Abstract
Background
Fondaparinux sodium has been compared with low‐molecular‐weight heparins (LMWH) in randomized controlled trials for perioperative surgical thromboprophylaxis. However, the results from these studies are inconsistent in terms of efficacy and safety to reach a clinical decision. The objective of this study was to systematically review the randomized controlled trials comparing the efficacy and safety of fondaparinux and LMWH for perioperative surgical thromboprophylaxis.
Methods and Results
Systematic search in various databases was done to identify randomized controlled trials comparing fondaparinux and LMWH published during the years 2000 to 2017. Outcomes of interest in this study included venous thromboembolism up to day 15, all‐cause mortality up to day 90, major bleeding, and minor bleeding during the treatment period. Analyses were performed with the relative odds based on a random‐effects model using Mantel‐Haenszel statistics. Results were presented as odds ratios with their 95% CIs. The assessment of study quality was performed as per Cochrane collaboration. After screening 10 644 articles, 12 randomized controlled trials including 14 906 patients were included in the final analyses. Pooled analyses showed the odds of venous thromboembolism in the fondaparinux group were 0.49 times the odds in LMWH group (OR=0.49 [0.38–0.64]). However, the odds of major bleeding in the fondaparinux group were 1.48 times the odds in the LMWH group (OR=1.48 [1.15–1.90]).
Conclusions
Fondaparinux was associated with a superior efficacy in terms of reduction of venous thromboembolism in this meta‐analysis. However, it was also associated with increased odds of major bleeding.
Keywords: effectiveness, fondaparinux, low molecular weight heparin, prevention, safety, systematic review, venous thromboembolism
Subject Categories: Thrombosis, Anticoagulants, Meta Analysis, Embolism, Primary Prevention
Clinical Perspective
What Is New?
Meta‐analyses during the past years have compared fondaparinux sodium with low molecular weight heparins (LMWH) for thromboprophylaxis in orthopedic surgeries; however, the literature lacks a systematic comparison of these drugs in different types of surgeries altogether.
Among the randomized controlled trials, both drugs have been compared for surgical thromboprophylaxis; nevertheless, the findings are not consistent in terms of efficacy and safety.
This study systematically reviews the randomized controlled trials comparing safety and efficacy of fondaparinux and LMWHs for perioperative surgical thromboprophylaxis for better clinical judgment.
What Are the Clinical Implications?
In this study, fondaparinux was associated with lower odds of venous thromboembolism compared with LMWH, but both groups were similar in terms of reduction of symptomatic venous thromboembolism and all‐cause mortality.
Fondaparinux was also associated with increased risk of major bleeding, especially surgical site bleeding, compared with LMWH.
This analysis suggested a trade‐off between safety and efficacy when fondaparinux is used over LMWH; however, net clinical benefit (venous thromboembolism + major bleeding) was in favor of fondaparinux compared with LMWH.
Introduction
Venous thromboembolism (VTE) is a leading cause of preventable death among patients undergoing surgical intervention.1 Major factors contributing to development of VTE among surgical patients may include variations in the flow of blood in the veins (circulatory stasis), changes in the vessel wall because of any injury during the procedure (vascular damage), and any variation in the composition of blood (hypercoagulability). These complex mechanisms interplay in the progression of VTE.2 A majority of patients undergoing surgeries possess at least 1 risk factor of VTE,3 making VTE a significant cause of healthcare and financial burden.4, 5
VTE is the most common postoperative complication following surgeries.2 VTE has been reported to be associated with long‐term sequelae such as chronic leg ulcers and venous insufficiency,6 which could be life threatening. Thus, surgical thromboprophylaxis is strongly recommended by the American College of Chest Physicians.7 Pharmacological prophylaxis is effective, yet VTE still occurs frequently.8 Hence, there is a need to improve thromboprophylaxis for surgical patients at risk for VTE.
Among patients undergoing surgical interventions, the management of anticoagulation is challenging, as underlying surgical procedures are associated with bleeding complications, which could be augmented further by anticoagulation for thromboprophylaxis. Thus, a balance between thrombosis prevention and devastating operative site bleeding is crucial in this population.9 Conventional therapy such as low‐molecular‐weight heparins (LMWHs) has been used for many years; nevertheless, factor Xa inhibitors are desired because they are more selective in their action.10 Fondaparinux is the first approved drug among factor Xa inhibitors,11 which has been compared with LMWH in various clinical trials; however, results are inconsistent in terms of efficacy and safety.6, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 This meta‐analysis was performed to examine existing clinical evidence on efficacy and safety of parenteral fondaparinux and LMWH for perioperative surgical thromboprophylaxis in patients at high risk for VTE.
Methods
The authors declare that all supporting data are available within the tables, figures, and online supplemental material of this manuscript. This systematic review and meta‐analysis followed the guidelines published by Cochrane Collaboration. We used the Preferred Reporting Items for Systematic Review and Meta‐analysis 200922 checklist (Table S1) for transparent reporting of this study.
Study Protocol
We developed a protocol for systematic review and meta‐analysis to assess overall risk factors as per Virchow's triad (Data S1). However, this paper was developed to specifically address perioperative surgical thromboprophylaxis considering its management very crucial and challenging for this patient population.9
Eligibility Criteria
Type of Studies
Only head‐to‐head randomized controlled trials (RCTs) comparing fondaparinux with LMWHs published in English, and meeting the study inclusion criteria were taken into consideration. LMWHs included enoxaparin sodium, dalteparin sodium, nadroparin calcium, parnaparin sodium, and tinzaparin sodium. Potential studies in other languages were searched for abstracts in English. Studies were included if published between January 2000 and December 2017. This period was selected because the first clinical trial of fondaparinux comparing LMWH was published in 2001.11
Participants
RCTs were considered eligible if they included both male and female adults aged ≥18 years old. RCTs enrolling patients undergoing major orthopedic surgeries (eg, total hip replacement, total knee replacement, or hip fracture surgeries), major general surgeries (eg, abdominal surgery, cancer surgery), and related surgical immobility proven to be a risk factor for the development of VTE were included in the analyses.
Interventions
The interventions compared in this review were subcutaneous fondaparinux and subcutaneous LMWHs at titrated dose/manufacturer's recommended dose as per body weight or at the standard dose as per the country of interest. Studies in which the assessment was done up to 15 days after surgery were only taken into consideration for inclusion. Assessment period was chosen as 15 days, as the increased risk of VTE has been cited to be during the first 2 weeks.23, 24, 25
Information Sources
We conducted a systematic search of the databases (Embase, PubMed, The Cochrane Central Register of Control Trials, ProQuest‐Direct, Science Direct, Clinicaltrials.gov), and conference proceedings to find RCTs evaluating fondaparinux and LMWH for the prophylaxis of VTE. Trials presented in conference proceedings but not published were searched for full‐text results. When full‐text results were not available, the reviewers contacted the authors of the unpublished studies via email to request trial results and full‐text manuscripts if available. The reference lists of all identified trials and review articles were hand searched to find any additional trials.
Search
The complete search strategy can be found in Table S2.
Study Selection
Covidence,26 an online systematic review platform (www.covidence.org) was used to initially screen articles on the basis of the title and abstract. Records were imported from various databases into Covidence, where duplicates were removed. Two review authors (A.K., A.T.) independently screened the titles and abstracts of identified citations for potential eligibility. Full texts of articles retrieved were judged potentially eligible by at least 1 review author.
Data Collection Process
Both review authors then independently screened the full‐text article for eligibility using an explicit inclusion and exclusion criteria. The screening process was documented in Preferred Reporting Items for Systematic Review and Meta‐analysis flow chart of study selection (Figure 1). A data extraction form was developed in the Covidence web platform to extract the information from relevant clinical trials. Both review authors independently extracted data from each included study. Any disagreements were resolved by discussion or by consulting a third reviewer (WW). After consensus, both reviewers analyzed the resulting papers in full text independently.
Figure 1.

Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) flow chart for study selection.
Data Items
The primary efficacy outcome of this study was VTE defined as the composite of symptomatic and asymptomatic deep vein thrombosis (DVT) and pulmonary embolism (PE). We also reported symptomatic VTE and PE as an independent outcome, considering it to be an important predictor of death. Considering the differential risk of proximal and distal DVT on PE and death, the events of proximal and distal DVT were also reported separately. Included studies assessed patients for DVT by systematic bilateral ascending venography of the legs, magnetic resonance venography, or d‐dimer test. PE was assessed by a lung scan, pulmonary angiography, and helical computed tomography or at autopsy. All‐cause mortality at day 90 after surgery was also reported. Mortality assessment at 90 days was chosen, as increased risk of VTE‐related mortality has been indicated as 60 to 90 days following surgeries.12 The safety outcome was the incidence of major bleeding, a composite outcome that included fatal bleeding; bleeding that was retroperitoneal, intracranial, or intraspinal or that involved any other critical organ; bleeding leading to reoperation; or overt bleeding, with hemoglobin level declined >2 g/dL, or requirement of transfusion of ≥2 units of blood as reported in major clinical trials.11, 12, 15, 27 Safety measures also included minor bleeding, which included any bleeding event not qualified as major bleeding. To assist clinical decision making, we also reported net clinical benefit in which we combined VTE and major bleeding events for each study.
Risk of Bias in Individual Studies
The assessment of methodological quality of included studies was done using The Cochrane Collaboration's “Risk of Bias” tool. Domains were classified as “low risk,” “high risk,” or “unclear risk” as per Cochrane Handbook for Systematic Reviews of Interventions.28
Summary Measures
All the outcomes in this analysis were binary. Relative treatment effects for each comparison were expressed as odds ratio (OR) with 95% CI. The pooled data for each outcome were used to create a “Forest Plot.” The individual participant was the unit of analysis. Finally, we also presented the primary outcomes using L'Abbe plots as a tool to look at the direction of pooled effect graphically and to compare the event rates in fondaparinux compared with LMWH.
Synthesis of Results
Data were analyzed using Mantel‐Haenszel statistics. Chi‐square tests and I2 statistics were used to assess heterogeneity. As the studies included in this meta‐analysis were from different countries, which might cause unexplained heterogeneity, we used a random‐effects model for summary statistics.29
Risk of Bias Across Studies
The reporting bias was assessed by use of a “funnel plot” to examine the relationship between treatment effects and their inverted standard errors.30 Funnel plots were presented only if there were at least 10 studies in any subgroup to maintain power to distinguish chance from real symmetry.28
Additional Analyses
Subgroup Analysis
Subgroup analysis was conducted in which studies from different continents were pooled together to see any difference in the results compared with primary analyses. We also reported a subgroup analysis comparing only postoperative thromboprophylaxis to control for possible bias caused by different timings of anticoagulation.
Sensitivity Analysis
The primary analyses included data from all patients in the trials during the period of randomly allocated treatment. A sensitivity analysis was performed to explore the effect that risk of bias had on estimates of treatment effects. The effect on the primary outcome was explored using sensitivity analyses by eliminating studies that were at a high risk of bias, had used a dose other than that recommended, did not clearly list the dose, or did not have the full text available. As some included studies in this meta‐analysis had a small number of events, we used the recommended Peto method31 for pooled analyses during sensitivity analyses. All statistical analyses in this meta‐analyses were performed using Review Manager32 (RevMan 5.3) and STATA33 version 15.1.
Results
Study Selection
A total of 10 644 citations were identified; 5923 duplicate studies were removed, leaving 4721 studies for screening. After screening the full text, 18 studies were retrieved and reviewed. Six of these 18 studies were subsequently excluded because they did not meet the RCT study design criteria or were duplicate publications. The final analysis included 12 studies (Figure 1).
Study Characteristics
The 12 studies6, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 captured data on 14 906 patients for analysis. All 12 studies compared fondaparinux with LMWHs at titrated dose/manufacturer recommendation for surgical thromboprophylaxis. Six of these studies6, 11, 12, 15, 18, 19 were multicenter RCTs while the remaining 613, 14, 16, 17, 20, 21 were single‐center RCTs (Table). Four studies each were conducted in North American11, 12, 16, 18 and Asian countries.13, 14, 17, 20 Three studies11, 12, 21 were conducted in European countries, and 1 study was multinational. Average assessment day of VTE was the ninth day in this meta‐analysis.
Table 1.
Description of Included Studies in the Meta‐analysis
| Characteristics of the Included Studies | Number of Patients | Mean Age in Years | Sex (M/F) | Mean Weight/BMI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Type of Surgery | N=14 906 | Clinical Trial Setting | FPX Dose | LMWH Dose | Assessment Day Post‐Surgery | Duration of Prophylaxis | FPX | LMWH | FPX | LMWH | FPX | LMWH | FPX | LMWH |
| Turpie, 200119 | Elective hip replacement surgery | n=437 | Multicentered, double blind RCT | 3.0 mg OD | Enoxaparin 30 mg BD | 10th day | 5–10 days | 177 | 260 | 66 | 66 | 80/97 | 123/137 | 80/NA | 81/NA |
| Bauer, 200118 | Elective major knee surgery | n=1034 | Multicentered, double‐blind RCT | 2.5 mg OD | Enoxaparin 30 mg BD | 11th Day | 5–9 days | 517 | 517 | 67.5 | 67.5 | 204/313 | 223/294 | 89/31.5 | 88/30.9 |
| Erikss‐on, 200111 | Hip‐fracture surgery | n=1673 | Multi‐centered, double‐blind RCT | 2.5 mg OD | Enoxaparin 40 mg OD | 11th day | 5–9 days | 831 | 842 | 76.8 | 77.3 | 187/644 | 224/618 | 64.3/23.8 | 64.223.6 |
| Turpie, 200212 | Total hip replacement | n=3841 | Multicentered, double‐blind RCT | 2.5 mg OD | Enoxaparin 30 mg BD | 11th day | 5–9 days | 1915 | 1926 | 67 | 67 | 942/973 | 897/1029 | 80.6/28 | 79.6/27.6 |
| Lassen, 20026 | Elective hip replacement surgery | n=4100 | Multicentered, double‐blind RCT | 2.5 mg OD | Enoxaparin 40 mg OD | 11th day | 5–9 days | 2048 | 2052 | 66.4 | 67 | 889/1159 | 875/1177 | 75/26 | 75/26.55 |
| Agnelli, 200515 | High‐risk abdominal surgery | n=2858 | Multicentered, double‐blind RCT | 2.5 mg OD | Dalteparin 5000 IU OD | 10th day | 5–9 days | 1433 | 1425 | 66 | 65 | 788/645 | 796/629 | 74.2/26.3 | 74.3/26.3 |
| Sasaki, 200914 | Hip fracture surgery | n=76 | Single‐centered, open‐label RCT | 2.5 mg OD | Not disclosed | 14th day | 14 days | 38 | 38 | 79.2 | 80.2 | 8/30 | 9/29 | NA/21.3 | NA/20.35 |
| Yokote, 201117 | Total hip replacement | n=167 | Single‐centered, single‐blind RCT | 2.5 mg OD | Enoxaparin 20 mg BD | 11th day | 10 days | 84 | 83 | 63 | 64 | 14/70 | 16/67 | NA/22.5 | NA/23.0 |
| Argun, 201321 | Elective Hip and Knee Arthroplasty | n=108 | Single‐centered, open‐label RCT | 2.5 mg OD | Nadroparin 2850 IU OD | 5th day | Not disclosed | 55 | 53 | 58.7 | 60 | 34/21 | 33/20 | NA/NA | NA/NA |
| Shen, 201420 | Esophagectomy | n=116 | Single‐centered, open‐label RCT | 2.5 mg OD | Nadroparin 4100 IU OD | Undisclosed‐Abstract only | Not disclosed | NA | NA | NA | NA | NA | NA | NA/NA | NA/NA |
| Steele, 201516 | Bariatric surgical patients | n=198 | Single‐centered, double‐blind RCT | 5.0 mg OD | Enoxaparin 40 mg BD | 14th day | Duration of hospital stay (Average: 2.5 days) | 100 | 98 | 40.4 | 41.8 | 16/84 | 16/82 | NA/NA | NA/NA |
| Hata, 201613 | Uro‐oncological surgery | n=298 | Single‐centered, single‐blind RCT | 2.5 mg OD | Enoxaparin 2000 IU BD | 5th day | 5 Days | 152 | 146 | 63.9 | 64.7 | 144/8 | 138/8 | NA/23.9 | NA/23.7 |
BMI indicates body mass index; FPX, fondaparinux sodium; LMWH, low‐molecular‐weight heparin; RCT, randomized controlled trial.
Risk of Bias Within Studies
Eight of the studies6, 11, 12, 13, 15, 16, 18, 19 were funded by pharmaceutical industries, which could have been a source of bias. These studies were indicated to be at high risk of bias. Review authors’ judgment about each risk is presented by a “risk of bias graph” and a “risk of bias summary” for each study in Figures 2 and 3, respectively. A detailed description of the risk of bias can be found in Data S2 and Data S3.
Figure 2.

Risk‐of‐bias graph: review authors’ judgments about each risk‐of‐bias item presented as percentages across all included studies.
Figure 3.

Risk‐of‐bias summary: review authors’ judgments about each risk‐of‐bias item for each included study.
Results of Individual Studies
Summary data for each study are indicated in Table and Data S3.
Synthesis of Results
Efficacy Outcome
Venous thromboembolism up to postoperative day 15
All 12 trials reported on the outcome measure of VTE; 243 of 4309 patients had VTE in the fondaparinux group versus 471 of 4357 patients in the LMWH group. The odds of VTE in the fondaparinux group were 0.49 times the odds of VTE in the LMWH group (OR, 0.49; 95% CI, 0.38–0.64; P<0.001; Figure 4. Sensitivity analysis (Figure S1) did not impact the effect size (OR, 0.50; 95% CI, 0.42–0.58; P<0.001). The L'Abbe plot (Figure 5) shows that most of the studies lie in the bottom right (under the null effect line) of the plot. This indicates that the VTE event rates were higher in the LMWH group compared with fondaparinux. The overall trendline (OR line) shows that fondaparinux has a protective effect against VTE compared with LMWH. Reporting bias was not evident, as the funnel plot was symmetric (Figure S2).
Figure 4.

Fondaparinux compared with low‐molecular‐weight heparins (LMWH) for venous thromboembolism up to postoperative day 15.
Figure 5.

Comparison of events rates of venous thromboembolism in fondaparinux and low‐molecular‐weight heparins (LMWH) group.
Deep vein thrombosis up to postoperative day 15
The odds of total DVT in the fondaparinux group was 0.48 times the odds in the LWMH group (OR, 0.48; 95% CI, 0.38–0.61; P<0.001; Figure S3). The odds for proximal DVT in the fondaparinux group were 0.49 times the odds in the LMWH group (OR, 0.49; 95% CI, 0.29–0.84; P=0.009; Figure S4). As far as distal DVT was concerned, the odds in the fondaparinux group were 0.50 times the odds in the LMWH group (OR, 0.50; 95% CI, 0.39–0.64; P<0.001; Figure S5). Not much impact on effect size was observed during sensitivity analysis (Figures S6, S7, and S8). Reporting bias was not evident as presented by funnel plot (Figure S9).
Symptomatic VTE up to postoperative day 15
We observed 29 events among 5152 patients in the fondaparinux arm and 20 events among 5153 patients on LMWH. We did not observe any statistically significant difference between the 2 groups (OR, 1.33; 95% CI, 0.62–2.86; P=0.47; Figure S10).
Pulmonary embolism up to postoperative day 15
Sixteen events each among 5373 and 5425 patients on fondaparinux and LMWH, respectively, were observed. There was no difference between the PE events of the two arms (OR, 1.01; 95% CI, 0.49–2.11; P=0.97; Figure S11).
All‐cause mortality up to postoperative day 90
Six studies reported on number of deaths. Odds in the fondaparinux group were 0.87 times the odds in the LMWH group (OR, 0.87; 95% CI, 0.62–1.23; P=0.44), but results were statistically insignificant (Figure S12). Sensitivity analysis (Figure S13) did not impact the effect size.
Safety Outcome
Major bleeding during the treatment period
Nine studies reported on the incidences of major bleeding. The odds in the fondaparinux group for major bleeding were 1.48 times the odds in the LMWH group (OR, 1.48; 95% CI, 1.15–1.90; P=0.002; Figure 6. Sensitivity analysis did not have much impact on the effect size (OR, 1.49; 95% CI, 1.17–1.91; P=0.001; Figure S14). The L'Abbe plot (Figure 7) showed that most of the studies lie above the null effect line of the plot, which indicates that major bleeding event rates were higher in the fondaparinux group compared with the LMWH group. The overall trendline shows that LMWH has a protective effect against major bleeding compared with fondaparinux.
Figure 6.

Fondaparinux compared with low‐molecular‐weight heparins (LMWH) for major bleeding during the treatment period.
Figure 7.

Comparison of events rates of major bleeding in fondaparinux and low‐molecular‐weight heparin (LMWH) group.
We also specifically looked at events of fatal bleeding and bleeding at surgical site between the 2 groups. For fatal bleeding, Agnelli et al15 reported 2 events each in both arms. Eriksson et al11 reported 1 event of fatal bleeding in LMWH, but no event of fatal bleeding was observed with fondaparinux in this study. As very few studies reported on fatal bleeding, pooled analysis could not be performed. However, for bleeding at the surgical site, we observed 82 events and 54 events in the fondaparinux arm and LMWH arm, respectively. In the pooled analysis, the odds of surgical site bleeding in fondaparinux were 1.43 times the odds in the LMWH arm (OR, 1.43; 95% CI, 1.01–2.04; P=0.05). The association was found to be statistically significant (Figure 8). The L'Abbe plot (Figure 9) shows that of 7 studies included in this analysis, 3 studies lie on the null effect line. However, the overall trendline shows that LMWH administration has a protective effect against surgical site bleeding compared with fondaparinux.
Figure 8.

Fondaparinux compared with low‐molecular‐weight heparins (LMWH) for surgical site bleeding.
Figure 9.

Comparison‐of‐events rates of surgical site bleeding in fondaparinux and low‐molecular‐weight heparins (LMWH) group.
Minor bleeding during the treatment period
Nine studies reported minor bleeding (any bleeding event that did not meet the criteria for major bleeding previously defined). In the summary statistics, it was indicated that fondaparinux odds for minor bleeding were 1.13 times the odds in LMWH (OR, 1.13; 95% CI, 0.89–1.43; P=0.31); however, the results were not significant (Figure S15). Results were consistent following sensitivity analysis (Figure S16).
Net clinical benefit
Eight studies were included in this analysis. Pooled analysis showed significant difference in net clinical benefit in favor of the fondaparinux arm (OR, 0.66; 95% CI, 0.51–0.84; P=0.001; Figure 10. The L'Abbe plot (Figure 11) shows that most of the studies lie under the null effect line. This indicates that the net clinical benefit is higher in the fondaparinux group compared with LMWH. The overall trendline shows that fondaparinux is better than LMWH in terms of net clinical benefit.
Figure 10.

Fondaparinux compared with low‐molecular‐weight heparins (LMWH) for net clinical benefit.
Figure 11.

Comparison‐of‐events rates of net clinical benefit in fondaparinux and low‐molecular‐weight heparins (LMWH) group.
Risk of bias across studies
Reporting bias was presented with funnel plots in the outcome variables above.
Subgroup Analysis
A subgroup analysis was performed stratifying studies within each of the different continents to examine any difference in the effect sizes.
North American and Australian Population
Four studies were conducted on the North American and Australian continents. For VTE, the odds in the fondaparinux group were 0.47 times the odds in the LMWH group (OR, 0.47; 95% CI, 0.27–0.84; P=0.01; Figure 12. For major bleeding, the odds in the fondaparinux group were 2.09 times the odds in the LMWH group, but the association was not statistically significant (OR, 2.09; 95% CI, 0.89–4.89; P=0.09; Figure 13.
Figure 12.

Fondaparinux compared with low‐molecular‐weight heparins (LMWH) for venous thromboembolism in the North American and Australian population.
Figure 13.

Fondaparinux compared with low‐molecular‐weight heparins (LMWH) for major bleeding in the North American and Australian population.
European Population
Three studies were conducted on the European continent. Odds in the fondaparinux group were 0.40 times the odds in LMWH group for VTE (OR, 0.40; 95% CI, 0.31–0.52; P<0.001; Figure 14. For major bleeding, the odds in the fondaparinux group were 1.27 times the odds in the LMWH group but were not statistically significant (OR, 1.27; 95% CI, 0.85–1.91; P=0.25; Figure 15.
Figure 14.
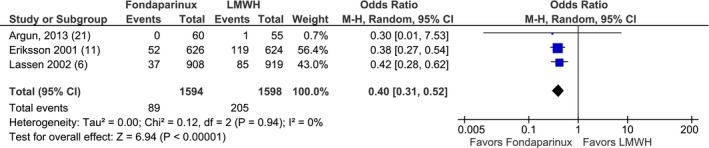
Fondaparinux compared with low‐molecular‐weight heparins (LMWH) for venous thromboembolism prophylaxis in the European population.
Figure 15.

Fondaparinux compared with low‐molecular‐weight heparins (LMWH) for major bleeding in the European population.
Asian Population
Four of the trials were conducted on the Asian continent. The summary statistics indicated that the odds for VTE in the fondaparinux group were 0.52 times the odds in the LMWH group (OR, 0.52; 95% CI, 0.19–1.41; P=0.20; Figure 16. As only 2 studies with very few events of major bleeding were estimable for the meta‐analysis, summary statistics for major bleeding were not performed for Asian studies.
Figure 16.

Fondaparinux compared with low‐molecular‐weight heparins (LMWH) for venous thromboembolism in the Asian population.
Fondaparinux Versus LMWH in Postoperative Thromboprophylaxis
To estimate the effect of “timing of dose” on the results, we included only the studies in which thromboprophylaxis was given postoperatively in this subgroup analysis. We continue to find results similar to the main analyses. The odds in the fondaparinux group for VTE were 0.49 times the odds in the LMWH group (OR, 0.49; 95% CI, 0.28–0.87; P=0.02; Figure 17. At the same time, fondaparinux was associated with an increased risk of major bleeding compared with LMWH (OR, 1.95; 95% CI, 1.02–3.76; P=0.04; Figure 18.
Figure 17.

Postoperative fondaparinux compared with low‐molecular‐weight heparins (LMWH) for venous thromboembolism.
Figure 18.

Postoperative fondaparinux compared with low‐molecular‐weight heparins (LMWH) for major bleeding.
Discussion
Our study suggests a significant reduction of the risk of VTE associated with fondaparinux compared with LMWH for perioperative surgical thromboprophylaxis. This is consistent with the findings of a previous meta‐analysis conducted on 4 RCTs by Turpie et al27 in which better efficacy of fondaparinux over LMWH was indicated with a 45.3% reduction of risk of total VTE. In their meta‐analysis, Turpie et al focused only on the patients undergoing major orthopedic surgeries, whereas our meta‐analysis extends the current existing knowledge to overall surgical interventions. Among the RCTs and large cohort studies, PE has been cited as a major cause of death in patients with VTE.34, 35 To the reviewers’ knowledge, previous studies have not reported PE as an independent outcome. PE events were reported independently in our study, which could be useful in clinical decision making. Our results suggest no significant difference in the incidence of PE between fondaparinux and LMWH combining data across available published studies. Because the patients after randomization in each trial were given prophylaxis treatment, events of PE in these trials might have been small compared with real clinical practice.
This meta‐analysis reported a significant reduction in the risk of total DVT associated with fondaparinux compared with LMWH, which is consistent with the results of previous meta‐analyses.27, 36, 37 Studies have indicated proximal DVT as a substantial risk factor for PE and mortality.38 As almost half of the patients with proximal DVT suffer fatal PE if untreated,39, 40 detection and treatment of proximal DVT is important.40 Fondaparinux significantly reduced the risk of proximal DVT compared with LMWH in our study. To the best of our knowledge, the recent meta‐analyses did not report the results pertinent to the proximal DVT;36, 37 thus, our study improves the current knowledge in this discipline. Also, although proximal DVT has a 3‐fold higher risk of recurrent VTE than distal DVT,41 there is a high probability that distal DVT may extend to proximal DVT and increase the risk of PE and death.40 Our study indicated a significant reduction of distal DVT by fondaparinux. The observed differences in the efficacy in both drugs might be related to the selective inhibition of factor Xa by fondaparinux compared with LMWH (by which both factor Xa and IIa are inhibited). It could also be attributable to longer half‐life and the linear pharmacokinetic profile of fondaparinux.42
Major bleeding in institutionalized surgical patients in VTE prevention trials is a strong predictor of mortality.43 Our pooled analysis indicated a greater risk of major bleeding with fondaparinux compared with LMWH consistent with previous studies.36, 37 More importantly, we observed a significantly increased risk of bleeding at the surgical site with fondaparinux, which is of high clinical relevance.27 Turpie et al pointed out a significant risk of major bleeding by fondaparinux;27 however, they reported no difference in clinically relevant bleeding between the 2 groups. Major bleeding in our study was reported as per the standard definition reported in the first major clinical trials assessing the efficacy and safety of these comparators11 to produce the most current and comprehensive evidence. The association of fondaparinux with major bleeding might be linked to differences in timing of the administration of fondaparinux and LMWH as reported in the previous study.27 We conducted a subgroup analysis in which we included only postsurgical thromboprophylaxis to control for bias, which may be caused by different timings of fondaparinux and LMWH. However, we found the results similar to the main analysis.
Prior studies have pointed out heterogeneity in definitions of major bleeding across different clinical trials,44 which might lead to confusion among clinicians about bleeding risk associated with VTE prophylaxis.45 To assist with clinical decision making, the incidences of minor bleeding were also compared in our study, which has not been reported in any previous meta‐analyses36, 37 to the best of our knowledge. We observed a nonsignificant increased risk of minor bleeding with fondaparinux in comparison with LMWH. In case the patients undergoing surgeries are at increased risk of bleeding, these results may help the clinicians in decision making.
In our analysis, the incidence of all‐cause mortality up to day 90 was also compared between treatment arms, which was not reported in the recent meta‐analyses.36, 37 Although not statistically significant, reduction in the odds of mortality was associated with fondaparinux compared with LMWH. Another meta‐analysis that was conducted to report the effect on mortality considered LMWH and placebo in the same group compared with fondaparinux46 and reported a statistically nonsignificant 21% reduction in the odds associated with fondaparinux. This difference in the results might be attributable to the fact that of the 8 studies included in the meta‐analysis by Eikelboom et al,46 2 studies compared fondaparinux with placebo. These 2 studies accounted for 2158 patients among the 13 085 patients, which might have caused the difference in mortality between our meta‐analysis and meta‐analysis by Eikelboom et al.
A meta‐analysis of 173 case‐control studies reported a significant association of genetic factors with VTE.47 As our analysis included trials from different continents, we also tested if there were any differences in the outcomes by the population. In our subgroup analysis, a significant 60% and 53% reduction in the odds of VTE was associated with fondaparinux among the European and North American and Australian populations, respectively. We also reported a 48% reduction in VTE in the Asian population, which was nonsignificant. The North American and Australian population was found to be at a greater risk of major bleeding compared with other races. These findings might be related to the differences in genetic factors related to venous thrombosis such as factor V, prothrombin G20210A, prothrombin G11991A, PAI‐1 4G/5G, or alpha‐fibrinogen Thr312Ala in different populations.47 As gene mutations may lead to variable risk of VTE in different populations, we hypothesize that exposure to anticoagulants in different populations may also differentiate the risk of major bleeding. This particular finding of our study could act as a hypotheses for future researchers to explore more on the effectiveness and safety of these drugs in different patient populations using real‐world data, which could be a significant addition to the existing literature. It may also be hypothesized that the low dose of LMWH in Japanese studies might have contributed, at least to some extent, to difference in the odds ratios across countries.
Limitations
This study had several limitations. Reviewers had access to the full text of all of the studies included in this meta‐analysis except 1 study from China.20 Efforts to contact the principal investigators of this study were made but were unsuccessful; consequently, reviewers labeled this study as having “unclear bias” during study bias assessment. This study was excluded during the sensitivity analysis as well. The clinical trial by Sasaki et al14 compared the fondaparinux with the non‐fondaparinux group. This non‐fondaparinux group was considered to be the LMWH group, as all the major clinical trials11, 12, 18 considered LMWH as the comparator to fondaparinux. However, in the sensitivity analysis, this study was excluded and the results were consistent. The clinical trial by Steele et al16 used a double dose of both fondaparinux and LMWH as a titration to the weight (body mass index, 35–59 kg/m2) of the participants randomized in the clinical trial. This study was included in the analysis because the dose was doubled in both arms. Nevertheless, this study was excluded as well during the sensitivity analysis, with no meaningful change to the results. In the clinical trial by Hata et al,13 randomized patients received low‐dose (5000 IU) unfractionated heparin for thromboprophylaxis during the first 24 hours after surgery. Low‐dose unfractionated heparin was administered initially because in Japan neither fondaparinux nor LMWH are approved to be prescribed immediately after surgery.13 After 24 hours, patients received either fondaparinux or LMWH for 5 days postoperatively. As patients in both arms received low‐dose unfractionated heparin, outcomes might have been affected similarly in each group.
Included studies in our meta‐analysis were from different countries. Thus, we reported pooled analysis of these studies, which were from different clinical settings, that might have impacted the results. Studies were also subgrouped by population (eg, North American and Australian, European, and Asian population) as well to address this issue. Two RCTs12, 19 conducted in North America had some of their centers in Australia. We could not separate the American population from the Australian population because of the limitation to data access. Thus, we presented pooled analyses of these 2 populations. Our study considered all LMWHs in 1 group whether it was enoxaparin, nadroparin, or dalteparin. Nevertheless, it has been indicated by a meta‐analysis of 20 RCTs that all LMWHs produce similar relative safety and efficacy when compared for VTE prophylaxis.48 There was a difference in the dose of the LMWH in studies from different countries, which might have impacted the outcomes as well. Also, there was a difference in the duration of the prophylaxis. This limitation is justified because of the fact that with the time there is a variation in the guidelines for the prophylaxis of VTE.7, 49, 50, 51
Strengths
To our knowledge, this is the most current comprehensive meta‐analysis comparing subcutaneous fondaparinux with LMWH for perioperative surgical thromboprophylaxis. To maintain the high quality of systematic review, this study considered only randomized clinical trials in the analyses as per the Cochrane Handbook for Systematic Reviews of Interventions.28 We kept the definition of our outcomes consistent while analyzing the results. The cutoff point for the assessment of our study was set to 15 days. If any study among the included studies assessed the outcomes after 15 days, we excluded the event from our analysis. For example, Argun et al21 reported an event of proximal DVT at day 19 with LMWH, which was not considered in our summary statistics. Thus, we maintained consistency throughout our study. To present the most comprehensive and robust results, we conducted several sensitivity and subgroup analyses as well to assist with the best clinical decision.
Conclusion
The results of this systematic review and meta‐analysis suggest that fondaparinux is significantly better in terms of reduction of VTE (composite of DVT and PE) for perioperative surgical thromboprophylaxis. However, for the symptomatic VTE and all‐cause mortality, fondaparinux was not found to be superior to LMWH in this study. Clinicians should be aware of the higher risk of major bleeding, especially surgical site bleeding with fondaparinux compared with LMWH. Nevertheless, net clinical benefit as per this study was in favor of fondaparinux compared with LMWH.
Disclosures
None.
Supporting information
Data S1. Study Protocol
Data S2. Description of “Risk of Bias” Among the Included Studies
Data S3. Detailed Description of Each Included Study (DATA EXTRACTION FORM)
Table S1. PRISMA 2009 Checklist
Table S2. Search Strategy for Study Inclusion
Figure S1. Fondaparinux vs LMWH for venous thromboembolism up to day 15 (sensitivity analysis).
Figure S2. Funnel plot for “reporting bias” of venous thromboembolism up to day 15.
Figure S3. Fondaparinux vs LMWH for total deep vein thrombosis up to day 15.
Figure S4. Fondaparinux vs LMWH for proximal deep vein thrombosis up to day 15.
Figure S5. Fondaparinux vs LMWH for distal deep vein thrombosis up to day 15.
Figure S6. Fondaparinux vs LMWH for total deep vein thrombosis up to day 15 (sensitivity analysis).
Figure S7. Fondaparinux vs LMWH for proximal deep vein thrombosis up to day 15 (sensitivity analysis).
Figure S8. Fondaparinux vs LMWH for distal deep vein thrombosis up to day 15 (sensitivity analysis).
Figure S9. Funnel plot for “reporting bias” of total deep vein thrombosis up to day 15.
Figure S10. Symptomatic VTE up to post‐operative day 15.
Figure S11. Pulmonary embolism up to post‐operative day 15.
Figure S12. Fondaparinux vs LMWH for all‐cause mortality at day 90.
Figure S13. Fondaparinux vs LMWH for all‐cause mortality at day 90 (sensitivity analysis).
Figure S14. Fondaparinux vs LMWH for major bleeding during the treatment period (sensitivity analysis).
Figure S15. Fondaparinux vs LMWH for minor bleeding during the treatment period.
Figure S16. Fondaparinux vs LMWH for minor bleeding during the treatment period (sensitivity analysis).
Acknowledgments
This study represents original work. It was completed recently and has not been published elsewhere. All of the authors of this paper contributed to the work, contributed in preparation of the manuscript, and have provided their final approval for the manuscript submission. The authors thank Dr. Wendy St. Peter, Professor, University of Minnesota (College of Pharmacy) for her contribution in preparation of the manuscript.
(J Am Heart Assoc. 2019;8:e012184. DOI: 10.1161/JAHA.119.012184.)
Preliminary results of this work were presented at the meeting of the International Society of Pharmacoeconomics and Outcomes Research, May 20 to 24, 2017 in Boston, MA.
References
- 1. Pai M, Douketis JD. Venous thromboembolism prophylaxis for hospitalized medical patients In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine. 2nd ed. New York, NY: McGraw‐Hill Education; 2017:2077. [Google Scholar]
- 2. Dahlbäck B. Advances in understanding pathogenic mechanisms of thrombophilic disorders. Blood. 2008;112:19–27. [DOI] [PubMed] [Google Scholar]
- 3. Solaiman FA, Kim ESH. Venous thromboembolism and hypercoagulable states In: Griffin BP, ed. Manual of Cardiovascular Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2013:446. [Google Scholar]
- 4. Wilbur J, Shian B. Diagnosis of deep venous thrombosis and pulmonary embolism. Am Fam Physician. 2012;86:913–919. [PubMed] [Google Scholar]
- 5. Lin J, Lingohr‐Smith M, Kwong WJ. Incremental health care resource utilization and economic burden of venous thromboembolism recurrence from a U.S. Payer perspective. J Manag Care Pharm. 2014;20:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lassen MR, Bauer KA, Eriksson BI, Turpie AG. Postoperative fondaparinux versus preoperative enoxaparin for prevention of venous thromboembolism in elective hip‐replacement surgery: a randomised double‐blind comparison. Lancet. 2002;359:1715–1720. [DOI] [PubMed] [Google Scholar]
- 7. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141:e419S–e494S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kodadek LM, Haut ER. Screening and diagnosis of VTE: the more you look, the more you find? Current Trauma Reports. 2016;2:29–34. [Google Scholar]
- 9. Lip GY, Douketis JD. Perioperative management of patients receiving anticoagulants. 2018. Available at: https://www.uptodate.com/contents/perioperative-management-of-patients-receiving-anticoagulants. Accessed November 20, 2018.
- 10. Alexander JH, Singh KP. Inhibition of factor Xa: a potential target for the development of new anticoagulants. Am J Cardiovasc Drugs. 2005;5:279–290. [DOI] [PubMed] [Google Scholar]
- 11. Eriksson BI, Bauer KA, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after hip‐fracture surgery. N Engl J Med. 2001;345:1298–1304. [DOI] [PubMed] [Google Scholar]
- 12. Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip‐replacement surgery: a randomised double‐blind trial. Lancet. 2002;359:1721–1726. [DOI] [PubMed] [Google Scholar]
- 13. Hata K, Kimura T, Tsuzuki S, Ishii G, Kido M, Yamamoto T, Sasaki H, Miki J, Yamada H, Furuta A, Miki K, Egawa S. Safety of fondaparinux for prevention of postoperative venous thromboembolism in urological malignancy: a prospective randomized clinical trial. Int J Urol. 2016;23:923–928. [DOI] [PubMed] [Google Scholar]
- 14. Sasaki S, Miyakoshi N, Matsuura H, Saitoh H, Kudoh D, Shimada Y. Prospective randomized controlled trial on the effect of fondaparinux sodium for prevention of venous thromboembolism after hip fracture surgery. J Orthop Sci. 2009;14:491–496. [DOI] [PubMed] [Google Scholar]
- 15. Agnelli G, Bergqvist D, Cohen AT, Gallus AS, Gent M. Randomized clinical trial of postoperative fondaparinux versus perioperative dalteparin for prevention of venous thromboembolism in high‐risk abdominal surgery. Br J Surg. 2005;92:1212–1220. [DOI] [PubMed] [Google Scholar]
- 16. Steele KE, Canner J, Prokopowicz G, Verde F, Beselman A, Wyse R, Chen J, Streiff M, Magnuson T, Lidor A, Schweitzer M. The effort trial: preoperative enoxaparin versus postoperative fondaparinux for thromboprophylaxis in bariatric surgical patients: a randomized double‐blind pilot trial. Surg Obes Relat Dis. 2015;11:672–683. [DOI] [PubMed] [Google Scholar]
- 17. Yokote R, Matsubara M, Hirasawa N, Hagio S, Ishii K, Takata C. Is routine chemical thromboprophylaxis after total hip replacement really necessary in a Japanese population?. J Bone Joint Surg Br. 2011;93:251–256. [DOI] [PubMed] [Google Scholar]
- 18. Bauer KA, Eriksson BI, Lassen MR, Turpie AG. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med. 2001;345:1305–1310. [DOI] [PubMed] [Google Scholar]
- 19. Turpie AGG, Gallus AS, Hoek JA. A synthetic pentasaccharide for the prevention of deep‐vein thrombosis after total hip replacement. N Engl J Med. 2001;344:619–625. [DOI] [PubMed] [Google Scholar]
- 20. Shen Y, Zhong M, Tan L. Fondaparinux versus low molecular weight heparin following esophagectomy: results from a randomized and controlled trial. Chest. 2014;145:535D. [Google Scholar]
- 21. Argun M, Oner M, Saglamoglu M, Karaman I, Guney A, Halici M, Halil Kafadar I. Fondaparınux versus nadroparın for preventıon of venous thromboembolısm after electıve hıp and knee arthroplasty. Curr Ther Res Clin Exp. 2013;74:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 23. Huber O, Bounameaux H, Borst F, Rohner A. Postoperative pulmonary embolism after hospital discharge: an underestimated risk. Arch Surg. 1992;127:310–313. [DOI] [PubMed] [Google Scholar]
- 24. McNally MP, Burns CJ. Venous thromboembolic disease in colorectal patients. Clin Colon Rectal Surg. 2009;22:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson Jr FA, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–I16. [DOI] [PubMed] [Google Scholar]
- 26. Babineau J. Product review: Covidence (systematic review software). J Can Health Libr Assoc. 2014;35:68–71. [Google Scholar]
- 27. Turpie AG, Bauer KA, Eriksson BI, Lassen MR; for the Steering Committees of the Pentasaccharide Orthopedic Prophylaxis S . Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta‐analysis of 4 randomized double‐blind studies. Arch Intern Med. 2002;162:1833–1840. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 30. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions Chichester, West Sussex: John Wiley & Sons Ltd; 2008:266. [Google Scholar]
- 32. Cochrane‐Collaboration . Review manager (version 5.3) [computer software]. Copenhagen, Denmark: The Nordic Cochrane Centre, Cochrane Collaboration; 2014.
- 33. StataCorp . Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC; 2017. [Google Scholar]
- 34. Laporte S, Mismetti P, Décousus H, Uresandi F, Otero R, Lobo JL, Monreal M. Clinical predictors for fatal pulmonary embolism in 15 520 patients with venous thromboembolism: findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE) Registry. Circulation. 2008;117:1711–1716. [DOI] [PubMed] [Google Scholar]
- 35. Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER). Lancet. 1999;353:1386–1389. [DOI] [PubMed] [Google Scholar]
- 36. Dong WJ, Qian HJ, Qian Y, Zhou L, Hu SL. Fondaparinux vs. enoxaparin for the prevention of venous thromboembolism after total hip replacement: a meta‐analysis. Exp Ther Med. 2016;12:969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li H, Wang J, Xiao J, Shi Z. [Efficacy and safety of fondaparinux versus enoxaparin for preventing venous thromboembolism after major orthopedic surgery: a meta‐analysis]. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:370–375. [PubMed] [Google Scholar]
- 38. Galanaud J‐P, Sevestre‐Pietri M‐A, Bosson J‐L, Laroche J‐P, Righini M, Brisot D, Boge G, van Kien AK, Gattolliat O, Bettarel‐Binon C. Comparative study on risk factors and early outcome of symptomatic distal versus proximal deep vein thrombosis: results from the OPTIMEV study. Thromb Haemost. 2009;102:493–500. [DOI] [PubMed] [Google Scholar]
- 39. Hogg K, Linkins L‐A, Kearon C. Diagnosis and Treatment of Venous Thromboembolism. In: McKean SC, Ross JJ, Dressler DD, Scheurer DB, eds. Principles and Practice of Hospital Medicine, 2 ed. New York, NY: McGraw‐Hill Education; 2017:2081. [Google Scholar]
- 40. Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:I22–I30. [DOI] [PubMed] [Google Scholar]
- 41. Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med. 2001;110:515–519. [DOI] [PubMed] [Google Scholar]
- 42. Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral anticoagulants: American College of Chest Physicians evidence‐based clinical practice guidelines (8th edition). Chest. 2008;133:141S–159S. [DOI] [PubMed] [Google Scholar]
- 43. Eikelboom J, Quinlan D, O'Donnell M. Major bleeding, mortality, and efficacy of fondaparinux in venous thromboembolism prevention trials. J Vasc Surg. 2010;51:1585. [DOI] [PubMed] [Google Scholar]
- 44. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 45. Leonardi MJ, McGory ML, Ko CY. The rate of bleeding complications after pharmacologic deep venous thrombosis prophylaxis: a systematic review of 33 randomized controlled trials. Arch Surg. 2006;141:790–799. [DOI] [PubMed] [Google Scholar]
- 46. Eikelboom JW. Effect of fondaparinux 2.5 mg once daily on mortality: a meta‐analysis of phase III randomized trials of venous thromboembolism prevention. Eur Heart J Suppl. 2008;10:C8–C13. [Google Scholar]
- 47. Gohil R, Peck G, Sharma P. The genetics of venous thromboembolism a meta‐analysis involving approximately 120,000 cases and 180,000 controls. Thromb Haemost. 2009;102:360–370. [DOI] [PubMed] [Google Scholar]
- 48. Dooley C, Kaur R, Sobieraj DM. Comparison of the efficacy and safety of low molecular weight heparins for venous thromboembolism prophylaxis in medically ill patients. Curr Med Res Opin. 2014;30:367–380. [DOI] [PubMed] [Google Scholar]
- 49. Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S–400S. [DOI] [PubMed] [Google Scholar]
- 50. Eikelboom JW, Karthikeyan G, Fagel N, Hirsh J. American association of orthopedic surgeons and American College of Chest Physicians guidelines for venous thromboembolism prevention in hip and knee arthroplasty differ: what are the implications for clinicians and patients? Chest. 2009;135:513–520. [DOI] [PubMed] [Google Scholar]
- 51. Huo MH, Spyropoulos AC. The eighth American College of Chest Physicians guidelines on venous thromboembolism prevention: implications for hospital prophylaxis strategies. J Thromb Thrombolysis. 2011;31:196–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Study Protocol
Data S2. Description of “Risk of Bias” Among the Included Studies
Data S3. Detailed Description of Each Included Study (DATA EXTRACTION FORM)
Table S1. PRISMA 2009 Checklist
Table S2. Search Strategy for Study Inclusion
Figure S1. Fondaparinux vs LMWH for venous thromboembolism up to day 15 (sensitivity analysis).
Figure S2. Funnel plot for “reporting bias” of venous thromboembolism up to day 15.
Figure S3. Fondaparinux vs LMWH for total deep vein thrombosis up to day 15.
Figure S4. Fondaparinux vs LMWH for proximal deep vein thrombosis up to day 15.
Figure S5. Fondaparinux vs LMWH for distal deep vein thrombosis up to day 15.
Figure S6. Fondaparinux vs LMWH for total deep vein thrombosis up to day 15 (sensitivity analysis).
Figure S7. Fondaparinux vs LMWH for proximal deep vein thrombosis up to day 15 (sensitivity analysis).
Figure S8. Fondaparinux vs LMWH for distal deep vein thrombosis up to day 15 (sensitivity analysis).
Figure S9. Funnel plot for “reporting bias” of total deep vein thrombosis up to day 15.
Figure S10. Symptomatic VTE up to post‐operative day 15.
Figure S11. Pulmonary embolism up to post‐operative day 15.
Figure S12. Fondaparinux vs LMWH for all‐cause mortality at day 90.
Figure S13. Fondaparinux vs LMWH for all‐cause mortality at day 90 (sensitivity analysis).
Figure S14. Fondaparinux vs LMWH for major bleeding during the treatment period (sensitivity analysis).
Figure S15. Fondaparinux vs LMWH for minor bleeding during the treatment period.
Figure S16. Fondaparinux vs LMWH for minor bleeding during the treatment period (sensitivity analysis).


