Abstract
Background:
Non-pharmacologic therapies have been deemed as potentially beneficial for patients with systemic lupus erythematosus (SLE). We conducted an updated review to determine the effects of these therapies to inform practice.
Methods:
Literature search was performed using PubMed (MEDLINE), EMBASE, Cochrane, PsychINFO, CINAHL, Web of Science, and Google Scholar until August 2018. We included randomized controlled trials (RCTs) of non-pharmacologic therapies in SLE patients with sample size ≥ 10. SLE was defined by 1982 or 1997 ACR criteria. Studies were synthesized separately by patient-reported outcomes and disease activity. Due to the heterogeneity of interventions and comparisons, a meta-analysis was not performed.
Results:
Fifteen RCTs involving 846 participants met the inclusion criteria. Of the 15 trials, 8 used exercise interventions, 6 used psychological interventions (1 group psychotherapy, 3 cognitive behavioral therapies, 1 psychoeducation, 1 mindfulness-based cognitive therapy) and 1 used electro-acupuncture. Five of 15 studies utilized control groups consisting of usual medical care. Other studies included control interventions of relaxation, attention placebo, symptom monitoring support, education, minimal needling, isotonic and resistance exercise. Compared with the control conditions, non-pharmacological interventions were associated with a significant improvement in fatigue in 3 out of 6 studies. Three out of 8 studies reported improved anxiety and depression, and 1 study reported improved pain after interventions. Seven out of 11 studies reported improvement in overall quality of life in at least one domain of the SF-36. Of note, no studies demonstrated an improvement in disease activity after 5–52 weeks of non-pharmacological therapies.
Conclusion:
This review showed promising results for physical exercise and psychological interventions as adjuncts to traditional medical therapy for improvement in fatigue, depression, pain and quality of life for SLE. Further high-quality RCTs with longer follow-up periods are warranted.
Keywords: Systemic lupus erythematosus, Non-pharmacologic therapies, Fatigue, Depression, Pain, Quality of life
Introduction:
Patients with Systemic lupus erythematosus (SLE) report high levels of cognitive difficulties, depression, pain, and fatigue (1–5). More than 80% of SLE patients experience fatigue and up to 90% of SLE patients experience pain at one point during the disease course (1–3). Psychological disorders are also common in SLE patients. Several studies have found that depression is highly prevalent in SLE ranging from 17–75% (4, 5) which is higher than in the general population. A study by Bachen et al found that up to 65% of Caucasian SLE patients had mood or anxiety disorders (5). These factors play an important role in the psychological and physical well-being of SLE patients.
Although pharmacologic treatments have improved overall survival, SLE continues to have a profound impact on quality of life (6). Despite several new therapies, there are significant unmet needs that remain to be addressed such as fatigue, pain, and psychological symptoms managements (7, 8). In addition, conventional pharmacological therapies can cause a wide range of side effects. Therefore, non-pharmacologic therapies may be important adjunctive options for SLE patients.
Over the last two decades, interest in non-pharmacologic therapies has increased in patients with SLE. A study of health resource utilization cohort of 707 SLE patients showed that 50% used alternative therapies and at similar rates across Canada, the United States, and the United Kingdom (9). Despite a high rate of integrative medical therapies used by SLE patients, the scientific study of various types of non-pharmacologic therapies in SLE is very limited.
Several non-pharmacologic remedies have been studied as potentially beneficial for patients with SLE, including physical, psychological, complementary and integrative interventions. A previous systematic review and meta-analysis by Zhang et al compared the effects of psychological interventions among SLE patients (10). The authors identified 6 RCTs and found that psychological interventions significantly reduced anxiety, depression, stress and disease activity, compared to controls. No statistically significant differences were observed in mental health, fatigue and physical function. In terms of exercise interventions, a systematic review with meta-analysis by O’Dwyer et al showed that exercise intervention improved cardiorespiratory capacity, reduced fatigue, and improved psychological functions, compared to controls. Moreover, exercise interventions were safe, did not adversely affect disease activity and were well tolerated by a majority of SLE patients (11). Another systematic review by Pino-Sedeno et al identified 7 randomized control trials (RCTs), 1 nonrandomized trial and 4 prospective observational studies. This review specifically focused on fatigue as an outcome in SLE. They concluded that aerobic exercise was effective and suitable for reducing fatigue, but results were not always consistent across instruments used. It seems premature to confirm the efficacy of psychological interventions, acupuncture, diets and ultraviolet A radiation in improving fatigue (12). The current review differs from previous reviews of non-pharmacologic interventions for SLE in that it is limited to RCTs and is focused on five specific outcomes: fatigue, depression, pain, quality of life and disease activity. In addition, our review also includes various types of non-pharmacologic interventions including exercise interventions, psychological interventions and mind-body intervention.
Methods:
We searched PubMed, EMBASE, Cochrane Library, PsychINFO, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, and Google Scholar from their inception to August 2018. The search strategies used both controlled vocabulary terms and keywords for “Systemic Lupus Erythematosus” and “Non-pharmacologic therapy” and related methods. We did not restrict by date.
We included RCTs of non-pharmacologic interventions in SLE patients with sample size ≥ 10; measuring fatigue, depression, pain, quality of life or disease activity. SLE was defined by 1982 or 1997 ACR criteria. In order to narrow the focus of this review, we include interventions that utilized physical activity, psychological or mind–body approaches in conjunction with or as a part of the interventions. We delineate literature categories into exercise, psychological/educational and mind-body interventions.
Data extraction and quality assessment were performed by one investigator and confirmed by at least one other investigator. Disagreements were resolved by consensus among team members. We extracted information on study characteristics, population characteristics, type, duration, frequency of interventions and outcomes.
We qualitatively synthesized all included studies and grouped them into the following five categories of outcomes including fatigue, depression, pain, quality of life and disease activity. Due to the heterogeneity of interventions and comparisons, a meta-analysis was not performed.
Results:
Literature search
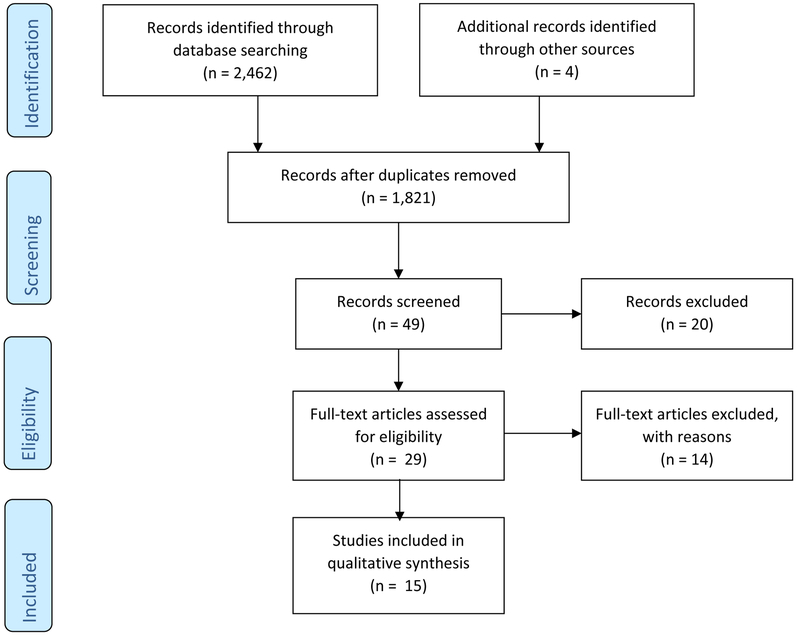
Figure 1 summarizes the flow of the literature search and publication selection process following PRISMA guidelines. A total of 2,466 references were identified by our search. After title and abstract screening, forty-nine articles remained for full-text screening. Twenty articles were excluded because they did not meet our inclusion criteria. We acquired 29 full text articles for further review. Of these 29 studies, 14 were excluded for the following reasons: non-RCTs (n=8), the SLE diagnosis was not defined by ACR criteria (n=3), the outcomes of interest were not assessed (n=1), poor methodologic design (n=1), and the sample size was less than the minimum of 10 (n=1). Finally, 15 RCTs were included in our systematic review for data abstraction and critical appraisal.
Figure 1.
The flow of the literature search and publication selection process following PRISMA guidelines.
Participant characteristics and study setting
Fifteen RCTs involving 846 participants met the inclusion criteria and were included in this review (13–27). One study also included patients with chronic cutaneous lupus (CCL) (24). SLE was defined by 1982 or 1997 ACR criteria. SLE disease duration ranged between 2.5 to 21 years. SLE disease activity was low in all studies that measured. Thirteen out of 15 studies were of adult subjects, two studies included only pediatric subjects (17, 25). The mean age ranged from 13 to 53 years. 95.2 percent of subjects included in the 15 studies were female, with 8 studies enrolling only females.
Intervention and control group characteristics
Non-pharmacologic interventions varied in their content, dosage, duration, and intensity. Of the 15 trials, 8 used exercise interventions, 6 used psychological interventions (1 group psychotherapy, 3 cognitive behavioral therapies (CBT), 1 psychoeducation, 1 mindfulness-based cognitive therapy (MBCT)) and 1 used electro-acupuncture. Individual sessions varied from 15 to 90 minutes, with session frequency ranging from 1 to 3 times per week. Lengths of the overall programs ranged from 5 to 52 weeks. Five of 15 studies utilized control groups consisting of usual medical care. Other studies included control interventions of relaxation, attention placebo, symptom monitoring support, education, minimal needling, and home, isotonic and resistance exercise.
Outcome measures
Table 1 presents a summary of the overall effects of non-pharmacologic interventions on each of the outcomes. The characteristics of studies included in the review are presented in Table 2. Our reported results correspond to the data on each outcome and are summarized below.
Table 1.
Summary of Evidence and Effect of Non-pharmacologic Interventions for SLE
| Interventions | Clinical Domains and No. of studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fatigue (n=6) |
Depression (N=8) |
Pain (N=3) |
Quality of
Life (N=11) |
Disease
activity (N=12) |
||||||
| Exercise N = 8 | 2+ | 2− | 4− | 3+ | 2− | 7− | ||||
| Psychological & Counselling N = 6 | 1+ | 3+ | 1− | 1+ | 1− | 4+ | 2− | 4− | ||
| Acupuncture N = 1 | 1− | 1− | 1− | |||||||
N=Number of Studies; + overall beneficial effect; − no effect; Blank – No study
Table 2.
Non-pharmacologic Interventions in Systemic Lupus Erythematosus (SLE)
| Author Yr, Country | N | Intervention | Control | Outcomes Measure | Duration (Weeks) | Results |
|---|---|---|---|---|---|---|
| Exercise Intervention (N=8) | ||||||
| Robb-Nicholson 1989, USA (1) | 23 | Home Aerobic exercise, 30 mins × 3 times/wk × 8 wks | Non-aerobic stretching exercise, 30 mins × 3 times/wk × 8 wks |
|
8 |
|
| Ramsey-Goldman 2000, USA (2) | 10 | Aerobic exercise
|
Range of motion/muscle strengthening
exercise
|
|
32 |
|
| Tench 2003, UK (3) | 93 | Home exercise (walking, cycling, swimming), 30–50 mins × 3 times/wk × 12 wks |
|
|
12 |
|
| Miossi 2012, Brazil (4) | 28 | Supervised aerobic exercise + resistance training, 80 mins × 2 times/wk × 12 wks |
|
|
12 |
|
| Prado 2013, Brazil (5) | 19 | Supervised aerobic exercise, 30–60 mins × 2 times/wk × 12 wks | Usual medical care |
|
12 |
|
| Bogdanovic 2015, Serbia (6) | 60 | Aerobic exercise, 15 mins × 3 times/wk × 6 wks | Isotonic exercises, 30 mins × 3 times/wk × 6 wks |
|
6 |
|
| Abrahão 2016, Brazil (7) | 63 | Cardiovascular exercise (CT), 50 mins × 3 times/wk × 12 wks |
|
|
12 |
|
| Bostrom 2016, Sweden (8) | 35 |
|
Usual medical care |
|
52 |
|
| Psychological & Counselling Intervention (N=6) | ||||||
| Dobkin 2002, Canada (9) | 133 | Group psychotherapy, 90 mins × 1 time/wk × 3 months + booster session, 1 time/month × 3 months | Usual medical care |
|
12 |
|
| Karlson 2004, USA (10) | 122 | Psychoeducational intervention, 1 time, followed by a phone call once a month × 5 months | Attention Placebo + Video presentation about lupus, 45 mins once |
|
20 |
|
| Greco 2004, USA (11) | 92 | Biofeedback-assisted/Cognitive-behavioral therapy + relaxation techniques (BF/CBT), 6 sessions × 3 months |
|
|
12 |
|
| Navarrete-Navarrete 2010, Spain (12) | 45 | Cognitive-behavioral therapy + relaxation techniques + social skill training, 120 min/wk × 10 wks | Usual medical care |
|
10 |
|
| Brown 2012, USA (13) | 53 | Cognitive-behavioral therapy (CBT) + home computer modules, 3 sessions × 6 wks |
|
|
6 |
|
| Solati 2017, Iran (14) | 46 | Mindfulness-based cognitive therapy (MBCT), 45–60 mins × 1 time/wk × 8 wks | Usual medical care |
|
8 |
|
| Acupuncture Intervention (N=1) | ||||||
| Greco 2008, USA (15) | 24 | Electrical Acupuncture, 30 mins × 10 sessions × 5 weeks |
|
|
5 |
|
VAS = Visual Analog Scale; POMS = the Profile of Mood States; NIMH = the National Institutes of Mental Health Depression Scale; SLE-AI = SLE lupus activity index; FSS = Fatigue Severity Score; SF-36-PF = Short-Form Health Survey Physical Function subscale; SLAM = Systemic Lupus Activity Measure; CFS = Chalder Fatigue Scale; HADS = Hospital Anxiety and Depression Scale; SF-36 = Short-Form Health Survey; SLEDAI = The Systemic Lupus Erythematosus Disease Activity Index; SLEDAI-2K = Systemic Lupus Erythematosus Disease Activity Index 2000; BDI = Beck Depression Inventory; SCL-90-R = The Symptom Checklist 90-Revised; SLAM-R = The Systemic Lupus Activity Measure-Revised; SLAQ = Systemic lupus activity questionnaire for population studies; CES-D = The Center for Epidemiological Studies Depression scale; STRESS = Cohen’s Perceived Stress Scale; ASES = Arthritis Self-Efficacy Scale; AIMS2-pain = The Revised Arthritis Impact Measurement Scales; MPI = Multidimensional Pain Inventory; SVI = Stress Vulnerability Inventory; STAI = Spielberger’s State-Trait Anxiety Inventory; SF-MPQ = The McGill Pain Questionnaire - Short Form; PedsQL = Pediatric Health-related Quality of Life; GHQ-28 = The self-reported General Health Questionnaire
Fatigue
The effect of non-pharmacologic interventions on fatigue was evaluated in 6 studies (13–15, 18, 22, 27). Sample sizes ranged from 10 to 122 with a total of 332 subjects. In 4 studies, all subjects were female (13–15, 18). In the remaining 2 studies, the majority of the subjects were female, 98% and 96% (22, 27). Of the 6 studies, 4 used exercise interventions (2 home exercise, 2 aerobic exercise), 1 used psychological intervention (psychoeducation) and 1 used electro-acupuncture. The fatigue outcome measurement tools used in these studies were the Fatigue Severity Scale (FSS), Chalder Fatigue Scale (CFS), Visual Analog Scale (VAS), the Profile of Mood States (POMS), SF-36 vitality and one study used a fatigue scale designed specifically for lupus patients (22). Compared with the control conditions, non-pharmacologic interventions were associated with a significant improvement in fatigue in 3 out of 6 studies (2 exercise and 1 psychological intervention).
Four studies evaluated the effects of exercise on fatigue. Two studies used home exercise intervention and 2 studies used aerobic exercise intervention. Both home exercise studies demonstrated the potential benefit on fatigue (13, 15). The 2 RCTs evaluating aerobic exercise revealed no significant difference in fatigue measured by the FSS compared to muscle strengthening or isotonic exercises (14, 18). However, both studies demonstrated statistically significant improvement in fatigue after the implementation of physical activity intervention in both aerobic and isotonic exercise groups.
The RCT examined the effect of 5 months of a psychoeducation intervention on fatigue in SLE patients and showed statistically significant reduction in fatigue score measured by a fatigue scale designed specifically for lupus patients in the experimental group compared to the attention placebo control (22). In a study of electro-acupuncture intervention, 5 weeks of electro-acupuncture may improve fatigue compared to usual medical care control but not statistically significant (27).
Depression
Eight RCTs evaluated the benefits of mind-body interventions on depression or psychological function in SLE patients (13, 15, 18, 19, 21, 23, 24, 26). Sample sizes ranged from 23 to 133 with a total of 555 subjects. A majority of study subjects were female (67 – 100%) with 4 studies including only female subjects (13, 15, 18, 21). These studies used various forms of the evaluation instruments including the National Institutes of Mental Health Depression Scale (NIMH), Hospital Anxiety and Depression Scale (HADS), Beck Depression Inventory (BDI), Symptom Checklist 90-Revised (SCL-90-R), Center for Epidemiological Studies Depression scale (CES-D), Cohen’s Perceived Stress Scale (STRESS), Stress Vulnerability Inventory (SVI), Spielberger’s State-Trait Anxiety Inventory (STAI) and self-reported General Health Questionnaire (GHQ-28).
Of the 8 studies, 4 used exercise interventions (2 home exercise, 2 aerobic exercise) and 4 used psychological interventions (1 group psychotherapy, 2 CBT with relaxation techniques, 1 MBCT). Out of these eight studies, three studies reported improved psychological function. These 3 positive studies used psychological interventions including biofeedback-assisted CBT, CBT and MBCT. A RCT using biofeedback-assisted CBT intervention observed a greater improvement in psychological function compared with the control and the symptom monitoring support intervention and the improvements in psychological function persisted at 9-month follow-up, (23). Another RCT using MBCT intervention also reported similar results. The study reported significant improvement in psychological symptoms measured by GHQ-28 and mental health domains of the SF36 in MBCT groups immediately after the intervention and at 6 months follow-up (26). Similar positive findings of CBT demonstrated improvement in stress, anxiety, and depression after the intervention compared with patients in a no-intervention control. In addition, patients in the CBT also benefited in mental health domains of the SF36 (24).
In contrast, five studies found no improvement in emotional health after interventions. A RCT by Dobkin did not demonstrate a significant benefit in the psychotherapy group using the psychological measuring instruments (SCL-90-R) for SLE patients (21). This study did not find any clinically important improvement in any of the parameters including psychological distress, quality of life and disease activity compared to usual care. All four studies on exercise interventions did not demonstrate benefit in psychological function in SLE patients compared to control group (13, 15, 18,19). However, a RCT by Bogdanovic et al used isotonic exercise as a control group and showed significant improvement in depression after all physical activity interventions in both experiment and control group (18).
Pain
Three studies (2 CBT and 1 electro-acupuncture) involving 169 patients evaluated pain (23, 25, 27). The pain evaluation instruments used in these trials were the Revised Arthritis Impact Measurement Scale-Pain Subscale (AIMS2-Pain), the Multidimensional Pain Inventory (MPI), the Bodily Pain scale of the SF-36 (SF-36 BP) and the McGill Pain Questionnaire - Short Form (SF-MPQ). One out of three studies reported pain reduction after interventions (23). The study of 3 months of biofeedback-assisted CBT with relaxation techniques revealed that the CBT intervention significantly greater reductions in pain measured by the AIMS2-Pain and MPI compared with the control group (23). In contrast, the study by Brown et al found that when compared with the control group, the patients in the CBT with home computer module group had no improvement in pain measured by the SF-MPQ. However, this study did not find any significant improvement in any outcomes (25). The third RCT evaluated the effect of 5 weeks of electro-acupuncture intervention by using the AIMS2-Pain, MPI and the SF-36 BP to assess pain severity found that electro-acupuncture is feasible and safe, and may benefit for pain. Although this pilot study did not have the appropriate sample size and power to determine statistical significance of treatment benefits (27).
Quality of Life
Eleven studies with 752 participants assessed quality of life of SLE patients before and after non-pharmacologic interventions (14, 15, 18–26). Six studies involved only female subjects and majority of the SLE subjects evaluated in other studies were female. Five studies on exercise interventions (1 home exercise, 4 aerobic exercise) and 6 studies on psychological interventions (1 group psychotherapy, 3 CBT, 1 psychoeducation, 1 MBCT) were identified. Ten studies used SF-36 to evaluate quality of life. One study used only the SF-36 physical function subscale (14). One study used the Pediatric Health-related Quality of Life (PedsQL) as a measurement tool (25). The results of these studies showed that 7 out of 11 studies (3 aerobic exercise, 4 psychological interventions) indicated improvement in at least one subscale of quality of life as measured by SF-36, compared to control. Four studies (1 aerobic exercise, 1 home exercise, 2 psychological interventions) showed no significant improvement in any subscales of quality of life between the intervention groups and control groups.
Five studies evaluated the effects of exercise interventions on quality of life. Three out of these five studies demonstrated the benefit of exercise on quality of life. All of these 3 studies used aerobic exercise intervention. Bogdanovic et al reported that 6 weeks of aerobic exercise significantly improved in pain, general health and mental health subscales of SF-36 compared to isotonic exercises control (18). A three-armed, RCT reported similar results. Twelve weeks of cardiovascular exercise compared to the resistant exercise and no intervention control, statistically significantly improved on the role physical and vitality subscales of SF-36 scores (19). These 2 studies also showed that the patients in both exercise groups had significant improvement of all areas of quality of life measured by the SF-36 after physical activity intervention compared with baseline (18, 19). In addition, a RCT of aerobic exercise intervention followed by self-managed physical activity provided improvements in mental health subscale of SF-36 compared with no intervention control (20). In contrast, these findings were not confirmed in the other 2 exercise studies (14, 15). There were interesting points to address in these 2 negative studies. One study evaluated only physical function subscale of SF-36 and used a strength-training exercise as a control group. However, in this study the authors observed that both aerobic and strength-training exercises showed improvement in SF-36 physical function subscale after exercise intervention but no significant different between group (14). Another study allocated 93 SLE patients to three groups (home exercise, relaxation, and control). This was the only study that evaluated the effect of home exercise intervention on quality of life. The authors reported no significant differences in SF-36 physical function, role physical and vitality subscales between the groups after 12 weeks of intervention (15). We may conclude that aerobic exercise intervention improved quality of life in SLE patient. The effect of home exercise on quality of life remains unclear.
Six studies evaluated the beneficial effects of psychological interventions on quality of life. Four of the studies (1 psychoeducation, 2 CBT, 1 MBCT) indicated improvement in at least one of the SF-36 subscales (22–24, 26). In contrast to the results from 2 RCTs that could not demonstrate the benefit of psychological interventions on quality of life (21, 25). One RCT evaluated quality of life measured by SF-36 after 3 months of psychoeducation intervention (21). Another RCT used CBT with home computer modules. This study used the Pediatric Health-related Quality of Life (PedsQL) as a measurement tool (25). However, these 2 studies did not find any clinically important improvement in any of the outcomes.
Disease Activity
Twelve RCTs examined the effects of non-pharmacologic interventions on SLE disease activity in a total of 687 SLE patients (13–17, 19–24, 27). Seven studies used exercise interventions (2 home exercise, 5 aerobic exercise) and 4 used psychological interventions (1 group psychotherapy, 2 CBT, 1 psychoeducation) and 1 used electro-acupuncture. Indices for assessing SLE disease activity that used in these studies were SLE lupus activity index (SLE-AI), Systemic Lupus Activity Measure (SLAM), Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), Systemic Lupus Activity Measure-Revised (SLAM-R) and Systemic lupus activity questionnaire for population studies (SLAQ). None of the studies demonstrated an improvement in disease activity with 5 to 52 weeks of non-pharmacologic interventions.
Discussion
Our review indicated that several non-pharmacologic treatments showed promising results as adjuncts to current medical therapy for improving fatigue, depression, pain and quality of life. However, none of the interventions demonstrated a decrease in SLE disease activity.
Fatigue is the most prevalent symptom in SLE and affects up to 80% of patients (28) with approximately 50% of patients considering fatigue their most disabling disease symptom (29). Fatigue in SLE is complex with multifaceted origins including disease activity, mood disorders, sleep disturbance, physical inactivity and chronic pain (2, 28, 30–34). Our study found that non-pharmacological interventions were associated with a significant improvement in fatigue especially the exercise intervention with home exercise is a preferable method. Our review also suggested that psychological interventions improved depression and pain which also have a profound impact on fatigue. Non-pharmacologic therapies may be adjunctive options for improving fatigue patients with SLE.
Several studies show that the quality of life in patients with SLE is lower than in the general population (6). Pharmacologic treatments that are effective for SLE disease activity may not improve quality of life in SLE patients since quality of life is an independent outcome from SLE disease activity (35). The patients and physicians may rate SLE disease activity differently (36) with patients prioritizing quality of life over physician-derived outcomes, resulting in an unacceptable persistence of care needs not being met. Our study provides important evidence to support aerobic exercise and psychological interventions as efficacious options for promoting overall quality of life in patients with SLE. The effect of home exercise on quality of life remains unclear.
In our review, there was only one Mind-Body Practices (acupuncture) study that met inclusion criteria. This study did not have the appropriate sample size and power to determine statistical significance of treatment benefits. However, the non-pharmacologic interventions in this review are multifaceted interventions that include several components. The mind-body component including mindfulness-based meditation, are among the most frequently used mind-body interventions in SLE, however evidence to support these practices in SLE is still lacking (9). While there was one MBCT study which found considerable improvement in psychological symptoms and quality of life in patients with SLE (26), to date there is no study of Mindfulness-Based Stress Reduction (MBSR) training, one of the most recognized forms of relaxation or mindfulness-based training as developed by Kabat-Zinn (37).
Tai Chi and yoga are also widely used mind-body interventions. While the effectiveness of Tai Chi has been demonstrated in RCTs and observational studies in various rheumatic conditions such as osteoarthritis, rheumatoid arthritis, and fibromyalgia (38) its impact on patient-reported outcomes has not been evaluated in SLE patients. Yoga has also yet to be studied in SLE patients. This indicates the need for further research to evaluate the effects of the complementary or integrative therapies including MBSR, tai chi, and yoga given the lack of existing literature in this area uncovered by this study.
Strengths and limitations
Strengths
The previous reviews of non-pharmacologic interventions in SLE have included quasi-RCT, non-RCT and prospective observational studies. Our review was limited to RCTs and we included additional articles that were not included in previous reviews. A strength of this review is that we included a wide variety of non-pharmacologic interventions including exercise, psychological interventions and acupuncture. In addition, mind-body interventions for SLE have not been included in previous reviews of exercise or psychological interventions. This review also examined a wide variety of outcomes including fatigue, depression, pain, quality of life and disease activity, presenting a fuller picture of the potential benefits of these interventions.
Limitations
Many studies of non-pharmacologic interventions lack rigorous scientific methods and randomized control design, as well as have small sample size. The duration of non-pharmacologic interventions in most studies we evaluated was short. Therefore, long-term effects of non-pharmacologic interventions are still unknown. The length and intensity of interventions should be evaluated to determine optimal dose. It is also difficult to obtain overall quantitative estimates of treatment effects due to the heterogeneity of details of the intervention, and outcomes definition and measurements. Most of the outcome measures in these studies are not disease specific. Other co-morbidities and medications can confound the results. Choice of comparison groups in RCTs is a study design element that deserves careful consideration. Several studies used different types of exercise, education or relaxation interventions for the control group (13–15, 18, 19, 22, 23, 25). These control interventions sometimes prove to be efficacious and affect the outcomes so it is critical to specify the components of the comparisons. Engagement in non-pharmacologic interventions outside of the research setting is likely to enhance treatment effects both during and following study interventions. In addition, the mechanisms of the benefits from these interventions in SLE patients are not well understood.
Conclusion
This review showed promising results for physical exercise and psychological interventions as adjuncts to traditional medical therapy for improving fatigue, depression, pain and quality of life. However, many studies had small sample sizes and short intervention durations. Importantly, many complementary and alternative therapies with proven benefit in other rheumatic diseases have not been evaluated. Future high-quality RCTs studies that examine specific components of interventions, report effectiveness, short- and long-term risks and benefits of these non-pharmacologic interventions for SLE patient are needed to better integrate these interventions into the care of the SLE patient population. In addition, future studies should attempt to measure engagement in non-pharmacologic treatments outside of the research setting such as home practice and conduct follow-up assessments to evaluate the impact of continued practice on symptoms over time.
Supported by:
National Center for Complementary and Integrative Health (K24AT007323 and K23AT009374) (CW and RB) and National Center for Advancing Translational Sciences (1KL2TR002545) (SK) at the National Institutes of Health. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
References:
- 1.Tench CM, McCurdie I, White PD, D’Cruz DP. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology (Oxford). 2000;39(11):1249–54. [DOI] [PubMed] [Google Scholar]
- 2.Zonana-Nacach A, Roseman JM, McGwin G Jr., Friedman AW, Baethge BA, Reveille JD, et al. Systemic lupus erythematosus in three ethnic groups. VI: Factors associated with fatigue within 5 years of criteria diagnosis. LUMINA Study Group. LUpus in MInority populations: NAture vs Nurture. Lupus. 2000;9(2):101–9. [DOI] [PubMed] [Google Scholar]
- 3.Petri M Musculoskeletal complications of systemic lupus erythematosus in the Hopkins Lupus Cohort: an update. Arthritis Care Res. 1995;8(3):137–45. [DOI] [PubMed] [Google Scholar]
- 4.Palagini L, Mosca M, Tani C, Gemignani A, Mauri M, Bombardieri S. Depression and systemic lupus erythematosus: a systematic review. Lupus. 2013;22(5):409–16. [DOI] [PubMed] [Google Scholar]
- 5.Bachen EA, Chesney MA, Criswell LA. Prevalence of mood and anxiety disorders in women with systemic lupus erythematosus. Arthritis and rheumatism. 2009;61(6):822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McElhone K, Abbott J, Teh LS. A review of health related quality of life in systemic lupus erythematosus. Lupus. 2006;15(10):633–43. [DOI] [PubMed] [Google Scholar]
- 7.Danoff-Burg S, Friedberg F. Unmet needs of patients with systemic lupus erythematosus. Behav Med. 2009;35(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moses N, Wiggers J, Nicholas C. Persistence of unmet need for care among people with systemic lupus erythematosus: a longitudinal study. Qual Life Res. 2008;17(6):867–76. [DOI] [PubMed] [Google Scholar]
- 9.Moore AD, Petri MA, Manzi S, Isenberg DA, Gordon C, Senecal JL, et al. The use of alternative medical therapies in patients with systemic lupus erythematosus. Trination Study Group. Arthritis and rheumatism. 2000;43(6):1410–8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Wei W, Wang CM. Effects of psychological interventions for patients with systemic lupus erythematosus: a systematic review and meta-analysis. Lupus. 2012;21(10):1077–87. [DOI] [PubMed] [Google Scholar]
- 11.O’Dwyer T, Durcan L, Wilson F. Exercise and physical activity in systemic lupus erythematosus: A systematic review with meta-analyses. Semin Arthritis Rheum. 2017;47(2):204–15. [DOI] [PubMed] [Google Scholar]
- 12.del Pino-Sedeno T, Trujillo-Martin MM, Ruiz-Irastorza G, Cuellar-Pompa L, de Pascual-Medina AM, Serrano-Aguilar P, et al. Effectiveness of Nonpharmacologic Interventions for Decreasing Fatigue in Adults With Systemic Lupus Erythematosus: A Systematic Review. Arthritis Care Res (Hoboken). 2016;68(1):141–8. [DOI] [PubMed] [Google Scholar]
- 13.Robb-Nicholson LC, Daltroy L, Eaton H, Gall V, Wright E, Hartley LH, et al. Effects of aerobic conditioning in lupus fatigue: a pilot study. Br J Rheumatol. 1989;28(6):500–5. [DOI] [PubMed] [Google Scholar]
- 14.Ramsey-Goldman R, Schilling EM, Dunlop D, Langman C, Greenland P, Thomas RJ, et al. A pilot study on the effects of exercise in patients with systemic lupus erythematosus. Arthritis Care Res. 2000;13(5):262–9. [DOI] [PubMed] [Google Scholar]
- 15.Tench CM, McCarthy J, McCurdie I, White PD, D’Cruz DP. Fatigue in systemic lupus erythematosus: a randomized controlled trial of exercise. Rheumatology (Oxford). 2003;42(9):1050–4. [DOI] [PubMed] [Google Scholar]
- 16.Miossi R, Benatti FB, Luciade de Sa Pinto A, Lima FR, Borba EF, Prado DM, et al. Using exercise training to counterbalance chronotropic incompetence and delayed heart rate recovery in systemic lupus erythematosus: a randomized trial. Arthritis Care Res (Hoboken). 2012;64(8):1159–66. [DOI] [PubMed] [Google Scholar]
- 17.Prado DM, Benatti FB, de Sa-Pinto AL, Hayashi AP, Gualano B, Pereira RM, et al. Exercise training in childhood-onset systemic lupus erythematosus: a controlled randomized trial. Arthritis research & therapy. 2013;15(2):R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogdanovic G, Stojanovich L, Djokovic A, Stanisavljevic N. Physical Activity Program Is Helpful for Improving Quality of Life in Patients with Systemic Lupus Erythematosus. Tohoku J Exp Med. 2015;237(3):193–9. [DOI] [PubMed] [Google Scholar]
- 19.Abrahao MI, Gomiero AB, Peccin MS, Grande AJ, Trevisani VF. Cardiovascular training vs. resistance training for improving quality of life and physical function in patients with systemic lupus erythematosus: a randomized controlled trial. Scand J Rheumatol. 2016;45(3):197–201. [DOI] [PubMed] [Google Scholar]
- 20.Bostrom C, Elfving B, Dupre B, Opava CH, Lundberg IE, Jansson E. Effects of a one-year physical activity programme for women with systemic lupus erythematosus - a randomized controlled study. Lupus. 2016;25(6):602–16. [DOI] [PubMed] [Google Scholar]
- 21.Dobkin PL, Da Costa D, Joseph L, Fortin PR, Edworthy S, Barr S, et al. Counterbalancing patient demands with evidence: results from a pan-Canadian randomized clinical trial of brief supportive-expressive group psychotherapy for women with systemic lupus erythematosus. Ann Behav Med. 2002;24(2):88–99. [DOI] [PubMed] [Google Scholar]
- 22.Karlson EW, Liang MH, Eaton H, Huang J, Fitzgerald L, Rogers MP, et al. A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis and rheumatism. 2004;50(6):1832–41. [DOI] [PubMed] [Google Scholar]
- 23.Greco CM, Rudy TE, Manzi S. Effects of a stress-reduction program on psychological function, pain, and physical function of systemic lupus erythematosus patients: a randomized controlled trial. Arthritis and rheumatism. 2004;51(4):625–34. [DOI] [PubMed] [Google Scholar]
- 24.Navarrete-Navarrete N, Peralta-Ramirez MI, Sabio-Sanchez JM, Coin MA, Robles-Ortega H, Hidalgo-Tenorio C, et al. Efficacy of cognitive behavioural therapy for the treatment of chronic stress in patients with lupus erythematosus: a randomized controlled trial. Psychother Psychosom. 2010;79(2):107–15. [DOI] [PubMed] [Google Scholar]
- 25.Brown RT, Shaftman SR, Tilley BC, Anthony KK, Kral MC, Maxson B, et al. The health education for lupus study: a randomized controlled cognitive-behavioral intervention targeting psychosocial adjustment and quality of life in adolescent females with systemic lupus erythematosus. Am J Med Sci. 2012;344(4):274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solati K, Mousavi M, Kheiri S, Hasanpour-Dehkordi A. The Effectiveness of Mindfulness-based Cognitive Therapy on Psychological Symptoms and Quality of Life in Systemic Lupus Erythematosus Patients: A Randomized Controlled Trial. Oman Med J. 2017;32(5):378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greco CM, Kao AH, Maksimowicz-McKinnon K, Glick RM, Houze M, Sereika SM, et al. Acupuncture for systemic lupus erythematosus: a pilot RCT feasibility and safety study. Lupus. 2008;17(12):1108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krupp LB, LaRocca NG, Muir J, Steinberg AD. A study of fatigue in systemic lupus erythematosus. The Journal of rheumatology. 1990;17(11):1450–2. [PubMed] [Google Scholar]
- 29.Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria for F. Measurement of fatigue in systemic lupus erythematosus: a systematic review. Arthritis and rheumatism. 2007;57(8):1348–57. [DOI] [PubMed] [Google Scholar]
- 30.Wysenbeek AJ, Leibovici L, Weinberger A, Guedj D. Fatigue in systemic lupus erythematosus. Prevalence and relation to disease expression. Br J Rheumatol. 1993;32(7):633–5. [DOI] [PubMed] [Google Scholar]
- 31.Jump RL, Robinson ME, Armstrong AE, Barnes EV, Kilbourn KM, Richards HB. Fatigue in systemic lupus erythematosus: contributions of disease activity, pain, depression, and perceived social support. The Journal of rheumatology. 2005;32(9):1699–705. [PubMed] [Google Scholar]
- 32.Da Costa D, Dritsa M, Bernatsky S, Pineau C, Menard HA, Dasgupta K, et al. Dimensions of fatigue in systemic lupus erythematosus: relationship to disease status and behavioral and psychosocial factors. The Journal of rheumatology. 2006;33(7):1282–8. [PubMed] [Google Scholar]
- 33.McKinley PS, Ouellette SC, Winkel GH. The contributions of disease activity, sleep patterns, and depression to fatigue in systemic lupus erythematosus. A proposed model. Arthritis and rheumatism. 1995;38(6):826–34. [DOI] [PubMed] [Google Scholar]
- 34.Taylor J, Skan J, Erb N, Carruthers D, Bowman S, Gordon C, et al. Lupus patients with fatigue-is there a link with fibromyalgia syndrome? Rheumatology (Oxford). 2000;39(6):620–3. [DOI] [PubMed] [Google Scholar]
- 35.Gladman D, Urowitz M, Fortin P, Isenberg D, Goldsmith C, Gordon C, et al. Systemic Lupus International Collaborating Clinics conference on assessment of lupus flare and quality of life measures in SLE. Systemic Lupus International Collaborating Clinics Group. The Journal of rheumatology. 1996;23(11):1953–5. [PubMed] [Google Scholar]
- 36.Alarcon GS, McGwin G Jr., Brooks K, Roseman JM, Fessler BJ, Sanchez ML, et al. Systemic lupus erythematosus in three ethnic groups. XI. Sources of discrepancy in perception of disease activity: a comparison of physician and patient visual analog scale scores. Arthritis and rheumatism. 2002;47(4):408–13. [DOI] [PubMed] [Google Scholar]
- 37.J K- Z. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Dell Publishing; 1990. [Google Scholar]
- 38.Wang C Role of Tai Chi in the treatment of rheumatologic diseases. Current rheumatology reports. 2012;14(6):598–603. [DOI] [PubMed] [Google Scholar]