 outcomes in shunt‐dependent SV defects. We report overall lower interstage mortality, with a probability of interstage death decreasing by nearly 30% in the ISVMP group compared with historical controls. The interstage period was shorter in the ISVMP group compared with the historical control group because stage 2 was completed at a younger age in the former. Based on our analysis, the significant reduction in risk of mortality did not appear to be associated solely with younger age at stage 2. Younger age at stage 2 was not associated with higher postoperative mortality or longer length of stay after the stage 2 operation.
Our center has previously reported interstage mortality of 10% for infants with HLHS and variants undergoing the Norwood operation between 1998 and 2005.2 Similarly, the SVR (Single Ventricle Reconstruction) trial reported interstage mortality of 12% for participants with HLHS and related right ventricular anomalies undergoing the Norwood operation between 2005 and 2008.14 In the SVR trial, there was a survival difference based on shunt type in participants with less than moderate atrioventricular valve regurgitation, with better survival among those who received a right‐ventricle‐to‐pulmonary‐artery shunt. In this study, even though the ISVMP group included a greater proportion of participants with a right‐ventricle‐to‐pulmonary‐artery shunt, based on our analysis, there was no difference in interstage mortality based on shunt type alone for participants undergoing the Norwood operation as the initial palliation.
In the first single‐center publication of the use of home monitoring, there was a reduction in interstage mortality in infants with HLHS from 15% to 0%.5 Since that time, multiple institutions have created similarly modeled HMPs in efforts to reduce interstage mortality, with varying results. Some studies demonstrated significant reductions in interstage mortality, including no mortality at all, whereas others reported no change.3, 15, 16, 17 The NPCQIC has reported a decrease in cumulative mortality among its centers from 9.5% between 2008 and May 2013 to 5.4% between June 2013 and August 2014. With one of the largest single‐center cohorts of any study to date, we report a 29% reduction in probability of interstage mortality in participants with HLHS by implementation of ISVMP. Our program was able to achieve this reduction in mortality without a dedicated SV clinic; rather, participants were seen in the interstage period by primary cardiologists within and outside our center. It is possible that the success of the program can largely be attributed to the nurse practitioner dedicated to the ISVMP, by virtue of close communication not only with the care teams but with the families. Interestingly, the ISVMP did not afford a protective effect on the subgroup of participants with a Blalock–Taussig shunt only. A majority of deaths (6/9) occurred in participants with pulmonary atresia with intact ventricular septum. This high‐risk group constituted nearly a third of the shunt‐only group.
We report a significant increase in the number of interstage readmissions in the ISVMP group compared with historical controls. Other studies have also observed frequent readmissions with implementation of HMP. In a multicenter report from the NPCQIC, 66% of patients experienced at least 1 interstage readmission, a majority of which were short admissions for observation and other limited diagnostic testing to rule out life‐threatening alterations in anatomy or physiology.18, 19 It is conceivable that readmission to the hospital for careful observation, assessment, and, in some cases, intervention prevented death in this subset of patients.
Patients in the ISVMP group were younger at the stage 2 operation than those in the historical control group, similar to previous reports including our own.20, 21 This finding was expected given that as part of the ISVMP, participants were consistently scheduled for the stage 2 operation at ≈4 months of age. Although this strategy led to younger age at the time of the stage 2 operation, our analysis suggests that younger age at stage 2 was not the only factor contributing to decreased mortality in the ISVMP. A recent multicenter report demonstrated that survival appears to be greatest when completion of the stage 2 operation occurs after 3 months of age for low‐ and intermediate‐risk patients.22 Others, however, have found that stage 2 performed before age 4 months had no impact on early or late outcomes.23
At the time of discharge from the initial neonatal hospitalization or the beginning of the interstage period, participants in the ISVMP and historical control groups had similar weight and WAZ. Despite younger age at stage 2 in the ISVMP group, participants had the same median weight and higher WAZ at the time of the stage 2 operation. This improvement in growth more than likely reflects standardized nutritional surveillance and management throughout the interstage period as part of the ISVMP. In contrast, minimal if any nutritional surveillance was available in the interstage period for our historical control group before implementation of the ISVMP. This finding is similar to findings of other studies that have reported better weight gain during the interstage period as a benefit of HMPs.19, 24 It has been suggested that weight gain is an important factor for stage 2 and long‐term neurodevelopmental outcomes.19, 25 It is possible that the better WAZ of our participants contributed favorably to stage 2 outcomes despite younger age, but this aspect of the ISVMP was outside the scope of this study.
We report increased incidence of cardiac catheterization after the stage 2 operation in the ISVMP group, but no other differences in morbidity or mortality were noted during the stage 2 admission. Although the reason for increased frequency in cardiac catheterizations after stage 2 is difficult to ascertain, it may reflect a practice change in our center, with a lower threshold for performing a cardiac catheterization when patients are not recovering as clinically expected.
We found similar survival and length of hospital stay at stage 2 and no difference in mortality beyond stage 2 up to 1 year of age. This finding was limited by the small number of deaths after stage 2 and warrants further study to investigate whether the ISVMP can exert an effect on longer term outcome. Contrary to our finding, others have reported a protective effect on mortality to 1 year, despite no impact on interstage survival.17
Limitations
Our study is one of the largest single‐center studies to evaluate the impact of an HMP on interstage mortality in the recent era. We acknowledge several limitations of the study. Our study design is retrospective and quasi‐experimental, with a before versus after analysis plan, making it difficult to fully account for era bias or some degree of misappropriation of benefit of instituting the ISVMP. As such, our results reflect an association with introduction of the ISVMP, with findings not directly attributed to causation. Assignment to the ISVMP was not randomized; therefore, our results are not quite generalizable to the population of patients with SV heart disease. Although we used robust statistical methods, the effect of younger age at stage 2 or shorter interstage period on the beneficial effect of the program will continue to be a challenging question to answer. We report more frequent readmissions in the ISVMP group but acknowledge that additional details are not included in this article. Readmissions will be the focus of a future analysis. Finally, we were unable to quantify the beneficial effects of the individual elements of the ISVMP, particularly the role of the nurse practitioner dedicated to the program. Future efforts should focus on trying to identify program components that have the most impact on outcomes. Given these results and HMPs becoming standard of care at many institutions, a randomized clinical trial to assess the impact of home monitoring is improbable.
Conclusions
In this single‐center study, we report a significantly lower interstage mortality in patients with HLHS in the ISVMP group. Younger age at stage 2 or participation in the ISVMP did not increase mortality or length of stay after the stage 2 operation. These results validate the NPCQIC's recommendation of standardization of patient care during the interstage period with HMPs. Future studies will further assess readmissions and growth in the interstage period, resource utilization associated with the ISVMP, and the impact of the ISVMP on long‐term survival.
Sources of Funding
Dr Mercer‐Rosa is supported in part by National Institutes of Health grant K01HL125521.
Disclosures
Dr Ravishankar has received honoraria from Dannone Medical. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e010783 DOI: 10.1161/JAHA.118.010783.)
References
- 1. Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar‐Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW; Pediatric Heart Network Investigators . Comparison of shunt types in the Norwood procedure for single‐ventricle lesions. N Engl J Med. 2010;362:1980–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hehir DA, Dominguez TE, Ballweg JA, Ravishankar C, Marino BS, Bird GL, Nicolson SC, Spray TL, Gaynor JW, Tabbutt S. Risk factors for interstage death after stage 1 reconstruction of hypoplastic left heart syndrome and variants. J Thorac Cardiovasc Surg. 2008;136:94–99.e3. [DOI] [PubMed] [Google Scholar]
- 3. Furck AK, Uebing A, Hansen JH, Scheewe J, Jung O, Fischer G, Rickers C, Holland‐Letz T, Kramer H‐H. Outcome of the Norwood operation in patients with hypoplastic left heart syndrome: a 12‐year single‐center survey. J Thorac Cardiovasc Surg. 2010;139:359–365. [DOI] [PubMed] [Google Scholar]
- 4. Hehir DA, Ghanayem NS. Single‐ventricle infant home monitoring programs. Curr Opin Cardiol. 2013;28:97–102. [DOI] [PubMed] [Google Scholar]
- 5. Ghanayem NS, Hoffman GM, Mussatto KA, Cava JR, Frommelt PC, Rudd NA, Steltzer MM, Bevandic SM, Frisbee SJ, Jaquiss RDB, Litwin SB, Tweddell JS. Home surveillance program prevents interstage mortality after the Norwood procedure. J Thorac Cardiovasc Surg. 2003;126:1367–1375. [DOI] [PubMed] [Google Scholar]
- 6. Ghanayem NS, Cava JR, Jaquiss RDB, Tweddell JS. Home monitoring of infants after stage one palliation for hypoplastic left heart syndrome. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:32–38. [DOI] [PubMed] [Google Scholar]
- 7. Anderson JB, Beekman RH3, Kugler JD, Rosenthal GL, Jenkins KJ, Klitzner TS, Martin GR, Neish SR, Brown DW, Mangeot C, King E, Peterson LE, Provost L, Lannon C. Improvement in interstage survival in a National Pediatric Cardiology Learning Network. Circ Cardiovasc Qual Outcomes. 2015;8:428–436. [DOI] [PubMed] [Google Scholar]
- 8. Angrist JD, Imbens GW, Rubin DB. Identification of causal effects using instrumental variables. J Am Stat Assoc. 1996;91:444–455. [Google Scholar]
- 9. McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. J Am Med Assoc. 1994;272:859–866. [PubMed] [Google Scholar]
- 10. Baiocchi M, Cheng J, Small DS. Instrumental variable methods for causal inference. Stat Med. 2014;33:2297–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. J Am Med Assoc. 2007;297:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schaubel DE, Wei G. Fitting semiparametric additive hazards models using standard statistical software. Biom J. 2007;49:719–730. [DOI] [PubMed] [Google Scholar]
- 13. Tchetgen Tchetgen EJ, Walter S, Vansteelandt S, Martinussen T, Glymour M. Instrumental variable estimation in a survival context. Epidemiology. 2015;26:402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, Eghtesady P, Frommelt PC, Gruber PJ, Hill KD, Kaltman JR, Laussen PC, Lewis AB, Lurito KJ, Minich LL, Ohye RG, Schonbeck JV, Schwartz SM, Singh RK, Goldberg CS; Pediatric Heart Network Investigators . Interstage mortality after the Norwood procedure: results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansen JH, Furck AK, Petko C, Buchholz‐Berdau R, Voges I, Scheewe J, Rickers C, Kramer H‐H. Use of surveillance criteria reduces interstage mortality after the Norwood operation for hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2011;41:1013–1018. [DOI] [PubMed] [Google Scholar]
- 16. Dobrolet NC, Nieves JA, Welch EM, Khan D, Rossi AF, Burke RP, Zahn EM. New approach to interstage care for palliated high‐risk patients with congenital heart disease. J Thorac Cardiovasc Surg. 2011;142:855–860. [DOI] [PubMed] [Google Scholar]
- 17. Petit CJ, Fraser CD, Mattamal R, Slesnick TC, Cephus CE, Ocampo EC. The impact of a dedicated single‐ventricle home‐monitoring program on interstage somatic growth, interstage attrition, and 1‐year survival. J Thorac Cardiovasc Surg. 2011;142:1358–1366. [DOI] [PubMed] [Google Scholar]
- 18. Hanke SP, Joy B, Riddle E, Ravishankar C, Peterson LE, King E, Mangeot C, Brown DW, Schoettker P, Anderson JB, Bates KE. Risk factors for unanticipated readmissions during the interstage: a report from the National Pediatric Cardiology Quality Improvement Collaborative. Semin Thorac Cardiovasc Surg. 2017;28:803–814. [DOI] [PubMed] [Google Scholar]
- 19. Oster ME, Ehrlich A, King E, Petit CJ, Clabby ML. Association of interstage home monitoring with mortality, readmissions, and weight gain. Circulation. 2015;132:502–508. [DOI] [PubMed] [Google Scholar]
- 20. Rogers LS, Glatz AC, Ravishankar C, Spray TL, Nicolson SC, Rychik J, Rush CH, Gaynor JW, Goldberg DJ. 18 years of the Fontan operation at a single institution: results from 771 consecutive patients. J Am Coll Cardiol. 2012;60:1018–1025. [DOI] [PubMed] [Google Scholar]
- 21. Hill GD, Rudd NA, Ghanayem NS, Hehir DA, Bartz PJ. Center variability in timing of stage 2 palliation and association with interstage mortality: a report from the National Pediatric Cardiology Quality Improvement Collaborative. Pediatr Cardiol. 2016;37:1516–1524. [DOI] [PubMed] [Google Scholar]
- 22. Meza JM, Hickey E, McCrindle B, Blackstone E, Anderson B, Overman D, Kirklin JK, Karamlou T, Caldarone C, Kim R, DeCampli W, Jacobs M, Guleserian K, Jacobs JP, Jaquiss R. The optimal timing of stage‐2‐palliation after the Norwood operation. Ann Thorac Soc. 2018;105:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghanayem NS, Tweddell JS, Hoffman GM, Mussatto K, Jaquiss RDB. Optimal timing of the second stage of palliation for hypoplastic left heart syndrome facilitated through home monitoring, and the results of early cavopulmonary anastomosis. Cardiol Young. 2006;16(suppl 1):61–66. [DOI] [PubMed] [Google Scholar]
- 24. Anderson JB, Beekman RH3, Kugler JD, Rosenthal GL, Jenkins KJ, Klitzner TS, Martin GR, Neish SR, Darbie L, King E, Lannon C. Use of a learning network to improve variation in interstage weight gain after the Norwood operation. Congenit Heart Dis. 2014;9:512–520. [DOI] [PubMed] [Google Scholar]
- 25. Ravishankar C, Zak V, Williams IA, Bellinger DC, Gaynor JW, Ghanayem NS, Krawczeski CD, Licht DJ, Mahony L, Newburger JW, Pemberton VL, Williams RV, Sananes R, Cook AL, Atz T, Khaikin S, Hsu DT; Pediatric Heart Network Investigators . Association of impaired linear growth and worse neurodevelopmental outcome in infants with single ventricle physiology: a report from the pediatric heart network infant single ventricle trial. J Pediatr. 2013;162:250–256.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]


