Abstract
Background
This study assessed the effect of blockading neural transmission in the ganglionated plexi by injecting lidocaine into fat pads in the vagal nerve stimulation canine model and patients with persistent atrial fibrillation (AF).
Methods and Results
An efficacy test of lidocaine injection was performed in 7 canines. During vagal nerve stimulation, AF was sustained for >5 minutes. The lidocaine was injected into ganglionated plexi during sinus rhythm and reinduction of AF was attempted. Six patients with persistent AF were studied at open heart surgery. Lidocaine was injected into ganglionated plexi. Atrial electrograms were recorded from 96 epicardial electrodes covering Bachmann's bundle and atrial appendages. In the canine vagal nerve stimulation AF model, AF was not inducible in 4 of 7 after lidocaine injection. In patients with persistent AF, during baseline AF, there was a left atrium (LA)‐to‐right atrium (RA) frequency gradient (LA, mean cycle length [CL] 175±17 ms; RA, mean CL 192±17 ms; P<0.01). After lidocaine injection, AF persisted in all patients, and the LA‐to‐RA frequency gradient disappeared (LA, mean CL 186±13 ms; RA, mean CL 199±23 ms; P=0.08). Comparison of mean CLs before and after lidocaine demonstrated prolongation of LA CLs (P<0.05) with no effect on RA CLs.
Conclusions
In the canine vagal nerve stimulation AF model, lidocaine injection decreased inducibility of AF. In patients with persistent AF, atrial electrograms from the LA had shorter CLs than RA, indicating an LA‐to‐RA frequency gradient. Lidocaine injection significantly prolonged only LA CLs, explaining disappearance of the LA‐to‐RA frequency gradient. The mechanism of localized atrial electrogram CL prolongation in patients with persistent AF is uncertain.
Keywords: atrial fibrillation, cardiac mapping, ganglionated plexi, lidocaine
Subject Categories: Arrhythmias, Atrial Fibrillation, Electrophysiology
Short abstract
See Editorial Hanna et al
Clinical Perspective
What Is New?
In the canine vagal nerve stimulation atrial fibrillation (AF) model, injection of lidocaine (a neural blocker) into the epicardial fat pads consisting of ganglionated plexi suppressed inducibility of AF.
In patients with persistent and long‐standing persistent AF, lidocaine injection into the epicardial fat pads (ganglionated plexi) intraoperatively prolonged only the left atrial cycle lengths, resulting in elimination of the left‐to‐right atrial frequency gradient observed during baseline AF.
What Are the Clinical Implications?
In the present study, we demonstrated a potential role of ganglionated plexi in the maintenance of persistent and long‐standing persistent AF.
Our study suggests a potential pharmacological approach for targeting ganglionated plexi in patients with persistent and long‐standing persistent AF.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in the western world. The mechanism(s) of AF is/are not well understood. Recent experimental and clinical studies have shown that ganglionated plexi (GPs), that is, collections of neurons located mostly in the fat pads on the epicardial surface of both atria,1, 2, 3 play an important role in the initiation and maintenance of AF.4, 5, 6, 7 It has been suggested that one of the mechanisms of AF could be attributed to hyperactivity of GPs.8, 9 Investigators have targeted GPs using endocardial catheter ablation,10, 11, 12, 13, 14 surgical ablation,15, 16, 17, 18 or pharmacological agents19, 20, 21 with variable results. A recent study demonstrated that there is a spatial relationship between GPs and localized AF sources in human AF.22 However, the role of GPs in the genesis of AF in patients with persistent and long‐standing persistent (LSP) AF is not well understood. It is postulated that AF ablation, that is, pulmonary vein isolation, unavoidably ablates GPs, thus affecting the neuronal contribution to AF.7, 23 During the AF ablation procedure, the gradual prolongation of AF cycle length (CL) and disappearance of left‐to‐right frequency gradient was consistently observed.24, 25, 26 Local injection of a neuronal blocker (ie, lidocaine) blocks transmission of nerve conduction,27 thus affecting AF CL and left‐to‐right frequency gradient. We hypothesized that blocking neural transmission from the GPs by injecting lidocaine into the fat pads would affect AF CL and frequency gradient in patients with persistent and LSP AF. Before human persistent and LSP AF studies, we performed efficacy testing of lidocaine injection into GPs in the canine vagal nerve stimulation (VNS) AF model to affect inducibility of AF.
Methods
The authors declare that all data supporting the findings of this study are available within the article.
Canine VNS Model
All studies were performed in accord with the guidelines specified by our Institutional Animal Care and Use Committee, the American Heart Association Policy on Research Animal Use, the United States Public Health Service Policy on Use of Laboratory Animals, and “Guiding Principles in the Care and Use of Animals” of the American Physiological Society.
Seven adult mongrel canines weighing 18 to 23 kg were studied. Using standard techniques previously described,28 a pair of plunge wire electrodes was placed in the epicardial surface of the right atrial appendage (RAA), posteroinferior left atrium, and the mid‐portion of Bachmann's bundle (BB). These electrodes were used for monitoring heart rhythms, and for cardiac pacing as needed. The right cervical vagus nerve was identified, isolated by a midline neck incision, and prepared for electrical stimulation using a custom‐made, tripolar, flat interface nerve electrode. As a physiological test, the vagus nerve was stimulated (frequency, 20 Hz; pulse width, 2 ms; amplitude, 2.0–7.5 mA) to produce sinus CL prolongation of at least 50%. During VNS resulting to achieve at least 50% sinus CL prolongation, AF was induced by rapid atrial pacing for 3 to 7 beats at a CL of 75 ms from 1 of the atrial epicardial electrodes (RAA, BB, or posteroinferior left atrium) and maintained by constant VNS (>5 minutes). After VNS was stopped, sustained AF spontaneously terminated within 1 minute. The entire protocol from AF onset through termination of AF was recorded using a Bard LabSystem PRO (Bard Electrophysiology, Lowell, MA) to record ECG lead II simultaneously with atrial electrograms (AEGs) from the electrodes placed at the RAA, BB, and posteroinferior left atrium sites.
Efficacy testing of lidocaine injection into the GPs
Up to 1 cc of 2% lidocaine was injected into each of 5 GPs (superior vena cava–aorta GP, anterior right GP, inferior right GP, superior left GP, and inferior left GP) during sinus rhythm (Figure 1A). To avoid inadvertent injection into the atrial muscle, lidocaine was injected into the fat pad just above the epicardial surface in order to infiltrate the GPs that were accomplished by orienting the needle parallel to the surface of the epicardium. During VNS resulting in at least 50% sinus CL prolongation, reinduction of AF was attempted for half an hour until successful induction of AF during VNS immediately after lidocaine injection.
Figure 1.

Gray area shows locations of the ganglionated plexi (GP) in the canine (A) and human (B) hearts. ARGP indicates anterior right GP; BB, Bachmann's bundle; ILGP, inferior left GP; IRGP, inferior right ganglionated plexi; IVC, inferior vena cava; LA, left atrium; LAA, left atrial appendage; LPVs, left pulmonary veins; RA, right atrium; RAA, right atrial appendage; RPVs, right pulmonary veins; SLGP, superior left GP; SVC, superior vena cava; SVC‐Ao‐GP, SCV‐aorta GP.
Patient Studies
We studied 6 patients with persistent and LSP AF during open heart surgery for valvular and/or coronary artery bypass grafting surgery (Table 1). The research protocol was approved by the committee on the conduct of human research at University Hospitals Cleveland Medical Center. All patients gave written informed consent before their surgery.
Table 1.
Patient Characteristics
| Patient No. | Age, y | Sex | AF Duration | Valvular Disease | CAD | Injection of Lidocaine |
|---|---|---|---|---|---|---|
| 1 | 60 | M | >1 y | MR | − | ALL |
| 2 | 57 | M | >1 y | MS | − | ALL |
| 3 | 70 | M | 9 y | AS, TR | − | ONLY ARGP |
| 4 | 70 | F | 8.5 y | AS | − | ALL |
| 5 | 80 | F | 2.5 y | TR | − | ALL |
| 6 | 63 | M | >1 y | MR, TR | + | ALL |
− indicates absent; +, present; AF, atrial fibrillation; ARGP, anterior right ganglionated plexi; AS, aortic stenosis; CAD, coronary artery disease; MR, mitral regurgitation; MS, mitral stenosis; TR, tricuspid regurgitation.
Data acquisition
During open heart surgery, AEGs were recorded from an array (1.5×9.4 cm) of 96 electrodes placed on the epicardial surface covering the left atrial appendage (LAA), BB, and RAA before and during the lidocaine study. This mapping array can continuously record throughout the lidocaine study because its location does not interfere with the lidocaine injection into GPs. The interelectrode distance of each bipolar pair in each array was 1.5 mm, and the distance between the center of each bipolar electrode pair and its neighbor ranged from 5.8 to 6.0 mm. Data were digitally recorded and processed with an Active Two system (BioSemi, Amsterdam, The Netherlands). All AEGs were sampled at 1024 Hz and digitized at 24 bits. Data were transferred in real time and stored on a personal computer for further analysis (CEPAS; Cuoretech Pty Ltd, Sydney, Australia).
Injection of lidocaine into the GPs
A dose of 1 cc of 2% lidocaine was injected into each of 4 GPs (anterior right GP, inferior right GP, inferior left GP, and Marshall tract GP) near the pulmonary veins during AF (Figure 1B). However, 1 patient (#3) only had an injection in the anterior right GPs because of an oversized heart (impossible to access left‐sided GPs). AEGs were recorded before, during, and after lidocaine injection.
Characterizations of AF from AEGs
Sequential activation maps of persistent and LSP AF were constructed for a period of 10 consecutive beat‐to‐beat activations per patient using a custom‐designed algorithm that has been previously described.28, 29, 30 All isochronal lines were determined by activation times from recorded bipolar AEGs. All bipolar AEGs were subjected to CL variation and dominant frequency (DF) analyses to detect mean CL and DF before and after lidocaine injection.31 Each analysis of bipolar AEGs was performed on 10‐second segments of data. DF maps during baseline AF and after injection of lidocaine were generated. Data are presented as the mean CL±SD because 1000/DF is equivalent to the mean CL.
Statistical analysis
Data are presented as the mean±SD using Minitab (Minitab Inc, State College, PA). A Student paired t test was used to compare differences in mean CL and DF in LA and RA of before and after lidocaine injection. Also, a 2‐sample t test was used to compare changes in LA‐to‐RA frequency gradient. A value of P<0.05 was considered statistically significant.
Results
Efficacy of Lidocaine Injection Into GPs in Canine VNS Model
In all studies, AF was induced and sustained per protocol, and recording was performed from onset of AF through its termination following cessation of VNS. After the injection of lidocaine into all 5 GPs, AF was not inducible (<5 seconds) in 4 of 7 canines during VNS. In the 4 dogs in which induction of AF failed immediately after injection of lidocaine, sustained AF was again inducible during VNS after a period of 0.5 to 2.0 hours.
Studies in Patients With Persistent and LSP AF
Left‐to‐right frequency gradients during baseline AF
In 6 patients with persistent and LSP AF, during baseline AF, there was an LA‐to‐RA frequency gradient (LA, mean CL 175±17 ms; RA, mean CL 192±17 ms, P<0.01, Figure 2). Figure 3A shows DF maps in 10‐second segments during baseline AF for all patients. DF maps show each DF Hz as a color recorded by the electrode array, which covers the LAA, BB, and RAA. An isochronal color bar in 0.5‐Hz increments from 3 to 8 Hz is shown on the right. Most of the DF (light green to green DF color; range, 5.5–7.5 Hz) in the LA was higher than in the RA (light red to yellow DF color; range, 4–5.5 Hz). However, in patient #3, there was no LA‐to‐RA frequency gradient (same light green DF color).
Figure 2.
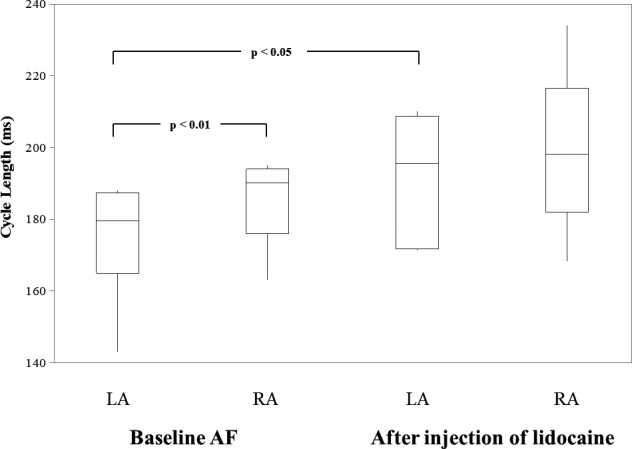
Box plots illustrating the range of mean CL (the box represents the 25th to the 75th percentile; the horizontal line in the box represents the median, the vertical line represents the minimum and maximum values) of all patients combined for left (LA) and right atria (RA) during baseline atrial fibrillation (AF) and after injection of lidocaine. See text for Discussion.
Figure 3.

DF maps from an array of 96 electrodes placed on the epicardial surface covering from the LAA to RAA during baseline AF (A) and after injection of lidocaine (B). DF color bar is shown on right (3–8 Hz). Black arrow indicates an LA‐to‐RA frequency gradient (P<0.05). See text for Discussion. BB indicates Bachmann's bundle; LAA, left atrial appendage; RAA, right atrial appendage.
Disappearance of left‐to‐right frequency gradients after injection of lidocaine
After injection of lidocaine into the 4 GPs during AF, AF persisted in all patients. Also, the LA‐to‐RA frequency gradient disappeared (LA, mean CL 186±13 ms; RA, mean CL 199±23 ms; P=0.08; Figure 2). Figure 3B shows DF maps in 10‐second segments after injection of lidocaine for 5 patients because we excluded data in patient #3 who only had an injection in the anterior right GP. Most of the DF maps in both atria had disappearance of the LA‐to‐RA frequency gradient. However, in patient #5, the LA‐to‐RA frequency gradient continued after injection of the lidocaine because of prolongation of the CLs in both atria. Overall, comparison of mean CLs before and after lidocaine injection demonstrated prolongation of the LA CLs (P<0.05), but there was no effect on the RA CLs (P=0.37; Figure 2). Table 2 shows the summary data of DFs from 48 bipolar AEGs in each of the 6 patients before and after lidocaine injection during persistent and LSP AF.
Table 2.
Summary Data of Dominant Frequency Analysis During Persistent and Long‐Standing Persistent AF
| Patient No. | LA DF (Hz) | RA DF (Hz) | LA‐to‐RA Frequency Gradient | |||||
|---|---|---|---|---|---|---|---|---|
| Before Lidocaine | After Lidocaine | P Value | Before Lidocaine | After Lidocaine | P Value | Before Lidocaine | After Lidocaine | |
| 1 | 5.7±0.4 | 5.3±0.2 | <0.01a | 5.2±0.4 | 5.2±0.4 | 0.98 | P<0.01a | P=0.15 |
| 2 | 5.3±0.3 | 5.1±0.6 | <0.05a | 4.8±0.2 | 5.0±0.5 | <0.01a | P<0.01a | P=0.93 |
| 3 | 5.7±0.7 | 5.7±0.7 | P=0.88 | |||||
| 4 | 5.3±0.4 | 5.3±0.6 | 0.7 | 5.0±0.4 | 5.0±0.7 | 0.4 | P<0.01a | P=0.2 |
| 5 | 5.4±0.7 | 5.2±0.6 | <0.05a | 4.8±0.6 | 4.3±0.5 | <0.05a | P<0.01a | P<0.01a |
| 6 | 7.0±0.7 | 6.1±0.6 | <0.01a | 5.9±0.5 | 5.9±0.2 | 0.47 | P<0.01a | P=0.13 |
For LA‐to‐RA frequency gradient, 2‐sample t test (LA vs RA) was used. AF indicates atrial fibrillation; DF, dominant frequency; LA, left atrium; RA, right atrium.
indicates statistical significance (p < 0.05).
Figure 4 is a representative example from patient #6 demonstrating the disappearance of the LA‐to‐RA frequency gradient after injection of lidocaine. The top panel of Figure 4 shows the activation sequence maps of 8 consecutive 140‐ms windows during baseline AF and after lidocaine injection recorded from the electrode array, which covers the LAA, BB, and RAA. A 10‐ms isochronal color bar from 0 to 140 ms is shown in the right. The bottom panel of Figure 4 shows selected bipolar AEGs (a, b) with CLs and DF analysis recorded from the LA and RA during baseline AF and after injection of lidocaine. As seen in the top left panel (baseline AF), in LA, wavefronts from LAA consistently propagate toward the mid‐portion of BB, where it collides with another wavefront. In the RA, wavefronts from the mid‐portion of BB and the RAA largely collided and merged with each other at continuously varying sites or with a functional line of block. As seen in the top right panel (injection of lidocaine), in the LA, wavefronts from the LAA continuously propagate toward the mid‐portion of BB and collide with other wavefronts. This repetitive wavefront occurred at a reduced rate compared with baseline. In the RA, the activation patterns were similar to baseline AF.
Figure 4.
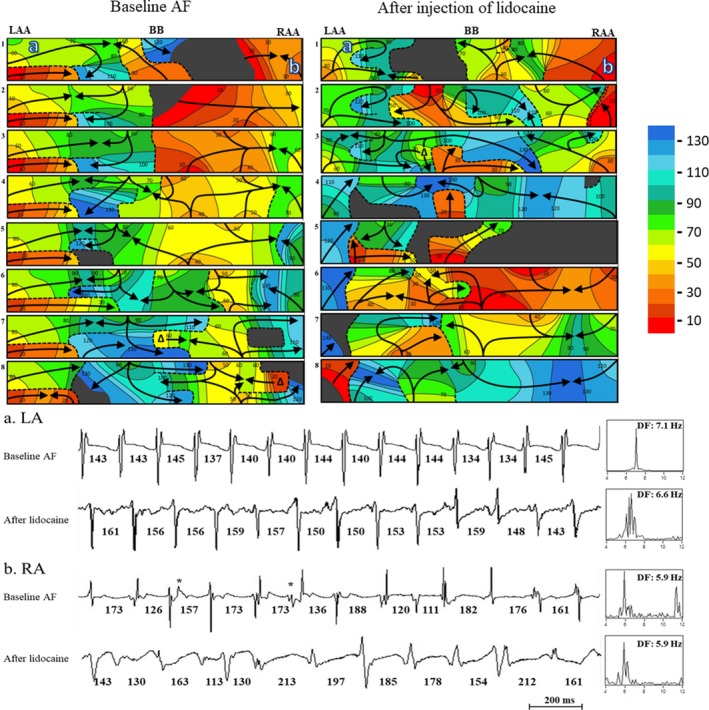
A representative example (patient #6) of an activation sequence map illustrating the disappearance of the LA‐to‐RA frequency gradient after injection of lidocaine. Top panel: Activation sequence maps of 8 consecutive beats during atrial fibrillation (AF) recorded from the LAA, BB, and RAA, with the locations of recording sites a and b. A 10‐ms isochronal color bar is shown on right. The black arrows indicate activation wavefronts. Black areas indicate nonactivation. Dashed lines indicate a functional line of block. T‐bars ( ) indicate block attributed to effective refractory period (ERP). Bottom panel: Bipolar AEGs from selected sites a (LA) and b (RA) with dominant frequency (DF) during baseline AF and after injection of lidocaine. The power spectrum is shown to the right of the traces with the dominant frequency. See text for Discussion. AF, atrial fibrillation; BB indicates Bachmann's bundle; DF, dominant frequency; ERP, effective refractory period; LAA, left atrial appendage; RAA, right atrial appendage.
) indicate block attributed to effective refractory period (ERP). Bottom panel: Bipolar AEGs from selected sites a (LA) and b (RA) with dominant frequency (DF) during baseline AF and after injection of lidocaine. The power spectrum is shown to the right of the traces with the dominant frequency. See text for Discussion. AF, atrial fibrillation; BB indicates Bachmann's bundle; DF, dominant frequency; ERP, effective refractory period; LAA, left atrial appendage; RAA, right atrial appendage.
In the bottom panel of Figure 4, selected AEGs (site a) from the LA during baseline AF show constant CLs with DF 7.1 Hz. However, after injection of lidocaine, the site a shows prolonged CLs with a DF of 6.6 Hz. As a result, in the top panel of Figure 4, during baseline AF, wavefronts from LAA appear 8 times during eight 140‐ms windows, but after injection of lidocaine, wavefronts from the LAA appear 7 times during another eight 140‐ms window. However, selected AEGs (site b) from the RA has the same DF (5.9 Hz) both during baseline AF and after injection of lidocaine.
Discussion
Major Findings
We studied the local injection of lidocaine into GPs for a neuronal blocker in the canine VNS AF model and in patients with persistent and LSP AF during open heart surgery. In the canine VNS AF model, we tested inducibility of AF after injection of lidocaine. In patients with persistent and LSP AF, we also characterized AEGs recorded from the electrode array (96 electrodes) covering the LAA, BB, and RAA. Using activation sequence maps and DF analysis, we compared baseline AF with AF after injection of lidocaine. Our results demonstrated that (1) in the canine VNS AF model, injection of lidocaine suppressed inducibility of AF; (2) in patients with persistent and LSP AF, AEGs from the LA had shorter CLs than the RA, indicating a left‐to‐right frequency gradient; and (3) injection of lidocaine significantly prolonged only the LA CLs, with no effect on the RA, explaining the observed disappearance of the left‐to‐right frequency gradient.
Hyperactivity of GPs Promotes AF
There is evidence from experimental and clinical studies that the autonomic nervous system plays an important role in the pathophysiology of AF. It has been shown that the GPs are critical for both initiation and maintenance of AF.4, 5, 6, 7 Hyperactivity of GPs using fat pad stimulation increases both parasympathetic (shortening of the action potential duration) and sympathetic tone (increasing calcium loading), which could initiate triggered firing and/or reentry.4, 9 AF in the rapid atrial pacing model can be prevented by GP ablation8, 32, 33 and hence thought to be attributed to hyperactivity of the GPs. Interestingly, a recent study demonstrated that there is a spatial relationship between GPs and localized AF sources (ie, focal and reentrant) in patients with AF.22 Several studies have shown that targeting GPs may improve the long‐term outcome of AF.10, 12, 14, 16, 17, 21 However, there is a need to investigate better ways to target GPs because of the difficulty in accurately identifying locations and sizes of GPs between patients. Also, it is postulated that pulmonary vein isolation unavoidably ablates GPs, thus affecting the neuronal contribution to AF.14
Comparison With Previous Similar Studies
In the canine fat pad stimulation AF model,27 injections of lidocaine into fat pads resulted in the loss of AF inducibility using the same stimulus threshold, but some episodes showed AF inducibility with a higher stimulus threshold. Also, injection of botulinum toxin was performed in animal AF models19, 20 and a patient with paroxysmal AF21 and showed atrial tachyarrhythmia suppression. In comparison with our study, our finding of noninducibility of AF after injection of lidocaine into GPs in the canine VNS AF model concurs with another study27 using a higher threshold. However, injection of lidocaine into GPs in patients with persistent and LSP AF is, to our knowledge, the first such study. During AF, the left‐to‐right frequency gradient is the same shown by others.24 Interestingly, after injection of lidocaine, increased CL or disappearance of a left‐to‐right frequency gradient are similar to the results observed after AF ablation.24, 25, 26
Implications
Recently, investigators considered that GPs could be used as a target during AF ablation. In patients with paroxysmal AF, ablation of GPs with pulmonary vein isolation resulted in better outcomes.14 However, in patients with persistent and LSP AF, efficacy of GP ablation showed variable results. Also, there are many questions regarding how to achieve complete GP ablation using either an endocardial catheter or surgical ablation. Our study suggests a potential short‐term pharmacological approach for targeting GPs. Injection of lidocaine in patients with persistent and LSP AF prolongs only the LA CLs, resulting in eliminating the left‐to‐right frequency gradient. We should consider injections of botulinum toxin for the long‐term pharmacological approach.
Study Limitation
In canine studies, we performed injections of lidocaine in normal hearts during sinus rhythm. It is unclear what would have resulted had these canine studies been done in structurally abnormal hearts, as is so often the case in the human AF model. However, the canine studies were an efficacy test for lidocaine injection into GPs in vagally mediated AF to affect inducibility of AF preceding our human AF studies. Also, we did not perform control studies by injection of saline. The locations of GPs in the canine and patient studies were selected in fad pad areas visualized by eye, not a functional approach which uses selective high‐frequency stimulation. Likely, some of GPs in the fat pad might not be affected by the injection of lidocaine. Because we performed studies in patients with persistent and LSP AF, our findings cannot be assumed to apply to patients with paroxysmal AF. Additionally, the fact that our study population is small and all patients had valvular heart disease might make this patient population unique when considering the contribution of GPs to AF. Also, we did not record from the rest of the atria, including fat pads, the endocardium, pulmonary veins, and atrial septum. Therefore, we were unable to demonstrate how the injection of lidocaine decreased LA CL.
Conclusions
In the canine VNS AF model, injection of lidocaine into GPs suppressed inducibility of AF. In patients with persistent and LSP AF, AEGs from the LA had shorter CLs than the RA, indicating an LA‐to‐RA frequency gradient. Injection of lidocaine into the GPs significantly prolonged only the LA CLs with no effect in the RA, explaining the disappearance of the LA‐to‐RA frequency gradient. The mechanism of localized AEG CL prolongation in patients with persistent and LSP AF is uncertain.
Sources of Funding
This work was supported from The Prentiss Foundation, the Jennie Zoline, Blue Dot, Glenstone, Frank & Gerry Pearl, and MCJ Amelior Foundations.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e011401 DOI: 10.1161/JAHA.118.011401.)
References
- 1. Armour JA, Murphy DA, Yuan B‐X, MacDonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–298. [DOI] [PubMed] [Google Scholar]
- 2. Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000;259:353–382. [DOI] [PubMed] [Google Scholar]
- 3. Tan AY, Li H, Wachsmann‐Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein‐atrial junction: implications for catheter ablation of atrial‐pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–143. [DOI] [PubMed] [Google Scholar]
- 4. Stavrakis S, Nakagawa H, Po SS, Scherlag BJ, Lazzara R, Jackman WM. The role of the autonomic ganglia in atrial fibrillation. JACC Clin Electrophysiol. 2015;1:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nishida K, Datino T, Macle L, Nattel S. Atrial fibrillation ablation: translating basic mechanistic insights to the patient. J Am Coll Cardiol. 2014;64:823–831. [DOI] [PubMed] [Google Scholar]
- 6. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114:1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishida K, Maguy A, Sakabe M, Comtois P, Inoue H, Nattel S. The role of pulmonary veins vs. autonomic ganglia in different experimental substrates of canine atrial fibrillation. Cardiovasc Res. 2011;89:825–833. [DOI] [PubMed] [Google Scholar]
- 8. Choi EK, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin SF, Chen PS. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. [DOI] [PubMed] [Google Scholar]
- 10. Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:1186–1189. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Wang Z, Wang W, Wang J, Gao M, Hou Y. Efficacy of cardiac autonomic denervation for atrial fibrillation: a meta‐analysis. J Cardiovasc Electrophysiol. 2012;23:592–600. [DOI] [PubMed] [Google Scholar]
- 12. Katritsis DG, Pokushalov E, Romanov A, Giazitzoglou E, Siontis GC, Po SS, Camm AJ, Ioannidis JP. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–2325. [DOI] [PubMed] [Google Scholar]
- 13. Pokushalov E, Romanov A, Katritsis DG, Artyomenko S, Shirokova N, Karaskov A, Mittal S, Steinberg JS. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long‐standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm. 2013;10:1280–1286. [DOI] [PubMed] [Google Scholar]
- 14. Katritsis DG, Giazitzoglou E, Zografos T, Pokushalov E, Po SS, Camm AJ. Rapid pulmonary vein isolation combined with autonomic ganglia modification: a randomized study. Heart Rhythm. 2011;8:672–678. [DOI] [PubMed] [Google Scholar]
- 15. Zheng S, Li Y, Han J, Zhang H, Zeng W, Xu C, Jia Y, Wang J, Guo K, Jiao Y, Meng X. Long‐term results of a minimally invasive surgical pulmonary vein isolation and ganglionic plexi ablation for atrial fibrillation. PLoS One. 2013;8:e79755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Onorati F, Curcio A, Santarpino G, Torella D, Mastroroberto P, Tucci L, Indolfi C, Renzulli A. Routine ganglionic plexi ablation during maze procedure improves hospital and early follow‐up results of mitral surgery. J Thorac Cardiovasc Surg. 2008;136:408–418. [DOI] [PubMed] [Google Scholar]
- 17. Doll N, Pritzwald‐Stegmann P, Czesla M, Kempfert J, Stenzel MA, Borger MA, Mohr FW. Ablation of ganglionic plexi during combined surgery for atrial fibrillation. Ann Thorac Surg. 2008;86:1659–1663. [DOI] [PubMed] [Google Scholar]
- 18. Driessen AHG, Berger WR, Krul SPJ, van den Berg NWE, Neefs J, Piersma FR, Chan Pin Yin DRPP, de Jong JSSG, van Boven WP, de Groot JR. Ganglion plexus ablation in advanced atrial fibrillation: the AFACT study. J Am Coll Cardiol. 2016;68:1155–1165. [DOI] [PubMed] [Google Scholar]
- 19. Nazeri A, Ganapathy AV, Massumi A, Massumi M, Tuzun E, Stainback R, Segura AM, Elayda MA, Razavi M. Effect of botulinum toxin on inducibility and maintenance of atrial fibrillation in ovine myocardial tissue. Pacing Clin Electrophysiol. 2017;40:693–702. [DOI] [PubMed] [Google Scholar]
- 20. Oh S, Choi EK, Zhang Y, Mazgalev TN. Botulinum toxin injection in epicardial autonomic ganglia temporarily suppresses vagally mediated atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:560–565. [DOI] [PubMed] [Google Scholar]
- 21. Pokushalov E, Kozlov B, Romanov A, Strelnikov A, Bayramova S, Sergeevichev D, Bogachev‐Prokophiev A, Zheleznev S, Shipulin V, Lomivorotov VV, Karaskov A, Po SS, Steinberg JS. Long‐term suppression of atrial fibrillation by botulinum toxin injection into epicardial fat pads in patients undergoing cardiac surgery: one year follow up of a randomized pilot study. Circ Arrhythm Electrophysiol. 2015;8:1334–1341. [DOI] [PubMed] [Google Scholar]
- 22. Baykaner T, Zografos TA, Zaman JAB, Pantos I, Alhusseini M, Navara R, Krummen DE, Narayan SM, Katritsis DG. Spatial relationship of organized rotational and focal sources in human atrial fibrillation to autonomic ganglionated plexi. Int J Cardiol. 2017;240:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lemola K, Chartier D, Yeh YH, Dubuc M, Cartier R, Armour A, Ting M, Sakabe M, Shiroshita‐Takeshita A, Comtois P, Nattel S. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation. 2008;117:470–477. [DOI] [PubMed] [Google Scholar]
- 24. Atienza F, Almendral J, Jalife J, Zlochiver S, Ploutz‐Snyder R, Torrecilla EG, Arenal A, Kalifa J, Fernandez‐Aviles F, Berenfeld O. Real‐time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left‐to‐right frequency gradients predicts long‐term maintenance of sinus rhythm. Heart Rhythm. 2009;6:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Filgueiras‐Rama D, Price NF, Martins RP, Yamazaki M, Avula UM, Kaur K, Kalifa J, Ennis SR, Hwang E, Devabhaktuni V, Jalife J, Berenfeld O. Long‐term frequency gradients during persistent atrial fibrillation in sheep are associated with stable sources in the left atrium/clinical perspective. Circ Arrhythm Electrophysiol. 2012;5:1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haïssaguerre M, Sanders P, Hocini M, Hsu LF, Shah DC, Scavée C, Takahashi Y, Rotter M, Pasquié JL, Garrigue S, Clémenty J, Jaïs P. Changes in atrial fibrillation cycle length and inducibility during catheter ablation and their relation to outcome. Circulation. 2004;109:3007–3013. [DOI] [PubMed] [Google Scholar]
- 27. Scherlag BJ, Yamanashi W, Patel U, Lazzara R, Jackman WM. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol. 2005;45:1878–1886. [DOI] [PubMed] [Google Scholar]
- 28. Lee S, Sahadevan J, Khrestian CM, Durand DM, Waldo AL. High density mapping of atrial fibrillation during vagal nerve stimulation in the canine heart: restudying the Moe hypothesis. J Cardiovasc Electrophysiol. 2013;24:328–335. [DOI] [PubMed] [Google Scholar]
- 29. Lee S, Sahadevan J, Khrestian CM, Cakulev I, Markowitz A, Waldo AL. Simultaneous biatrial high‐density (510–512 electrodes) epicardial mapping of persistent and long‐standing persistent atrial fibrillation in patients: new insights into the mechanism of its maintenance. Circulation. 2015;132:2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee S, Sahadevan J, Khrestian CM, Markowitz A, Waldo AL. Characterization of foci and breakthrough sites during persistent and long‐standing persistent atrial fibrillation in patients: studies using high‐density (510–512 electrodes) biatrial epicardial mapping. J Am Heart Assoc. 2017;6:e005274 DOI: 10.1161/JAHA.116.005274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee S, Ryu K, Waldo AL, Khrestian CM, Durand DM, Sahadevan J. An algorithm to measure beat‐to‐beat cycle lengths for assessment of atrial electrogram rate and regularity during atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:199–206. [DOI] [PubMed] [Google Scholar]
- 32. Yu L, Scherlag BJ, Sha Y, Li S, Sharma T, Nakagawa H, Jackman WM, Lazzara R, Jiang H, Po SS. Interactions between atrial electrical remodeling and autonomic remodeling: how to break the vicious cycle. Heart Rhythm. 2012;9:804–809. [DOI] [PubMed] [Google Scholar]
- 33. Lu Z, Scherlag BJ, Lin J, Niu G, Fung KM, Zhao L, Ghias M, Jackman WM, Lazzara R, Jiang H, Po SS. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short‐term rapid atrial pacing. Circ Arrhythm Electrophysiol. 2008;1:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]


