Abstract
Background
We examined temporal trends, timing, and frequency, as well as adverse neonatal and maternal outcomes occurring in the first year postpartum among women experiencing syncope during pregnancy.
Methods and Results
This was a retrospective study of pregnancies between January 1, 2005, and December 31, 2014, in the province of Alberta, Canada. Of 481 930 pregnancies, 4667 had an episode of syncope. Poisson regression analysis found a 5% increase/year (rate ratio, 1.05; 95% CI, 1.04–1.06) in the age‐adjusted incidence of syncope. Overall, 1506 (32.3%) of the syncope episodes first occurred in the first trimester, 2058 (44.1%) in the second trimester, and 1103 (23.6%) in the third trimester; and 8% (n=377) of pregnancies had >1 episode of syncope. Compared with women without syncope, women who experienced syncope were younger (age <25 years; 34.7% versus 20.8%; P<0.001), and primiparous (52.1% versus 42.4%; P<0.001). The rate of preterm birth was higher in pregnancies with syncope during the first trimester (18.3%), compared with the second (15.8%) and third trimesters (14.2%) and pregnancies without syncope (15.0%; P<0.01). The incidence of congenital anomalies among children born of pregnancies with multiple syncope episodes was significantly higher (4.9%) compared with children of pregnancies without syncope (2.9%; P<0.01). Within 1 year after delivery, women with syncope during pregnancy had higher rates of cardiac arrhythmias and syncope episodes than women with no syncope during pregnancy.
Conclusions
Pregnant women with syncope, especially when the syncopal event occurs during the first trimester, may be at a higher risk of adverse pregnancy outcomes as well as an increased incidence of cardiac arrhythmia and syncope postpartum.
Keywords: outcomes, pregnancy, syncope, trends
Subject Categories: Pregnancy
Clinical Perspective
What Is New?
Syncope during pregnancy, especially when it occurs during the first trimester, may be associated with higher rates of adverse outcomes including preterm births, congenital anomalies, and an increased incidence of maternal cardiac arrhythmias and syncope in the first‐year postpartum.
What Are the Clinical Implications?
Given the observed higher rates of adverse outcomes among women experiencing syncope in pregnancy, closer monitoring both during pregnancy and in the postpartum period may be necessitated.
Syncope is the sudden, transient loss of consciousness that occurs as a result of global cerebral hypoperfusion.1 It is a relatively common clinical problem, presenting in a bimodal distribution, with the highest incidence occurring between ages 10 to 30 and in patients older than 65 years.2 The most common cause of syncope in adolescents and young adults, including women of childbearing age, is vasovagal syncope.
Syncope may be a manifestation of a number of clinical conditions occurring on a spectrum of severity from deadly underlying heart conditions such as arrhythmia to benign conditions such as vasovagal episodes. This is reflected in the prognosis of 1‐year mortality estimates ranging from as low as 0% for vasovagal syncope up to 30% in the context of cardiac syncope.3 Moreover, it has been shown that there is a significant association between syncope and the presence of cardiovascular disease, and the relationship appears to be stronger among young people.4 The pregnant mother undergoes a number of hemodynamic changes5 including reduced systemic vascular resistance, increased blood volume and heart rate, and eccentric hypertrophy of the left ventricle. These changes may predispose her to the development of syncope.6
There are currently limited data on the incidence of syncope in pregnancy.7 Moreover, the effect of a transient fall in blood pressure on maternal and fetal outcomes is not well described. While most case reports suggest a benign course for both mother and child, there are no long‐term follow‐up data. Given this paucity of evidence, we sought to describe temporal trends in the incidence of syncope during pregnancy using a large, contemporary, population‐based cohort of women in a defined geographic area with a single‐payer healthcare system with universal access. Additionally, neonatal outcomes, including rates of preterm birth, small for gestational age (SGA), large for gestational age, and congenital anomalies were examined, overall, and according to the timing (first occurrence in first, second, or third trimester), and number (≥1) of syncope episodes during pregnancy. The frequency of syncope and other cardiovascular events in the mother in the year following the delivery was also evaluated.
Methods
Data for the study were received from the Alberta Ministry of Health (Alberta Health). The data are proprietary and not available publicly. Aggregate data are available upon request. Study approval was received from the University of Alberta Ethics Board. Due to the retrospective, deidentified nature of the study, informed consent from participants was not required.
Study Design and Data Source
This retrospective cohort study used the longitudinal Alberta Pregnancy‐Birth cohort,8 consisting of all live births between January 1, 2005, and December 31, 2014, in the province of Alberta, Canada. The cohort has been described in detail previously.8, 9, 10 In brief, the cohort was identified from the Alberta Vital Statistics—Birth Database, which contains detailed birth information, including birth date, sex, birth weight, gestational age, maternal age at delivery, singleton/multiple birth status, and parity. Data from the Alberta Vital Statistics—Birth Database were linked with the following data for mothers and their offspring using a unique scrambled identifier: (1) hospitalization data from the Discharge Abstract Database; (2) ambulatory care data, including visits to the emergency departments and hospital‐based outpatient clinics, from the National Ambulatory Care Reporting System database; (3) physician office visits from the practitioners claims database; (4) demographic data from the Alberta Health Care Insurance Population registry; and (5) date of death from the Vital Statistics—Death Database. Data on the neighborhood‐level household income were obtained from the 2010 Population Census data.11
Study Population
All pregnant women with a gestational age of 15 to 43 weeks who delivered live births between January 1, 2005, and December 31, 2014, and their offspring, were included in the study. Women who were not residents of Alberta at the time of delivery were excluded. A flowchart (diagram) of the study cohort selection is provided as Figure S1. Women with syncope during pregnancy were identified as those having an International Classification of Disease, Ninth Revision (ICD‐9) code 780.2 or Tenth Revision (ICD‐10) code R55 in any diagnosis field of their inpatient, outpatient, or physician office visit record during the gestation period. The ICD‐9 and ICD‐10 codes of syncope have a moderate sensitivity of 63%, high specificity of 98% to 99%, and a positive predictive value of 83% to 95%.12, 13
Demographic, Clinical, and Obstetric Data
In addition to maternal age at the time of delivery, the Alberta Health Care Insurance Population data were used to identify women who had identified themselves as Status Aboriginal. This flag was available for all residents before 2009. Previously developed lists of surnames were applied to the surnames in the Alberta Health Care Insurance Population registry to identify pregnancies of women of South Asian and Chinese ethnicity.14, 15, 16 These algorithms have been validated against self‐identified ethnicity in the Canadian Community Health Survey. Based on the ethnic distribution of the population of Alberta, women who were not South Asian or Chinese were most likely white and categorized as General Population.17 Women were categorized as living in rural or urban areas based on the first numerical digit of their postal code. The annual household income at the neighborhood level from census data was used as a measure of socioeconomic status.
Women with preexisting diabetes mellitus or cardiovascular disease or other medical conditions before pregnancy, such as lupus, epilepsy, chronic obstructive pulmonary disease, or liver disease, were identified on the basis of the delivery hospitalization and any previous hospitalization (Discharge Abstract Database) or ambulatory (National Ambulatory Care Reporting System) record as of April 1, 1997. Pregnancy complications, including gestational diabetes mellitus and hypertensive disorders during pregnancy (including preeclampsia and eclampsia), were identified if they were coded on the delivery hospitalization records. The complete list of ICD codes used to identify clinical comorbidities is provided in Table S1.
The delivery hospitalization record was used to identify children who were born via cesarean section. On the basis of previous studies, large for gestational age and SGA were defined as birth weights <10% and >90%, respectively, compared with neonates of the same gestational age and sex.18
Outcomes
We examined the annual incidence of syncope during pregnancy, as well as its overall incidence by trimester. Neonatal outcomes, including rates of preterm birth (defined as gestational age <37 weeks), SGA, low birth weight (defined as birth weight <2500 g), large for gestational age, admission rates to the neonatal intensive care unit during the delivery hospitalization, and congenital abnormalities were compared in pregnancies with and without syncope, overall, and by timing and frequency of syncope episodes. Maternal outcomes of interest included the presentation of 1‐year postpregnancy syncope and cardiovascular events in the mothers.
Statistical Analysis
The unit of analysis was the pregnancy, so women with >1 pregnancy within the study time period were included multiple times. Pregnancies were first categorized into 2 groups according to the presence or absence of syncope and then further categorized as follows: (1) into 4 groups according to the timing of the first syncope episode during pregnancy as none, first trimester, second trimester, and third trimester; and (2) into 3 groups based on the number of syncope episodes during the pregnancy as none, 1, and >1 episode.
All categorical variables are described as percentages and were compared across groups using chi‐square tests. Continuous variables are presented as medians with interquartile range and were compared between groups using a nonparametric Mann–Whitney U test. We calculated incidence rates of syncope during pregnancy per 1000 pregnancies for each year of the study period. A Poisson regression model, with year and maternal age as covariates, was used to assess whether incidence rates had changed over time.
The unit of analysis for neonatal and congenital anomaly outcomes was the child. The number of children included in the study was larger than the number of pregnancies as a result of nonsingleton pregnancies (ie, twins, triplets, etc). Children were categorized according to whether they were born to pregnancies of mothers with syncopal episodes, as described above. All hospitalization (Discharge Abstract Database), ambulatory (National Ambulatory Care Reporting System), and practitioner claims records from birth to March 31, 2014, were examined to identify children with congenital anomalies.
Because of the longitudinal nature of the pregnancy‐birth cohort, women may have had multiple pregnancies during the study time period or may have had multiple births for the same pregnancy. To account for correlations across pregnancies, we conducted supplementary analyses using generalized estimating equation models with binomial distribution and logit function to examine the association between syncope during pregnancy and neonatal and congenital anomaly outcomes in the offspring (Data S1).
Finally, hospitalization (Discharge Abstract Database), ambulatory (National Ambulatory Care Reporting System), and practitioner claims records in the year after delivery were used to examine 1‐year postpartum rates of syncope and other cardiovascular conditions (see Table S1 for full list of codes).
All analyses were performed using SAS statistical software (Version 9.4, SAS Institute, Cary, NC).
Results
Between January 1, 2005, and December 31, 2014, there were 496 159 live births in Alberta, Canada. After excluding births of women who were not residents of Alberta (n=5747) and births with incorrect or incomplete data (n=351), the final study population consisted of 490 061 live births of 481 930 pregnancies (Figure S1).
Overall, 4667 of the 481 930 pregnancies had an episode of syncope for an overall incidence rate of 9.7 per 1000 pregnancies (95% CI, 9.4–10.0 per 1000 pregnancies). The incidence rate of syncope during pregnancy increased from 7.6 per 1000 pregnancies in 2005 to 11 per 1000 pregnancies in 2014 (Figure 1), equivalent to an age‐adjusted 5% increase per year (rate ratio, 1.05; 95% CI, 1.04–1.06; P<0.01).
Figure 1.
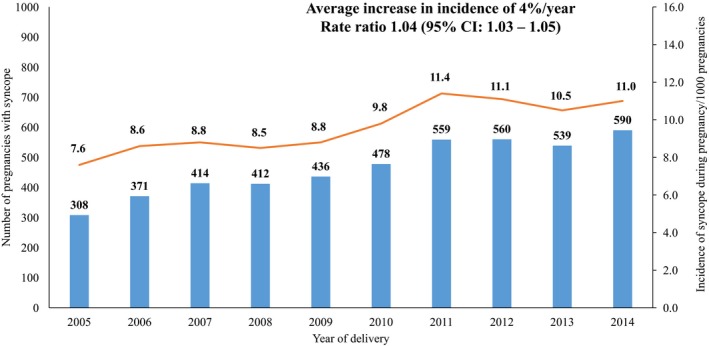
Incidence of syncope during pregnancy by year of delivery.
Compared with women with pregnancies without syncope, women who experienced syncope were younger (age <25 years; 34.7% versus 20.8%; P<0.01), more likely primiparous (52.1% versus 42.4%; P<0.01), less likely to be married (57.7% versus 69.8%; P<0.01) and had higher rates of preexisting medical conditions (1.8% versus 1.0%; P<0.01; Table 1). Women with syncope during pregnancy were also more likely to have a history of syncope before pregnancy compared with women without syncope during pregnancy (11.5% versus 3.2%, P<0.01, Table 1). Although the rates of gestational diabetes mellitus were slightly lower in women with syncope during pregnancy, there was no difference in the rates of hypertensive disorders during pregnancy between the 2 groups (Table 3).
Table 1.
Baseline Characteristics and Outcomes of Women With Pregnancies With and Without Syncope
| Characteristics | No Syncope During Pregnancy | Syncope During Pregnancy | P Value |
|---|---|---|---|
| Pregnancy, N | 477 263 | 4667 | |
| Women, N | 314 576 | 4584 | |
| Age at delivery, y | |||
| <25, % (N) | 20.8 (99 268) | 34.7 (1621) | <0.001 |
| 25–34, % (N) | 57.9 (276 232) | 51.6 (2410) | |
| >34, % (N) | 21.3 (101 743) | 13.6 (636) | |
| Ethnicity | |||
| General population, % (N) | 87.1 (415 531) | 89.3 (4166) | <0.001 |
| Aboriginal, % (N) | 6.7 (31 868) | 5.8 (269) | |
| Chinese, % (N) | 3.4 (16 254) | 2.2 (102) | |
| South Asian, % (N) | 2.9 (13 610) | 2.8 (130) | |
| Rural residence, % (N) | 16.8 (79 927) | 16.0 (745) | 0.15 |
| Married, % (N) | 69.8 (332 988) | 57.7 (2691) | <0.001 |
| Annual household income, CAD, median (IQR) | 75 857 (68 280–96 257) | 76 315 (68 090–94 410) | 0.25 |
| Preexisting diabetes mellitus, % (N) | 0.9 (4399) | 0.9 (44) | 0.88 |
| Preexisting cardiovascular disease, % (N) | 2.8 (13 177) | 4.7 (221) | <0.01 |
| Other preexisting medical conditions, % (N)a | 1.0 (4956) | 1.8 (85) | <0.001 |
| Previous syncope, % (N) | 3.2 (15 178) | 11.5 (537) | <0.01 |
| Primiparous, % (N) | 42.4 (202 515) | 52.1 (2432) | <0.001 |
| Multiple pregnancy, % (N) | 1.7 (8065) | 2.1 (96) | 0.06 |
CAD indicates Canadian dollar; IQR, interquartile range.
Including chronic obstructive pulmonary disease, liver disease, epilepsy, and lupus.
Table 3.
Maternal Pregnancy, Delivery, and Postpartum (Within 1 Year of Delivery) Outcomes Among Women With Pregnancies With and Without Syncope
| Maternal Outcomes | No Syncope During Pregnancy | Syncope During Pregnancy | P Value |
|---|---|---|---|
| Pregnancy, N | 477 263 | 4667 | |
| Pregnancy and delivery outcomes | |||
| Gestational diabetes mellitus, % (N) | 5.7 (27 021) | 4.4 (204) | <0.001 |
| Hypertensive disorders of pregnancy, % (N) | 5.9 (28 151) | 4.8 (223) | 0.001 |
| Preterm delivery, % (N) | 15.0 (71 520) | 16.3 (759) | 0.015 |
| Cesarean section delivery, % (N) | 27.3 (130 463) | 28.0 (1305) | 0.34 |
| Postpartum outcomes | |||
| Congestive heart failure, % (N) | 0.07 (333) | 0.04 (2) | 0.49 |
| Cardiac arrhythmia, % (N) | 0.22 (1064) | 0.77 (36) | <0.01 |
| Valvular heart disease, % (N) | 0.05 (244) | 0.04 (2) | 0.80 |
| Ischemic heart disease, % (N) | 0.08 (362) | 0.09 (4) | 0.81 |
| Hypertension, % (N) | 1.64 (7846) | 1.18 (55) | 0.01 |
| Pulmonary circulation disorder, % (N) | 0.16 (787) | 0.28 (13) | 0.06 |
| Syncope, % (N) | 0.22 (1072) | 1.35 (63) | <0.01 |
When grouped by timing of first episode of syncope, we found that in 32.2% (1506) of pregnancies the first episode of syncope occurred in the first trimester, in 44.1% (2058) it occurred in the second trimester, and in 23.6% (1103) it occurred in the third trimester. Overall, 8% (377/4667) of pregnancies with syncope had >1 episode.
Women experiencing a syncope episode had a higher rate of preterm birth compared with women with no syncope episode (16.3% versus 15.0%; P=0.02; Table 3). The rates of preterm birth differed significantly by the time of the first syncope episode: women who experienced syncope during the first trimester had the highest rates of preterm birth (18.3%) compared with those with syncope during the second (15.8%) and third (14.2%) trimesters (P<0.01) (Figure 2). Although higher than the rate of preterm birth in pregnancies without syncope, there was no difference in preterm birth rates among pregnancies with 1 versus >1 episode of syncope.
Figure 2.
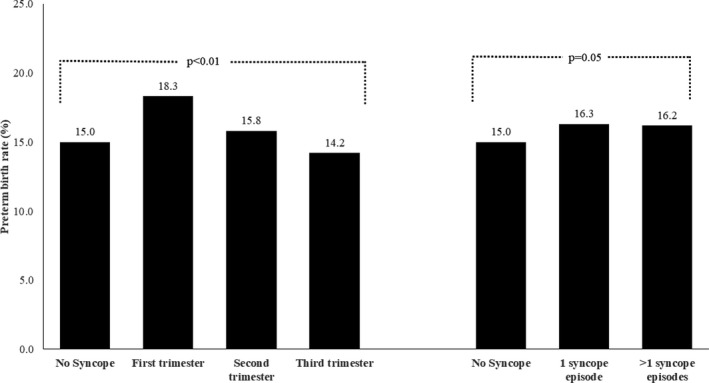
Rates of preterm birth in pregnancies with no syncope and pregnancies with syncope (categorized according to timing and frequency). Comparison between preterm birth rate in pregnancies with no syncope vs in pregnancies with syncope in first, second, and third trimesters statistically significant at P<0.01 (left panel), and comparison between preterm birth rate in pregnancies with no syncope vs in pregnancies with ≥1 syncope episode statistically significant at P=0.05 (right panel).
With respect to neonatal outcomes, the slightly higher rates of SGA and low birth weight in children of pregnancies with syncope (10.5% and 7.5%, respectively) than in children of pregnancies without syncope (9.7% and 6.8%, respectively) was largely attributed to higher rates of these outcomes in pregnancies with syncope in the first and second trimesters (Table 2). Multiple episodes of syncope during pregnancy was not associated with higher rates of adverse neonatal outcomes.
Table 2.
Neonatal Outcomes and Congenital Anomalies by Incidence, Timing, and Frequency of Syncope During Pregnancy
| Outcomes | Any Syncope During Pregnancy | Timing of Syncope | Frequency of Syncope Episodes in a Given Pregnancy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | P Valuea | First Trimester | Second Trimester | Third Trimester | P Valueb | 1 | >1 | P Valuec | |
| Children, N | 485 297 | 4764 | 1537 | 2104 | 1123 | 4379 | 385 | |||
| SGA, % (N)d | 9.7 (46 809) | 10.5 (500) | 0.05 | 11.3 (173) | 11.1 (233) | 8.4 (94) | <0.01 | 10.4 (457) | 11.7 (43) | 0.13 |
| LBW, % (N)e | 6.8 (33 143) | 7.5 (355) | 0.09 | 7.8 (120) | 8.5 (179) | 5.0 (56) | <0.01 | 7.7 (338) | 4.4 (17) | 0.01 |
| LGA, % (N)d | 9.3 (44 886) | 7.7 (365) | <0.01 | 6.5 (100) | 8.2 (172) | 8.3 (93) | <0.01 | 7.2 (343) | 5.7 (22) | <0.01 |
| NICU admission, % (N) | 12.6 (61 114) | 13.2 (628) | 0.23 | 13.4 (205) | 13.4 (282) | 12.6 (141) | 0.59 | 13.5 (589) | 10.1 (39) | 0.08 |
| Congenital anomalies, % (N) | 2.6 (12 435) | 3.1 (147) | 0.023 | 3.4 (52) | 3.0 (63) | 2.9 (32) | 0.11 | 2.9 (128) | 4.9 (19) | <0.01 |
LBW indicates low birth weight; LGA, large for gestational age; NICU, neonatal intensive care unit; SGA, small for gestational age.
Compares outcomes across no syncope vs syncope.
Compares outcomes across timing of syncope (no syncope, syncope event during first, second, and third trimesters).
Compares outcomes across frequency of syncope (no syncope, 1 syncope event, and >1 syncope event).
Data were not available for 571 pregnancies without syncope and for 2 pregnancies with syncope.
Data were not available for 26 pregnancies without syncope.
Looking beyond the neonatal period, over a median follow‐up period of 4.6 years (95% CI, 2.7–7.1 years) for children of pregnancies with syncope and 5.1 years (95% CI, 2.9–7.5 years) for children of pregnancies without syncope, the rates of presentation of congenital anomalies was higher in the former group (3.1%) than in the latter group (2.6%; P=0.023; Table 2). The highest prevalence of congenital anomalies was observed among children of pregnancies in which the first episode of syncope occurred during the first trimester (3.4%) (Table 2). The incidence of congenital anomalies was significantly higher in children of pregnancies with multiple syncope episodes during pregnancy than children of pregnancies with only one episode (4.9% versus 2.9%; P<0.01).
Overall, 130 317 women had >1 pregnancy or birth during the study time period. Results of generalized estimating equation modeling accounting for correlations between outcomes of mothers with multiple pregnancies/births are presented in Figure S2. These analyses, adjusted for maternal age, confirmed that women who experience the first syncope episode during the first trimester had higher odds of preterm birth (odds ratio, 1.3; 95% CI, 1.1, 1.4; P=0.001), SGA (odds ratio, 1.2; 95% CI, 1.0, 1.4; P=0.04), and congenital anomalies (odds ratio, 1.4; 95% CI, 1.0, 1.8; P=0.036) compared with women with no syncope during pregnancy. The odds of congenital anomalies were significantly higher in children of pregnant women with multiple syncope episodes during pregnancy than children of those with no syncope during pregnancy (odds ratio, 2.0; 95% CI, 1.3, 3.2; P=0.003).
Women with syncopal episodes during pregnancy had higher rates of cardiac arrhythmias (0.8%) and syncope episodes (1.4%) within 1 year following delivery compared with women with pregnancies without syncope (0.2% for cardiac arrhythmias and 0.2% for syncope; P<0.01 for both; Table 3). Among the 36 women who had syncope during pregnancy and presented with cardiac arrhythmias postpartum, 17 (47%) were new‐onset cases.
Discussion
In a population‐based study of 481 930 pregnancies between 2005 and 2014, we found the incidence of syncope during pregnancy to be ≈1.0% or 10 per 1000 pregnancies. The incidence of syncope during pregnancy appears to be increasing over time by 5% each year. The timing of syncope during pregnancy may offer some prognostic information, as rates of preterm birth were higher in pregnancies in which the first episode of syncope occurred during the first trimester. Although >1 syncope episode during pregnancy did not appear to be associated with a higher rate of adverse neonatal outcomes relative to pregnancies with only 1 syncopal episode, the 5‐year incidence of congenital anomalies was significantly higher in the former group. Women with syncopal episodes during pregnancy had higher rates of cardiac arrhythmias and syncope in the year after delivery compared with women with no syncope during pregnancy.
Our study is the first to provide data on the population‐level incidence of syncope during pregnancy. The only other estimate (published only in abstract form) is from a cross‐sectional survey of 174 postpartum women in which 4.6% self‐report experiencing syncope during pregnancy.7 However, no information about the cohort that was surveyed is available. In our population‐based analysis, we found the incidence of syncope during pregnancy to be ≈1.0% or 10 per 1000 pregnancies. In 7814 participants from the general population enrolled in the Framingham study, Soteriades et al19 found 822 patient‐reported cases of syncope over a 17‐year follow‐up period, for an age‐adjusted incidence of first syncope of 7.2 per 1000 person‐years. In this study, the incidence of syncope among women aged 20 to 59 years ranged from 3.2 to 4.7 per 1000 person‐years. More recently, Sandhu et al20 reported an age‐sex standardized hospitalization rate (for a primary diagnosis of syncope) of 0.54 per 1000 population. Our findings suggest that the incidence of syncope during pregnancy may be slightly higher than in the general population. The increased rate of syncope in pregnancy may be attributable to physiological changes occurring during pregnancy, but may also in part be attributable to pregnant women being more likely to seek medical attention for syncope during pregnancy.
We found a significant increase in the incidence of syncope during pregnancy over time. This finding is consistent with that reported in the general population. Ruwald et al4 found that the incidence rate of syncope increased from 13.8 to 19.4 per 100 person‐years over a 12‐year study period from 1997 to 2009. The highest rates of syncope in the general population are observed among young (<30 years) and older (>65 years) individuals.2 Although mean maternal age increased slightly over time (by 0.21 years per year in women with syncope during pregnancy and 0.13 years per year in women without syncope during pregnancy), there was no significant increase in the comorbidity burden over time in our cohort of relatively young pregnant women (data not shown).
During pregnancy, the maternal circulation undergoes hemodynamic, electrical, and structural changes.5 These include reduced systemic vascular resistance attributable to a vasodilatory state, increased blood volume and heart rate, and eccentric hypertrophy of the left ventricle. These changes may contribute to an exaggerated vasovagal response during pregnancy.6 Other possible mechanisms include stimulation of the nerve plexus located behind the uterus, causing reflex‐mediated tachycardia followed by bradycardia, or compression of the inferior vena cava by the gravid uterus, causing reduced venous return to the heart.21 More detailed clinical data are needed to identify potential causes for the observed increase in syncope during pregnancy in our study.
In the only case report to document adverse fetal outcome, maternal vasovagal syncope was associated with severe prenatal encephalopathy.22 Our data suggest that syncope during pregnancy may not be a benign occurrence, as it was found that syncope during the first trimester of pregnancy was associated with higher rates of preterm birth, SGA, and low birth weight. The exact mechanisms explaining the association between syncope during the early stages of pregnancy and adverse neonatal outcomes remain unclear. It is known, however, that a hypoxic maternal environment may be detrimental to placental development as well as fetal growth and may lead to potential consequences, including intrauterine fetal growth restriction, premature delivery, and preeclampsia.23 Our findings of the long‐term consequences of syncope during pregnancy, including the higher incidence of congenital anomalies among children of pregnancies with multiple syncope episodes as well as the higher maternal rates of cardiac arrhythmias in the year following delivery in women with syncope during pregnancy, require further study and confirmation in other independent cohorts.
Although our study provides novel real‐world data on the incidence of syncope during pregnancy in a large population‐based cohort with universal health, it has a few limitations. Our study may underestimate the true incidence of syncope, as it does not account for women with symptoms of syncope who may not have sought medical attention. Our diagnosis of syncope was based on algorithms using ICD codes with moderate sensitivity in administrative data sets,12, 13 and the true incidence of syncope may thus have been underestimated. Additionally, pregnancies that resulted in stillbirths or miscarriages were not included in our database, thus leading to potential further underestimation of the true incidence and adverse neonatal outcomes of syncope in pregnancy.
Conclusions
In a large, contemporary, population‐based cohort, we found that the incidence of syncope during pregnancy is ≈1.0%, and is increasing over time. Pregnancies with syncope, especially when it occurs during the first trimester, may be associated with higher rates of adverse outcomes, including higher rates of preterm births and congenital anomalies and increased incidence of maternal cardiac arrhythmia and syncope postpartum. Whether women who experience syncope during pregnancy may benefit from closer monitoring during the obstetric and postpartum periods requires further study.
Author Contributions
Kaul conceived of the study. Kaul and Sandhu obtained funding. Chatur, Islam, Moore, and Kaul drafted the manuscript. Islam conducted all analyses. All authors edited subsequent versions of the manuscript and approved the final manuscript for submission.
Sources of Funding
This study was funded by a peer‐reviewed research grant from the Cardiac Arrhythmia Network of Canada. The funding agency did not have input into study design, data collection, interpretation of results, manuscript preparation, or approval for submission.
Disclosures
None.
Supporting information
Data S1. Supplemental analysis.
Table S1. ICD Codes for Preexisting Medical Conditions and Congenital Anomalies
Figure S1. Flow diagram for study cohort selection.
Figure S2. Maternal age‐adjusted odds ratios and 95% CIs for the association of syncope and neonatal/congenital anomaly outcomes based on generalized estimating equation models accounting for correlation between pregnancies/births of the same mother.
Acknowledgments
This study is based on data provided by Alberta Health. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta. Guarantor: PK takes responsibility for the contents of the article.
(J Am Heart Assoc. 2019;8:e011608 DOI: 10.1161/JAHA.118.011608.)
This study was presented at the American Heart Association's Scientific Sessions, November 10 to 12, in Chicago, IL.
References
- 1. Walsh K, Hoffmayer K, Hamdan MH. Syncope: diagnosis and management. Curr Probl Cardiol. 2015;40:51–86. [DOI] [PubMed] [Google Scholar]
- 2. Task Force for the D, Management of S, European Society of C, European Heart Rhythm A, Heart Failure A, Heart Rhythm S , Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, Deharo JC, Gajek J, Gjesdal K, Krahn A, Massin M, Pepi M, Pezawas T, Ruiz Granell R, Sarasin F, Ungar A, van Dijk JG, Walma EP, Wieling W. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Costantino G, Perego F, Dipaola F, Borella M, Galli A, Cantoni G, Dell'Orto S, Dassi S, Filardo N, Duca PG, Montano N, Furlan R. Short‐ and long‐term prognosis of syncope, risk factors, and role of hospital admission: results from the STePS (Short‐Term Prognosis of Syncope) study. J Am Coll Cardiol. 2008;51:276–283. [DOI] [PubMed] [Google Scholar]
- 4. Ruwald MH, Hansen ML, Lamberts M, Hansen CM, Hojgaard MV, Kober L, Torp‐Pedersen C, Hansen J, Gislason GH. The relation between age, sex, comorbidity, and pharmacotherapy and the risk of syncope: a Danish nationwide study. Europace. 2012;14:1506–1514. [DOI] [PubMed] [Google Scholar]
- 5. Bett GC. Hormones and sex differences: changes in cardiac electrophysiology with pregnancy. Clin Sci (Lond). 2016;130:747–759. [DOI] [PubMed] [Google Scholar]
- 6. Schwarzwald S, Kersten D, Mitrache A, Cohen T. Syncope in pregnancy: case report and review of the literature. EP Lab Dig. 2015;15 Available at: https://www.eplabdigest.com/articles/Syncope-Pregnancy-Case-Report-and-Review-Literature. [Google Scholar]
- 7. Gibson PS, Powrie R, Peipert J. Prevalence of syncope and recurrent presyncope during pregnancy. Obstet Gynecol. 2001;97:S41–S42. [Google Scholar]
- 8. Thanh NX, Toye J, Savu A, Kumar M, Kaul P. Health service use and costs associated with low birth weight—a population level analysis. J Pediatr. 2015;167:551–556.e1‐3. [DOI] [PubMed] [Google Scholar]
- 9. Alberta Health, Analytics and Performance Reporting Branch, Overview of Administrative Health Datasets. 2017. Available at: https://open.alberta.ca/dataset/657ed26d-eb2c-4432-b9cb-0ca2158f165d/resource/38f47433-b33d-4d1e-b959-df312e9d9855/download/research-health-datasets.pdf. Updated April 28, 2017. Accessed March 20, 2019.
- 10. Tran DT, Ohinmaa A, Thanh NX, Welsh RC, Kaul P. The healthcare cost burden of acute myocardial infarction in Alberta, Canada. Pharmacoecon Open. 2018;2:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Statistics Canada . 2006 population census. Available at: http://www23.statcan.gc.ca/imdb/p2SV.plFunction=getSurvey&SurvId=30216&InstaId=30219&SDDS=3901. Updated October 24, 2007. Accessed March 20, 2019.
- 12. Furlan L, Solbiati M, Pacetti V, Dipaola F, Meda M, Bonzi M, Fiorelli E, Cernuschi G, Alberio D, Casazza G, Montano N, Furlan R, Costantino G. Diagnostic accuracy of ICD‐9 code 780.2 for the identification of patients with syncope in the emergency department. Clin Auton Res. 2018;28:577–582. [DOI] [PubMed] [Google Scholar]
- 13. Ruwald MH, Hansen ML, Lamberts M, Kristensen SL, Wissenberg M, Olsen AM, Christensen SB, Vinther M, Kober L, Torp‐Pedersen C, Hansen J, Gislason GH. Accuracy of the ICD‐10 discharge diagnosis for syncope. Europace. 2013;15:595–600. [DOI] [PubMed] [Google Scholar]
- 14. Quan H, Wang F, Schopflocher D, Norris C, Galbraith PD, Faris P, Graham MM, Knudtson ML, Ghali WA. Development and validation of a surname list to define Chinese ethnicity. Med Care. 2006;44:328–333. [DOI] [PubMed] [Google Scholar]
- 15. Cummins C, Winter H, Cheng KK, Maric R, Silcocks P, Varghese C. An assessment of the Nam Pehchan computer program for the identification of names of south Asian ethnic origin. J Public Health Med. 1999;21:401–406. [DOI] [PubMed] [Google Scholar]
- 16. Harding S, Dews H, Simpson SL. The potential to identify South Asians using a computerised algorithm to classify names. Popul Trends. 1999:46–49. Available at: https://www.ncbi.nlm.nih.gov/pubmed/10549044. [PubMed] [Google Scholar]
- 17. Statistics Canada . Census profile, 2016 census. Available at: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E. Updated May 18, 2018. Accessed: March 20, 2019.
- 18. Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, Blondel B, Breart G; Fetal/Infant Health Study Group of the Canadian Perinatal Surveillance System . A new and improved population‐based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:E35. [DOI] [PubMed] [Google Scholar]
- 19. Soteriades ES, Evans JC, Larson MG, Chen MH, Chen L, Benjamin EJ, Levy D. Incidence and prognosis of syncope. N Engl J Med. 2002;347:878–885. [DOI] [PubMed] [Google Scholar]
- 20. Sandhu RK, Sheldon RS, Savu A, Kaul P. Nationwide trends in syncope hospitalizations and outcomes from 2004 to 2014. Can J Cardiol. 2017;33:456–462. [DOI] [PubMed] [Google Scholar]
- 21. Schmitt D. The hypotensive syndrome in the supine position in late pregnancy. N Engl J Med. 1958;259:1219–1221. [DOI] [PubMed] [Google Scholar]
- 22. Gonce‐Mellgren A, Tamayo‐Rojas O, Sanchez‐Martinez M, Salvia‐Roiges D, Ramirez‐Ruz J, Cararach‐Ramoneda V. [Severe neonatal encephalopathy, secondary to a prolonged vasovagal episode in a woman 31 weeks pregnant]. Rev Neurol. 2002;34:833–835. [PubMed] [Google Scholar]
- 23. Thompson LP, Crimmins S, Telugu BP, Turan S. Intrauterine hypoxia: clinical consequences and therpeutic persepctives. Res Rep Neonatol. 2015;5:79–89. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental analysis.
Table S1. ICD Codes for Preexisting Medical Conditions and Congenital Anomalies
Figure S1. Flow diagram for study cohort selection.
Figure S2. Maternal age‐adjusted odds ratios and 95% CIs for the association of syncope and neonatal/congenital anomaly outcomes based on generalized estimating equation models accounting for correlation between pregnancies/births of the same mother.


