Abstract
Background
Beyond the controlled setting of trials, scarce information exists on the burden, predictors, and outcomes associated with elevated hsCRP (high‐sensitivity C‐reactive protein) in “real‐world” patients with myocardial infarction (MI).
Methods and Results
We included all‐coming MI survivors undergoing hsCRP testing >30 days after an MI during routine health care in Stockholm, Sweden (2006–2011). hsCRP tests measured during hospitalization/emergency department visits, followed by antibiotics or indicative of acute illness, were excluded, together with patients with ongoing/recent cancer, chronic infections, or immunosuppression. Inflammation was defined over a 3‐month baseline window and associated with subsequent death and major adverse cardiovascular events (composite of MI, ischemic stroke, or cardiovascular death). Included were 17 464 patients (63% men; mean age, 72.6 years) with a median hsCRP level of 2.2 (interquartile range, 1.0–6.0) mg/L and a median of 2.2 (interquartile range, 0.8–4.9) years since their MI. Most (66%) had hsCRP ≥2 mg/L, and 40% had hsCRP >3 mg/L. Lower hemoglobin, lower estimated glomerular filtration rate, and comorbidities (eg, heart failure, peripheral vascular disease, stroke, atrial fibrillation, diabetes mellitus, and rheumatoid diseases) were associated with higher odds of hsCRP ≥2 mg/L. Conversely, previous percutaneous coronary intervention, ongoing renin‐angiotensin blockade, and statins were associated with lower hsCRP ≥2 mg/L odds. Patients with hsCRP ≥2 mg/L were at higher risk of major adverse cardiovascular events (n=3900; adjusted hazard ratio, 1.28; 95% CI, 1.18–1.38) and death (n=4138; adjusted hazard ratio, 1.42; 95% CI, 1.31–1.53). Results were robust across subgroups of patients and after exclusion of events occurring during the first 6 to 12 months. On a continuous scale, the association between hsCRP and outcomes was linear until hsCRP >5 mg/L, plateauing thereafter.
Conclusions
Most patients with MI exhibit elevated hsCRP levels. Besides identifying populations at high‐inflammatory risk, this study extends the prognostic validity of this biomarker from trial evidence to real‐world healthcare settings.
Keywords: acute coronary syndrome, inflammation, monitoring, outcome
Subject Categories: Cardiovascular Disease, Secondary Prevention, Risk Factors
Clinical Perspective
What Is New?
hsCRP (high‐sensitivity C‐reactive protein), a marker of systemic inflammation, is clinically elevated in most healthcare‐managed patients with myocardial infarction.
hsCRP is associated with subsequent risk of major adverse cardiovascular events and death, with associations being linear for hsCRP ranging between 1 and 5 mg/L and plateauing thereafter to a sustained increased risk.
What Are the Clinical Implications?
Our results suggest that healthcare providers monitor and pay attention to the information on hsCRP in patients with myocardial infarction.
Our results identify patient groups who may benefit from more aggressive guideline‐based treatment of risk factors, helping patient discussions to ecourage lifestyle changes to reduce their inflammation.
Introduction
The contribution of inflammation to the pathophysiological features of atherosclerosis is well established,1, 2 as well as the prognostic usefulness of biomarker surrogates, such as hsCRP (high‐sensitivity C‐reactive protein), for predicting the risk of vascular events in primary cardiovascular prevention.3 Although there is also evidence supporting the use of hsCRP in patients with established cardiovascular disease (eg, secondary prevention), this comes primarily from post hoc analyses of clinical trials.4, 5, 6 The controlled settings of trials, subjected to strict inclusion/exclusion criteria and monitoring protocols, may, however, not reflect the heterogeneous reality of clinical practice.
Screening for inflammation in secondary prevention has remained infrequent. One contributing factor could be that, until recently, no data were available to provide direct evidence that reducing hsCRP would reduce rates of recurrent cardiovascular events. The CANTOS (Canakinumab Anti‐Inflammatory Thrombosis Outcome Study) convincingly showed that, among patients with prior myocardial infarction (MI) and hsCRP ≥2 mg/L, treatment with a monoclonal antibody targeting interleukin‐1β was associated with fewer cardiovascular events.7 The demonstration that low‐dose methotrexate in patients with MI failed to reduce levels of interleukin‐1β, interleukin‐6, or hsCRP and did not result in fewer cardiovascular events than placebo8 points perhaps future pharmacologic attention away from broad‐spectrum anti‐inflammatory treatments and toward targeted inhibition of the interleukin‐1/interleukin‐6 pathway of innate immunity.9 While still awaiting for the results of other inflammation‐targeted trials, such as those of tocilizumab10, 11 or colchicine,12, 13 it is conceivable to expect a paradigm shift in the treatment of human atherosclerosis and cardiovascular disease.
At present, there is limited or no information on hsCRP distribution among stable patients with MI from routine health care and on whether hsCRP has prognostic value in contemporary secondary cardiovascular prevention. Such evidence would inform clinical decisions on the usefulness of screening/monitoring inflammation after MI and on the size and target populations who may benefit from inflammation‐targeted therapies and be of assistance in both health service planning and risk management. The objective of this study was to estimate the background/residual inflammatory risk reflected by hsCRP in healthcare‐managed patients with MI, identify clinical predictors of elevated hsCRP, and explore purportedly associated risks of death and of major adverse cardiovascular events (MACEs).
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Data Sources
This study is based on the Stockholm Creatinine Measurements healthcare‐use cohort,14 which includes all Stockholm residents (Sweden) accessing health care and undertaking at least one plasma creatinine test during 2006 to 2011. Given the commonness of plasma creatinine testing, the Stockholm Creatinine Measurements healthcare‐use cohort captured 68% of the complete adult population census of the region. Because plasma creatinine is routinely ordered during the characterization and follow‐up of patients with MI, the Stockholm Creatinine Measurements healthcare‐use cohort was estimated to capture the virtually complete (99% coverage) population with MI of the region.14 Laboratory data were linked with regional and national administrative databases for complete information on healthcare use, both in outpatient and inpatient care, and including diagnoses, procedures, dispensed drugs, and follow‐up for death, with no loss to follow‐up. The study used only deidentified data and, thus, was deemed not to require informed consent. It was approved by regional ethical review boards and the Swedish National Board of Welfare and adheres to the Declaration of Helsinki.
Patient Selection, Study Design, and Study Exposure
We included all adult (>18 years old) patients with MI accessing health care and undertaking hsCRP testing (at least 30 days after the MI event) from January 2007 to December 2011. Patients with MI were identified by relevant International Classification of Diseases, Tenth Revision (ICD‐10), codes (I21, acute MI; I22, subsequent MI; and I252, old MI), registered in outpatient or inpatient care since 1997. All consecutive hsCRP measurements performed in Stockholm health care (in either outpatient or inpatient care) during the Stockholm Creatinine Measurements healthcare‐use cohort data collection period were then extracted.
The study exposure is hsCRP, as measured in clinical practice. In analyses from JUPITER (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin)11 or US National Health and Nutrition Examination Survey,15 hsCRP levels were found stable over time as long as they were not measured during acute infection. Nonetheless, real‐world health care differs from controlled settings, with hsCRP testing being done by judgement of the attending physician. Given the multiple indications of hsCRP tests, several patient‐ and test‐specific exclusion criteria were applied to avoid, as much as possible, healthcare‐use–related biases. To minimize confounding by indication bias, we excluded hsCRP levels occurring within the 30 days after the (most recent) MI event, hsCRP tests taken during an inpatient stay or emergency department visit (±1 day, but allowed hsCRP tests taken at admission of an elective surgery), and elevated hsCRP levels (>20 mg/L), presumably indicative of acute illness. We also excluded hsCRP tests followed by the prescription of antibiotics, antivirals, or antimycotics within 7 days on the assumption that infection was the reason for hsCRP testing. Likewise, we excluded hsCRP levels during the following 3 months as they may relate to the monitoring and/or resolution of the infection event (see definitions in Tables S1 and S2). After applying these hsCRP‐exclusion criteria, we selected the first eligible hsCRP level per patient to define a 3‐month baseline window. At this point, we excluded patients with comorbidities and/or long‐term medications that modify systemic inflammatory levels. These included ongoing/recent cancer (diagnosed within the previous 3 years), chronic infections (hepatitis, tuberculosis, or HIV infection), and undertaking corticosteroids or immunosuppressive drugs.
Finally, to minimize the possibility of reverse causality, we required patients to survive a minimum of 3 months from the first eligible hsCRP level, and we defined the background/residual inflammation of each patient as the geometric mean of all eligible hsCRP tests during a 3‐month baseline window. The end of that 3‐month window was considered as the index date of the study, the date at which study covariates were constructed and follow‐up for clinical outcomes was initiated. At this point, we estimated intraindividual hsCRP variability in the year after the index date to evaluate if our coefficients of variation approximated to previous reports from controlled settings.11, 15
Study Covariates
Study covariates were calculated at the index date and included age, sex, comorbidities and undertaken surgical procedures, ongoing medications, and laboratory values. Comorbid conditions are listed in Table S1 and were assessed through ICD‐10 diagnostic codes issued at the time of or before the index date. Information on comorbidities comes from the Regional Healthcare register. Comorbidities identified in this study used established algorithms with an 85% to 95% validity.16 We collected information on coronary artery bypass grafting and percutaneous coronary intervention (PCI) by identification of issued Nordic Medico‐Statistical Committee surgical procedure codes (Table S1).
Ongoing medications are listed in Table S2 and were assumed to be concomitant if there was a pharmacy dispensation at the time of or within the previous 3 months from index date or after 15 days. Information on drug dispensations was obtained from the Dispensed Drug Registry, a nationwide register with complete information on all prescribed drugs dispensed at Swedish pharmacies. The coverage of this register is considered virtually complete, as outpatient drug prescriptions and dispensations in Sweden are done via each citizen's unique personal identification number.
In Stockholm health care, laboratory tests are measured by 3 different laboratories (Aleris, Unilabs, and Karolinska), which are frequently audited to ensure reproducibility and consistency of determinations across the region. hsCRP levels were measured in plasma by either immunochemistry or turbidimetry, both with a minimum level of detection of 1 mg/L. Other laboratory values considered in this analysis were measurements of plasma creatinine, hemoglobin, total cholesterol, low‐density lipoprotein cholesterol, and serum albumin, as performed in health care. Laboratory concentrations were defined as the geometric mean of all available laboratory tests performed in connection with an outpatient consultation at time of or within the 12 months preceding index date. Missing values (ie, the laboratory test was not ordered during the defined window) were coded as “missing.” Serum creatinine was measured with either enzymatic or corrected Jaffe method (alkaline picrate reaction) and used to obtain estimated glomerular filtration rate (eGFR) with the Chronic Kidney Disease (CKD)–Epidemiology Collaboration equation.17 CKD was defined as eGFR <60 mL/min per 1.73 m2. The severity of CKD17 was then categorized as eGFR <60 to 45, eGFR <45 to 30, and eGFR <30 mL/min per 1.73 m2. Patients undergoing renal replacement therapy (ie, maintenance dialysis or renal transplantation) at index date were categorized as having eGFR <30 mL/min per 1.73 m2.
Study Outcomes
The primary study outcomes were time to (cardiovascular) death and MACEs. Deaths were retrieved from the Swedish death registry, which is updated monthly and has complete national coverage. We calculated both the risk association with all‐cause mortality and with cause‐specific mortality. Cardiovascular‐related mortality was defined as any death with an ICD‐10 code of the I family as main cause of death, and the remaining deaths were considered noncardiovascular. MACE was defined as the composite of nonfatal MI (ICD‐10 code I21, I22, or I23), nonfatal ischemic stroke (ICD‐10 code I63), or cardiovascular death. Nonfatal events were identified from diagnostic codes issued in primary or secondary position at a hospitalization discharge. Finally, we also evaluated the incidence rate of (any) hospitalizations and length of hospital stay as a more general surrogate of healthcare consumption/disease complications. Patients were followed up from index date until occurrence of event, migration from the region, or end of follow‐up (December 31, 2012), whichever occurred first.
Statistical Analysis
We present descriptive values as median and interquartile range (IQR), mean and SD, or counts with proportions. We report baseline demographics and clinical characteristics for all MI cases, stratified according to increased hsCRP levels. Because the optimal definition of inflammation in patients after MI is not established, we chose the cutoff hsCRP ≥2 mg/L as this was the hsCRP cutoff in post‐MI randomized controlled trials. We used logistic regression analysis to identify clinical predictors of this hsCRP threshold. As a sensitivity analysis, we also explore general characteristics and predictors, according to the hsCRP cutoffs, to define low, middle, and high cardiovascular risk in the general population (hsCRP level, <1, 1–3 and >3–10, and >10 mg/L, respectively). We also redefined our baseline hsCRP level as the minimum hsCRP level encountered per patient during the 3‐month eligibility window. Within‐person variability of hsCRP was estimated as coefficient of variation during the first year after the index hsCRP measurement.
Crude cumulative survival rates for all‐cause mortality and MACEs were calculated as Kaplan‐Meier curves stratified over the hsCRP categories. Multivariable‐adjusted hazard ratios and 95% CIs were estimated using Cox proportional hazard models to determine the association between serum hsCRP level (both continuous and categorical) and study outcomes. We graphically assessed and found satisfying the proportional hazards assumption by plotting Schoenfeld residuals against ranks of time. We also investigated nonlinear relationships between serum hsCRP level and outcomes using restricted cubic splines. A smooth function of the logarithm of hsCRP, because of the skewed distribution, was added, where the optimal number of 4 knots was determined by generalized cross validation. P value for nonlinearity was obtained by testing the coefficient of the second spline transformation equal to 0. Interaction terms in a priori specified strata were used to evaluate the consistency of observed associations and explore effect modifications. To assess the impact of reverse causation bias, we repeated the main analyses, excluding events occurring within the first 6 or 12 months of follow‐up. All analyses were performed using R, version 3.4.1 (http://www.rproject.org), with packages “survival,” version 2.43 to 3 (https://github.com/therneau/survival), and “mgcv,” version 1.8 to 17, for the spline regressions.
Results
Patient Selection and hsCRP Levels
The patient selection flow chart is depicted in Figure 1. Of 50 931 adult MI cases identified, 34 057 had at least 1 hsCRP tested >30 days after MI during 2007 to 2011 and were considered for analysis. After applying patient‐ and hsCRP‐specific exclusion criteria, a total of 17 464 adult MI cases with at least 1 eligible hsCRP during the baseline 3‐month eligibility window were included in the study.
Figure 1.
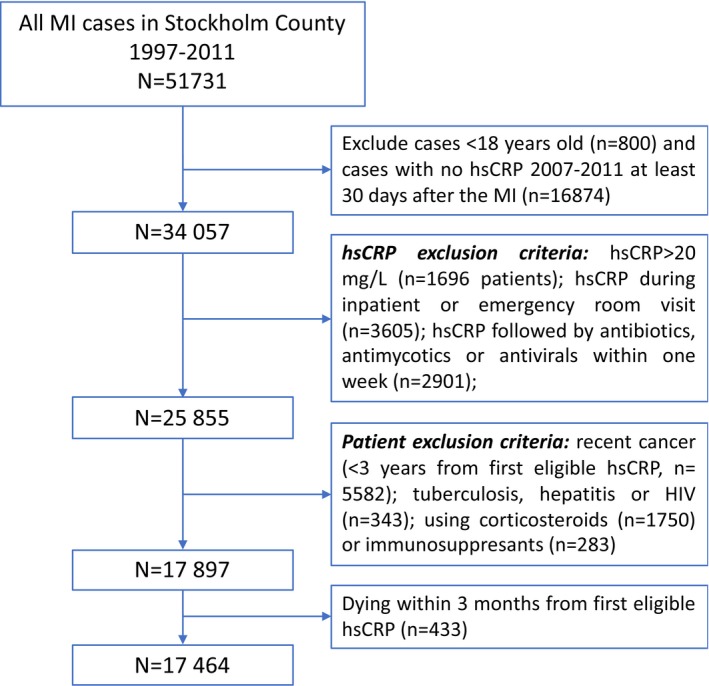
Patient selection flow chart. hsCRP indicates high‐sensitivity C‐reactive protein; MI, myocardial infarction.
For included individuals, a total of 22 408 eligible hsCRP measurements were considered in the calculation of the baseline residual inflammatory risk. Most patients (81%) only had 1 test during the 3‐month baseline window, and the remaining 19% had between 2 and 22 tests taken (Figure S1). We defined the baseline hsCRP as the geometric mean of all eligible measurements per participant, for it rendered the lowest SD and reduced the impact of possible outliers. The within‐person coefficient of variation during the first year after the index hsCRP measurement was estimated as 0.4 (IQR, 0.0–0.8). There was no difference in variability stratified on age and sex (Table S3).
Baseline Characteristics and Clinical Predictors of hsCRP
Demographics and clinical characteristics are presented in Table 1. A total of 63.5% of patients were men, with a mean age of 72.6 (SD, 12.4) years and a mean time since the MI of 3.2 (median, 2.2; IQR, 0.8–4.9) years. The mean baseline hsCRP level was 4.27 (median, 2.24; SD, 4.28; IQR, 1–6) mg/L. Hypertension (66%), heart failure (41%), atrial fibrillation (26%), diabetes mellitus (27%), and CKD (27%) were common comorbidities. A total of 39% of patients had undergone PCI, and 18% had undergone coronary artery bypass grafting. Aspirin, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, β blockers, and statins were the most commonly prescribed medications, present in >50% of participants.
Table 1.
General Characteristics of Adult Patients After MI in Stockholm, Sweden, From 2007 to 2011, Overall and by hsCRP Categories
| Characteristics | Overall | hsCRP Level <2 mg/L | hsCRP Level ≥2 mg/L |
|---|---|---|---|
| No. (%) | 17 464 | 5924 (33.9) | 11 540 (66.1) |
| Age, y | 72.6 (12.4) | 70.3 (12.4) | 73.7 (12.2) |
| Men, % | 63.5 | 67.0 | 61.7 |
| Time since MI, y | 3.2 (3.0) | 3.2 (3.0) | 3.2 (3.0) |
| Hemoglobin, g/dL (n=13 605) | 135.1 (16.1) | 137.7 (15.1) | 133.9 (16.4) |
| Cholesterol, mmol/L (n=4575) | 4.46 (1.10) | 4.39 (1.03) | 4.51 (1.12) |
| LDL‐C, mmol/L (n=3211) | 2.53 (0.92) | 2.47 (0.86) | 2.57 (0.95) |
| Albumin, mg/dL (n=5069) | 36.2 (4.5) | 37.9 (3.8) | 35.7 (4.6) |
| eGFR, mL/min per 1.73 m2 (n=13 562) | 69.3 (23.3) | 74.1 (21.3) | 67.0 (23.8) |
| ≥60, % | 51.2 | 54.4 | 49.5 |
| <60–45, % | 13.1 | 10.5 | 14.4 |
| <45–30, % | 8.7 | 5.4 | 10.4 |
| <30, % | 4.7 | 2.4 | 5.9 |
| Missing, % | 22.3 | 27.3 | 19.8 |
| Diabetes mellitus, % | 27.7 | 22.3 | 30.5 |
| Hypertension, % | 67.3 | 62.5 | 69.8 |
| COPD, % | 15.4 | 10.5 | 17.9 |
| Cancer, % | 8.7 | 7.2 | 9.4 |
| Dementia, % | 2.7 | 2.2 | 3.0 |
| Heart failure, % | 41.9 | 31.6 | 47.1 |
| Peripheral vascular disease, % | 13.4 | 9.2 | 15.6 |
| Stroke, % | 15.7 | 11.2 | 18.0 |
| Atrial fibrillation, % | 26.6 | 20.0 | 30.0 |
| Inflammatory bowel disease, % | 1.5 | 1.3 | 1.6 |
| Rheumatoid diseases, % | 12.1 | 8.1 | 14.1 |
| CABG, % | 17.3 | 15.9 | 18.0 |
| PCI, % | 38.2 | 44.3 | 35.0 |
| Aspirin, % | 66.9 | 72.4 | 64.1 |
| NSAIDs, % | 9.6 | 8.6 | 10.2 |
| ACEIs/ARBs, % | 53.4 | 53.3 | 53.4 |
| MRA, % | 7.3 | 4.8 | 8.6 |
| β Blockers, % | 69.1 | 71.3 | 67.9 |
| Diuretics, % | 34.5 | 24.3 | 39.7 |
| Calcium channel blockers, % | 19.2 | 17.8 | 20.0 |
| Digoxin, % | 3.9 | 2.2 | 4.7 |
| Statins, % | 59.0 | 67.0 | 54.9 |
| Ezetimibe, % | 2.0 | 2.4 | 1.7 |
| Fibrates, resins, and nicotinic acid, % | 1.3 | 1.1 | 1.4 |
| Other blood pressure drugs, % | 0.7 | 0.5 | 0.8 |
Data are given as mean (SD) unless otherwise indicated. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction; MRA, mineralocorticoid‐receptor antagonist; PCI, percutaneous coronary intervention.
Most (66%) of patients had an hsCRP level ≥2 mg/L, and 40% had an hsCRP level >3 mg/L (Figure 2). Patients with an hsCRP level ≥2 mg/L (Table 1) were older, were more often women, and had a lower eGFR. The prevalence of most comorbidities increased in patients with inflammation, notably cardiovascular and rheumatoid diseases. Although diuretics and NSAIDs were more often dispensed to inflamed patients, statins, aspirin, and βblockers were less often dispensed. Table S4 shows patient characteristics, according to hsCRP levels of <1, 1 to 3, >3 to 10, and >10 mg/L, observing similar trends across increasing hsCRP categories.
Figure 2.
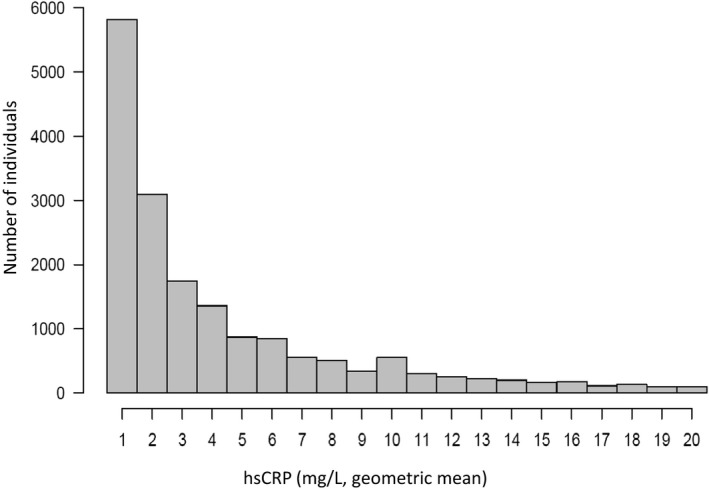
Distribution of baseline hsCRP (high‐sensitivity C‐reactive protein) levels in real‐world patients after myocardial infarction from Stockholm, Sweden, from 2007 to 2011. Baseline hsCRP was defined as the geometric mean of all eligible tests per patient during a 3‐month eligibility window (see Methods).
The multivariable predictors of hsCRP ≥2 mg/L are shown in Table S5. Briefly, lower hemoglobin, lower eGFR, comorbidities (heart failure, peripheral vascular disease, stroke, atrial fibrillation, diabetes mellitus, chronic obstructive pulmonary disease, and rheumatoid diseases), and ongoing medications (digoxin, diuretics, and NSAIDs) were associated with higher odds of hsCRP ≥2 mg/L. Conversely, those with PCI and taking angiotensin‐converting enzyme inhibitors/angiotensin receptor blocker or statins were associated with lower odds of elevated hsCRP. Multivariable predictors of hsCRP >3 mg/L were essentially the same (Table S6).
As a sensitivity analysis, we redefined the baseline hsCRP with the minimum hsCRP per patient within the 3‐month baseline window (Figure S1). The minimum hsCRP was similar and not statistically different from the main analysis: mean minimum hsCRP, 4.02 (median, 2.00; SD, 4.16; IQR, 1–5) mg/L. Baseline characteristics and predictors remained essentially unchanged (Tables S7 through S9).
Risk of Mortality and MACEs Associated With hsCRP
During a median follow‐up of 3.2 (IQR, 1.8–4.7) years, 4138 deaths (annual incidence rate, 7.4%, including 2343 cardiovascular and 1795 noncardiovascular deaths) and 3900 MACEs (incidence rate, 7.9%, composite of 1689 cardiovascular‐related deaths, 1390 nonfatal MIs, and 821 nonfatal stroke events) were recorded.
In crude analyses (Figure 3 and Table 2), hsCRP was highly predictive of the incidence of deaths and MACEs. In multivariable‐adjusted Cox analyses, patients with hsCRP ≥2 mg/L compared with hsCRP <2 mg/L had a 1.42 (95% CI, 1.31–1.53) higher risk of death, similarly attributed to both cardiovascular and noncardiovascular causes (Table S10). Compared with hsCRP <2 mg/L, patients with hsCRP ≥2 mg/L also had a 1.28 (95% CI, 1.18–1.38) higher risk of MACEs. This risk association was similarly consistent for some of the single components of MACEs (MI and cardiovascular mortality), but there was no clear association between hsCRP and the risk of nonfatal stroke (Table S11).
Figure 3.

Kaplan‐Meier curves showing the cumulative incidence of death (A and B) or major adverse cardiovascular events (MACEs; C and D) for different hsCRP (high‐sensitivity C‐reactive protein) categories in real‐world patients after myocardial infarction.
Table 2.
Number of Events, Incidence Rates, and HRs for the Risk of All‐Cause Mortality and MACEs Associated With hsCRP Level
| Variable | No. of Events/No. of Patients | Annual Incidence Rate, % | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| All‐cause mortality | ||||
| hsCRP <2 mg/L | 842/5924 | 4.1 | 1.00 | 1.00 |
| hsCRP ≥2 mg/L | 3296/11 540 | 9.3 | 2.26 (2.10–2.44) | 1.42 (1.31–1.53) |
| hsCRP ≤1 mg/L | 765/5543 | 4.0 | 1.00 | 1.00 |
| hsCRP <1–3 mg/L | 972/4938 | 6.0 | 1.49 (1.36–1.64) | 1.20 (1.09–1.32) |
| hsCRP >3–10 mg/L | 1638/5161 | 10.5 | 2.64 (2.42–2.87) | 1.57 (1.44–1.72) |
| hsCRP >10 mg/L | 763/1826 | 15.8 | 3.96 (3.58–4.37) | 1.63 (1.47–1.81) |
| MACEs | ||||
| hsCRP <2 mg/L | 928/5924 | 4.8 | 1.00 | 1.00 |
| hsCRP ≥2 mg/L | 2972/11 540 | 9.1 | 1.86 (1.72–2.00) | 1.28 (1.18–1.38) |
| hsCRP ≤1 mg/L | 844/5543 | 4.7 | 1.00 | 1.00 |
| hsCRP <1–3 mg/L | 1005/4938 | 6.6 | 1.41 (1.28–1.54) | 1.18 (1.08–1.29) |
| hsCRP >3–10 mg/L | 1433/5161 | 10.0 | 2.10 (1.93–2.29) | 1.37 (1.25–1.50) |
| hsCRP >10 mg/L | 618/1826 | 13.9 | 2.89 (2.60–3.21) | 1.40 (1.25–1.56) |
Models were adjusted for age, sex, time since myocardial infarction, hemoglobin, estimated glomerular filtration rate, comorbidities (diabetes mellitus, hypertension, chronic obstructive pulmonary disease, cancer, dementia, heart failure, peripheral vascular disease, stroke, atrial fibrillation, inflammatory bowel diseases, and rheumatoid diseases), undertaken procedures (coronary artery bypass grafting and percutaneous coronary intervention), and ongoing medications (aspirin, NSAIDs, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, mineralocorticoid‐receptor antagonists, β blocker, other diuretics, calcium channel blockers, other blood pressure drugs, digoxin, statins, ezetimibe, fibrates, resins, and nicotinic acid). HR indicates hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; MACE, major adverse cardiovascular event.
When stratifying hsCRP into 4 categories (≤1, 1–3, >3–10, and >10 mg/L), we observed similar dose‐dependent associations for the 3 first categories, but not for the highest stratum. This flattening of risk association at higher levels was also observed in cubic splines (Figure 4), depicting an exponential association between hsCRP (as a continuous variable) and study outcomes. The magnitude of the risk association plateaued at an hsCRP value of ≈5 to 6 mg/L for all‐cause mortality, and of 4 to 5 mg/L for MACEs.
Figure 4.

Restricted cubic splines depicting the adjusted hazard ratios (and 95% CIs) of all‐cause mortality (A) and major adverse cardiovascular events (B) associated with hsCRP (high‐sensitivity C‐reactive protein; a continuous variable). Models were adjusted for age, sex, time since myocardial infarction, hemoglobin, estimated glomerular filtration rate, comorbidities (diabetes mellitus, hypertension, chronic obstructive pulmonary disease, cancer, dementia, heart failure, peripheral vascular disease, stroke, atrial fibrillation, inflammatory bowel diseases, and rheumatoid diseases), undertaken procedures (coronary artery bypass grafting and percutaneous coronary intervention), and ongoing medications (aspirin, NSAIDs, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, mineralocorticoid‐receptor antagonists, β blocker, other diuretics, calcium channel blockers, digoxin, statins, ezetimibe, fibrates, resins, nicotinic acid, and other blood pressure drugs).
To test the robustness of our findings, stratified analyses were conducted in high‐risk populations, and multiplicative interaction terms were tested to explore risk modifications. The association between hsCRP ≥2 mg/L and study outcomes was found robust and similar in magnitude through the studied subgroups (Figures 5 and 6), with hazard ratios between 1.20 and 1.60. Some multiplicative interactions were noted, suggesting that the association between hsCRP and outcomes was slightly stronger among patients aged <65 years and among men. The association was also stronger in the absence, rather than in the presence, of some comorbidities (hypertension, CKD, heart failure, and atrial fibrillation) and medications (statins). Nonetheless, differences in risk magnitude within these substrata were marginal. Exclusion of events occurring during the first 6 or 12 months of follow‐up minimally attenuated the magnitude of the estimates (Table S12), suggesting reverse causation bias to be low. Finally, we explored hospitalization risks in these individuals, observing that patients with MI with elevated hsCRP levels had a higher hospitalization rate over time and a significantly longer in‐hospital stay compared with patients with MI with low hsCRP levels (Table S13).
Figure 5.
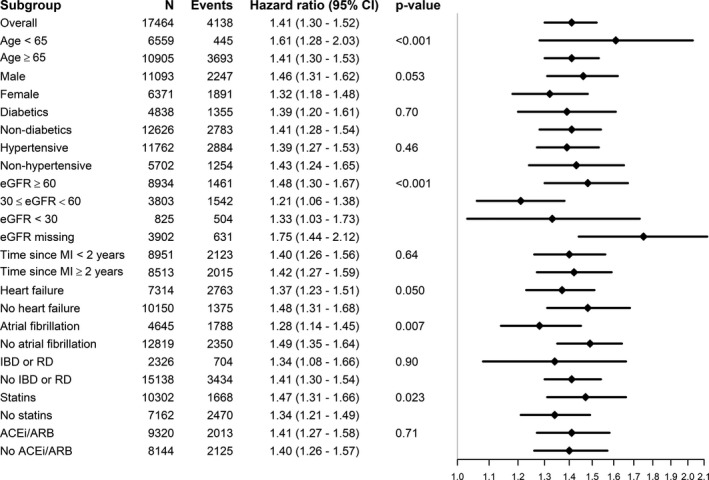
Forest plots of hsCRP (high‐sensitivity C‐reactive protein) ≥2 mg/L and all‐cause mortality, overall and in subgroups. Models were adjusted (when appropriate) for age, sex, time since myocardial infarction (MI), hemoglobin, estimated glomerular filtration rate (eGFR), comorbidities (diabetes mellitus, hypertension, chronic obstructive pulmonary disease, cancer, dementia, heart failure, peripheral vascular disease, stroke, atrial fibrillation, inflammatory bowel diseases, and rheumatoid diseases), undertaken procedures (coronary artery bypass grafting and percutaneous coronary intervention), and ongoing medications (aspirin, z NSAIDs, angiotensin‐converting enzyme [ACE] inhibitors/angiotensin receptor blockers [ARBs], mineralocorticoid‐receptor antagonists, β blocker, other diuretics, calcium channel blockers, digoxin, statins, ezetimibe, fibrates, resins, nicotinic acid, and other blood pressure drugs). IBD indicates inflammatory bowel disease; RD, rheumatoid disease.
Figure 6.

Forest plots of hsCRP (high‐sensitivity C‐reactive protein) ≥2 mg/L and risk of major adverse cardiovascular events, overall and in subgroups. Models were adjusted (when appropriate) for age, sex, time since myocardial infarction(MI), hemoglobin, estimated glomerular filtration rate (eGFR), comorbidities (diabetes mellitus, hypertension, chronic obstructive pulmonary disease, cancer, dementia, heart failure, peripheral vascular disease, stroke, atrial fibrillation, inflammatory bowel diseases, and rheumatoid diseases), undertaken procedures (coronary artery bypass grafting and percutaneous coronary intervention), and ongoing medications (aspirin, NSAIDs, angiotensin‐converting enzyme [ACE] inhibitors/angiotensin receptor blockers [ARBs], mineralocorticoid‐receptor antagonists, β blocker, other diuretics, calcium channel blockers, digoxin, statins, ezetimibe, fibrates, resins, nicotinic acid, and other blood pressure drugs). IBD indicates inflammatory bowel disease; RD, rheumatoid disease.
Discussion
This healthcare‐based study of stable patients with MI has the following novel findings: (1) Inflammation, as reflected by hsCRP ≥2 mg/L, was pervasive and present in >60% of patients. (2) Inflammation was particularly common among those with comorbidities and, notably, among those with low kidney function and anemia. Conversely, the use of statins was associated with lower inflammation risk. (3) Inflammation was a strong predictor of subsequent risk of MACEs and death, with associations being linear for hsCRP ranging between 1 and 5 mg/L and plateauing thereafter to a sustained increased risk. Collectively, our study comprehensively characterizes the prevalence, predictors, and prognostic validity of this condition, thus expanding trial evidence to real‐world clinical settings of secondary prevention.
Two thirds of the patients in our study had hsCRP ≥2 mg/L, and 40% had hsCRP >3 mg/L. Evaluating the frequency of inflammation in routine clinical practice is important to size and increase awareness of this problem. However, epidemiological evidence for patients with MI to date derived mainly from historical proof‐of‐concept studies performed in the 1990s18, 19, 20 or from post hoc analyses of trials4, 5, 6 with inclusion/exclusion criteria that could not, per definition, estimate the burden of this phenomenon. Our results are the largest healthcare‐based analysis to date, and the external validity of the cross‐sectional part of our study is supported by a recent report of 1296 participants from the US National Health and Nutrition Examination Survey that reaches similar prevalence figures.21 Also, recently, Kalkman and colleagues22 estimated, in a retrospective registry study of 7026 patients undergoing PCI, that, although 38% had persistently hsCRP ≥2 mg/L, 10% more increased their hsCRP over a 4‐week period. If inflammation is in the causal pathway of cardiovascular disease,1, 2 this high prevalence has important clinical implications.
The chief finding in our study is the consistent association between hsCRP and MACEs or risk of death, expanding to routine health care the observations from clinical trials4, 5, 6 and patients undergoing PCI.22 Another addition to the literature is our spline analyses, showing on a continuous scale the dynamic outcome association for this biomarker. The plateauing risk magnitude for hsCRP >5 mg/L may have implications for understanding risks and potentially directing treatment, and we suggest this cutoff as a potential infliction point to test and validate in future studies. We speculate that to improve patient outcomes, hsCRP levels in highly inflamed patients should be brought down to the sloping part (ie, <5 mg/L). Such speculation is in line with results from CANTOS, whereby individuals most likely to achieve the target hsCRP concentration of <2 mg/L at 3 months were, naturally, those who started out with lower baseline values.23 Those who started out with higher baseline values and did not reach the target had only small and nonsignificant benefits (hazard ratio, 0.95; 95% CI, 0.84–1.09; P=0.48).23 Attaining specific levels of a risk marker, as hsCRP in this case, may help guiding conversations toward personalized medicine and rational resource use.
Finally, our multivariate risk analyses confirm the diversity of risk factors involved in this condition; diabetes mellitus, cardiovascular comorbidities, and other proinflammatory conditions, such as chronic obstructive pulmonary disease and rheumatoid diseases, credibly contribute to the patient's inflammatory risk, along with some of the medications used to treat them, such as NSAIDs, digoxin, or diuretics.24, 25, 26 Lower kidney function and anemia were progressively associated with the risk of inflammation in our study. Suppression of erythropoiesis by inflammation, together with alterations of the erythrocyte membrane that impair its survival and impaired iron homeostasis, is thought to cause anemia of inflammation in a wide range of chronic diseases.27 Although inflammation is prevalent in people with advanced kidney disease,28 a wealth of evidence suggests that multiple processes related to renal injury and repair induce unique changes in innate and adaptive immunity.28 It is, however, unknown if resolving inflammation may retard kidney disease progression.29
The use of statins emerged as a notable clinical predictor (with an overall risk reduction of 33%), in line with the demonstrated effects of this therapy in reducing inflammation,29, 30 as lipids activate the knot‐like receptor protein 3 inflammasome that leads to interleukin‐1β activation.31 We also found that the risk of inflammation was lower among users of angiotensin‐converting enzyme inhibitors/angiotensin receptor blocker. Disentangling the risk attributed to medications from that of comorbidities is complex in observational analyses, as comorbidities and medications are intricately connected. However, such an association again agrees with convincing evidence indicating that RAS (Renin Angiotensin System) blockade, on balance, improves parameters of innate and adaptive immunity.32, 33, 34, 35 As discussed in recent editorials, hsCRP remains often unmonitored in secondary cardiovascular prevention by internists and cardiologists.36, 37 The characterization of inflammation risk in our study, thus, serves to identify patient groups who may benefit from more aggressive and guideline‐based treatment of risk factors. Although our findings can constitute the basis for risk scores for use in settings where hsCRP testing is not available, this information can motivate the need for inflammation‐preventive strategies, such as lipid‐lowering agents of other inflammation‐specific drugs. Furthermore, it can help patient discussions conveying risk and encouraging lifestyle changes related to diet, exercise, and smoking cessation to reduce their inflammation.35
Strengths of our study include a large sample size, richness of information on risk factors and confounders, solid outcome definition, and virtually no losses to follow‐up. Our study also has limitations; the studied population was representative of the patients with MI of Stockholm, Sweden, during 2007 to 2011, which counts with uniform and tax‐funded health care. Extrapolation of our findings to other areas or periods should be done with caution. As any observational study, our associations preclude any inference on causality. Because hsCRP tests were taken in connection to a healthcare encounter, factors that predict blood testing may predict detected inflammation. Despite the fact that our careful study design tried to avoid confounding by indication, residual and unknown confounding remains intrinsic to observational analyses. In this sense, we acknowledge the lack of information on MI size, troponins, body mass index, or smoking habits. Causes of death were ascertained by ICD‐10 codes and not always confirmed by autopsies; thus, there was the possibility of misclassification. Finally, as our exposure depends on laboratory test ordering in health care, it was unfortunate that there were not sufficient participants with concurrent blood lipid testing to study the combined risks and prognosis of high low‐density lipoprotein cholesterol and high hsCRP.
To conclude, by characterizing hsCRP levels among stable patients with MI accessing routine health care, we demonstrate its commonness and prognostic significance for the risk of death and MACEs in secondary prevention. Our analysis also identifies populations at high risk of persistent inflammation who may particularly benefit from targeted anticytokine therapies.
Sources of Funding
This study was supported by an institutional grant from Novartis Pharma AG, the manufacturer of canakinumab, to Karolinska Institutet. In addition, we acknowledge grant support from the Swedish Heart and Lung Foundation, the Stockholm County Council, Martin Rind's and Westman's Foundations.
Disclosures
Carrero reports grant funding from Novartis, AstraZeneca, ViforPharma, Merck‐Sharp and Dome, and Astellas; and consulting from Astellas, AstraZeneca, and Baxter Healthcare. Gabrielsen was previously employed by Novartis; and is currently employed by AstraZeneca. Obergfell is an employee of Novartis. Jernberg reports grant funding from MSD and Novartis; and fees for lecturing and consulting for Astra Zeneca, MSD, Aspen, and Bayer. Franko has no disclosures to report.
Supporting information
Table S1. Definitions for Comorbidities and Undertaken Surgical Procedures
Table S2. Definitions for Ongoing Medications
Table S3. Within‐Person Coefficient of Variation (with interquartile ranges) of hsCRP During the First Year After the Index hsCRP Measurement, Stratified By Baseline hsCRP Levels, Age and Sex
Table S4. General Characteristics of Real‐World Post‐MI Patients in Stockholm 2006–2011, Across Increasing hsCRP Categories
Table S5. Multinomial Logistic Regressions Exploring Clinical Predictors of hsCRP ≥2 mg/L Among Post‐MI Patients
Table S6. Multinomial Logistic Regressions Exploring Clinical Predictors of hsCRP >3 mg/L Among Post‐MI Patients
Table S7. Patient Characteristics Overall and By hsCRP < and ≥2 mg/L Defining the Baseline hsCRP as the Minimum hsCRP Encountered Within the 3‐Month Eligibility Window
Table S8. Patient Characteristics Across Increasing hsCRP Categories Defining the Baseline hsCRP as the Minimum hsCRP Encountered Per Patient Within the 3‐Month Eligibility Window
Table S9. Multinomial Logistic Regression Exploring Clinical Predictors of hsCRP ≥2 mg/L and hsCRP >3 mg/L Among Post‐MI Patients Defining the Baseline hsCRP as the Minimum hsCRP Encountered Per Patient Within the 3‐Month Eligibility Window
Table S10. Number of Events, Incidence Rate and Hazard Ratios for the Risk of Cardiovascular and Non‐Cardiovascular Mortality Associated to hsCRP
Table S11. Number of Events, Incidence Rate and Hazard Ratios for the Risk of Myocardial Re‐Infarction and Stroke Associated to hsCRP
Table S12. Sensitivity Analysis to Address the Risk of Reverse Causation Bias: Exclusion of Events Occurring During the First 6 or 12 Months of Follow Up
Table S13. Number of Hospitalizations, Incidence Rate and Average (or median) Length of Stay (in days) Associated to hsCRP
Figure S1. Number of eligible hsCRP measurements per participant during the baseline 3‐month eligibility window (A); Distribution of baseline hsCRP levels defined as the minimum hsCRP encountered during the 3‐month eligibility window (B).
(J Am Heart Assoc. 2019;8:e012638 DOI: 10.1161/JAHA.119.012638.)
References
- 1. Gomez D, Baylis RA, Durgin BG, Newman AAC, Alencar GF, Mahan S, St Hilaire C, Muller W, Waisman A, Francis SE, Pinteaux E, Randolph GJ, Gram H, Owens GK. Interleukin‐1beta has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med. 2018;24:1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tunon J, Back M, Badimon L, Bochaton‐Piallat ML, Cariou B, Daemen MJ, Egido J, Evans PC, Francis SE, Ketelhuth DF, Lutgens E, Matter CM, Monaco C, Steffens S, Stroes E, Vindis C, Weber C, Hoefer IE; ESC Working Group on Atherosclerosis and Vascular Biology . Interplay between hypercholesterolaemia and inflammation in atherosclerosis: translating experimental targets into clinical practice. Eur J Prev Cardiol. 2018;25:948–955. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM. Clinician's guide to reducing inflammation to reduce atherothrombotic risk: JACC review topic of the week. J Am Coll Cardiol. 2018;72:3320–3331. [DOI] [PubMed] [Google Scholar]
- 4. Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, Hsia J, Gersh BJ, Rifai N, Ridker PM, Pfeffer MA, Braunwald E; PEACE Investigators . Prognostic significance of the Centers for Disease Control/American Heart Association high‐sensitivity C‐reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. [DOI] [PubMed] [Google Scholar]
- 5. Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, Koenig W, Siegbahn A, Steg PG, Soffer J, Weaver WD, Ostlund O, Wallentin L; STABILITY Investigators . Inflammatory biomarkers interleukin‐6 and C‐reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) trial. J Am Heart Assoc. 2017;6:e005077 DOI: 10.1161/JAHA.116.005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L; FRISC Study Group (Fragmin During Instability in Coronary Artery Disease). Markers of myocardial damage and inflammation in relation to long‐term mortality in unstable coronary artery disease. N Engl J Med. 2000;343:1139–1147. [DOI] [PubMed] [Google Scholar]
- 7. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 8. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ; CIRT Investigators . Low‐dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ridker PM. Anti‐inflammatory therapy for atherosclerosis: interpreting divergent results from the CANTOS and CIRT clinical trials. J Intern Med. 2019;285:503–509. [DOI] [PubMed] [Google Scholar]
- 10. Kleveland O, Kunszt G, Bratlie M, Ueland T, Broch K, Holte E, Michelsen AE, Bendz B, Amundsen BH, Espevik T, Aakhus S, Damas JK, Aukrust P, Wiseth R, Gullestad L. Effect of a single dose of the interleukin‐6 receptor antagonist tocilizumab on inflammation and troponin T release in patients with non‐ST‐elevation myocardial infarction: a double‐blind, randomized, placebo‐controlled phase 2 trial. Eur Heart J. 2016;37:2406–2413. [DOI] [PubMed] [Google Scholar]
- 11.NCT03004703. Assessing the effect of anti‐IL‐6 treatment in myocardial infarction: the ASSAIL‐MI trial (ASSAIL‐MI). https://www.Clinicaltrials.Gov/ct2/show/nct03004703. Accessed December 17, 2018.
- 12. Martinez GJ, Celermajer DS, Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis‐associated inflammation. Atherosclerosis. 2018;269:262–271. [DOI] [PubMed] [Google Scholar]
- 13.NCT02551094. Colchicine cardiovascular outcomes trial (COLCOT). 2016. https://clinicaltrials.Gov/ct2/show/nct02551094. Accessed December 17, 2018.
- 14. Runesson B, Gasparini A, Qureshi AR, Norin O, Evans M, Barany P, Wettermark B, Elinder CG, Carrero JJ. The stockholm CREAtinine measurements (SCREAM) project: protocol overview and regional representativeness. Clin Kidney J. 2016;9:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bower JK, Lazo M, Juraschek SP, Selvin E. Within‐person variability in high‐sensitivity C‐reactive protein. Arch Intern Med. 2012;172:1519–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. [DOI] [PubMed] [Google Scholar]
- 19. Berk BC, Weintraub WS, Alexander RW. Elevation of C‐reactive protein in “active” coronary artery disease. Am J Cardiol. 1990;65:168–172. [DOI] [PubMed] [Google Scholar]
- 20. Liuzzo G, Biasucci LM, Gallimore JR, Grillo RL, Rebuzzi AG, Pepys MB, Maseri A. The prognostic value of C‐reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med. 1994;331:417–424. [DOI] [PubMed] [Google Scholar]
- 21. Pagidipati NJ, Hellkamp AS, Sharma PP, Wang TY, Fonarow GC, Pencina M. High‐sensitivity C‐reactive protein elevation in patients with prior myocardial infarction in the United States. Am Heart J. 2018;204:151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalkman DN, Aquino M, Claessen BE, Baber U, Guedeney P, Sorrentino S, Vogel B, de Winter RJ, Sweeny J, Kovacic JC, Shah S, Vijay P, Barman N, Kini A, Sharma S, Dangas GD, Mehran R. Residual inflammatory risk and the impact on clinical outcomes in patients after percutaneous coronary interventions. Eur Heart J. 2018;39:4101–4108. [DOI] [PubMed] [Google Scholar]
- 23. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ; CANTOS Trial Group . Relationship of C‐reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
- 24. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138:16–27. [DOI] [PubMed] [Google Scholar]
- 25. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. [DOI] [PubMed] [Google Scholar]
- 26. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. [DOI] [PubMed] [Google Scholar]
- 27. Fraenkel PG. Anemia of inflammation: a review. Med Clin North Am. 2017;101:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meuwese CL, Stenvinkel P, Dekker FW, Carrero JJ. Monitoring of inflammation in patients on dialysis: forewarned is forearmed. Nat Rev Nephrol. 2011;7:166–176. [DOI] [PubMed] [Google Scholar]
- 29. Ridker PM, MacFadyen JG, Glynn RJ, Koenig W, Libby P, Everett BM, Lefkowitz M, Thuren T, Cornel JH. Inhibition of interleukin‐1beta by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol. 2018;71:2405–2414. [DOI] [PubMed] [Google Scholar]
- 30. Bohula EA, Giugliano RP, Cannon CP, Zhou J, Murphy SA, White JA, Tershakovec AM, Blazing MA, Braunwald E. Achievement of dual low‐density lipoprotein cholesterol and high‐sensitivity C‐reactive protein targets more frequent with the addition of ezetimibe to simvastatin and associated with better outcomes in improve‐it. Circulation. 2015;132:1224–1233. [DOI] [PubMed] [Google Scholar]
- 31. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schieffer B, Bunte C, Witte J, Hoeper K, Boger RH, Schwedhelm E, Drexler H. Comparative effects of AT1‐antagonism and angiotensin‐converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J Am Coll Cardiol. 2004;44:362–368. [DOI] [PubMed] [Google Scholar]
- 33. Di Napoli M, Papa F. Angiotensin‐converting enzyme inhibitor use is associated with reduced plasma concentration of C‐reactive protein in patients with first‐ever ischemic stroke. Stroke. 2003;34:2922–2929. [DOI] [PubMed] [Google Scholar]
- 34. Crowley SD, Rudemiller NP. Immunologic effects of the renin‐angiotensin system. J Am Soc Nephrol. 2017;28:1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsikouris JP, Suarez JA, Simoni JS, Ziska M, Meyerrose GE. Exploring the effects of ACE inhibitor tissue penetration on vascular inflammation following acute myocardial infarction. Coron Artery Dis. 2004;15:211–217. [PubMed] [Google Scholar]
- 36. Ridker PM, Koenig W, Kastelein JJ, Mach F, Luscher TF. Has the time finally come to measure hsCRP universally in primary and secondary cardiovascular prevention? Eur Heart J. 2018;39:4109–4111. [DOI] [PubMed] [Google Scholar]
- 37. Candreva A, Matter CM. Is the amount of glow predicting the fire? Residual inflammatory risk after percutaneous coronary intervention. Eur Heart J. 2018. DOI: 10.1093/eurheartj/ehy729. Available at: https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehy729/5173735. Accessed May 18, 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definitions for Comorbidities and Undertaken Surgical Procedures
Table S2. Definitions for Ongoing Medications
Table S3. Within‐Person Coefficient of Variation (with interquartile ranges) of hsCRP During the First Year After the Index hsCRP Measurement, Stratified By Baseline hsCRP Levels, Age and Sex
Table S4. General Characteristics of Real‐World Post‐MI Patients in Stockholm 2006–2011, Across Increasing hsCRP Categories
Table S5. Multinomial Logistic Regressions Exploring Clinical Predictors of hsCRP ≥2 mg/L Among Post‐MI Patients
Table S6. Multinomial Logistic Regressions Exploring Clinical Predictors of hsCRP >3 mg/L Among Post‐MI Patients
Table S7. Patient Characteristics Overall and By hsCRP < and ≥2 mg/L Defining the Baseline hsCRP as the Minimum hsCRP Encountered Within the 3‐Month Eligibility Window
Table S8. Patient Characteristics Across Increasing hsCRP Categories Defining the Baseline hsCRP as the Minimum hsCRP Encountered Per Patient Within the 3‐Month Eligibility Window
Table S9. Multinomial Logistic Regression Exploring Clinical Predictors of hsCRP ≥2 mg/L and hsCRP >3 mg/L Among Post‐MI Patients Defining the Baseline hsCRP as the Minimum hsCRP Encountered Per Patient Within the 3‐Month Eligibility Window
Table S10. Number of Events, Incidence Rate and Hazard Ratios for the Risk of Cardiovascular and Non‐Cardiovascular Mortality Associated to hsCRP
Table S11. Number of Events, Incidence Rate and Hazard Ratios for the Risk of Myocardial Re‐Infarction and Stroke Associated to hsCRP
Table S12. Sensitivity Analysis to Address the Risk of Reverse Causation Bias: Exclusion of Events Occurring During the First 6 or 12 Months of Follow Up
Table S13. Number of Hospitalizations, Incidence Rate and Average (or median) Length of Stay (in days) Associated to hsCRP
Figure S1. Number of eligible hsCRP measurements per participant during the baseline 3‐month eligibility window (A); Distribution of baseline hsCRP levels defined as the minimum hsCRP encountered during the 3‐month eligibility window (B).


