Abstract
Background
There is heterogeneity in the severity of domains affected in patients with stroke, resulting in differences in health‐related quality of life (hrQoL). Identifying different clinical profiles of stroke patients may provide a means for selecting patients for tailored interventions to improve hrQoL.
Methods and Results
This was an observational study of 496 patients with ischemic stroke or intracerebral hemorrhage seen in a cerebrovascular clinic from October 12, 2015, through June 11, 2018, who completed patient‐reported outcome measures using Patient‐Reported Outcome Measurement Information System (PROMIS) tools within 1 month of stroke. Latent profile analysis identified groups based on PROMIS domain scores—pain, depression, cognitive function, fatigue, social role satisfaction, and physical function—as well as clinician‐reported modified Rankin Scale (mRS). Five distinct profiles were identified. Group 1 (“excellent hrQoL,” n=106) had fewer symptoms in all domains than the general population. Group 2 (“disabled with mixed hrQoL,” n=17) had fewer symptoms than the general population in all domains except social role satisfaction and physical function, despite having moderate disability (median mRS score: 3). Group 3 (“mild limitations with average hrQoL,” n=189) had scores similar to the general population for all domains and minimal disability (median mRS score: 1). Group 4 (“mild limitations with poor hrQoL,” n=152) also had a median mRS score of 1 but had worse scores than group 3 on all domains. Group 5 (“disabled with poor hrQoL,” n=32) had worse symptoms than patients in the other profiles and a median mRS score of 3.
Conclusions
Patients with recent stroke have distinct clinical symptom profiles, even with similar levels of clinician‐reported disability. Symptom profiles provide a means of understanding patterns of outcomes in patients with stroke.
Keywords: outcome, patient‐reported outcome, stroke, symptom cluster
Subject Categories: Quality and Outcomes
Clinical Perspective
What Is New?
Patients with recent stroke were classified into 5 distinct clinical symptom profiles based on patient‐reported outcomes and clinician‐reported disability.
Group membership was similar at 6 to 12 months after stroke.
What Are the Clinical Implications?
Symptom profiles provide a means of integrating outcomes across multiple dimensions of health and understanding patterns of outcomes in patients with stroke.
Categorization of patients by clinical symptom profiles may help target the provision of resources to patients who have the most need and would be more likely to benefit.
Introduction
Stroke is a leading cause of serious adult long‐term disability in the United States.1 In addition to physical impairment, it often affects multiple domains of health such as social roles,2 fatigue,3 depression,4 pain,5 and cognition.6 There is heterogeneity in the presence and severity of domains affected in patients with stroke, resulting in differences in health‐related quality of life (hrQOL), even among patients with the same level of disability.7 This heterogeneity is also a factor in the variable pattern and time course of recovery after stroke.8 Identifying symptom‐cluster phenotypes in patients with stroke may improve the ability to predict future outcomes and lead to tailored interventions to improve hrQOL.
Clinical profiles based on patterns of neurological impairment in the National Institutes of Health Stroke Scale (NIHSS) have been demonstrated to improve prediction of functional outcomes and mortality in patients enrolled in clinical stroke trials9 and those receiving intravenous thrombolysis.10 Cluster analysis has been used to identify stroke rehabilitation profiles that include the variables of caregiver support, disability, and cognitive impairment.11 Data are limited, however, on symptom profiles that utilize patient‐reported outcome measures (PROMs).
The Patient‐Reported Outcome Measurement Information System (PROMIS) uses computer‐adaptive testing to efficiently measure patient status for different domains of health in people with a wide variety of diseases and symptoms.12 Importantly, all PROMIS domain scales are measured on the same scale, allowing direct comparison of scores across domains.
Our study objective was to identify distinct symptom‐cluster phenotypes after stroke using latent profile analysis (LPA) of PROMIS scale scores in a cohort study of patients discharged from the hospital with a principal diagnosis of ischemic stroke and intracerebral hemorrhage (ICH).
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Design
We performed a retrospective cohort study of incident stroke patients seen in the Cleveland Clinic cerebrovascular center from October 12, 2015, through June 11, 2018. Our patient‐reported data collection infrastructure has been described previously.7 As part of routine care, both patient‐ and clinician‐reported scales are collected through the Knowledge Program, an electronic platform for systematic collection of patient‐reported information.13 PROMs are administered on tablets at the time of the ambulatory visit or through the electronic health record patient portal (MyChart; Epic Systems) before the appointment. Clinicians completed the NIHSS and modified Rankin Scale (mRS) during each visit and recorded the date of the last cerebrovascular event.
Patients were included in the study cohort if they were aged ≥18 years, completed PROMs within 1 month of their incident stroke, and had provider documentation of ischemic stroke or ICH in structured fields of the encounter note or had a visit diagnosis of one of these cerebrovascular events as defined by the following codes from the 9th and 10th revisions of International Classification of Diseases, Clinical Modification (ICD‐CM): ischemic stroke—433.x1, 434.x1, 436, I63.x; ICH—432, I61. We included patients with ischemic stroke and patients with ICH in the analysis, as previous research indicated that patterns of symptoms were similar between these stroke subtypes.14 Patients who had multiple event types were excluded. For patients who had >1 visit during the study period, scores from the first visit with the most complete data were included in analyses.
Study Variables
Patient‐reported outcome measures
The PROMIS measures patient status for different domains of health along a continuous scale in people with a wide variety of diseases and symptoms,12 including ischemic stroke and ICH.14, 15, 16 PROMIS scales were administered to patients or their proxies using computer‐adaptive testing. With this type of testing, the most informative questions are selected from an item bank of questions based on the patient's prior responses. This improves score precision, reduces patient burden, and minimizes ceiling and floor effects. Scores are standardized to the general US adult population as a T‐score with a mean of 50 (SD: 10). Quality of Life in Neurological Disorders (NeuroQoL) is a closely related set of scales spanning similar domains of health designed for use in patients with neurological conditions. NeuroQoL was developed with the same psychometric methods as PROMIS and uses overlapping item banks and the same standardized scoring system.17
The NeuroQoL cognitive function scale and 4 PROMIS scales—physical function, satisfaction with social roles, pain interference, and fatigue—were completed by patients and used in the symptom‐cluster profiles. These domains have been previously reported to be affected by stroke2, 3, 4, 5, 6, 18 and are considered important domains to measure when performing a comprehensive evaluation of patient health.19
The Patient Health Questionnaire 9 (PHQ‐9) is a 9‐item depression screen that is frequently used in stroke and is the standard depression assessment tool at Cleveland Clinic.20 The PHQ‐9 score was cocalibrated to the PROMIS metric, providing equivalent PROMIS depression scores.21
In our analyses, scales were oriented so higher domain scores indicated worse hrQOL. Additional questions assessed whether patients had help completing the PROMs (proxy respondents) and, if so, whether patients could have completed the PROMs on their own.
Clinician‐reported measures
The NIHSS is the standard scale for measuring neurological impairment. It consists of 15 items with scores ranging from 0 to 42, with higher scores indicating greater impairment.22
The mRS is a 1‐item measure of global disability with scores ranging from 0 to 6, with 0 representing no symptoms and 6 representing death.23 It is the most commonly used outcome measure in clinical stroke trials.24 To optimize the interobserver reliability of mRS in our practice, all providers underwent standardized training and have been certified in completion of the mRS.25
Patient demographics were obtained from the electronic health record. Approximate household income was estimated by zip code based on 2010 census data.
Statistical Analysis
Patient demographics, clinical characteristics, and PROMs were summarized using descriptive statistics. Demographics and clinical characteristics were compared between incident stroke patients included in the study cohort versus patients excluded for not completing PROMs. Categorical variables were compared using the χ2 test, and continuous variables were compared using the t test or Mann–Whitney U test, as appropriate.
To identify symptom‐cluster profiles, we conducted LPA. This analytic approach uses model‐based probabilities to group patients into similar symptom and functional profiles.26 The PROMIS domains of pain, depression, fatigue, social role satisfaction, and physical function and the NeuroQoL cognitive function domain were utilized to empirically categorize subgroups of patients’ symptoms and functional status. These items were identified a priori based on their severity and prevalence in patients with stroke.14 These 6 symptoms are also among the most common symptoms used in prior symptom‐cluster research.27 Depression was included despite its modest severity compared with many other domains because of its known association with worse outcomes and the particular attention to this symptom in stroke. Provider‐reported mRS scores were also included as a measure of global disability to categorize patients. An LPA model was initially constructed with a single profile and then successively built with an increasing number of profiles (ie, the second model had 2 domain profiles, the third model had 3 profiles, etc). The optimal number of profiles was determined by iteratively by comparing the model with k profiles with the model with (k−1) profiles using multiple fit indexes: the Akaike and sample‐size‐adjusted Bayesian information criterion indexes, entropy, the Vuong–Lo–Mendell–Rubin likelihood ratio test, and the Lo–Mendell–Rubin likelihood ratio test.28, 29 On selection of the model with the optimal number of profiles, patients were grouped into their most likely latent profiles using estimated posterior membership probabilities. Average posterior probabilities within the subgroups were compared to assess how well the model classified patients. Missing data were minimal and handled using a full‐information maximum‐likelihood method. Mean domain scores per profile were plotted for visual interpretation.
Demographics, clinical characteristics, and PROMs were compared across identified profile subgroups using the χ2 test, ANOVA with the Tukey post hoc test, or the Kruskal–Wallis test with the Dunn post hoc test (nonparametric), as appropriate.
A sensitivity analysis was conducted to confirm results after excluding PROMs from proxy respondents. A subset analysis evaluated the stability of profile memberships in the first 6 to 12 months after stroke, within the subset of patients with follow‐up data. Change in PROMs were evaluated between baseline and follow‐up using a paired t test or Wilcoxon signed rank test, as appropriate.
Statistical significance was established throughout at P<0.05. LPA was conducted in MPlus v8.0,30 with all other statistical analyses conducted using SAS v9.4 (SAS Institute).
Standard Protocol Approvals, Registration, and Patient Consent
This study was approved by the Cleveland Clinic institutional review board. Because the study consisted of analyses of preexisting data, the requirement for patient informed consent was waived.
Results
In total, 1079 incident ischemic and ICH stroke patients seen in the Cleveland Clinic's cerebrovascular clinic during the study period. Of these, 496 (46.0%) patients completed a PROM screen and were included in the study cohort. Compared with the 583 excluded patients, patients included in the cohort were significantly younger (61.2±15.9 versus 63.9±14.6), were white (81.9% versus 64.4%), were married (61.4% versus 50.1%), had higher median household income ($50 545 versus $45 787), and had less diabetes mellitus and hypertension (Table 1). The study cohort had significantly less clinician‐rated disability compared with excluded patients (median [interquartile range]: mRS: 1 [0–2] versus 1 [1–2], respectively; NIHSS: 0 [0–1] versus 1 [0–2], respectively).
Table 1.
Demographics and Clinical Characteristics for Incident Stroke Patients (n=1079)
| Study Cohort | Excluded | P Value | |
|---|---|---|---|
| Patients | 496 (46.0) | 583 (54.0) | |
| Conceptual model of patient outcomes, female | 227 (45.8) | 267 (45.8) | 0.99 |
| Age, y, mean±SD | 61.2±15.9 | 63.9±14.6 | 0.005 |
| White | 394 (81.9) | 364 (64.4) | <0.001 |
| Married | 298 (61.4) | 284 (50.1) | <0.001 |
| Household income (×$10 000), median (IQR) | 5.1 (4.0–6.5) | 4.6 (3.5–6.0) | <0.001 |
| Comorbidities (n=807) | |||
| Cancer | 107 (27.6) | 97 (23.0) | 0.14 |
| Chronic renal failure | 42 (10.9) | 62 (14.7) | 0.12 |
| Coronary artery disease | 80 (20.7) | 96 (22.7) | 0.50 |
| Diabetes mellitus | 115 (29.7) | 182 (43.1) | <0.001 |
| Hypertension | 261 (65.7) | 306 (72.5) | 0.036 |
| Depression | 59 (15.3) | 74 (17.5) | 0.41 |
| Clinical characteristics | |||
| Ischemic stroke (vs ICH) | 427 (86.1) | 514 (88.2) | 0.27 |
| Days since stroke, median (IQR) | 27 (16, 38) | 28 (16, 39) | 0.19 |
| mRS score, median (IQR) | 1 (0, 2) | 1 (1, 2) | <0.001 |
| NIHSS score, median (IQR) | 0 (0, 1) | 1 (0, 2) | <0.001 |
| Patients with deficits by NIHSS item | |||
| Level of consciousness (items 1a–1c) | 19 (4.3) | 43 (8.0) | 0.028 |
| Best gaze | 7 (1.6) | 18 (3.3) | 0.14 |
| Visual | 42 (9.5) | 56 (10.4) | 0.73 |
| Facial palsy | 58 (13.1) | 110 (20.4) | 0.003 |
| Motor arm (left or right) | 50 (11.3) | 97 (18.0) | 0.003 |
| Motor leg (left or right) | 36 (8.1) | 91 (17.0) | <0.001 |
| Limb ataxia | 14 (3.2) | 26 (4.8) | 0.19 |
| Sensory | 43 (9.7) | 77 (14.3) | 0.030 |
| Best language | 39 (8.8) | 72 (13.4) | 0.045 |
| Dysarthria | 37 (8.4) | 77 (14.3) | 0.004 |
| Extinction and inattention | 11 (2.5) | 40 (7.4) | <0.001 |
Data are shown as n (%) except as noted. Study cohort included patients with ischemic stroke or ICH who completed patient‐reported scales within 1 month of their stroke. Patients not included in the cohort did not complete patient‐reported scales. ICH indicates intracerebral hemorrhage; IQR, interquartile range; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Patient‐reported outcomes are presented in Table 2. Scores ranged from 49.4±9.7 for depression to 59.2±10.4 for physical function. A total of 119 (28.7%) patients had help from a proxy to complete their PROMs, although 69 (58.0%) of these patients could have completed PROMs on their own.
Table 2.
PROMs in the Stroke Cohort (n=496)
| PROMIS/NeuroQoL Domain | Patients, n | Score, Mean±SD |
|---|---|---|
| Depressiona | 462 | 49.4±9.7 |
| Pain | 460 | 50.2±10.8 |
| Cognitive function | 434 | 52.4±10.5 |
| Fatigue | 484 | 53.1±10.3 |
| Social role satisfaction | 443 | 54.8±11.3 |
| Physical function | 496 | 59.2±10.4 |
All PROMIS and NeuroQoL scores are oriented so higher scores indicate worse health‐related quality of life. NeuroQol indicates Quality of Life in Neurological Disorders; PROMIS, Patient‐Reported Outcome Measurement Information System; PROMs, patient‐reported outcome measures.
Depression was measured with the Patient Health Questionnaire 9 depression screen, which was cocalibrated on the PROMIS metric to provide an equivalent PROMIS depression score.
In LPA, comparing the indexes of fit, 5 distinct patient symptom profiles were determined to best fit the study sample (data available on request). The model classified patients well, with average posterior probabilities within the identified profile groups ranging from 0.89 (SD: 0.15) to 0.94 (SD: 0.12).
Figure 1 presents the 5 identified latent profiles in a line graph. The y‐axis represents the average PROMIS score, and the x‐axis shows the 6 PROMIS domains. Average PROMIS scores are connected across the domains to visually display the 5 profiles: patients in group 1 (n=106), labeled as “excellent hrQoL,” had fewer symptoms in all domains than the general population and a median mRS score of 0 (Table 3). Group 2 (n=17), labeled as “disabled with mixed hrQoL,” on average had fewer symptoms than the general population in all domains except satisfaction with social roles and physical function and had higher disability (median mRS score: 3). Group 3 (n=189), labeled as “mild limitations with average hrQoL,” had similar scores relative to each other and to those of the general population for all domains and had low disability (median mRS score: 1). Group 4 (n=152), labeled as “mild limitations with poor hrQoL,” had relatively worse scores than group 3 on all domains, despite having levels of disability similar to group 3 (median mRS score: 1). Group 5 (n=32), labeled as “disabled with poor hrQoL,” had worse symptom severity and functioning than patients in the other profiles, with a median mRS score of 3. The distribution of mRS scores across profiles is shown in Table 4.
Figure 1.
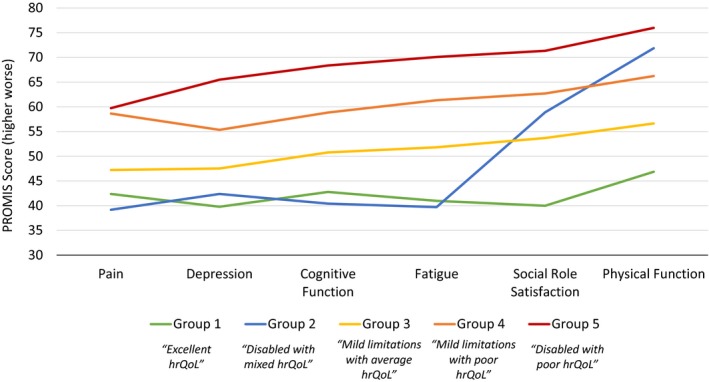
Patient‐reported outcomes by latent profile group (n=496). Latent profile analysis identified 5 subgroups based on PROMIS/NeuroQoL domains of pain, depression, cognitive function, fatigue, social role satisfaction, function, and modified Rankin Scale score. Depression was measured with the Patient Health Questionnaire 9 depression screen, which was cocalibrated on the PROMIS metric to provide an equivalent PROMIS depression score. PROMIS and NeuroQoL scores are oriented so that higher scores indicated worse symptoms or function. Mean score of the US general population is 50. hrQoL indicates health‐related quality of life; NeuroQoL, Quality of Life in Neurological Disorders; PROMIS, Patient‐Reported Outcome Measurement Information System.
Table 3.
Patient‐ and Clinician‐Reported Outcomes Across Latent Profile Group (n=496)
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | P Valuea | |
|---|---|---|---|---|---|---|
| Patients, n (%) | 106 (21.4) | 17 (3.4) | 189 (38.1) | 152 (30.6) | 32 (6.5) | |
| PROMIS/NeuroQoL domain scores, mean±SD | ||||||
| Depressiona | 39.8±4.9 | 42.4±7.4 | 47.5±6.1 | 55.3±7.5 | 65.5±8.8 | <0.001b , c , d |
| Pain | 42.4±5.9 | 39.2±2.1 | 47.2±8.5 | 58.6±9.0 | 59.7±12.7 | <0.001e , b , c, e , b , c , d |
| Fatigue | 41.0±5.8 | 39.7±5.7 | 51.8±5.1 | 61.3±5.5 | 70.1±5.4 | <0.001e , b , c , d |
| Social role satisfaction | 40.0±6.6 | 58.9±12.4 | 53.7±6.3 | 62.7±6.5 | 71.3±6.1 | <0.001c , d , f |
| Physical function | 46.8±5.2 | 71.9±5.7 | 56.6±6.3 | 66.2±5.9 | 76.0±4.8 | <0.001e , b , d , f |
| mRS score, median (IQR) | 0 (0, 1) | 3 (2, 3) | 1 (1, 2) | 1 (1, 2) | 3 (2, 4) | <0.001e , b , d , f |
All scores oriented so higher scores indicate worse health‐related quality of life. IQR indicates interquartile range; NeuroQoL, Quality of Life in Neurological Disorders; PROMIS, Patient‐Reported Outcome Measurement Information System.
Group 1 differed significantly from groups 3–5. Group 3 differed significantly from groups 4 and 5.
Depression was measured with the Patient Health Questionnaire 9 depression screen, which was cocalibrated on the PROMIS metric to provide an equivalent PROMIS depression score.
Group 2 differed significantly from group 4.
Group 2 differed significantly from group 5.
Group 4 differed significantly from group 5.
Group 2 differed significantly from group 3.
Group 1 differed significantly from group 2.
Table 4.
Distribution of mRS Scores Across Latent Profile Groups
| Group | mRS 0 | mRS 1 | mRS 2 | mRS ≥3 |
|---|---|---|---|---|
| 1 | 54 (44.6) | 42 (22.5) | 6 (5.7) | 2 (3.2) |
| 2 | 0 | 2 (1.1) | 2 (1.9) | 11 (17.7) |
| 3 | 45 (37.2) | 82 (43.8) | 46 (43.8) | 8 (12.9) |
| 4 | 22 (18.2) | 59 (31.5) | 45 (42.9) | 21 (33.9) |
| 5 | 0 | 2 (1.1) | 6 (5.7) | 20 (32.3) |
| Total, n | 121 | 187 | 105 | 62 |
Data are shown as n (%). mRS indicates modified Rankin Scale.
Table 5 presents demographics and clinical characteristics across symptom profiles. There were differences between groups for multiple characteristics. When comparing group 3 (“mild limitations with average hrQoL”) and group 4 (“mild limitations with poor hrQoL”), which both had a median mRS score of 1, patients in group 4 were more likely to be female, to have a higher NIHSS score, and to have PROMs completed by a proxy. A higher proportion of group 4 patients had depression and coronary artery disease compared with group 3. When comparing group 2 (“disabled with mixed hrQoL”) and group 5 (“disabled with poor hrQoL”), which both had a median mRS score of 3, patients in group 5 were more likely to be female and to have PROMs complete by a proxy.
Table 5.
Demographics and Clinical Characteristics Compared Across Latent Profile Group (n=496)
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | P Value | |
|---|---|---|---|---|---|---|
| Patients | 106 (21.4) | 17 (3.4) | 189 (38.1) | 152 (30.6) | 32 (6.5) | |
| Female | 35 (33.0) | 5 (29.4) | 81 (42.9) | 84 (55.3) | 22 (68.8) | <0.001a , b , c , d , e , f |
| Age, y, mean±SD | 58.2±14.3 | 63.2±15.8 | 61.5±15.2 | 62.1±17.1 | 68.7±17.6 | 0.025b |
| White | 80 (77.7) | 15 (88.2) | 152 (83.1) | 123 (83.1) | 24 (80.0) | 0.72 |
| Married | 67 (66.3) | 11 (68.8) | 114 (60.6) | 93 (62.0) | 13 (43.3) | 0.23b |
| Proxy responder | 6 (6.7) | 4 (30.8) | 34 (21.0) | 55 (44.7) | 20 (74.1) | <0.001a , b , d , e , f , g , h , i |
| Patient could have responded | 6 (100.0) | 2 (50.0) | 28 (82.4) | 30 (54.6) | 3 (15.0) | <0.001b , e , f , i |
| Household income (×$10 000), median (IQR) | 5.4 (4.1, 6.9) | 5.3 (4.2, 6.1) | 5.1 (4.2, 6.5) | 4.8 (4.0, 6.3) | 4.6 (3.7, 6.2) | 0.17b |
| Comorbidities (n=397) | ||||||
| Cancer | 20 (23.0) | 5 (35.7) | 36 (24.8) | 37 (31.1) | 9 (39.1) | 0.38 |
| Chronic renal failure | 5 (5.8) | 1 (7.1) | 16 (11.2) | 18 (15.1) | 2 (8.7) | 0.31a |
| Coronary artery disease | 13 (15.1) | 4 (30.8) | 24 (16.8) | 33 (27.3) | 6 (25.0) | 0.12a , e |
| Diabetes mellitus | 13 (15.1) | 6 (40.0) | 39 (27.1) | 47 (39.8) | 10 (41.7) | 0.002a , b , e , g , h |
| Hypertension | 48 (55.2) | 10 (66.7) | 94 (63.5) | 90 (73.2) | 19 (79.2) | 0.048a , b |
| Depression | 8 (9.3) | 1 (7.1) | 16 (11.1) | 32 (27.1) | 2 (8.3) | <0.001a , e , i |
| Clinical characteristics | ||||||
| Ischemic stroke (vs ICH) | 97 (91.5) | 12 (70.6) | 164 (86.8) | 133 (87.5) | 21 (65.6) | 0.002b , f , g , i |
| Days since stroke, median (IQR) | 26 (18–40) | 31 (26–36) | 23 (16–35) | 28 (15–38) | 34 (23–41) | 0.24f |
| mRS score, median (IQR) | 0 (0–1) | 3 (2–3) | 1 (1–2) | 1 (1–2) | 3 (2–4) | <0.001a , b , j , c , e , f , g , h , i |
| NIHSS, median (IQR) | 0 (0–0) | 3.5 (1–5) | 0 (0–1) | 1 (0–2) | 2 (1–4) | <0.001a , b , j , c , e , f , g , h , i |
| Patients with deficits by NIHSS item | ||||||
| Level of consciousness (items 1a–1c) | 0 (0.0) | 3 (21.4) | 5 (3.1) | 3 (2.2) | 8 (28.6) | <0.001b , j , c , f , g , i |
| Best gaze | 1 (1.0) | 0 (0.0) | 2 (1.2) | 3 (2.2) | 1 (3.7) | 0.79 |
| Visual | 5 (5.0) | 1 (7.1) | 14 (8.6) | 16 (11.8) | 6 (21.4) | 0.087b , f |
| Facial palsy | 8 (7.9) | 9 (64.3) | 13 (8.0) | 18 (13.2) | 10 (34.5) | <0.001b , j , c , f , g , i |
| Motor arm (left or right) | 3 (3.0) | 7 (50.0) | 9 (5.5) | 20 (14.6) | 11 (37.9) | <0.001a , b , j , c , e , f , g , i |
| Motor leg (left or right) | 1 (1.0) | 4 (28.6) | 4 (2.5) | 15 (11.0) | 12 (42.9) | <0.001a , b , j , e , f , g , i |
| Limb ataxia | 2 (2.0) | 0 (0.0) | 5 (3.1) | 6 (4.4) | 1 (3.6) | 0.80 |
| Sensory | 4 (4.0) | 1 (7.1) | 10 (6.1) | 23 (16.9) | 5 (17.9) | 0.002a , b , e , f |
| Best language | 3 (3.0) | 3 (21.4) | 18 (11.0) | 11 (8.1) | 4 (14.3) | 0.057b , g , h |
| Dysarthria | 4 (4.0) | 4 (28.6) | 9 (5.5) | 15 (11.0) | 5 (18.5) | 0.002a , b , j , f , g |
| Extinction and inattention | 1 (1.0) | 1 (7.1) | 2 (1.2) | 3 (2.2) | 4 (14.8) | <0.001b , f , i |
Data shown as n (%) except as noted. ICH indicates intracerebral hemorrhage; IQR, interquartile range; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale.
Group 1 differed significantly from group 4.
Group 1 differed significantly from group 5.
Group 2 differed significantly from group 4.
Group 2 differed significantly from group 5.
Group 3 differed significantly from group 4.
Group 3 differed significantly from group 5.
Group 1 differed significantly from group 2.
Group 1 differed significantly from group 3.
Group 4 differed significantly from group 5.
Group 2 differed significantly from group 3.
A sensitivity analysis excluded responses from proxies and was conducted with LPA of the 296 patients who completed PROMs on their own. Five symptom profiles were determined with severity groupings similar to the full sample (Table 6). The only difference was that the highest severity group, group 5, included only 5 patients, and their median mRS score was 1.
Table 6.
Patient‐ and Clinician‐Reported Outcomes Across Latent Profile Group After Excluding Proxy Responses (n=296)
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|
| Patients, n (%) | 86 (29.0) | 10 (3.4) | 131 (44.3) | 64 (21.6) | 5 (1.7) |
| PROMIS/NeuroQoL domain scores, mean±SD | |||||
| Depressiona | 39.9±4.8 | 40.8±7.1 | 47.9±5.8 | 54.0±6.1 | 72.6±3.5 |
| Pain | 42.7±6.1 | 39.3±2.4 | 47.6±7.9 | 59.7±8.0 | 68.4±8.1 |
| Cognitive function | 42.6±7.4 | 39.5±9.5 | 50.2±7.9 | 56.7±6.9 | 66.9±5.6 |
| Fatigue | 41.1±5.9 | 36.7±3.9 | 52.4±5.3 | 61.2±5.6 | 70.3±5.8 |
| Social role satisfaction | 39.9±6.6 | 56.6±13.1 | 54.5±6.5 | 63.5±6.3 | 74.9±0 |
| Physical function | 47.5±5.0 | 71.2±7.3 | 56.5±6.0 | 65.9±6.6 | 69.6±3.7 |
| mRS score, median (IQR) | 0 (0–1) | 2.5 (1.5–3) | 1 (0–1) | 1 (1–2) | 1 (1–2) |
IQR indicates interquartile range; mRS, modified Rankin Scale; NeuroQoL, Quality of Life in Neurological Disorders; PROMIS, Patient‐Reported Outcome Measurement Information System.
Depression was measured with the Patient Health Questionnaire 9 depression screen, which was cocalibrated on the PROMIS metric to provide an equivalent PROMIS depression score.
To evaluate the stability of profile memberships over time, a subset analysis of 125 (25.2%) patients with follow‐up data 6 to 12 months following stroke was conducted. Overall, PROMs improved over time, with depression, fatigue, social role satisfaction, physical function, and mRS scores improving significantly. Group memberships remained stable (Table 7, Figure 2).
Table 7.
Patient‐ and Clinician‐Reported Outcomes at 6–12 Months of Follow‐up Compared Across Latent Profile Groups (n=125)
| n | Total | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|---|---|
| Patients, n (%) | 125 | 20 (16.0) | 6 (4.8) | 46 (36.8) | 48 (38.4) | 5 (4.0) | |
| PROMIS/NeuroQoL domain scores, mean±SD | |||||||
| Depressiona | 84 | 47.0±8.4b | 40.1±8.0 | 40.9±7.9 | 45.2±6.0b | 52.3±8.0 | 50.5±7.0b |
| Pain | 79 | 50.0±10.0 | 41.1±5.7 | 41.5±4.0 | 47.2±8.4 | 56.9±7.8 | 52.7±16.3 |
| Cognitive function | 78 | 50.5±10.5 | 41.4±6.0 | 39.3±8.2 | 48.2±9.5 | 56.2±8.1 | 60.4±14.2 |
| Fatigue | 83 | 51.1±9.7b | 39.5±6.1 | 44.0±5.6 | 49.7±8.4 | 56.9±8.1 | 57.5±5.5 |
| Social role satisfaction | 79 | 54.4±12.0b | 38.3±6.7 | 55.5±11.3 | 50.4±10.4 | 61.6±7.6b | 68.7±5.5 |
| Physical function | 85 | 57.0±9.6b | 47.5±4.3 | 59.0±5.8b | 53.8±8.5b | 61.1±8.3b | 73.5±4.4 |
| mRS, median (IQR) | 119 | 1 (0–2)b | 0 (0–1) | 2 (1–3) | 1 (0–1)b | 1 (1–2) | 3.5 (1.5–4) |
IQR indicates interquartile range; mRS, modified Rankin Scale; NeuroQoL, Quality of Life in Neurological Disorders; PROMIS, Patient‐Reported Outcome Measurement Information System.
Depression was measured with the Patient Health Questionnaire 9 depression screen, which was cocalibrated on the PROMIS metric to provide an equivalent PROMIS depression score.
Significant improvement from baseline, P<0.05.
Figure 2.
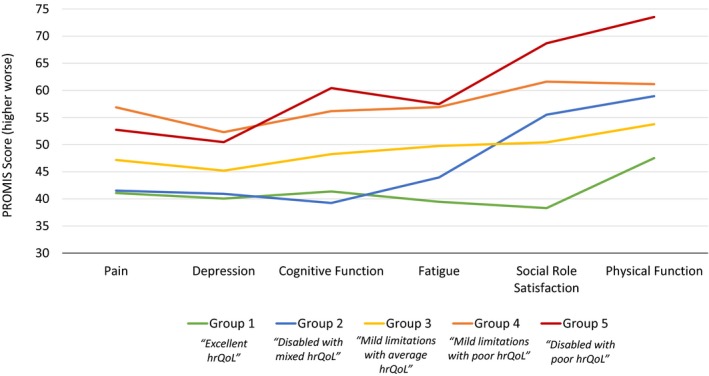
Patient‐reported outcomes by latent profile group in patients with 6 to 12 months of follow‐up (n=125). Latent profile groups are based on PROMIS/NeuroQoL domains of pain, depression, cognitive function, fatigue, social role satisfaction, function, and modified Rankin Scale score. Depression was measured with the Patient Health Questionnaire 9 depression screen, which was cocalibrated on the PROMIS metric to provide an equivalent PROMIS depression score. PROMIS and NeuroQoL scores are oriented so that higher scores indicate worse symptoms or function. Mean score of the US general population is 50. hrQoL indicates health‐related quality of life; NeuroQoL, Quality of Life in Neurological Disorders; PROMIS, Patient‐Reported Outcome Measurement Information System.
Discussion
Our study results suggest that patients with recent stroke fall within distinct clinical phenotypes. Profiles of patients were similar for the 125 patients who had follow‐up scores, suggesting that categorization remained stable for at least the first year after stroke. These profiles provide insights into the different patterns of outcomes of stroke survivors.
Group 1 (“excellent hrQoL”) consisted of patients with no or minimal disability (median mRS score: 0) who had PROMIS symptoms that were meaningfully better than the average of the general US population. Approximately 44.6% of all patients with an mRS score of 0 fell into this category, suggesting that this pattern is common for patients with no residual symptoms. These patients may have had positive perceptions of their health predating their stroke, but it is also conceivable that the perspectives of some patients in this group were affected by their experience, resulting in a response shift with a more favorable perception of their current health. Patients may have been more appreciative of their health status having escaped significant deficits. Recalibration of expectation for health is known to occur after significant health events.31
Patients in group 3 (“mild limitations with average hrQoL”) and group 4 (“mild limitations with poor hrQoL”) had similar mild levels of clinician‐reported disability (median mRS score: 1), although the patients in group 4 had more severe symptoms than those in group 3 in all domains of health. Patients in group 4 had more comorbid conditions including depression and higher NIHSS scores, both of which likely contributed to worse scores. In addition, patients in group 4 were more likely to be female, which has been associated with worse patient‐reported symptoms in the domains composing the clinical profiles.14, 32 Although more patients in group 4 had scores completed by proxies, the study results were similar after repeating the analyses excluding proxy responses. Apart from the differences in characteristics already described, some health perceptions of patients in these different profiles may have been influenced by their level of social support, mental outlook, and self‐defined standards for health.33 Categorization of patients by clinical symptom profiles can help target the provision of resources to patients who, despite having only minimal clinician‐reported disability, have significant patient‐reported symptoms in multiple domains of health.
Patients in group 2 (“disabled with mixed hrQoL”) and 5 (“disabled with poor hrQoL”) both had moderate clinician‐reported disability with median mRS score of 3. Both groups also had similar patient‐reported physical function and social role satisfaction scores, which were meaningfully worse than the average for the general US population. However, patients in group 2 had less pain, fatigue, and cognitive symptoms than even the general population. Patients in group 2 were younger, more likely to be male, white, and married than patients in group 5, which may explain some of these findings. The minimal level of certain symptoms in group 2 may also reflect these patients’ positive mental outlook or internal resilience. Only 13.1% (n=62) of the patients in the study cohort had mRS scores ≥3, but of those, 17.7% (n=11) fell into group 2. Although this requires confirmation in future studies with additional patients with moderate disability, this profile appears to occur in an appreciable subgroup of patients with moderate–severe deficits. The discordance between the severity of self‐reported emotional symptoms and mRS scores across different groups suggest that including both patient‐reported symptoms and clinician‐reported disability in clinical profiles of patients with stroke is warranted.
Patient‐reported symptoms improved over time within all groups, particularly for physical function and satisfaction with social roles—the domains most affected at baseline. Interestingly, even patients in group 1 (“excellent hrQoL”), which had better scores than the general population at baseline, demonstrated some improvement in symptoms for most domains in the clinical profiles. Group membership was similar at 6 to 12 months after stroke.
Symptom profiles in stroke have several potential clinical implications. First, categorization of patients into clinical symptom profiles can aid clinicians in quickly assimilating scores from multiple scales in a clinically meaningful way and provide information on symptom patterns that cannot be retrieved with composite scores. Second, symptom profiles can identify patients who may benefit from additional interventions that focus on the relevant domains of health in a particular profile. Third, by differentiating between patients with similar disability scores but distinct patterns of clinical symptoms, these profiles may predict longer term outcomes, such as return to work and future disability, and healthcare utilization, which is more informative than clinician‐reported disability alone. Proof of concept for this approach in stroke has been seen using the NIHSS impairment score. LPA was successfully applied to improve prognostication of stroke outcomes based on patterns of initial impairment defined by individual items of the NIHSS compared with the total NIHSS score.10
An important strength of this study is the use of PROMIS tools, which are well suited for assessment of symptom clusters. PROMIS tools have the characteristics of an ideal measure for evaluation of symptoms clusters34: consistent scaling, assessment of parallel dimensions (eg, severity, impact), consistent time frame, consistent clinical context, and reasonable response burden. Additional strengths of our study are the large number of patients with recent stroke and the availability of information on both severity of impairment using the NIHSS and clinician‐reported disability with the mRS.
Several limitations also must be considered when interpreting our study results. Patients in the study cohort were included from an ambulatory clinic at one institution with a mild degree of disability overall, the majority having mRS scores of 0 to 2. Findings need to be validated in other populations and settings, especially those with moderate to severe disability. Not all patients seen in the ambulatory clinic completed PROMs, and patients who did not participate differed in the severity of disability and several other characteristics. Generalizability of symptom profiles to these patients is unclear. A larger sample size is needed to confirm group memberships in patients with ICH. Finally, given the limited number of patients with follow‐up scores in our cohort, our findings of stability of group membership over time should be considered preliminary and requires confirmation. After validation of our findings in patients, especially in patients with moderate to severe disability and those with ICH, these symptom clusters can be used in patients with recent stroke who are seen in the ambulatory setting and who complete PROMs.
In conclusion, patients with recent stroke have distinct clinical symptom profiles that provide a means of integrating outcomes across multiple dimensions of health and an opportunity to improve our understanding of the different patterns of outcomes seen in patients with stroke. The presence of different symptom profiles among patients with similar clinician‐reported disability demonstrates the discordance that can occur between clinician‐reported disability and patient‐reported outcomes. This discrepancy supports of the use of clinician‐reported measures along with PROMs to provide a comprehensive assessment of outcomes after stroke. Further research on the utility of these profiles for use in clinical care and research is indicated.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e012421 DOI: 10.1161/JAHA.119.012421.)
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mayo NE, Wood‐Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Arch Phys Med Rehabil. 2002;83:1035–1042. [DOI] [PubMed] [Google Scholar]
- 3. Choi‐Kwon S, Kim JS. Poststroke fatigue: an emerging, critical issue in stroke medicine. Int J Stroke. 2011;6:328–336. [DOI] [PubMed] [Google Scholar]
- 4. De Wit L, Putman K, Baert I, Lincoln NB, Angst F, Beyens H, Bogaerts K, Brinkmann N, Connell L, Dejaeger E, De Weerdt W, Jenni W, Kaske C, Komarek A, Lesaffre E, Leys M, Louckx F, Schuback B, Schupp W, Smith B, Feys H. Anxiety and depression in the first six months after stroke. A longitudinal multicentre study. Disabil Rehabil. 2008;30:1858–1866. [DOI] [PubMed] [Google Scholar]
- 5. Jonsson AC, Lindgren I, Hallstrom B, Norrving B, Lindgren A. Prevalence and intensity of pain after stroke: a population based study focusing on patients’ perspectives. J Neurol Neurosurg Psychiatry. 2006;77:590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shatzman S, Mahajan S, Sundararajan S. Often overlooked but critical: poststroke cognitive impairment in right hemispheric ischemic stroke. Stroke. 2016;47:e221–e223. [DOI] [PubMed] [Google Scholar]
- 7. Katzan IL, Thompson NR, Uchino K, Lapin B. The most affected health domains after ischemic stroke. Neurology. 2018;90:e1364–e1371. [DOI] [PubMed] [Google Scholar]
- 8. Munyombwe T, Hill KM, Knapp P, West RM. Mixture modelling analysis of one‐month disability after stroke: stroke outcomes study (SOS1). Qual Life Res. 2014;23:2267–2275. [DOI] [PubMed] [Google Scholar]
- 9. Sucharew H, Khoury J, Moomaw CJ, Alwell K, Kissela BM, Belagaje S, Adeoye O, Khatri P, Woo D, Flaherty ML, Ferioli S, Heitsch L, Broderick JP, Kleindorfer D. Profiles of the National Institutes of Health Stroke Scale items as a predictor of patient outcome. Stroke. 2013;44:2182–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abdul‐Rahim AH, Fulton RL, Sucharew H, Kleindorfer D, Khatri P, Broderick JP, Lees KR; SITS‐MOST Steering Committee . National Institutes of Health Stroke Scale item profiles as predictor of patient outcome: external validation on safe implementation of thrombolysis in stroke‐monitoring study data. Stroke. 2015;46:2779–2785. [DOI] [PubMed] [Google Scholar]
- 11. Perez LM, Inzitari M, Quinn TJ, Montaner J, Gavalda R, Duarte E, Coll‐Planas L, Cerda M, Santaeugenia S, Closa C, Gallofre M. Rehabilitation profiles of older adult stroke survivors admitted to intermediate care units: a multi‐centre study. PLoS One. 2016;11:e0166304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. PROMIS Network . PROMIS—dynamic tools to measure health outcomes from the patient perspective. Available at: www.nihpromis.org. Accessed June 29, 2018.
- 13. Katzan I, Speck M, Dopler C, Urchek J, Bielawski K, Dunphy C, Jehi L, Bae C, Parchman A. The Knowledge Program: an innovative, comprehensive electronic data capture system and warehouse. AMIA Annu Symp Proc. 2011;2011:683–692. [PMC free article] [PubMed] [Google Scholar]
- 14. Katzan IL, Schuster A, Newey C, Uchino K, Lapin B. Patient‐reported outcomes across cerebrovascular event types: more similar than different. Neurology. 2018;91:e2182–e2191. [DOI] [PubMed] [Google Scholar]
- 15. Katzan IL, Fan Y, Uchino K, Griffith SD. The PROMIS physical function scale: a promising scale for use in patients with ischemic stroke. Neurology. 2016;86:1801–1807. [DOI] [PubMed] [Google Scholar]
- 16. Sangha RS, Caprio FZ, Askew R, Corado C, Bernstein R, Curran Y, Ruff I, Cella D, Naidech AM, Prabhakaran S. Quality of life in patients with TIA and minor ischemic stroke. Neurology. 2015;85:1957–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cella D, Lai JS, Nowinski CJ, Victorson D, Peterman A, Miller D, Bethoux F, Heinemann A, Rubin S, Cavazos JE, Reder AT, Sufit R, Simuni T, Holmes GL, Siderowf A, Wojna V, Bode R, McKinney N, Podrabsky T, Wortman K, Choi S, Gershon R, Rothrock N, Moy C. Neuro‐QOL: brief measures of health‐related quality of life for clinical research in neurology. Neurology. 2012;78:1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hermann DM, Bassetti CL. Sleep‐related breathing and sleep‐wake disturbances in ischemic stroke. Neurology. 2009;73:1313–1322. [DOI] [PubMed] [Google Scholar]
- 19. Craig BM, Reeve BB, Brown PM, Cella D, Hays RD, Lipscomb J, Simon Pickard A, Revicki DA. US valuation of health outcomes measured using the PROMIS‐29. Value Health. 2014;17:846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choi SW, Schalet B, Cook KF, Cella D. Establishing a common metric for depressive symptoms: linking the BDI‐II, CES‐D, and PHQ‐9 to PROMIS depression. Psychol Assess. 2014;26:513–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, Rorick M, Moomaw CJ, Walker M. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. [DOI] [PubMed] [Google Scholar]
- 23. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 24. Quinn TJ, Dawson J, Walters MR, Lees KR. Functional outcome measures in contemporary stroke trials. Int J Stroke. 2009;4:200–205. [DOI] [PubMed] [Google Scholar]
- 25. Quinn TJ, Lees KR, Hardemark HG, Dawson J, Walters MR. Initial experience of a digital training resource for modified Rankin scale assessment in clinical trials. Stroke. 2007;38:2257–2261. [DOI] [PubMed] [Google Scholar]
- 26. Collins LM, Lanza ST. Latent Class and Latent Transition Analysis: With Applications in the Social Behavioral, and Health Sciences. Hoboken, NJ: Wiley; 2010. [Google Scholar]
- 27. Miaskowski C, Barsevick A, Berger A, Casagrande R, Grady PA, Jacobsen P, Kutner J, Patrick D, Zimmerman L, Xiao C, Matocha M, Marden S. Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst. 2017;109:djw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- 29. McLachlan GJ, Peel D. Finite Mixture Models. xxii ed New York, NY: Wiley; 2000. [Google Scholar]
- 30. Muthén LK, Muthén BO. Mplus User's Guide . 8th ed Los Angeles, CA: Muthen & Muthen; 1998‐2017. [Google Scholar]
- 31. Barclay‐Goddard R, Lix LM, Tate R, Weinberg L, Mayo NE. Response shift was identified over multiple occasions with a structural equation modeling framework. J Clin Epidemiol. 2009;62:1181–1188. [DOI] [PubMed] [Google Scholar]
- 32. Bushnell CD, Reeves MJ, Zhao X, Pan W, Prvu‐Bettger J, Zimmer L, Olson D, Peterson E. Sex differences in quality of life after ischemic stroke. Neurology. 2014;82:922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilson IB, Cleary PD. Linking clinical variables with health‐related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]
- 34. Barsevick AM, Whitmer K, Nail LM, Beck SL, Dudley WN. Symptom cluster research: conceptual, design, measurement, and analysis issues. J Pain Symptom Manage. 2006;31:85–95. [DOI] [PubMed] [Google Scholar]


