Abstract
Background
There are limited outcome studies of hypertension among young adults, especially using the new blood pressure (BP) categories from the American College of Cardiology and the American Heart Association. We examined associations between the new BP categories and the risk of incident cardiovascular disease (CVD) in low‐risk and young adults.
Methods and Results
A cohort study was performed in 244 837 Korean adults (mean age, 39.0 years; SD, 8.9 years) who underwent a comprehensive health examination at Kangbuk Samsung Hospital from January 1, 2011, to December 31, 2016; they were followed up for incident CVD via linkage to the Health Insurance and Review Agency database until the end of 2016, with a median follow‐up of 4.3 years. BP was categorized according to the new American College of Cardiology/American Heart Association (ACC/AHA) hypertension guidelines. During 924 420.7 person‐years, 1435 participants developed new‐onset CVD (incidence rate of 16.0 per 104 person‐years). The multivariable‐adjusted hazard ratios (95% CIs ) for CVD comparing elevated BP, stage 1 hypertension, stage 2 hypertension, treated and strictly controlled (systolic BP/diastolic BP <130/80 mm Hg with antihypertensive use), treated and controlled (systolic BP 130–139 and diastolic BP 80 to 89 mm Hg with antihypertensive use), treated uncontrolled, and untreated hypertension to normal BP were 1.37 (1.11–1.68), 1.45 (1.26–1.68), 2.12 (1.74–2.58), 1.41 (1.12–1.78), 1.97 (1.52–2.56), 2.29 (1.56–3.37) and 1.93 (1.53–2.45), respectively.
Conclusions
In this large cohort of low‐risk and young adults, all categories of higher BP were independently associated with an increased risk of CVD compared with normal BP, underscoring the importance of BP management even in these low‐risk populations.
Keywords: cardiovascular outcomes, cohort study, high blood pressure, hypertension, incidence, risk factor
Subject Categories: Epidemiology, Myocardial Infarction, High Blood Pressure, Hypertension
Clinical Perspective
What Is New?
In this cohort study of 244 837 young to middle‐aged adults, higher blood pressure (BP) categories were independently associated with an increased incidence of cardiovascular disease compared with normal BP and that association began in the elevated BP category, even in adults aged <40 years and in those with low risk.
What Are the Clinical Implications?
These findings suggest that stratification using the new BP guidelines may help identify individuals at high risk for cardiovascular disease, even in low‐risk and young adults.
Early surveillance and proper management of high BP are required to prevent short‐ or intermediate‐term cardiovascular disease events, even in low‐risk and young adults.
Introduction
High blood pressure (BP) is a major contributing factor to cardiovascular disease (CVD) and a leading cause of morbidity and mortality worldwide.1 Despite an increasing incidence of hypertension in the younger population and continuous efforts to improve prevention and management of hypertension, its awareness, treatment, and control are often worse in young adults compared with middle‐aged and older adults.2, 3, 4 Low rates of hypertension awareness and control in young populations are a primary concern as they translate directly into increase in CVD risk in later life.5 There are some studies on prognostic implications of hypertension on CVD events, where follow‐up of most studies starts after the age of 40 years (eg, the ARIC [Atherosclerosis Risk in Communities] Study involved individuals aged 45–64 years at baseline, and the MESA [Multi‐Ethnic Study of Atherosclerosis] involved individuals aged 45–84 years).6, 7 The FHS (Framingham Heart Study) and several meta‐analyses have demonstrated the relationship of BP as a continuous variable with CVD event in adults aged ≥30 years8, 9, 10; however, there are limited studies to evaluate the short‐ or intermediate‐term risk of CVD events in hypertensive young adults aged 20 to 39 years on the basis of the recently revised hypertension categories.
The 2017 American College of Cardiology/American Heart Association (ACC/AHA) Task Force on Clinical Practice Guidelines for BP lowered the threshold for the definition of hypertension to 130/80 mm Hg.11 Application of these guidelines will have direct implications on the estimates of the association of hypertension categories with CVD outcomes, but the relative prognostic implications of BP categories, on the basis of the revised hypertension definition in low‐risk young adults, is unknown. The objective of this study was, thus, to investigate the association of BP categories according to the 2017 ACC/AHA guidelines with the risk of incident CVD, considering antihypertensive treatment in a large cohort of young and middle‐aged men and women free of CVD at baseline.
Methods
All data and supporting materials have been provided with the published article.
Study Population
The Kangbuk Samsung Health Study is a cohort study of Korean men and women, aged ≥18 years, who underwent a comprehensive annual or biennial health examination at the Kangbuk Samsung Hospital Total Healthcare Centers in Seoul and Suwon, South Korea.12 Most examinees (>80%) are employees of various companies and local governmental organizations and their spouses. In South Korea, the Industrial Safety and Health Law requires annual or biennial health screening examinations of all employees, free of charge. Other examinees voluntarily purchased a health checkup at the center.
Our analysis was restricted to Kangbuk Samsung Health Study participants who underwent a comprehensive health examination from January 1, 2011, to December 31, 2016, and provided informed consent for linkage to the Health Insurance Review and Assessment Service database (n=263 532; Figure 1). In Korea, health care is organized under a mandatory single‐payer nationwide insurance system (National Health Insurance) that collects all information on medical services use covering the entire Korean population under a comprehensive database operated by the Health Insurance Review and Assessment Service.13
Figure 1.
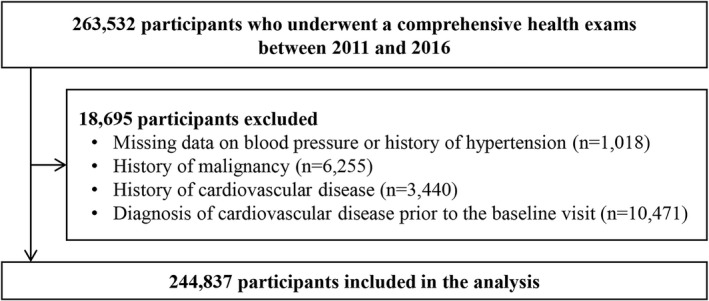
Flow diagram of the included participants.
We excluded participants with missing data on BP or history of hypertension (n=1018), with history of malignancy (n=6255), with history of CVD (n=3440), or with a diagnosis of CVD (n=10 471) at baseline. Because some participants met >1 exclusion criterion, the final sample size included in the analysis was 244 837 participants (mean [SD] age, 39.0 [8.9] years; interquartile range, 32.2–43.7 years; and young adults aged <40 years of 60.7 %).
Written informed consent was obtained from all participants. The study was approved by the Institutional Review Board of Kangbuk Samsung Hospital.
Measurements
Data on demographic characteristics, lifestyle factors, medical history, and family history of CVD were collected by standardized, self‐administered questionnaires.14 Smoking status was categorized as never, former, and current smoker. Alcohol intake was categorized as <20 and ≥20 g/d, as applied in previous studies.12, 15 Education level was categorized as less than college and college education or more. Physical activity was assessed using the validated Korean version of the International Physical Activity Questionnaire short form.16 Participants were classified as inactive, minimally active, and health‐enhancing physically active. Health‐enhancing physically active was defined as physical activity that meets either of 2 criteria: (1) vigorous‐intensity activity on ≥3 days per week, accumulating ≥1500 metabolic equivalent min/wk; or (2) 7 days of any combination of walking, moderate‐intensity activities, or vigorous‐intensity activities achieving at least 3000 metabolic equivalent min/wk.16 Usual dietary intake was assessed using a 103‐item, self‐administered food frequency questionnaire designed and validated for use in Korea.17 Daily intake of sodium was calculated by multiplying the frequency of consumption of each food by the portion size and sodium content of each food and summing across all relevant food items.18, 19
Height and weight were measured by trained nurses. Body mass index was calculated as weight (in kilograms) divided by height (in meters squared). BP was measured using an automated oscillometric device (53000; Welch Allyn, New York, NY) by trained nurses while participants were in a sitting position, with the arm supported at the heart level after a 5‐minute rest. We recoded 3 consecutive BP readings and used the average of the second and third readings in the analysis. BP levels were categorized according to the 2017 ACC/AHA hypertension guideline.11 Participants without a history of hypertension were categorized as normal BP (<120/80 mm Hg), elevated BP (120–129/<80 mm Hg), stage 1 hypertension (130–139/80–89 mm Hg), and stage 2 hypertension (≥140/90 mm Hg). Participants with a history of hypertension were categorized as treated and strictly controlled hypertension (<130/80 mm Hg on antihypertensive medication use), treated and controlled hypertension (130–139/80–89 mm Hg on antihypertensive medication use), treated but uncontrolled hypertension (≥140/90 mm Hg on antihypertensive medication use), and untreated hypertension (not using antihypertensive medications).
For CVD risk stratification, we calculated the Framingham risk score and the atherosclerotic CVD risk score on the basis of the Pooled Cohorts Equation.20, 21 Risk scores were considered low if <10% and high if ≥10%.11, 20, 22 The Charlson comorbidity index was calculated for the year before the baseline visit.23
Blood samples were drawn from the antecubital vein after at least 10 hours of fasting. Blood tests included total cholesterol, high‐density lipoprotein cholesterol, triglycerides, and fasting glucose.
Antihypertensive and Lipid‐Lowering Medications
Information on antihypertensive and lipid‐lowering medications was obtained through linkage to the Health Insurance Review and Assessment Service prescription database.13 Antihypertensive medications were classified into α‐adrenergic blockers, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, β‐adrenergic blockers, calcium channel blockers, and diuretics. For combination antihypertensive medications, each active component was counted separately. Statins included atorvastatin, rosuvastatin, simvastatin, pravastatin, lovastatin, fluvastatin, and pitavastatin.
Outcomes
The primary outcome was incident CVD, defined as the first hospitalization for CVD, including ischemic heart disease (International Classification of Diseases, Tenth Revision [ICD‐10], codes I20‐I25), stroke (ICD‐10 codes I60‐I64), and transient ischemic attack (ICD‐10 code G45), ascertained through linkage to the Health Insurance Review and Assessment Service database. As secondary outcomes, we also evaluated ischemic heart disease, myocardial infarction (ICD‐10 codes I21‐I24), stroke, ischemic stroke (ICD‐10 code I63), hemorrhagic stroke (ICD‐10 code I60‐I62), and transient ischemic attacks separately.24, 25
Statistical Analyses
Study participants were divided into 8 mutually exclusive categories: (1) normal BP; (2) elevated BP; (3) stage 1 hypertension; (4) stage 2 hypertension; (5) treated and strictly controlled hypertension; (6) treated and controlled hypertension; (7) treated but uncontrolled hypertension; and (8) untreated hypertension. Each participant was followed up from the baseline examination until the development of incident CVD or December 31, 2016, whichever came first. Hazard ratios and 95% CIs for CVD were estimated using Cox proportional hazards regression analysis. We assessed the proportional hazards assumption by examining graphs of estimated log (‐log) survival curves.
Cox models were initially adjusted for age and sex, and then further adjusted for study center (Seoul or Suwon), year of screening examination (1‐year categories), body mass index, smoking (never, past, current, or unknown), alcohol intake (0 g/d, <20 g/d, ≥20 g/d, or unknown), physical activity (inactive, minimally active, health‐enhancing physically active, or unknown), education level (less than college education, college education or more, or unknown), total calorie and sodium intake, history of diabetes mellitus, use of statins, and Charlson comorbidity index (model 1). Model 2 further adjusted for low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, and glucose (model 2).
We also examined the association between BP categories and CVD in subgroups defined by age (<40, 40‐<50, and ≥50 years), Framingham risk score (<10% and ≥10%), and atherosclerotic CVD risk score (<10% versus ≥10%). Interactions between BP categories and subgroup characteristics were tested using likelihood ratio tests comparing models with versus without multiplicative interaction terms.
All statistical analyses were conducted with Statistical Analysis Software (SAS) Enterprise Guide, Version 6.1 (SAS Institute, Inc, Cary, NC). P<0.05 was considered statistically significant.
Results
The average (SD) age of study participants was 39.0 (8.9) years, and 54.5% were men (Table 1). The prevalences of normal BP, elevated BP, stage 1 hypertension, stage 2 hypertension, treated and strictly controlled hypertension, treated and controlled hypertension, treated and uncontrolled hypertension, and untreated hypertension at baseline were 71.7%, 6.2%, 13.5%, 3.5%, 2.0%, 1.1%, 0.3%, and 1.7%, respectively. Higher BP levels were positively associated with older age, male sex, alcohol intake, diabetes mellitus, family history of CVD, and higher levels of total cholesterol, low‐density lipoprotein cholesterol, and triglycerides; and they were inversely associated with high‐density lipoprotein cholesterol. Participants with untreated hypertension were more likely to be younger and less likely to have diabetes mellitus than participants with treated hypertension.
Table 1.
Baseline Characteristics According to BP Category
| Characteristics | No History of Hypertension | History of Hypertension | ||||||
|---|---|---|---|---|---|---|---|---|
| SBP <120 mm Hg and DBP <80 mm Hg | SBP 120–129 mm Hg and DBP <80 mm Hg | SBP 130–139 mm Hg or DBP 80–89 mm Hg | SBP ≥140 mm Hg or DBP ≥90 mm Hg | SBP <130 mm Hg, DBP <80 mm Hg, and Medication | SBP 130–139 mm Hg, DBP 80–89 mm Hg, and Medication | SBP ≥140 mm Hg or DBP ≥90 mm Hg and Medication | No Medication | |
| No. | 175 458 | 15 172 | 33 086 | 8633 | 7589 | 7589 | 829 | 4070 |
| Age, y* | 37.7 (8.0) | 38.7 (9.4) | 40.8 (8.6) | 42.7 (8.8) | 51.5 (10.9) | 50.7 (9.9) | 50.6 (10.6) | 49.9 (9.9) |
| Men, % | 44.6 | 79.4 | 81.0 | 83.3 | 61.9 | 74.8 | 76.4 | 73.8 |
| Current smoker, % | 19.5 | 30.8 | 33.3 | 32.3 | 25.0 | 24.7 | 23.3 | 28.0 |
| Alcohol intake, %a | 19.0 | 33.8 | 39.0 | 45.9 | 32.5 | 40.7 | 42.4 | 41.1 |
| HEPA, % | 15.8 | 21.6 | 17.7 | 18.9 | 22.0 | 21.7 | 22.9 | 20.4 |
| High education level, %b | 80.0 | 79.5 | 78.6 | 75.7 | 60.2 | 63.4 | 64.1 | 67.3 |
| Diabetes mellitus, % | 4.2 | 6.7 | 8.2 | 12.2 | 31.2 | 29.7 | 34.8 | 27.7 |
| Family history of CVD, % | 10.6 | 10.7 | 12.9 | 14.1 | 20.8 | 20.4 | 21.2 | 20.8 |
| Statin medication, % | 0.9 | 1.3 | 1.4 | 1.2 | 27.0 | 23.9 | 19.2 | 20.2 |
| Obesity, %c | 19.9 | 45.3 | 45.5 | 57.0 | 46.4 | 56.2 | 58.7 | 57.1 |
| Body mass index, kg/m2* | 22.6 (3.0) | 24.9 (3.3) | 24.9 (3.3) | 25.8 (3.5) | 25.0 (3.3) | 25.7 (3.3) | 26.1 (3.7) | 25.8 (3.3) |
| Systolic BP, mm Hg* | 103.5 (8.6) | 123.0 (2.7) | 123.8 (7.3) | 138.5 (10.0) | 111.2 (9.0) | 125.1 (7.1) | 139.7 (10.4) | 119.5 (12.5) |
| Diastolic BP, mm Hg* | 65.8 (6.6) | 73.7 (4.3) | 82.5 (3.6) | 94.1 (7.2) | 70.3 (6.0) | 82.4 (4.2) | 92.0 (8.2) | 77.4 (9.5) |
| Total cholesterol, mg/dL* | 190.4 (33.1) | 200.8 (35.2) | 205.4 (34.9) | 210.5 (35.6) | 191.1 (36.0) | 197.0 (35.0) | 201.4 (34.5) | 195.6 (36.6) |
| LDL‐C, mg/dL* | 116.8 (31.1) | 128.7 (32.6) | 131.3 (32.5) | 135.1 (33.0) | 119.4 (32.7) | 123.6 (32.7) | 126.9 (32.1) | 123.5 (33.3) |
| HDL‐C, mg/dL* | 60.4 (15.2) | 55.1 (14.1) | 54.4 (14.2) | 53.4 (14.1) | 53.9 (14.0) | 53.2 (14.1) | 52.8 (14.1) | 52.8 (13.4) |
| Glucose, mg/dL* | 93.0 (12.0) | 97.6 (14.9) | 99.8 (16.8) | 103.9 (22.1) | 104.5 (21.4) | 107.8 (24.9) | 112.2 (27.7) | 106.4 (23.0) |
| Uric acid, mg/dL* | 5.0 (1.4) | 5.9 (1.4) | 5.9 (1.4) | 6.1 (1.5) | 5.5 (1.4) | 5.7 (1.5) | 5.8 (1.6) | 5.8 (1.5) |
| Triglycerides, mg/dL∥ | 81 (60–118) | 109 (77–158) | 123 (85–179) | 137 (95–199) | 113 (80.161) | 126 (88–182) | 136 (98–197) | 125 (90–180) |
| AST, U/L∥ | 18 (16–23) | 22 (18–27) | 22 (18–28) | 23 (19–30) | 23 (19–29) | 24 (19–30) | 24 (19–30) | 23 (19–30) |
| ALT, U/L∥ | 16 (12–23) | 23 (16–34) | 24 (17–36) | 26 (18–40) | 23 (16–34) | 25 (18–37) | 26 (18–38) | 25 (18–37) |
| GGT, U/L∥ | 17 (12–28) | 28 (18–47) | 32 (20–55) | 40 (24–69) | 28 (18–48) | 34 (22–59) | 38 (23–66) | 35 (22–59) |
| HOMA‐IR∥ | 1.10 (0.73–1.61) | 1.37 (0.90–2.05) | 1.44 (0.95–2.16) | 1.72 (1.13–2.58) | 1.52 (0.97–2.40) | 1.69 (1.10–2.55) | 1.91 (1.24–3.06) | 1.78 (1.13–2.74) |
| hsCRP, mg/L∥ | 0.4 (0.2–0.8) | 0.5 (0.3–1.1) | 0.6 (0.3–1.1) | 0.7 (0.4–1.4) | 0.6 (0.3–1.2) | 0.6 (0.4–1.3) | 0.7 (0.4–1.6) | 0.7 (0.4–1.3) |
| Total energy intake, kcal/d∥ , d | 1457.1 (1063.6–1863.8) | 1590.1 (1188.7–2018.7) | 1552.5 (1167.8–1966.8) | 1526.5 (1135.2–1924.6) | 1438.7 (1015.3–1820.0) | 1483.9 (1110.6–1871.6) | 1509.8 (1070.3–1899.7) | 1467.5 (1066.6–1857.5) |
| FRS >10 %, % | 3.5 | 10.1 | 16.2 | 30.7 | 42.7 | 57.5 | 67.3 | 34.5 |
| ASCVD risk >10%, %e | 0.9 | 3.4 | 4.5 | 9.3 | 15.9 | 20.3 | 28.0 | 13.4 |
| Charlson comorbidity index, % | ||||||||
| 1–2 | 8.8 | 8.4 | 8.6 | 8.5 | 12.5 | 12.3 | 10.7 | 12.3 |
| ≥3 | 3.5 | 4.0 | 4.4 | 4.3 | 21.4 | 16.4 | 19.7 | 14.0 |
ALT indicates alanine aminotransferase; ASCVD, atherosclerotic CVD; AST, aspartate aminotransferase; BP, blood pressure; CVD, cardiovascular disease; DBP, diastolic BP; FRS, Framingham risk score; GGT, gamma‐glutamyltransferase; HDL‐C, high‐density lipoprotein cholesterol; HEPA, health‐enhancing physically active; HOMA‐IR, homeostasis model assessment of insulin resistance; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic BP.
*Data are expressed as mean (SD).
≥20 g of ethanol per day.
College graduate or more.
Body mass index ≥25 kg/m2.
∥Data are expressed as median (interquartile range).
Among 199 592 participants with plausible estimated energy intake levels (within 3 SDs from the log‐transformed mean energy intake).
ASCVD risk based on the Pooled Cohorts Equation.
During 924 420.7 person‐years of follow‐up (median follow‐up, 4.3 years; interquartile range, 2.6–5.1 years), we observed 1435 incident cases of CVD (incidence rate, 16.0 per 10 000 person‐years; Table 2, Figure 2). In the fully adjusted model, the multivariable‐adjusted hazard ratios (95% CIs) for CVD comparing elevated BP, stage 1 hypertension, stage 2 hypertension, treated and strictly controlled hypertension, treated and controlled hypertension, treated and uncontrolled hypertension, and untreated hypertension to normal BP (reference) were 1.37 (1.11–1.68), 1.45 (1.26–1.68), 2.12 (1.74–2.58), 1.41 (1.12–1.78), 1.97 (1.52–2.56), 2.29 (1.56–3.37), and 1.93 (1.53–2.45), respectively.
Table 2.
Development of CVD by BP Category
| BP Categories | Person‐Years | Incident Cases | Incidence Density, per 10 000 Person‐Years | Age‐Sex Adjusted HR (95% CI) | Multivariable‐Adjusted HRa | |
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| Cardiovascular disease | ||||||
| No history of hypertension | ||||||
| SBP <120 mm Hg and DBP <80 mm Hg | 651 936.3 | 607 | 9.3 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| SBP 120–129 mm Hg and DBP <80 mm Hg | 58 157.1 | 108 | 18.6 | 1.47 (1.20–1.81) | 1.40 (1.14–1.72) | 1.37 (1.11–1.68) |
| SBP 130–139 mm Hg or DBP 80–89 mm Hg | 133 422.8 | 298 | 22.3 | 1.61 (1.40–1.85) | 1.52 (1.31–1.75) | 1.45 (1.26–1.68) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 33 205.7 | 132 | 39.8 | 2.42 (2.00–2.93) | 2.21 (1.82–2.69) | 2.12 (1.74–2.58) |
| History of hypertension | ||||||
| With antihypertensive medication | ||||||
| SBP <130 mm Hg and DBP <80 mm Hg | 19 210.0 | 100 | 52.1 | 1.75 (1.40–2.18) | 1.36 (1.08–1.72) | 1.41 (1.12–1.78) |
| SBP 130–139 mm Hg and DBP 80–89 mm Hg | 10 429.4 | 71 | 68.1 | 2.42 (1.88–3.12) | 1.93 (1.48–2.51) | 1.97 (1.52–2.56) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 3336.2 | 29 | 86.9 | 2.82 (1.93–4.12) | 2.27 (1.54–3.34) | 2.29 (1.55–3.37) |
| Without antihypertensive medication | 14 723.2 | 90 | 61.1 | 2.34 (1.86–2.94) | 1.87 (1.47–2.37) | 1.93 (1.53–2.45) |
| Ischemic heart disease | ||||||
| No history of hypertension | ||||||
| SBP <120 mm Hg and DBP <80 mm Hg | 652 351.4 | 387 | 5.9 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| SBP 120–129 mm Hg and DBP <80 mm Hg | 58 235.4 | 69 | 11.8 | 1.38 (1.07–1.79) | 1.28 (0.99–1.66) | 1.26 (0.97–1.63) |
| SBP 130–139 mm Hg or DBP 80–89 mm Hg | 133 621.9 | 198 | 14.8 | 1.54 (1.29–1.83) | 1.40 (1.17–1.68) | 1.36 (1.14–1.62) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 33 306.9 | 77 | 23.1 | 2.01 (1.57–2.58) | 1.79 (1.39–2.30) | 1.74 (1.35–2.24) |
| History of hypertension | ||||||
| With antihypertensive medication | ||||||
| SBP <130 mm Hg and DBP <80 mm Hg | 19 265.6 | 72 | 37.4 | 1.88 (1.44–2.45) | 1.38 (1.04–1.82) | 1.44 (1.09–1.91) |
| SBP 130–139 mm Hg and DBP 80–89 mm Hg | 10 459.2 | 53 | 50.7 | 2.61 (1.94–3.51) | 1.93 (1.42–2.63) | 2.00 (1.47–2.72) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 3365.8 | 17 | 50.5 | 2.32 (1.42–3.81) | 1.75 (1.06–2.89) | 1.78 (1.08–2.95) |
| Without antihypertensive medication | 14 761.7 | 68 | 46.1 | 2.57 (1.96–3.36) | 1.92 (1.46–2.54) | 2.02 (1.53–2.66) |
| Stroke or TIA | ||||||
| No history of hypertension | ||||||
| SBP <120 mm Hg and DBP <80 mm Hg | 652 649.2 | 223 | 3.4 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| SBP 120–129 mm Hg and DBP <80 mm Hg | 58 295.7 | 39 | 6.7 | 1.65 (1.17–2.32) | 1.64 (1.16–2.33) | 1.60 (1.13–2.27) |
| SBP 130–139 mm Hg or DBP 80–89 mm Hg | 133 816.3 | 102 | 7.6 | 1.78 (1.39–2.26) | 1.78 (1.39–2.28) | 1.69 (1.31–2.17) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 33 338.8 | 56 | 16.8 | 3.34 (2.47–4.52) | 3.20 (2.35–4.36) | 3.02 (2.21–4.13) |
| History of hypertension | ||||||
| With antihypertensive medication | ||||||
| SBP <130 mm Hg and DBP <80 mm Hg | 19 342.3 | 31 | 16.0 | 1.60 (1.08–2.38) | 1.42 (0.94–2.15) | 1.45 (0.96–2.20) |
| SBP 130–139 mm Hg and DBP 80–89 mm Hg | 10 524.9 | 19 | 18.1 | 2.03 (1.25–3.29) | 1.89 (1.15–3.10) | 1.88 (1.15–3.09) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 3385.7 | 12 | 35.4 | 3.71 (2.04–6.75) | 3.43 (1.86–6.30) | 3.39 (1.84–6.23) |
| Without antihypertensive medication | 14 866.9 | 22 | 14.8 | 1.78 (1.13–2.79) | 1.62 (1.02–2.57) | 1.64 (1.03–2.60) |
BP indicates blood pressure; CVD, cardiovascular disease; DBP, diastolic BP; HR, hazard ratio; SBP, systolic BP; TIA, transient ischemic attack.
Estimated from Cox proportional hazard model. Multivariable model 1 was adjusted for age, sex, center, year of screening examination, body mass index, smoking status, alcohol intake, physical activity, educational level, total calorie intake, history of diabetes mellitus, statin medication, Charlson comorbidity index, and sodium intake; model 2, model 1 plus adjustment for low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglyceride, and glucose.
Figure 2.
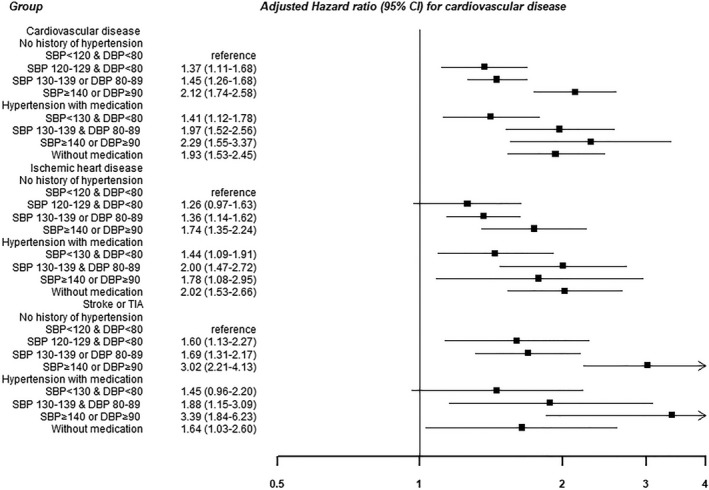
Development of cardiovascular disease by blood pressure (BP) category. Estimated from Cox proportional hazard model. Multivariable model was adjusted for age, sex, center, year of screening examination, body mass index, smoking status, alcohol intake, physical activity, educational level, total calorie intake, history of diabetes mellitus, statin medication, Charlson comorbidity index, sodium intake, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglyceride, and glucose. DBP indicates diastolic BP; SBP, systolic BP; TIA, transient ischemic attack.
The fully adjusted hazard ratios (95% CIs) for stroke or transient ischemic attack comparing elevated BP, stage 1 hypertension, stage 2 hypertension, treated and strictly controlled hypertension, treated and controlled hypertension, treated and uncontrolled hypertension, and untreated hypertension to normal BP (reference) were 1.60 (1.13–2.27), 1.69 (1.31–2.17), 3.02 (2.21–4.13), 1.45 (0.96–2.20), 1.88 (1.15–3.09), 3.39 (1.84–6.23), and 1.64 (1.03–2.60), respectively. A graded, progressive association between BP categories was also observed for individual outcomes (Table S1). The associations were particularly strong for cerebrovascular outcomes.
The absolute incidence rates of CVD were lower in younger participants (incidence rates of 5.5, 20.2, and 54.4 for those aged <40, 40‐<50, and ≥50 years, respectively), but the relative association between BP categories and CVD outcomes was stronger in younger compared with older participants (P=0.006 for interaction; Table 3, Figure 3). Notably, among young adults aged <40 years, the highest risk of CVD was observed in those with a history of hypertension but without antihypertensive medication.
Table 3.
Association Between BP Category and the Development of CVD by Different Age Strata
| BP Categories | Person‐Years | Incident Cases | Incidence Density, per 104 Person‐Years | Age‐Sex Adjusted HR (95% CI) | Multivariable‐Adjusted HRa | |
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| Aged <40 y | ||||||
| No history of hypertension | ||||||
| SBP <120 mm Hg and DBP <80 mm Hg | 414 594.3 | 159 | 3.8 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| SBP 120–129 mm Hg and DBP <80 mm Hg | 34 293.1 | 32 | 9.3 | 1.79 (1.21–2.65) | 1.71 (1.15–2.55) | 1.70 (1.14–2.53) |
| SBP 130–139 mm Hg or DBP 80–89 mm Hg | 64 792.9 | 56 | 8.6 | 1.52 (1.11–2.09) | 1.47 (1.06–2.04) | 1.43 (1.03–1.99) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 12 748.4 | 29 | 22.7 | 3.77 (2.51–5.67) | 3.21 (2.11–4.90) | 3.15 (2.06–4.82) |
| History of hypertension | ||||||
| With antihypertensive medication | ||||||
| SBP <130 mm Hg and DBP <80 mm Hg | 3023.2 | 4 | 13.2 | 2.35 (0.87–6.37) | 1.34 (0.47–3.81) | 1.39 (0.49–3.94) |
| SBP 130–139 mm Hg and DBP 80–89 mm Hg | 1415.9 | 2 | 14.1 | 2.04 (0.50–8.27) | 1.33 (0.32–5.55) | 1.37 (0.33–5.72) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 496.4 | 0 | <0.001 | … | … | … |
| Without antihypertensive medication | 2385.0 | 10 | 41.9 | 6.48 (3.39–12.39) | 3.93 (1.97–7.85) | 4.03 (2.03–8.01) |
| 40 y ≤ Age < 50 y | ||||||
| No history of hypertension | ||||||
| SBP <120 mm Hg and DBP <80 mm Hg | 185 467.7 | 250 | 13.5 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| SBP 120–129 mm Hg and DBP <80 mm Hg | 17 345.8 | 42 | 24.2 | 1.52 (1.09–2.11) | 1.56 (1.12–2.17) | 1.53 (1.09–2.13) |
| SBP 130–139 mm Hg or DBP 80–89 mm Hg | 51 596.8 | 154 | 29.8 | 1.78 (1.45–2.19) | 1.75 (1.41–2.16) | 1.71 (1.38–2.12) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 14 663.8 | 61 | 41.6 | 2.40 (1.80–3.18) | 2.30 (1.72–3.08) | 2.27 (1.70–3.05) |
| History of hypertension | ||||||
| With antihypertensive medication | ||||||
| SBP <130 mm Hg and DBP <80 mm Hg | 6501.8 | 20 | 30.8 | 1.71 (1.08–2.70) | 1.26 (0.78–2.04) | 1.33 (0.82–2.15) |
| SBP 130–139 mm Hg and DBP 80–89 mm Hg | 4383.0 | 20 | 45.6 | 2.32 (1.47–3.68) | 1.87 (1.16–3.02) | 1.99 (1.23–3.20) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 1418.3 | 6 | 42.3 | 2.22 (0.99–5.00) | 1.82 (0.80–4.13) | 1.91 (0.84–4.35) |
| Without antihypertensive medication | 6149.3 | 29 | 47.2 | 2.55 (1.73–3.76) | 1.98 (1.32–2.97) | 2.12 (1.42–3.19) |
| Aged ≥50 y | ||||||
| No history of hypertension | ||||||
| SBP <120 mm Hg and DBP <80 mm Hg | 51 874.4 | 198 | 38.2 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| SBP 120–129 mm Hg and DBP <80 mm Hg | 6518.2 | 34 | 52.2 | 1.18 (0.82–1.70) | 1.16 (0.81–1.68) | 1.15 (0.79–1.66) |
| SBP 130–139 mm Hg or DBP 80–89 | 17 033.1 | 88 | 51.7 | 1.23 (0.95–1.58) | 1.18 (0.91–1.53) | 1.15 (0.89–1.49) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 5793.4 | 42 | 72.5 | 1.66 (1.19–2.33) | 1.60 (1.14–2.24) | 1.54 (1.10–2.17) |
| History of hypertension | ||||||
| With antihypertensive medication | ||||||
| SBP <130 mm Hg and DBP <80 mm Hg | 9685.1 | 76 | 78.5 | 1.61 (1.22–2.11) | 1.38 (1.04–1.84) | 1.40 (1.05–1.86) |
| SBP 130–139 mm Hg and DBP 80–89 mm Hg | 4630.5 | 49 | 105.8 | 2.21 (1.61–3.03) | 1.90 (1.37–2.64) | 1.90 (1.37–2.65) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 1421.5 | 23 | 161.8 | 3.06 (1.98–4.75) | 2.59 (1.65–4.06) | 2.53 (1.61–3.97) |
| Without antihypertensive medication | 6188.9 | 51 | 82.4 | 1.76 (1.29–2.40) | 1.52 (1.11–2.10) | 1.54 (1.12–2.12) |
P=0.006 for the overall interaction between age and BP categories for development of CVD all event (adjusted model). Incidence density (per 10 000 person‐years): 5.5 for subjects with age <40 years, 20.2 for subjects with 40 years ≤ age <50 years, and 54.4 for subjects with age ≥50 years. BP indicates blood pressure; CVD, cardiovascular disease; DBP, diastolic BP; HR, hazard ratio; SBP, systolic BP.
Estimated from Cox proportional hazard model. Multivariable model 1 was adjusted for age, sex, center, year of screening examination, body mass index, smoking status, alcohol intake, physical activity, educational level, total calorie intake, history of diabetes mellitus, statin medication, Charlson comorbidity index, and sodium intake; model 2, model 1 plus adjustment for low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglyceride, and glucose.
Figure 3.
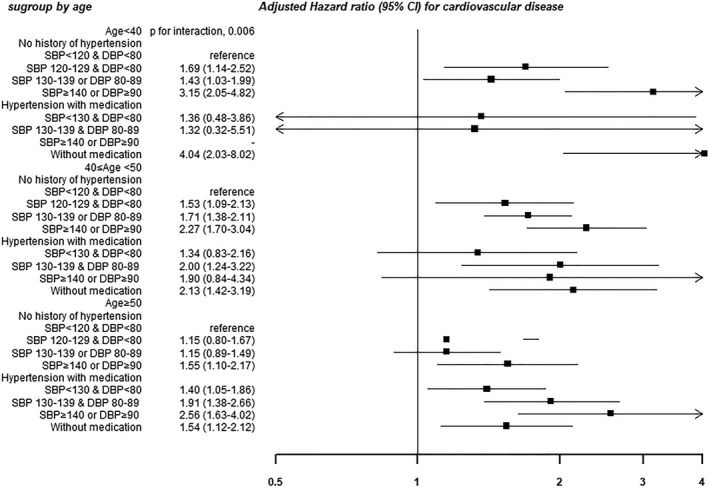
Association between blood pressure (BP) category and the development of cardiovascular disease in subgroup by age. Estimated from Cox proportional hazard model. Multivariable model was adjusted for age, sex, center, year of screening examination, body mass index, smoking status, alcohol intake, physical activity, educational level, total calorie intake, history of diabetes mellitus, statin medication, Charlson comorbidity index, sodium intake, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglyceride, and glucose. DBP indicates diastolic BP; SBP, systolic BP.
By risk score category, the absolute incidence rates of CVD were lower in participants with a Framingham risk score <10% than in those with a risk score ≥10% (10.1 versus 66.7 per 10 000 person‐years), but the association between BP categories and incident CVD was stronger in those with a Framingham risk score <10% (P<0.001 for interaction; Table 4). For atherosclerotic CVD risk categories, the absolute incidence rate of CVD was lower in those with <10% predicted CVD risk (12.8 versus 102.7 per 10 000 person‐years), but the association between BP categories and incidence did not significantly differ by risk category (P=0.21 for interaction).
Table 4.
Association Between BP Category and the Development of CVD by CVD Risk Stratification, on the Basis of Different Risk Scores
| BP Categories | ASCVD Risk Score, %a | Framingham Risk Score, %a | ||
|---|---|---|---|---|
| <10 | ≥10 | <10 | ≥10 | |
| No history of hypertension | ||||
| SBP <120 mm Hg and DBP <80 mm Hg | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| SBP 120–129 mm Hg and DBP <80 mm Hg | 1.47 (1.15–1.87) | 1.10 (0.62–1.95) | 1.53 (1.16–2.01) | 1.12 (0.77–1.65) |
| SBP 130–139 mm Hg or DBP 80–89 mm Hg | 1.59 (1.34–1.88) | 0.89 (0.57–1.38) | 1.74 (1.44–2.11) | 0.96 (0.73–1.26) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 2.16 (1.70–2.75) | 1.47 (0.92–2.37) | 1.91 (1.97–2.66) | 1.67 (1.24–2.25) |
| History of hypertension | ||||
| With antihypertensive medication | ||||
| SBP <130 mm Hg and DBP <80 mm Hg | 1.46 (1.08–1.97) | 1.06 (0.63–1.78) | 1.66 (1.13–2.44) | 1.03 (0.72–1.46) |
| SBP 130–139 mm Hg and DBP 80–89 mm Hg | 1.75 (1.21–2.52) | 1.81 (1.10–2.96) | 2.54 (1.53–4.22) | 1.44 (1.00–2.06) |
| SBP ≥140 mm Hg or DBP ≥90 mm Hg | 1.90 (1.01–3.59) | 1.41 (0.68–2.94) | 0.78 (0.11–5.53) | 1.60 (0.96–2.65) |
| Without antihypertensive medication | 1.95 (1.46–2.62) | 1.71 (1.03–2.83) | 1.85 (1.26–2.70) | 1.73 (1.22–2.44) |
P=0.208 for the overall interaction between ASCVD risk strata (<10% vs ≥10%) and BP categories for development of CVD all event (adjusted model); P<0.001 for the overall interaction between Framingham risk score strata (<10% vs ≥10%) and BP categories for development of CVD all event (adjusted model). Incidence density (per 10 000 person‐years): 12.8 for subjects with ASCVD risk <10% and 102.7 for subjects with ASCVD risk ≥10%; 10.1 for subjects with Framingham risk score <10% and 66.7 for subjects with Framingham risk score ≥10 %. ASCVD risk score was based on the Pooled Cohorts Equation. ASCVD indicates atherosclerotic CVD; BP, blood pressure; CVD, cardiovascular disease; DBP, diastolic BP; SBP, systolic BP.
Multivariable‐adjusted hazard ratios (95% CIs) were estimated from Cox proportional hazard model. Multivariable model 1 was adjusted for age, sex, center, year of screening examination, body mass index, smoking status, alcohol intake, physical activity, educational level, total calorie intake, history of diabetes mellitus, statin medication, Charlson comorbidity index, and sodium intake.
Discussion
In this large cohort study of relatively low‐risk, young and middle‐aged Korean adults, higher BP categories, based on the new BP guidelines, were significantly and progressively associated with an increased risk of developing CVD compared with the normal BP category. Although the absolute incidence of CVD was lower in younger participants, the association between the new BP categories and risk of CVD was stronger in individuals aged <40 years than in the older subjects, reaffirming that early surveillance and proper management of high BP are required to prevent short‐ or intermediate‐term CVD events, even in a young population.
To our knowledge, there is limited evidence of the prospective association of BP categories based on the new 2017 guidelines with the incidence risk of clinically manifest CVD in low‐risk and young adults. The rationale for this change is based on multiple individual studies and meta‐analyses of observational data, which have reported gradually and progressively higher CVD risk from normal BP to elevated BP and stage 1 hypertension.8, 9, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 According to previous meta‐analyses, prehypertension was associated with a greater risk of total CVD (relative risk, 1.44–1.55),26, 31 coronary heart disease (relative risk, 1.36–1.50),31, 34 and stroke (relative risk, 1.66–1.73)26, 27 compared with normal BP of <120/80 mm Hg, with higher CVD risk in high‐range prehypertension than in low‐range prehypertension. In contrast, studies in young adults are limited, with inconsistent findings.5, 44, 45, 46, 47, 48 A cohort study of 10 874 male employees, aged 18 to 39 years, showed that BP levels predicted increased 25‐year mortality for coronary heart disease, CVD, and all causes.46 A Swedish nationwide cohort study of >1.2 million military men (mean age, 18.4 years) showed that higher BP was associated with increased CVD mortality over a 24‐year follow‐up period, but no increased risk of CVD mortality was observed in elevated BP or stage 1 hypertension categories.44 These studies were restricted to male participants and lacked adjustment for important covariates, such as low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, glucose, alcohol intake, smoking, and family history of CVD. Furthermore, because of the use of different BP categories across studies, the prognostic implications of new BP categories remained unclear.
The relationship between new BP categories and short‐ or intermediate‐term CVD outcomes in young adults has been understudied because most studies have evaluated the association between young adult BP exposure and risk of CVD later in life after the age of 40 years.5, 44, 45, 46, 47, 48 Indeed, the absolute incidence of CVD events at this age is low, and studies in young adults require large sample sizes to observe sufficient CVD events compared with studies in middle‐aged and older populations. In the present large‐scale cohort, higher BP levels beginning at the elevated BP category were gradually and continuously associated with an increased risk of CVD during a median follow‐up of 4.3 years among young adults aged ≤40 years.
Recently, in the study of 5798 participants, aged 45 to 84 years, using data from the MESA, the results demonstrated that those with well‐controlled hypertension (<120/80 mm Hg) on antihypertensive medication still had twice the risk of incident CVD events in the next 9.5 years than participants with ideal BP levels without treatment.7 The authors suggested that antihypertensive treatment cannot restore CVD risk to ideal levels and emphasized primordial prevention of BP increases to further reduce CVD morbidity and mortality. Similarly, in our study, for a given BP category, the risk of CVD event tended to be higher in participants with antihypertensive medication than in those without a history of hypertension. However, in young adults, aged <40 years, with a history of hypertension, the risk of CVD event was much higher in participants without antihypertensive medication than those with antihypertensive medication. Our findings suggest that not only primordial prevention but also early detection and proper management are important in young adults, especially given that previous studies have reported a much lower prevalence of both awareness and control rate in young adults than in older subjects.2, 3, 5, 49, 50
In the current study, the association of new BP categories with risk of incident CVD was more evident in young adults aged <40 years than in the older subjects, which is in line with earlier studies that also reported a stronger association between BP and CVD outcomes in middle‐aged compared with elderly populations.8, 46, 51, 52, 53 The reasons for the stronger associations in young adults are unclear. The effect of BP on risk of CVD may be diluted with increasing age because both the prevalence of higher BP and other CVD risk factors also become more prevalent with age.
In South Korea, the Korean Society of Hypertension determined to use the previous hypertension criteria of systolic BP/diastolic BP ≥ 140/90 mm Hg because of a lack of clear evidence for additional benefit from lowering the hypertension threshold to 120/80 mm Hg for Koreans.54, 55 According to the Korean Hypertension Fact Sheet 2018, the number of people diagnosed with hypertension increased from 3 million in 2002 to 8.9 million in 2016, with only 5.7 million people with appropriate and persistent antihypertensive treatment in 2016.56 The treatment rate increased from 22% in 1998 to 59% in 2007 and to 61% in 2016, and the control rate increased from 5% in 1998 to 41% in 2007 and to 44% in 2016.56 In a recent study using the Korean National Health and Nutrition Examination Survey, the prevalence of hypertension and the number of adults who need antihypertensive treatment will be increased, being similar with other countries.55, 57, 58 According to recent studies addressing implementation of the new guidelines in multiple countries, including the United States, China, and Korea, the 2017 ACC/AHA hypertension guidelines will markedly increase the prevalence of hypertension and the number of patients who need antihypertensive treatment initiation and those who need treatment intensification globally.57, 58, 59, 60, 61, 62 In a recent study from the National Health and Nutrition Examination Survey, according to the 2017 ACC/AHA guideline, compared with the Seventh Joint National Committee guideline, the prevalence of hypertension has increased from 31.9% to 45.6%, the percentage of US adults recommended for antihypertensive medication has increased from 34.3% to 36.2%, and 53.4% of US adults taking antihypertensive medication need more intensive lowering of their BP.59 In case of China, adoption of the 2017 ACC/AHA hypertension guidelines would lead to the increment in the prevalence of hypertension from 25% to 50%.63 It should be evaluated if such changes in the diagnostic threshold and therapeutic targets from 140/90 to 130/80 mm Hg would improve BP control and its associated outcome. Future studies are also needed to confirm the association between BP and CVD risk in the young population with diverse ethnicity and to determine if the risk/benefit ratio for treatment is favorable in this low‐risk group.
The strengths of our study are its cohort study design, the large sample size, the use of carefully standardized clinical procedures, and the almost complete follow‐up for CVD events, as the National Health Insurance collects all medical services use covering the entire Korean population. This study also has several limitations. First, as with most previous studies, the determination of BP was based on a single‐day measurement, although 3 readings were taken. This approach may lead to a misclassification of BP categories and introduce dilution bias, possibly underestimating true associations. Second, we did not incorporate changes in BP categories and other covariates during follow‐up. Third, health behaviors were assessed via a self‐administered structured questionnaire used in health checkup programs in Korea, as part of the National Health Insurance plan. Measurement errors in these variables may introduce some degree of residual confounding, similar to most epidemiologic studies. Fourth, we used the Pooled Cohorts Equations in all participants; however, it was not validated in adults aged <40 years. Finally, this is an opportunistic cohort of individuals, who self‐presented for the health examination, and hence is not a representative sample of low cardiovascular risk young adults in the community. The study population of this cohort was relatively highly educated, young to middle‐aged Korean adults with high accessibility to healthcare resources. We compared our study population with a representative sample of the general Korean population (the Korea National Health and Nutrition Examination Survey). The age and sex standardization was performed using the direct method to the age structure of the Korean population, aged 20 to 80 years, in the year 2010. The age‐ and sex‐standardized prevalence of hypertension (defined as systolic BP ≥140 mm Hg, diastolic BP ≥90 mm Hg, or the use of antihypertensive medication), type 2 diabetes mellitus (defined as fasting serum glucose level ≥126 mg/dL or the use of blood glucose–lowering agents), obesity (body mass index ≥25 kg/m2), and current smoker was lower than those of the general population (16.6% versus 29.1%, 9.3% versus 10.5%, 27.6% versus 31.5%, and 16.8% versus 26.5%, respectively), indicating that our study population may be healthier than the general Korean population. Thus, our findings might not be generalizable to other ethnic groups or populations with different age, demographic, diet, and health behavior characteristics.
In conclusion, compared with the normal BP category, higher BP categories, based on new ACC/AHA guidelines, were positively associated with an increased risk of CVD, even in low‐risk, young adults. Our study suggests that stratification using the new BP guidelines can help identify young adults at high risk for adverse CVD outcomes. These findings also reiterate the importance of proper BP management for the primary prevention of CVD, even in low‐risk and young adults.
Sources of Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2018R1D1A1B07049620).
Disclosures
None.
Supporting information
Table S1. Development of Stroke Subtype by BP Category
(J Am Heart Assoc. 2019;8:e011946 DOI: 10.1161/JAHA.119.011946.)
Contributor Information
Seungho Ryu, Email: sh703.yoo@gmail.com.
Ki‐Chul Sung, Email: kcmd.sung@samsung.com.
References
- 1. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker‐Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan‐Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez‐Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez‐Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol. 2012;60:599–606. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Moran AE. Trends in the prevalence, awareness, treatment, and control of hypertension among young adults in the United States, 1999 to 2014. Hypertension. 2017;70:736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 5. De Venecia T, Lu M, Figueredo VM. Hypertension in young adults. Postgrad Med. 2016;128:201–207. [DOI] [PubMed] [Google Scholar]
- 6. Kshirsagar AV, Carpenter M, Bang H, Wyatt SB, Colindres RE. Blood pressure usually considered normal is associated with an elevated risk of cardiovascular disease. Am J Med. 2006;119:133–141. [DOI] [PubMed] [Google Scholar]
- 7. Liu K, Colangelo LA, Daviglus ML, Goff DC, Pletcher M, Schreiner PJ, Sibley CT, Burke GL, Post WS, Michos ED, Lloyd‐Jones DM. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels? The Coronary Artery Risk Development in Young Adults (CARDIA) study and the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Heart Assoc. 2015;4:e002275 DOI: 10.1161/JAHA.115.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 9. Rapsomaniki E, Timmis A, George J, Pujades‐Rodriguez M, Shah AD, Denaxas S, White IR, Caulfield MJ, Deanfield JE, Smeeth L, Williams B, Hingorani A, Hemingway H. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 11. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 12. Chang Y, Ryu S, Choi Y, Zhang Y, Cho J, Kwon M‐J, Hyun YY, Lee K‐B, Kim H, Jung H‐S. Metabolically healthy obesity and development of chronic kidney disease: a cohort study. Ann Intern Med. 2016;164:305–312. [DOI] [PubMed] [Google Scholar]
- 13. Kwon S. Payment system reform for health care providers in Korea. Health Policy Plan. 2003;18:84–92. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Chang Y, Ryu S, Cho J, Lee WY, Rhee EJ, Kwon MJ, Pastor‐Barriuso R, Rampal S, Han WK, Shin H, Guallar E. Thyroid hormone levels and incident chronic kidney disease in euthyroid individuals: the Kangbuk Samsung Health Study. Int J Epidemiol. 2014;43:1624–1632. [DOI] [PubMed] [Google Scholar]
- 15. Chang Y, Kim B‐K, Yun KE, Cho J, Zhang Y, Rampal S, Zhao D, Jung H‐S, Choi Y, Ahn J. Metabolically‐healthy obesity and coronary artery calcification. J Am Coll Cardiol. 2014;63:2679–2686. [DOI] [PubMed] [Google Scholar]
- 16. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 17. Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, Park C, Kim DH. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. 2007;61:1435–1441. [DOI] [PubMed] [Google Scholar]
- 18. Kim HJ, Paik HY, Lee SY, Shim JE, Kim YS. Salt usage behaviors are related to urinary sodium excretion in normotensive Korean adults. Asia Pac J Clin Nutr. 2007;16:122–128. [PubMed] [Google Scholar]
- 19. Kim K, Chang Y, Ahn J, Yang HJ, Jung JY, Kim S, Sohn CI, Ryu S. Smoking and urinary cotinine levels are predictors of increased risk for gastric intestinal metaplasia. Cancer Res. 2019;79:676–684. [DOI] [PubMed] [Google Scholar]
- 20. D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P; CHD Risk Prediction Group . Validation of the framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. [DOI] [PubMed] [Google Scholar]
- 21. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 22. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 23. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 24. Song YM, Cho HJ. Risk of stroke and myocardial infarction after reduction or cessation of cigarette smoking: a cohort study in Korean men. Stroke. 2008;39:2432–2438. [DOI] [PubMed] [Google Scholar]
- 25. Kim K, Park SM, Lee K. Weight gain after smoking cessation does not modify its protective effect on myocardial infarction and stroke: evidence from a cohort study of men. Eur Heart J. 2018;39:1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo X, Zhang X, Guo L, Li Z, Zheng L, Yu S, Yang H, Zhou X, Zhang X, Sun Z, Li J, Sun Y. Association between pre‐hypertension and cardiovascular outcomes: a systematic review and meta‐analysis of prospective studies. Curr Hypertens Rep. 2013;15:703–716. [DOI] [PubMed] [Google Scholar]
- 27. Huang Y, Cai X, Li Y, Su L, Mai W, Wang S, Hu Y, Wu Y, Xu D. Prehypertension and the risk of stroke: a meta‐analysis. Neurology. 2014;82:1153–1161. [DOI] [PubMed] [Google Scholar]
- 28. Huang Y, Cai X, Liu C, Zhu D, Hua J, Hu Y, Peng J, Xu D. Prehypertension and the risk of coronary heart disease in Asian and Western populations: a meta‐analysis. J Am Heart Assoc. 2015;4:e001519 DOI: 10.1161/JAHA.114.001519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang Y, Cai X, Zhang J, Mai W, Wang S, Hu Y, Ren H, Xu D. Prehypertension and incidence of ESRD: a systematic review and meta‐analysis. Am J Kidney Dis. 2014;63:76–83. [DOI] [PubMed] [Google Scholar]
- 30. Huang Y, Su L, Cai X, Mai W, Wang S, Hu Y, Wu Y, Tang H, Xu D. Association of all‐cause and cardiovascular mortality with prehypertension: a meta‐analysis. Am Heart J. 2014;167:160–168.e1. [DOI] [PubMed] [Google Scholar]
- 31. Huang Y, Wang S, Cai X, Mai W, Hu Y, Tang H, Xu D. Prehypertension and incidence of cardiovascular disease: a meta‐analysis. BMC Med. 2013;11:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee M, Saver JL, Chang B, Chang KH, Hao Q, Ovbiagele B. Presence of baseline prehypertension and risk of incident stroke: a meta‐analysis. Neurology. 2011;77:1330–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang S, Wu H, Zhang Q, Xu J, Fan Y. Impact of baseline prehypertension on cardiovascular events and all‐cause mortality in the general population: a meta‐analysis of prospective cohort studies. Int J Cardiol. 2013;168:4857–4860. [DOI] [PubMed] [Google Scholar]
- 34. Shen L, Ma H, Xiang MX, Wang JA. Meta‐analysis of cohort studies of baseline prehypertension and risk of coronary heart disease. Am J Cardiol. 2013;112:266–271. [DOI] [PubMed] [Google Scholar]
- 35. Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, Woodward M, MacMahon S, Turnbull F, Hillis GS, Chalmers J, Mant J, Salam A, Rahimi K, Perkovic V, Rodgers A. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta‐analysis. Lancet. 2016;387:435–443. [DOI] [PubMed] [Google Scholar]
- 36. Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta‐analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. [DOI] [PubMed] [Google Scholar]
- 37. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 2: effects at different baseline and achieved blood pressure levels–overview and meta‐analyses of randomized trials. J Hypertens. 2014;32:2296–2304. [DOI] [PubMed] [Google Scholar]
- 38. Sundstrom J, Arima H, Jackson R, Turnbull F, Rahimi K, Chalmers J, Woodward M, Neal B. Effects of blood pressure reduction in mild hypertension: a systematic review and meta‐analysis. Ann Intern Med. 2015;162:184–191. [DOI] [PubMed] [Google Scholar]
- 39. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo X, Zhang X, Zheng L, Guo L, Li Z, Yu S, Yang H, Zhou X, Zou L, Zhang X, Sun Z, Li J, Sun Y. Prehypertension is not associated with all‐cause mortality: a systematic review and meta‐analysis of prospective studies. PLoS One. 2013;8:e61796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet. 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 42. Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe‐Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (life): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 43. Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, Black HR, Hamilton BP, Holland J, Nwachuku C, Papademetriou V, Probstfield J, Wright JT Jr, Alderman MH, Weiss RJ, Piller L, Bettencourt J, Walsh SM. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4:393–404. [DOI] [PubMed] [Google Scholar]
- 44. Sundstrom J, Neovius M, Tynelius P, Rasmussen F. Association of blood pressure in late adolescence with subsequent mortality: cohort study of Swedish male conscripts. BMJ. 2011;342:d643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McCarron P, Smith GD, Okasha M, McEwen J. Blood pressure in young adulthood and mortality from cardiovascular disease. Lancet. 2000;355:1430–1431. [DOI] [PubMed] [Google Scholar]
- 46. Miura K, Daviglus ML, Dyer AR, Liu K, Garside DB, Stamler J, Greenland P. Relationship of blood pressure to 25‐year mortality due to coronary heart disease, cardiovascular diseases, and all causes in young adult men: the Chicago Heart Association Detection Project in Industry. Arch Intern Med. 2001;161:1501–1508. [DOI] [PubMed] [Google Scholar]
- 47. Pletcher MJ, Vittinghoff E, Thanataveerat A, Bibbins‐Domingo K, Moran AE. Young adult exposure to cardiovascular risk factors and risk of events later in life: the Framingham offspring study. PLoS One. 2016;11:e0154288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gray L, Lee IM, Sesso HD, Batty GD. Blood pressure in early adulthood, hypertension in middle age, and future cardiovascular disease mortality: HAHS (Harvard Alumni Health Study). J Am Coll Cardiol. 2011;58:2396–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johnson HM, Thorpe CT, Bartels CM, Schumacher JR, Palta M, Pandhi N, Sheehy AM, Smith MA. Undiagnosed hypertension among young adults with regular primary care use. J Hypertens. 2014;32:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Johnson HM, Thorpe CT, Bartels CM, Schumacher JR, Palta M, Pandhi N, Sheehy AM, Smith MA. Antihypertensive medication initiation among young adults with regular primary care use. J Gen Intern Med. 2014;29:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takashima N, Ohkubo T, Miura K, Okamura T, Murakami Y, Fujiyoshi A, Nagasawa SY, Kadota A, Kita Y, Miyagawa N, Hisamatsu T, Hayakawa T, Okayama A, Ueshima H. Long‐term risk of BP values above normal for cardiovascular mortality: a 24‐year observation of Japanese aged 30 to 92 years. J Hypertens. 2012;30:2299–2306. [DOI] [PubMed] [Google Scholar]
- 52. Hadaegh F, Mohebi R, Khalili D, Hasheminia M, Sheikholeslami F, Azizi F. High normal blood pressure is an independent risk factor for cardiovascular disease among middle‐aged but not in elderly populations: 9‐year results of a population‐based study. J Hum Hypertens. 2013;27:18–23. [DOI] [PubMed] [Google Scholar]
- 53. Gu D, Chen J, Wu X, Duan X, Jones DW, Huang JF, Chen CS, Chen JC, Kelly TN, Whelton PK, He J. Prehypertension and risk of cardiovascular disease in Chinese adults. J Hypertens. 2009;27:721–729. [DOI] [PubMed] [Google Scholar]
- 54. Lee H‐Y. New definition for hypertension. J Korean Med Assoc. 2018;61:485‐492. [Google Scholar]
- 55. Boo S, Yoon YJ, Oh H. Evaluating the prevalence, awareness, and control of hypertension, diabetes, and dyslipidemia in Korea using the NHIS‐NSC database: a cross‐sectional analysis. Medicine. 2018;97:e13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Korean Society Hypertension (KSH); Hypertension Epidemiology Research Working Group , Kim HC, Cho MC. Korea hypertension fact sheet. Clin Hypertens. 2018;24:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee JH, Kim SH, Kang SH, Cho JH, Cho Y, Oh IY, Yoon CH, Lee HY, Youn TJ, Chae IH, Kim CH. Blood pressure control and cardiovascular outcomes: real‐world implications of the 2017 ACC/AHA hypertension guideline. Sci Rep. 2018;8:13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Khera R, Lu Y, Lu J, Saxena A, Nasir K, Jiang L, Krumholz HM. Impact of 2017 ACC/AHA guidelines on prevalence of hypertension and eligibility for antihypertensive treatment in United States and china: nationally representative cross sectional study. BMJ. 2018;362:k2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential U.S. population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol. 2018;71:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kibria GMA, Swasey K, Kc A, Mirbolouk M, Sakib MN, Sharmeen A, Chadni MJ, Stafford KA. Estimated change in prevalence of hypertension in Nepal following application of the 2017 ACC/AHA guideline. JAMA Netw Open. 2018;1:e180606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Islam JY, Zaman MM, Haq SA, Ahmed S, Al‐Quadir Z. Epidemiology of hypertension among Bangladeshi adults using the 2017 ACC/AHA Hypertension Clinical Practice Guidelines and Joint National Committee 7 Guidelines. J Hum Hypertens. 2018;32:668–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Colantonio LD, Booth JN III, Bress AP, Whelton PK, Shimbo D, Levitan EB, Howard G, Safford MM, Muntner P. 2017 ACC/AHA blood pressure treatment guideline recommendations and cardiovascular risk. J Am Coll Cardiol. 2018;72:1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang JG, Liu L. Global impact of 2017 American College of Cardiology/American Heart Association Hypertension Guidelines: a perspective from China. Circulation. 2018;137:546–548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Development of Stroke Subtype by BP Category


