Abstract
Inflammatory bowel disease (IBD) is a heterogeneous group of inflammation-mediated pathologies that include Crohn’s disease and ulcerative colitis and primarily affects the colon and small intestine. Previous studies have shown that a disintegrin and metalloprotease (ADAM) 17, a membrane-bound sheddase, capable of cleaving the proinflammatory cytokine tumor necrosis factor and epidermal growth factor receptor ligands, plays a critical role in maintaining gut homeostasis and modulating intestinal inflammation during IBD. Rhomboid 5 homolog 2 (RHBDF2), a catalytically inactive member of the rhomboid family of intra-membrane serine proteinases, was recently identified as a crucial regulator of ADAM17. Here, we assessed the role of RHBDF2 in the development of colitis in the context of interleukin (IL) 10 deficiency. Il10−/−/Rhbdf2−/− mice developed spontaneous colitis and experienced severe weight loss starting at 8 weeks of age, without the need for exogenous triggers. Severity of disease pathology in Il10−/−/Rhbdf2−/− mice correlated with a dysbiotic gut microbiota and elevated Th1-associated immune responses with increased interferon gamma and IL2 production. In addition, Il10−/−/Rhbdf2−/− mice failed to maintain their epithelial cell homeostasis, although the intestinal epithelial barrier of Rhbdf2−/− mice is intact and loss of Rhbdf2 did not significantly exacerbate sensitivity to dextran sulfate sodium-induced colitis, suggesting differences in the underlying disease pathway of intestinal inflammation in this model. Taken together, our results demonstrate a critical regulatory role for RHBDF2 in the maintenance of the unique homeostasis between intestinal microbiota and host immune responses in the gut that is dysregulated during the pathogenesis of IBD.
Keywords: Inflammatory bowel disease (IBD), a disintegrin and metalloprotease (ADAM) 17, rhomboid 5 homolog 2 (RHBDF2), inactive rhomboid (iRHOM2), epidermal growth factor receptor (EGFR), tumor necrosis factor (TNF)
1. Introduction
Inflammatory bowel disease (IBD) represents a heterogeneous group of idiopathic inflammatory conditions of the colon and small intestine, affecting about 0.3% of the population [1]. Tumor necrosis factor (TNF)-mediated chronic intestinal inflammation and elevated activity of epidermal growth factor receptor (EGFR) signaling have been associated with the development of IBD [2–4]. Treatment options for IBD are relatively limited and often require long-term therapy with steroids, anti-inflammatory drugs, and/or anti-TNF biologics to slow the progression of disease [5]. Incomplete control can necessitate surgical removal of the damaged portion of the digestive tract. Because TNF levels are elevated in the serum, mucosa, and stool of IBD patients; therapeutic targeting of this signaling molecule is a seemingly logical treatment option for patients with IBD [6]. However, for reasons poorly defined, approximately 30% of all IBD patients fail to respond to anti-TNF treatment and up to 50% lose the response to anti-TNF or become intolerant over time [7, 8]. In addition, these biologic agents can increase the risk of infections and of malignancies in patients with IBD [9]. Due to these shortcomings, there is a need for the development of novel therapeutic approaches for the treatment of IBD.
The membrane-anchored metalloprotease a disintegrin and metalloprotease 17 (ADAM17) is essential for the release of a variety of membrane-anchored substrates, including TNF and EGFR ligands [10, 11], and increased expression of ADAM17 has been found in the colonic mucosa of IBD patients [12]. Although it has been shown that specific pharmacologic inhibition of ADAM17 can reduce clinical signs of chemically induced colitis [13], there are substantial concerns about therapeutically targeting ADAM17 due to its essential role in protecting the skin and intestinal barrier through activating the EGFR pathway [14]. Indeed, in a dextran sulfate sodium (DSS) mouse model of colitis, mice hypomorphic for Adam17 were highly susceptible to intestinal damage. Disease could be overcome by transforming growth factor alpha (TGFA)-mediated activation of the EGFR pathway, thus pointing to a function of ADAM17 in preserving the intestinal barrier [15].
Rhomboid 5 homolog proteins (RHBDF, also referred to as iRHOM proteins), a family of seven trans-membrane spanning molecules that are catalytically inactive members of the rhomboid family of intra-membrane serine proteases, were recently identified as crucial regulators required for ADAM17 maturation and function [16]. There are two RHBDFs (RHBDF1 and RHBDF2 ) which have a similar domain organization and overlapping as well as distinct functions [17]. The lack of robust antibodies to the RHBDF molecules has limited a systematic assessment of physiological protein expression, but databases of tissue mRNA levels indicate that RHBDF2 is enriched in hematopoietic cells relative to RHBDF1 [18]. Thus, inactivation of RHBDF2 allows tissue-specific regulation of ADAM17 by selectively preventing its maturation in immune cells, while RHBDF1 maintains homeostatic functions of ADAM17 in other tissues [16, 19, 20].
Inactivation of RHBDF2 in mice abolishes the release of TNF and consequently, Rhbdf2-deficient mice are protected from endotoxin-induced shock, which requires the release of soluble TNF from myeloid cells by ADAM17 [10, 16]. Additionally, our studies have shown that RHBDF2 is also required for the ADAM17-dependent release of heparin-binding EGF-like growth factor (HBEGF) and amphiregulin, whilst leaving the cleavage of other EGFR ligands such as TGFA intact [19], due to compensatory or redundant roles of RHBDF1 in these cell types [20].
Recent studies have shown that RHBDF2 is critical for the development of inflammation and for tissue-remodeling in a mouse model of lupus nephritis and that mice lacking RHBDF2 are protected from rheumatoid arthritis, similarly to mice lacking ADAM17 in myeloid cells [10, 20, 21]. Although these studies confirm that RHBDF2 plays a significant role in both autoimmune diseases and that RHBDF2 is involved in TNF-dependent septic shock, the role of RHBDF2 in IBD has only been perfunctorily explored [22].
Dysfunction of interleukin (IL) 10 signaling is associated with early onset IBD [23], and polymorphisms at the IL10 locus are associated with heightened risk of the development of both ulcerative colitis and Crohn’s disease [24, 25]. In mice with targeted deletion of IL10, lymphocyte development and antibody production remain unaffected; however, most mice suffer from chronic enterocolitis due to mucosal hyperplasia, inflammation, and aberrant expression of major histocompatibility complex class II expressed upon epithelial cells [26]. In this study, we have investigated the role of RHBDF2 in regulating the function of mucosal immune cells and intestinal epithelial cells using the Il10-deficient mouse model of colitis. Our findings show that loss of RHBDF2 in Il10-deficient mice dramatically increased susceptibility to spontaneous intestinal inflammation with an exaggerated Th1-associated immune response and alterations of the gut microbiota.
2. Materials and Methods
2.1. Reagents and antibodies
Reagents were obtained from the following sources: Calprotectin ELISA (MyBioSource); DSS (Ameresco); ProcartaPlex multiplex immunoassays for IL1B, IL4, IL17, IFNG, and ProcartaPlex simplex kit for TNF (Thermo Fisher Scientific); PMA (R&D Systems); RevertAid Reverse Transcriptase (Thermo Fisher Scientific); RNeasy Kit (Qiagen); SYBR Green PCR Mix (Quanta Biotech Ltd.); TGFA ELISA (Assay Biotechnology). Antibodies used for flow cytometry were purchased from Thermo Fisher Scientific or Tonbo Biosciences respectively: anti-TBX21-PE/Cy7, anti-TCRB-APC, anti-CD4-PerCP/Cy5.5, and anti-CD8-APC/Cy7. Zombie aqua fixable viability kit and True-Nuclear fix were from BioLegend. All other reagents including collagenase D, dispase, DNase, fluorescein (FITC)-dextran 4000 (FITC-dextran), kiCqStart primer sets, and Percoll were purchased from Sigma-Aldrich.
2.2. Generation of Il10−/−/Rhbdf2−/− mice
Wild type (WT) and Rhbdf2−/− mice have been described previously [16]. Il10−/− mice were purchased from Jackson laboratories (B6.129P2-Il10<tm1Cgn>/J; stock # 002251) and bred in house at the animal facility of the Carver College of Medicine, University of Iowa. Il10−/− /Rhbdf2−/− mice were generated by crossing the Rhbdf2−/− females with the Il10−/− males and then intercrossing F1 heterozygotes. For studies of spontaneous colitis, cohorts of mice were euthanized for histologic scoring of colon inflammation at predetermined time points or if they reached the humane endpoints of rectal prolapse or signs of pain and distress including poor grooming, decreased activity, and hunched posture. All animal experiments were conducted according to the institutional animal care and use committee of University of Iowa (#7031979).
2.3. Induction of DSS colitis
WT and Rhbdf2−/− mice received DSS in drinking water for 7 days as described previously [27]. Severity and inflammation of colitis were assessed based on bodyweight changes and histopathological examination. The bodyweights of all mice were measured from the date of administration to sacrifice four to five times a week. All mice were kept in a barrier facility at the Carver College of Medicine, University of Iowa with access to food and water ad libitum.
2.4. Histological analysis
Colons from WT, Rhbdf2−/−, Il10−/− and Il10−/−/Rhbdf2−/− mice were isolated and flushed with PBS to clear off the feces. Collected colons were then cut longitudinally, Swiss-rolled for formalin fixation, and paraffin-embedded [28]. Histological examination of hematoxylin and eosin-stained sections was performed in a blinded fashion by a pathologist using the standard scoring system. Tissue scoring was performed in a masked fashion using the post-examination masking method by following the principles of reproducible scoring in research [29]. Composite scores were assigned for degree of tissue inflammatory remodeling (0 – lack of lesions, 1 – mild, scattered leukocyte infiltration in lamina propria, increased height of proliferating crypts, 2 – moderate, multifocal aggregates of infiltrating leukocytes in lamina propria extending into the submucosa, increased height and proliferation of mucosa, and 3 – severe, coalescing aggregates of infiltrating leukocytes expanding lamina propria and submucosa that extend at times to the serosa, extensive proliferation and increased height of colonocytes with significant thickening of mucosa, erosions are possible along with ulcerations) and intestinal distribution (percentage affected). These scores were utilized to calculate the composite H-score for each tissue (range of 0 to 300). The H-scores were divided by 100 to yield a composite severity score for each tissue (small and large intestine) per animal.
2.5. FITC-dextran administration
To assess the effect of Rhbdf2 deficiency on intestinal permeability, we administered a non-metabolized FITC-dextran to the mice. FITC-dextran was administered by oral gavage (44 mg/100 g of bodyweight) 4 h before euthanization. Whole blood was obtained by cardiac puncture and serum was obtained by centrifugation at 5000 rpm for 15 min. Dilutions of FITC-dextran in PBS were used as a standard curve, and absorption of 100 μL of serum or standard was measured at 488 nm.
2.6. Flow cytometric analysis
Colonic mononuclear cells were isolated as described previously [30]. Briefly, colons were resected from WT, Rhbdf2−/−, Il10−/−, Il10−/−/Rhbdf2−/− mice, cleared from feces and subjected digestion buffer containing 0.5 g/L collagenase D, 0.5 g/L DNAse and 3 g/L dispase. Solutions containing digested samples were strained using a 40 µm strainer, and cells were collected by centrifugation at 1500 rpm for 7 min. Cells were resuspended in 40% Percoll solution and added to 80% Percoll solution to perform a gradient density centrifugation for 20 min at 2400 rpm. The ring obtained at the interface was collected as lymphocytes and the upper layer as epithelial cells. Cells were subsequently washed twice with FACS buffer (PBS, 1% BSA, and 0.01% NaN3). For the intracellular and nuclear staining, the cells were subjected to intracellular True-Nuclear fix and True-Nuclear perm buffers according to the manufacturers manual (BioLegend). The cells were stained with TCRB-APC, CD4-PerCP/Cy5.5, TBX21-PE/Cy7, and CD8-APC/Cy7 antibodies for 15 min (surface markers) and 30 min (intracellular markers). Cells were washed with 200 μL FACS buffer and analyzed by flow cytometry (BD LSR II). Data were acquired from 50,000 gated events per sample and further analyzed with the FlowJo software.
2.7. Quantitative real-time reverse transcription
Lymphocytes and epithelial cells were isolated from colon tissues as described previously [30], subjected to mechanical homogenization in RLT buffer, and mRNA was isolated using RNeasy Mini Kit according to the manufacturer’s protocol (Qiagen). mRNA was then reverse transcribed into cDNA using RevertAid M-MuLV reverse transcriptase, which was utilized for qPCR using a SYBR Green master mix on an Eppendorf RealPlex2 thermocycler. KiCqStart primer sets for various epidermal growth factor receptor (EGFR) ligands such as amphiregulin (AREG), betacellulin (BTC), epidermal growth factor (EGF), epiregulin (EREG), heparin-binding EGF-like growth factor (HBEGF), transforming growth factor alpha (TGFA), as well as epithelial cell-specific markers such as prostaglandin-endoperoxide synthase (PTGS) 2, keratin (KRT) 18, transglutaminase (TGM) 2, and fatty acid binding protein (FABP) 2 were utilized to quantify their gene expression in intestinal epithelial cells. mRNA expression of various inflammatory cytokines such as IL1B, IL2, IL4, IL6, IL12, IL17A, IL18, IL23, IFNG, was quantified in colonic lymphocytes utilizing KiCqStart primer sets and analyzed on an Eppendorf RealPlex2 thermocycler.
2.8. Protein expression analysis
The distal segment of colons was cut open longitudinally, followed by several washes in PBS containing penicillin and streptomycin. Colonic epithelial cells were isolated through Percoll gradient centrifugation as mentioned earlier and cultured on poly-l-lysine coated 96-well plates in the presence of DMEM supplemented with 10% calf serum, 1% penicillin/streptomycin (100 U/mL penicillium and 100 μg/mL streptomycin), and 250 μg/mL amphotericin B. The cells were cultured in the presence or absence of PMA for 2 h. The cell-based ELISA for TGFA was performed according to the manufacturer’s protocol (Assay Biotechnology). Serum and bone marrow-derived cells were isolated from WT, Rhbdf2−/−, Il10−/−, Il10−/−/Rhbdf2−/− mice as described previously [31]. TNF concentrations in the serum as well as in the supernatants from freshly isolated bone marrow cells were measured by ProcartaPlex simplex magnetic bead-based multiplex assays according to the manufacturer protocol [32]. Colons were homogenized in tissue lysis buffer [33], and centrifuged at 15000 x g for 4 min at 4oC to collect the supernatants. 50 μL of supernatants were utilized to measure the tissue-specific levels of IL1B, IL4, IL17 and IFNG by using the ProcartaPlex multiplex magnetic bead-based multiplex assay according to the manufacturer’s recommendations [32]. All samples were assayed in technical duplicates and results were analyzed using the Bio-Plex 200 system with Bio-Plex manager software version 6.1.
2.9. Fecal microbiota analysis
Experimental Rhbdf2/-, Il10−/− as well as the Il10−/− Rhbdf2/- mice were co-housed together with wild type controls at 3 weeks of age and fecal samples were collected and DNA was isolated at the indicated time points as described previously [34]. Briefly, 1–1.5 g of feces were washed in ethanol and minced. Pellets were collected by centrifuging the samples at 4000 x g for 2 min, then washed in TE buffer (10 mMol/L Tris, 1 mMol/L EDTA, pH 8.0) and incubated in TNE buffer (10 mMol/L Tris, 0.5% SDS, 1 mMol/L CaCl2) and proteinase K (50 μg/mL) at 55oC for 1 h. After incubation, samples were centrifuged and 0.4 g of starch was added to the collected supernatants. Solutions were centrifuged at 8000 x g for 3 min to pellet the starch. Next, 150 μL of a 3.5 Mol/L NaCl solution as well as 250 μL of CTAB buffer (100 mMol/L Tris, 1.4 Mol/L NaCl, 12.5 mMol/L EDTA, 20g/L CTAB, pH8.0) were added to the supernatants and incubated at 70oC for 10 min followed by addition of phenol:chloroform:isoamyl alcohol. The aqueous layer was mixed with binding buffer (4 Mol/L guanidine hydrochloride, 1 Mol/L potassium acetate, pH 5.5) and run through spin columns. After 2 washes of the filter membrane, the DNA was eluted in TE buffer. For characterization of the fecal microbiota, quantitative real-time PCR (real-time qPCR) analysis was performed with primers amplifying the genes encoding 16S rRNA from specific bacterial groups such as Enterobacteriaceae, Bacteroidaceae, Clostridiaceae Lactobacillaceae, Prevotellaceae, Bifidobacteriaceae, Verrucomicrobiaceae, Flexispira and Entamoebidae [35, 36]. The amplification and detection were performed with an Eppendorf RealPlex2 thermocycler.
2.10. Statistical analysis
All values are expressed as means ± standard error of the mean (s.e.m.). The standard error values indicate the variation between mean values obtained from at least three independent experiments. The analysis was performed with GraphPad Prism Version 7.0 and SigmaStat 3.1 software respectively. All statistical tests and outcomes are described in the corresponding figure legends. P values (p) of <0.05 were classified as statistically significant.
3. Results
3.1. Loss of Rhbdf2 expression exacerbates Il10−/− colitis.
To assess the role of RHBDF2 in the regulation of intestinal homeostasis and mucosal inflammation, we employed Il10−/− mice as an established genetic model for spontaneous IBD and crossed Il10−/− mice with Rhbdf2−/− mice. Il10−/−/Rhbdf2−/− mice were born at the expected Mendelian ratio (data not shown) and were viable but spontaneously developed severe intestinal inflammation starting at around 8 weeks of age. Although Rhbdf2−/− mice displayed no pathophysiological phenotype [16], Il10−/−/Rhbdf2−/− did not survive beyond 20 weeks (Fig. 1A). In comparison, Il10−/− mice displayed only mild signs of intestinal inflammation within 8–12 weeks of age, yielding a survival rate of more than 60% over 54 weeks (Fig. 1A). Il10−/−/Rhbdf2−/− mice developed normally for 8 weeks before experiencing accelerated weight loss with a mean ± s.e.m. decrement at 16 weeks of 17.8 ± 4.3 g versus 2.8 ± 0.2 g for Il10−/− mice compared to that of wild type (WT) controls, indicating the onset of systemic manifestations of colitis (Fig. 1B). Furthermore, Il10−/−/Rhbdf2−/− mice displayed substantially thicker and shorter colons (6.6 ± 0.5 cm) than did either Il10−/− (8.3 ± 1.1 cm) or Rhbdf2−/− mice (9.6 ± 0.6 cm), suggesting the presence of chronic inflammation with remodeling (Fig. 1C and D). To evaluate the systemic effects upon immune cell proliferation of Rhbdf2 deficiency in Il10-deficient mice, spleen weights were measured from WT, Rhbdf2−/−, Il10−/−, and Il10−/−/Rhbdf2−/− mice. Neither Rhbdf2−/− nor Il10−/− mice developed significantly larger spleens (0.095 ± 0.01 versus 0.08 ± 0.05 g) than did the WT controls (0.1 ± 0.01 g), suggesting that the Rhbdf2−/− or Il10−/− mice did not induce a strong systemic inflammatory response at this time point; however, the Il10−/−/Rhbdf2−/− mice yielded substantially larger spleens (0.2 ± 0.09 g) than did WT, Rhbdf2−/−, or Il10−/− mice (Fig. 1E).
Figure 1. RHBDF2 deficiency induced spontaneous colitis in Il10−/− mice.
Rhbdf2−/− mice were crossbred with Il10−/− mice to study the pathophysiological role of RHBDF2 in a genetic mouse model of IBD (A) Kaplan-Meier survival curves of WT, Rhbdf2−/−, Il10−/− and Il10−/−/Rhbdf2−/− mice (n = 10 per cohort, significant differences with p<0.01 between Il10−/− and Il10−/−/Rhbdf2−/− mice, log-rank test). (B) Measured bodyweights of WT, Rhbdf2−/−, Il10−/− and Il10−/−/Rhbdf2−/− mice from 1 to 16 weeks of age (n = 4 per cohort, significant differences with p<0.05 between Il10−/− and Il10−/−/Rhbdf2−/− mice, post hoc pairwise comparisons were made by using Bonferroni correction). (C) Representative colon photomicrographs from all four groups of mice at week 16. (D) Quantitative analysis of colon lengths. (E) Representative spleen images and quantitative analysis of spleen weights of mice at week 16. (F) Representative histopathological images of the large intestine of 16-week-old mice, hematoxylin and eosin (HE)-stained, bar = 150 μm. Note the inflammation separating colonic glands (*) and increased mucosa height of the right two panels. (G) Histopathological scores for the large intestines of mice at week 16. (H) Representative histopathological images of the small intestine of 16-week-old mice, HE-stained, bar = 150 μm. (I) Histopathological scores for the small intestines of 16-week-old mice. Data are representative of three independent experiments, n = 4 per cohort in D, E, G, and I. n.s., not significant, statistics were calculated using a 2-tailed, unpaired Student t test. P<0.05
Histological analysis of Il10−/−/Rhbdf2−/− colons revealed increased inflammation, with dramatically elevated levels of immune cell infiltration, submucosal edema, epithelial erosion, and distorted crypts in the large intestine (Fig. 1F). Total histological scores of the colon were 2.5 ± 0.3 and 0.4 ± 0.1 (scale 0–3) for Il10−/−/Rhbdf2−/− and Il10−/− mice, respectively (Fig. 1G), while the small intestine did not show any significant signs of inflammation and remodeling (Fig. 1H and I). These results suggest that the loss of RHBDF2 in Il10−/− mice led to a tissue-specific inflammatory phenotype in the colon, driving disease pathogenesis.
3.2. Characterization of the commensal microbiota composition in the gut of Il10−/−/Rhbdf2−/− mice.
As IL10 plays a critical role in the maintenance of gut homeostasis and deficiency of IL10 leads to a microbiota-dependent development of colitis in Il10−/− mice [37], we explored whether RHBDF2 together with IL10 played a role in gut microbiota homeostasis. We performed fecal microbiome profiling of 6-week-old as well as 16-week-old WT, Rhbdf2−/−, Il10−/−, and Il10−/−/Rhbdf2−/− mice by real-time qPCR-based quantifications of 11 bacterial species and 7 bacterial families (Fig. 2A), that not only play commensal roles in the bacterial homeostasis within the gut but are known to contribute to pathology during the development of IBD [38]. Closer investigation of the heat map analysis demonstrated an overall higher abundance of A. muciniphila, and γ-Proteobacteria in both Il10−/− and Il10−/−/Rhbdf2−/− mice compared to that of WT controls. Importantly, there were no significant differences in the analyzed bacterial groups between Rhbdf2−/− and WT controls. Extended bar plot analysis revealed significant differences in 3 out of the 18 examined bacterial groups between Il10−/− and Il10−/−/Rhbdf2−/− mice with Enterobacteriaceae (Fig. 2B), and E. coli (Fig. 2 C), being significantly higher in Il10/Rhbdf2−/− mice along with B. acidifaciens (Fig. 2D). Although we did not find any significant differences between Rhbdf2−/− mice and WT controls or between young (6 weeks) or aged mice (16 weeks), we detected significant differences in the gut microbiome of Il10/Rhbdf2−/− mice when compared to Il10−/− mice, even at an early age where a disease phenotype was not apparent.
Figure 2. Significant differences in the gut microbiota of Il10−/−/Rhbdf2−/− mice.
Quantitative representation of the various mucosa-associated bacterial populations in 6-week-old mice (A, upper panel) and 16-week-old WT, Rhbdf2−/−, Il10−/− and Il10−/−/Rhbdf2−/− mice. (A, lower panel) Normalized heat map analysis of data obtained from three independent cohorts with n = 4 mice per group comparing the relative abundance of various microbial families in fecal DNA by using 16S rRNA gene-targeted group specific primers for real-time qPCR. Dark red indicates the higher bacterial gene expression in the fecal DNA. Relative abundance of three most prevalent bacterial families (B) Enterobacteriaceae, (C) E. coli, and (D) B. acidifaciens in Il10−/−/Rhbdf2−/− mice compared to that of WT, Rhbdf2−/−, Il10−/− mice (three independent collections with n = 4 mice per group). The bars represent means ± s.e.m. of the relative difference which is the fold amplification of sequences for the target population relative to amplification of universal bacterial domain sequences. n.s., not significant. Pairwise multiple comparison procedures for (B-D) were calculated using one-way analysis of variance with Tukey post hoc test. * indicates a significant increase in 16S rRNA levels relative to Il10−/− mice. P<0.05
Taken together, our data suggest that the loss of RHBDF2 contributes to the dysregulation of previously shown colitogenic bacterial families in Il10-deficient mice at early stages of development, which could potentially lead to increased severity of disease over time.
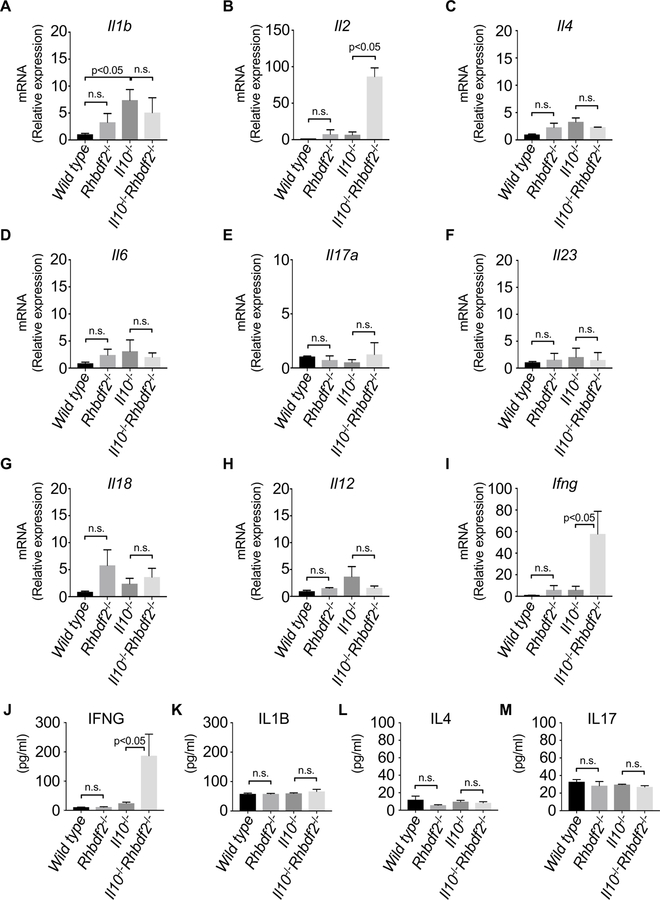
3.3. Increased expression of Th1-associated cytokine transcripts in Il10−/−/Rhbdf2−/− mononuclear cells.
Il10-deficient mice have an exaggerated CD4+ T cell response in the gut and increased production of the inflammatory cytokines IL12, IL17, and IFNG [39]. To assess the levels of inflammatory markers in the gut, we compared the cytokine expression patterns of mononuclear cells from Rhbdf2−/−, Il10−/−, or Il10−/−/Rhbdf2−/− with that of WT controls. Although Il1b mRNA expression was overall elevated in Rhbdf2−/−, Il10−/−, and Il10−/−/Rhbdf2−/− mice compared to that of WT controls, we observed no significant differences in Il1b mRNA expression between Il10−/− and Il10−/−/Rhbdf2−/− mice (Fig. 3A). In contrast, Il2 expression in Il10−/−/Rhbdf2−/− mice increased by nearly 100-fold (Fig. 3B), consistent with an exaggerated Th1-like immune response. However, we found no significant differences in Il4 (Fig. 3C) nor Il6 (Fig. 3D), Il17a (Fig. 3E) or Il23 (Fig. 3F) mRNA expression in Il10−/−/Rhbdf2−/− mice compared to that of Il10−/− mice. Additionally, we sought to determine whether cytokines associated with epithelial cell proliferation and repair were dysregulated in Il10−/−/Rhbdf2−/− mice. We found no significant difference in Il18 expression in Il10−/−/Rhbdf2−/− mice compared to that of Il10−/− mice but slightly increased levels Il18 expression in the Rhbdf2−/− mice (Fig. 3G). Although relative expression of Il12 (Fig. 3H), a well-established inducer of Th1 cells, did not show significant differences, the Th1-derived cytokine expression of Ifng was increased approximately 40 to 50-fold in Il10−/− /Rhbdf2−/− mice compared to that of Il10−/− mice (Fig. 3I). Additionally, we determined the levels of circulating cytokines of 16-week-old mice by a bead-based multiplex assay. In agreement with an increased mRNA expression of Ifng, we observed a marked increase in IFNG secretion in Il10−/− mice in the absence of RHBDF2 (Fig. 3J). Levels of IL1B (Fig. 3K), IL4 (Fig. 3L), and IL17 (Fig. 3M) were not significantly different in either group. Finally, we observed no significant differences in IL18 protein levels between WT and Rhbdf2−/− mice in contrast to mRNA expression analysis (data not shown). Taken together, these data demonstrated that Il10−/− /Rhbdf2−/− mice displayed a substantial and specific increase in the Th1-associated response that could potentially drive enhanced colitis and suggesting that RHBDF2 may safeguard gut homeostasis by curbing an exaggerated Th1 response to gut microbiota.
Figure 3. Increased expression of Th1-derived cytokines in colons of Il10−/−/Rhbdf2−/− mice.
Relative mRNA expression levels of various inflammatory mediators (A) Il1b, (B) Il2, (C) Il4, (D) Il6, (E) Il17a, (F) Il23, (G) Il18, (H) Il12, and (I) Ifng quantified using gene-specific primers in colons of WT, Rhbdf2−/−, Il10−/− and Il10−/−/Rhbdf2−/− mice at week 16 (three pooled experiments with n = 4 mice per group). Actin beta and TATA-box binding protein were used as internal controls for the normalization of the expression levels. Levels of (J) IFNG, (K) IL1B, (L) IL4, and (M) IL17 were determined by bead-based multiplex assay from colonic homogenates of 16-week-old WT, Rhbdf2−/−, Il10−/− and Il10−/−/Rhbdf2−/− mice. n.s., not significant, statistics were calculated using a 2-tailed, unpaired Student t test. P<0.05
3.4. Il10−/−/Rhbdf2−/− mice have an elevated Th1 T cell response.
To further characterize the elevated Th1 response that we observed in the Il10−/−/Rhbdf2−/− mice, we tested whether Rhbdf2 deficiency altered the composition of inflammatory effector T cells in the gut that ultimately culminated in severe colitis. Flow cytometric analysis confirmed an increase in immune cells, in agreement with our histopathological analysis. Specifically, we observed an increased frequency of CD4+TCRB+ T cells into the colon lamina propria of Il10−/− /Rhbdf2−/− mice (Fig. 4A). Among CD4+TCRB+ T cells, we observed a significant increase in the TBX21+ lymphocytes in the colons of Il10−/−/Rhbdf2−/− compared to that of WT, Rhbdf2−/−, or Il10−/− mice (Fig. 4B), suggesting that RHBDF2 played a critical role in specifically regulating a Th1-like response in this model.
Figure 4. RHBDF2 deficiency elicits Th1 immune response in Il10−/− mice.
Lamina propria mononuclear cells were isolated from colons of WT, Rhbdf2−/−, Il10−/− and Il10−/−/Rhbdf2−/− mice at week 16. (A) Representative dot plots of flow cytometric analysis of CD4+ T lymphocytes gated on live TCRB+ cell populations. Representative of three pooled experiments with n = 4 mice per group. (B) Summary analysis of the frequency of Th1, TCR+CD4+TBX21+ populations among the CD4+ cells from colons at week 16, three pooled experiments with n = 4. n.s., not significant. Pairwise multiple comparison procedures were calculated using one-way analysis of variance with Tukey post hoc test. * indicates a significant increase in TCR+CD4+TBX21+ cells relative to Il10−/− mice. P<0.05
3.5. The loss of Rhbdf2 expression yields epithelial cell damage leading to a dysfunctional immune barrier in the colons of Il10−/−/Rhbdf2−/− mice.
Given the severe colitis spontaneously contracted by Il10−/−/Rhbdf2−/− mice compared to Il10−/− controls, we examined whether Il10−/−/Rhbdf2−/− mice displayed a compromised epithelial cell barrier in the intestinal lumen. To assess the structural integrity of the epithelial barrier following the loss of Il10−/−/Rhbdf2−/− mice, we quantified intestinal permeability through the administration of FITC-dextran. Il10−/−/Rhbdf2−/− mice displayed substantially higher levels of FITC-dextran in serum than did Rhbdf2−/−, Il10−/−, or WT controls (Fig. 5A), suggesting that the loss of RHBDF2 in Il10−/− mice resulted in increased intestinal permeability. We next assessed levels of calprotectin, which is a biomarker of neutrophil activation and inflammation [40], in the serum and in feces from WT, Rhbdf2−/−, Il10−/−, and Il10−/−/Rhbdf2−/− mice. Both serum and fecal calprotectin levels were elevated in Il10−/−/Rhbdf2−/− mice, indicating the presence of elevated neutrophil activity in the gut (Fig. 5B and C). Next, we examined the expression levels of genes associated with epithelial cell repair and inflammation in gut pathophysiology. In the inflamed colon, prostaglandin-endoperoxide synthase 2 (PTGS2) is often upregulated with mucosal tissue damage repair [41]. Il10−/−/Rhbdf2−/− mice displayed an increase in Ptgs2 transcriptional levels compared to that in WT, Rhbdf2−/− or Il10−/− mice (Fig. 5D), suggesting that the loss of RHBDF2 in IBD activates transcriptional pathways associated with tissue repair as the intestinal epithelium becomes compromised. We also examined the expression levels of keratin (Krt) 18, an epithelial cell intermediate filament protein that has been implicated in the structural alteration of epithelial barrier function in IBD [42]. Krt18 transcriptional levels were also increased in Il10−/−/Rhbdf2−/− mice (Fig. 5E), further corroborating our observation that intestinal inflammation present in the colons of Il10−/−/Rhbdf2−/− mice led to upregulation of epithelial cell repair. Fatty acid binding protein 2 (FABP2) and transglutaminase 2 (TGM2) have both been implicated in the development and progression of IBD [43–45]. In addition, ADAM17/EGFR signaling mechanisms have been shown to regulate TGM2 activity during epithelial cell differentiation [46]. Transcriptional levels of FABP2 and TGM2 in Il10−/−/Rhbdf2−/− mice were found to be similar to that of Rhbdf2−/− or Il10−/− mice and WT controls, indicating that RHBDF2 may not be responsible for the regulation of FABP2 and TGM2 at the genetic level under chronic inflammation (Fig. 5F and G). Collectively, these data suggest that Il10−/−/Rhbdf2−/− mice display a severely compromised epithelial barrier function of the colon.
Figure 5. Il10−/−/Rhbdf2−/− mice exhibit severe colonic epithelial damage thereby permeability.
(A) Measurement of intestinal permeability by quantifying the serum FITC- dextran levels after 4 h of administration through oral gavage. Detection of neutrophil-specific marker calprotectin levels in (B) serum and (C) feces of WT, Rhbdf2−/−, Il10−/− and Il10−/−/Rhbdf2−/− mice using ELISA (three pooled experiments with n = 4 per group). Relative mRNA expression analysis of epithelial damage and repair marker genes (D) Prostaglandin-endoperoxide synthase 2 (Ptgs2), (E) Keratin 18 (Krt18), (F) Fatty acid binding protein (Fabp) 2, and (G) Transglutaminase (Tgm) 2 (three pooled experiments with n = 4 mice per group). Actin beta and TATA-box binding protein were used as internal controls for the normalization of the expression levels. n.s., not significant, statistics were calculated using a 2-tailed, unpaired Student t test.
3.6. Lack of RHBDF2 does not affect upregulation and shedding of epithelial TGFA in response to colitis-induced intestinal injury and ulceration
As ADAM17 plays a critical role in the cleavage of EGFR ligands that aid in tissue repair mechanisms of intestinal epithelium during chemically induced IBD [15], we sought to determine whether Il10−/−/Rhbdf2−/− mice have a deficiency in an EGFR-dependent tissue repair pathway. We evaluated the mRNA expression levels of various EGFR ligands in primary colonic epithelial cells isolated from the mucosa of 16-week-old mice. We first evaluated TGFA which has been shown to play a crucial role in tissue repair and regeneration of the epithelial barrier [47]. In agreement with an engagement of an active repair pathway in response to damage, Il10−/− /Rhbdf2−/− mice demonstrated a dramatic increase in Tgfa expression (Fig. 6A). Previous studies have shown that Hbegf mRNA transcript can be induced following intestinal injury and is involved in epithelial cell repair, proliferation, and regeneration in early stages of injury [48]. We observed a similar increase in Hbegf mRNA levels in Il10−/−/Rhbdf2−/− mice, further suggesting the induction of a transcriptional network geared toward intestinal epithelial mucosa repair (Fig. 6B). Il10−/−/Rhbdf2−/− mice also displayed upregulated mRNA levels of epiregulin (EREG) compared to that of controls (Fig. 6C); however, we did not detect elevated transcript levels of amphiregulin (AREG) upon the loss of RHBDF2 in Il10−/−/Rhbdf2−/− mice (Fig. 6D), suggesting specific regulation of EGFR ligands in response to inflammation. Betacellulin (BTC) and epidermal growth factor (EGF) are both substrates for ADAM10 which have been implicated in tissue repair and wound healing [47, 49, 50]. Interestingly, both Btc and Egf mRNA expression were dramatically upregulated in Il10−/−/Rhbdf2−/− mice, indicating that the loss RHBDF2 in Il10−/− /Rhbdf2−/− mice also led to the upregulation of ADAM10 substrates involved with tissue repair in the colonic epithelium (Fig. 6E and F). Taken together, these results support the hypothesis that a compromised immune barrier in the intestinal epithelium in Il10−/−/Rhbdf2−/− mice leads to the upregulation of multiple EGFR ligand genes to promote recovery of the intestinal epithelial barrier.
Figure 6. Il10−/−/Rhbdf2−/− mice do not exhibit impaired TGFA shedding.
Real-time qPCR analysis of mRNA expression levels of EGFR ligands (A) transforming growth factor alpha (Tgfa), (B) heparin-binding EGF-like growth factor (Hbegf), (C) epiregulin (Ereg), (D) amphiregulin (Areg), (E) betacellulin (Btc) and (F) epidermal growth factor (Egf) in colonic epithelial cells isolated from WT, Rhbdf2−/−, Il10−/− and Il10−/−/Rhbdf2−/− mice (two pooled experiments with n = 8 mice per group). (G) Detection of TGFA shedding by performing ELISA for the soluble TGFA after stimulation of colonic epithelial cells with PMA for 2 h. Constitutive TGFA was normalized to quantify the fold change increase after stimulation with PMA (representative of two independent experiments with n = 8 mice per group). n.s., not significant, statistics were calculated using a 2-tailed, unpaired Student t test. P<0.05
Recent work has demonstrated that TGFA aids in the proliferation of intestinal epithelial cells in an ADAM17-dependent manner and plays critical role in tissue repair of gut epithelium during pathogenesis of IBD [15]. However, lack of RHBDF2 does not affect the shedding of overexpressed TGFA from mouse embryonic fibroblasts [19]. To determine whether RHBDF2 plays a role in the regulation of endogenous TGFA shedding in the colonic epithelium during experimental IBD, we monitored the constitutive and stimulated shedding of TGFA from mucosal epithelial cells isolated from large intestine of 16-week-old mice. Following stimulation with the PKC-activating phorbol ester PMA, often used as a strong inducer of ADAM17, WT, Rhbdf2−/−, Il10−/−, and Il10−/−/Rhbdf2−/− intestinal epithelial cells all released increased levels of endogenous soluble TGFA (Fig. 6G). Il10−/−/Rhbdf2−/− epithelial cells produced the highest levels of TGFA shedding, concordant with its elevated expression levels in Il10−/−/Rhbdf2−/− mice, indicating that TGFA release was not affected by the loss of RHBDF2 in Il10−/−/Rhbdf2−/− mice (Fig. 6G). This finding suggests that Il10−/−/Rhbdf2−/− mice retained the capacity to initiate a TGFA-mediated epithelial repair mechanism during the pathogenesis of experimental IBD.
3.7. RHBDF2−/− mice demonstrate no differences from controls in DSS-induced colitis
The EGFR ligand TGFA plays a crucial role in the regeneration of intestinal epithelial cells through an ADAM17-dependent mechanism in chemically induced colitis [15]. As we did not find an impairment in TGFA shedding in the Il10−/−/Rhbdf2−/− deficient mice, we sought to determine whether RHBDF2 was required for intestinal repair during chemically induced colitis. WT controls and Rhbdf2−/− mice were treated with 5% DSS and bodyweight of each genotype was measured over 6 days. WT and Rhbdf2−/− mice experienced significant weight loss over the 6-day period indicating the onset of metabolic stress due to the development of colitis. However, we observed no significant difference in weight loss between the WT and Rhbdf2−/− mice, implying that RHBDF2 was not required for epithelial repair in this model. (Fig. 7A). When mice were allowed to recover after DSS treatment, all mice treated with 5% DSS died by day 2 of the recovery period (data not shown). To mitigate the morbid effects of high dose DSS administration and to enable our studies on the epithelial repair mechanism during the recovery phase, a decreased dose of 2% DSS treatment was used. Both WT controls and Rhbdf2−/− mice lost significant bodyweight, with no difference in weight loss between the genotypes (Fig. 7B). After DSS removal mice recovered to become free of symptoms with nearly complete recovery by week 2 as ascertained by histopathology (Fig. 7C and D). WT and Rhbdf2−/− colons from mice at 7 days of DSS treatment showed a similar injury composed of epithelial injury/loss and leukocyte infiltration (Fig. 7C, upper panels) as well as similar mucosal repair and disease recovery during 6 days after a single cycle of DSS (Fig. 7C, lower panels), with similar total histological scores (Fig. 7D). In conclusion, Rhbdf2 deficiency did not increase disease severity in DSS colitis and epithelial repair mechanisms appeared functional, in contrast to defective intestinal barrier healing in Adam17-hypomorphic mice treated with DSS [15], suggesting that the epithelial damage in Il10−/−/Rhbdf2−/− mice could be possibly due to increased inflammation rather than a defect in epithelial repair.
Figure 7. Lack of RHBDF2 does not affect the course of DSS-induced murine colitis.
Percent of initial bodyweights for WT and Rhbdf2−/− mice ingesting (A) 5% w/v dextran sulfate sodium (DSS) and (B) 2% w/v DSS is plotted. Representative of three independent experiments with n = 5 mice per group. n.s., not significant. (C) Representative large intestine photomicrographs of WT and Rhbdf2−/− mice treated with 2% w/v DSS at day 7 (d7) and day 13 (d13), respectively. Note the mucosal lesion (asterisks) composed of epithelial injury/loss and leukocyte infiltration at d7, that is repaired to near normal by d13, bars = 150 μm. (D) Histopathological scores in for images in (C). Pairwise multiple comparison procedures were calculated using two-way analysis of variance with Tukey post hoc test. P values are relative to day 0 (*) and day 7 (#) respectively. P<0.05
3.8. Il10−/−/Rhbdf2−/− mice display a decreased capacity to produce soluble TNF
TNF plays an essential role in both host defense against microbial infections as well as type 1 immunity responses [51], and loss of TNF signaling increases the risk of bacterial infections [52]. To determine whether loss of RHBDF2 results in lower levels of shed TNF, we monitored soluble TNF in serum as well as its release from bone marrow cells extracted from WT, Rhbdf2−/−, Il10−/−, Il10−/−/Rhbdf2−/− mice. We found a significant decrease in the serum TNF protein levels in both Rhbdf2−/− and Il10−/−/Rhbdf2−/− mice compared to that of WT and Il10−/− mice (Fig. 8A). In addition, we also observed moderately elevated levels of TNF in the Il10−/− mice. Bone marrow- derived cells from these mice also showed a similar pattern in TNF secretion (Fig. 8B). These results suggest that loss of RHBDF2-dependent shedding of TNF from myeloid cells in Il10−/− /Rhbdf2−/− mice might contribute to the dysregulation of Th1-associated immune responses leading to loss of intestinal epithelial integrity and tissue architecture.
Figure 8. Il10−/−/Rhbdf2−/− mice show reduced levels of circulating TNF.
Analysis of (A) serum levels of TNF and (B) TNF production in primary bone marrow cells from WT, Rhbdf2−/−, Il10−/− and Il10−/−/Rhbdf2−/− mice, as determined by bead-based multiplex assay. Analysis of three pooled experiments with n = 4. Multiple comparison procedures versus control group were calculated using one-way analysis of variance with Dunnett’s Method. P values are relative to WT controls (*). P<0.05
4. Discussion
Recent studies have associated the proinflammatory cytokine TNF with impaired intestinal homeostasis, compromised barrier function, and the pathogenesis of intestinal inflammation [53]. Membrane-bound TNF is proteolytically cleaved by the membrane-anchored metalloprotease ADAM17, releasing biologically active soluble TNF [10, 54, 55]. Recently, RHBDF2, a proteolytically inactive member of the rhomboid family, has been identified as a hematopoietic specific regulator of ADAM17-mediated shedding of TNF [16, 20], and prompted us to investigate its role in the pathogenesis of IBD.
Using a non-injury genetic model of IBD, we demonstrate that loss of RHBDF2 promotes an earlier onset and increased severity of colitis in Il10−/− mice. This enhanced colitis is associated with early colonization by potentially pathogenic bacteria and the subsequent development of a predominant Th1 response. In the absence of RHBDF2 and IL10, we observed evidence of chronic, widespread colonic inflammation and remodeling, indicating a critical role for RHBDF2 in maintaining the gut homeostasis in this model.
Previous studies have demonstrated that TNF modulates the composition of the gut microbiota and that the severity of disease is correlated with specific microbial shifts [56]. In our study, the relative abundance of colitogenic pathogens such as Enterobacteriaceae including E. coli, was significantly increased in Il10−/−/Rhbdf2−/− mice compared to that of Il10−/− mice. These results mirror previous findings in IBD patients, who harbor higher levels of Enterobacteriaceae than do healthy controls [38, 57, 58]. Similarly, B. acidifaciens, a Gram-negative, obligate anaerobic bacterium that has been identified as a phylotype of acute colitis and mucin degrader [59], was also enriched in Il10−/−/Rhbdf2−/− mice relative to Rhbdf2−/−, Il10−/− and WT controls. Furthermore, blockade of TNF in the Il10-deficient mouse model also leads to amelioration of disease [60]. Paradoxically however, mice with genetic deficiencies of both TNF and IL10 spontaneously develop severe colitis in response to microbial exposure soon after weaning, without the need for additional triggers [61], akin to what we report here in Il10−/−/Rhbdf2−/− mice. Our results support the idea that the loss of soluble TNF in Il10−/−/Rhbdf2−/− mice might contribute to microbial dysbiosis through a lack of RHBDF2-mediated-TNF signaling mechanism. Further studies are required to determine whether immune mechanisms are affected during early intestinal colonization in Il10−/−/Rhbdf2−/− and Il10−/−/Tnf−/− mice. It is also possible that altered bacterial composition and expansion in the colon drives the pathogenesis of IBD as a direct result of dysbiosis of gut microbiota that is associated with aberrant TNF signaling in the context of IL10 [62, 63].
Il10−/−/Rhbdf2−/− mice displayed a Th1-like lymphocyte response in the course of colitis. These results suggest that RHBDF2 may play a role in the development and maintenance of T lymphocyte homeostasis in IBD through the modulation of the Th1-associated response. It will be interesting to dissect out the mechanism by which RHBDF2 downregulates Th1-like responses, presumably in response to altered bacterial populations in the gut in the absence of IL10.
Previously, it has been shown that the shedding of EGFR ligands induces expression and activation of these same ligands in a feed-forward loop [64]. Il10−/−/Rhbdf2−/− mice showed enhanced expression levels of EGFR ligands, suggesting that RHBDF2/ADAM17-independent shedding events of EGFR ligands such as TGFA by the related RHBDF1/ADAM17 [19], may be sufficient to induce EGFR ligand expression for epithelial repair in IBD. Interestingly, Tgfa expression and stimulated shedding of TGFA were not significantly affected by the loss of RHBDF2 in Il10−/− mice, implying that tissue repair through the TGFA-activated EGFR pathway alone might be insufficient for maintaining an intact colonic epithelium, particularly under chronic colitis conditions. It is therefore reasonable to postulate that the loss of RHBDF2 in Il10−/− mice might result in the loss of specific RHBDF2-dependent shedding events that may contribute to the integrity of the intestinal barrier during IBD. However, given that the loss of ADAM17-mediated shedding of TGFA renders mice more susceptible to DSS-induced inflammation [15], while Rhbdf2−/− mice, similar to homozygous Rhbdf2sinecure mutant mice [22], showed no greater sensitivity to DSS than WT controls, our data suggest that disease pathology in Il10−/−/Rhbdf2−/− mice might result from an exaggerated Th1-like response. Nonetheless, it is also possible that increased Th1-associated responses in Rhbdf2−/− mice together with compromised intestinal epithelial barrier functions promote an earlier onset and increased severity of colitis in Il10−/− mice. Therefore, it will be interesting to dissect the unique properties of substrates that are involved in preserving the epithelial barrier under an IL10/TNF signaling axis.
Taken together, our study reveals that, unlike the deficiency of ADAM17, which results in multiple inflammatory manifestations including colitis [15, 65], loss of RHBDF2 alone does not compromise the integrity of the epithelial barrier at homeostasis. However, in the context of IL10 deficiency, and possibly other genetic susceptibilities, RHBDF2 plays an important role in safeguarding the integrity of the intestinal barrier. Further studies are warranted to examine the crosstalk between RHBDF2 and other signaling pathways that ensure homeostatic control of gut microbiota composition as well as barrier integrity early in life.
6. Acknowledgements
Support was provided in part by the American Cancer Society (Award Number ACS-IRG-15– 176-41) and by the Carver College of Medicine, University of Iowa Research Start-Up Funds to T.M. We thank Dr. Tak W. Mak from the Campbell Family Institute for Breast Cancer Research, Princess Margaret Cancer Center, University Health Network (Toronto, ON, Canada) for kindly providing us with the Rhbdf2−/− mice and the staff of The Office of Animal Resources of the University of Iowa for their help in taking care of these mice. The data presented herein were obtained in part at the Flow Cytometry Facility, which is a Carver College of Medicine / Holden Comprehensive Cancer Center core research facility at the University of Iowa. The facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran’s Administration Medical Center.
Abbreviations
- ADAM17
a disintegration and metalloprotease 17
- AREG
amphiregulin
- BTC
betacellulin
- DSS
dextran sulfate sodium
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EREG
epiregulin
- HBEGF
heparin-binding EGF-like growth factor
- IBD
inflammatory bowel disease
- IL
interleukin
- iRHOM
inactive rhomboid
- PMA
phorbol 12-myristate 13-acetate
- RHBDF2
rhomboid 5 homolog 2
- TGFA
transforming growth factor alpha
- TNF
tumor necrosis factor
Footnotes
Disclosures
The authors declare no conflicts of interest.
8. References
- 1.Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, Panaccione R, Ghosh S, Wu JCY, Chan FKL, Sung JJY, Kaplan GG (2018) Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778. [DOI] [PubMed] [Google Scholar]
- 2.Neurath MF (2014) Cytokines in inflammatory bowel disease. Nat Rev Immunol 14, 329–42. [DOI] [PubMed] [Google Scholar]
- 3.Svrcek M, Cosnes J, Tiret E, Bennis M, Parc Y, Flejou JF (2007) Expression of epidermal growth factor receptor (EGFR) is frequent in inflammatory bowel disease (IBD)-associated intestinal cancer. Virchows Arch 450, 243–4. [DOI] [PubMed] [Google Scholar]
- 4.Lu N, Wang L, Cao H, Liu L, Van Kaer L, Washington MK, Rosen MJ, Dube PE, Wilson KT, Ren X, Hao X, Polk DB, Yan F (2014) Activation of the epidermal growth factor receptor in macrophages regulates cytokine production and experimental colitis. J Immunol 192, 1013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triantafillidis JK, Merikas E, Georgopoulos F (2011) Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther 5, 185–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Heel DA, Udalova IA, De Silva AP, McGovern DP, Kinouchi Y, Hull J, Lench NJ, Cardon LR, Carey AH, Jewell DP, Kwiatkowski D (2002) Inflammatory bowel disease is associated with a TNF polymorphism that affects an interaction between the OCT1 and NF(-kappa)B transcription factors. Hum Mol Genet 11, 1281–9. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein GR (2013) Comprehensive review: antitumor necrosis factor agents in inflammatory bowel disease and factors implicated in treatment response. Therap Adv Gastroenterol 6, 269–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billmeier U, Dieterich W, Neurath MF, Atreya R (2016) Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J Gastroenterol 22, 9300–9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonovas S, Fiorino G, Allocca M, Lytras T, Nikolopoulos GK, Peyrin-Biroulet L, Danese S (2016) Biologic Therapies and Risk of Infection and Malignancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin Gastroenterol Hepatol 14, 1385–1397 e10. [DOI] [PubMed] [Google Scholar]
- 10.Horiuchi K, Kimura T, Miyamoto T, Takaishi H, Okada Y, Toyama Y, Blobel CP (2007) Cutting edge: TNF-alpha-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J Immunol 179, 2686–9. [DOI] [PubMed] [Google Scholar]
- 11.Blobel CP (2005) ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 6, 32–43. [DOI] [PubMed] [Google Scholar]
- 12.Brynskov J, Foegh P, Pedersen G, Ellervik C, Kirkegaard T, Bingham A, Saermark T (2002) Tumour necrosis factor alpha converting enzyme (TACE) activity in the colonic mucosa of patients with inflammatory bowel disease. Gut 51, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma M, Mohapatra J, Wagh A, Patel HM, Pandey D, Kadam S, Argade A, Deshpande SS, Shah GB, Chatterjee A, Jain MR (2014) Involvement of TACE in colon inflammation: a novel mechanism of regulation via SIRT-1 activation. Cytokine 66, 30–9. [DOI] [PubMed] [Google Scholar]
- 14.Franzke CW, Cobzaru C, Triantafyllopoulou A, Loffek S, Horiuchi K, Threadgill DW, Kurz T, van Rooijen N, Bruckner-Tuderman L, Blobel CP (2012) Epidermal ADAM17 maintains the skin barrier by regulating EGFR ligand-dependent terminal keratinocyte differentiation. J Exp Med 209, 1105–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalaris A, Adam N, Sina C, Rosenstiel P, Lehmann-Koch J, Schirmacher P, Hartmann D, Cichy J, Gavrilova O, Schreiber S, Jostock T, Matthews V, Hasler R, Becker C, Neurath MF, Reiss K, Saftig P, Scheller J, Rose-John S (2010) Critical role of the disintegrin metalloprotease ADAM17 for intestinal inflammation and regeneration in mice. J Exp Med 207, 1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIlwain DR, Lang PA, Maretzky T, Hamada K, Ohishi K, Maney SK, Berger T, Murthy A, Duncan G, Xu HC, Lang KS, Haussinger D, Wakeham A, Itie-Youten A, Khokha R, Ohashi PS, Blobel CP, Mak TW (2012) iRhom2 regulation of TACE controls TNF-mediated protection against Listeria and responses to LPS. Science 335, 229–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christova Y, Adrain C, Bambrough P, Ibrahim A, Freeman M (2013) Mammalian iRhoms have distinct physiological functions including an essential role in TACE regulation. EMBO Rep 14, 884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Maretzky T, Weskamp G, Monette S, Qing X, Issuree PD, Crawford HC, McIlwain DR, Mak TW, Salmon JE, Blobel CP (2015) iRhoms 1 and 2 are essential upstream regulators of ADAM17-dependent EGFR signaling. Proc Natl Acad Sci U S A 112, 6080–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maretzky T, McIlwain DR, Issuree PD, Li X, Malapeira J, Amin S, Lang PA, Mak TW, Blobel CP (2013) iRhom2 controls the substrate selectivity of stimulated ADAM17-dependent ectodomain shedding. Proc Natl Acad Sci U S A 110, 11433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Issuree PD, Maretzky T, McIlwain DR, Monette S, Qing X, Lang PA, Swendeman SL, Park-Min KH, Binder N, Kalliolias GD, Yarilina A, Horiuchi K, Ivashkiv LB, Mak TW, Salmon JE, Blobel CP (2013) iRHOM2 is a critical pathogenic mediator of inflammatory arthritis. J Clin Invest 123, 928–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qing X, Chinenov Y, Redecha P, Madaio M, Roelofs JJ, Farber G, Issuree PD, Donlin L, McLlwain DR, Mak TW, Blobel CP, Salmon JE (2018) iRhom2 promotes lupus nephritis through TNF-alpha and EGFR signaling. J Clin Invest 128, 1397–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siggs OM, Xiao N, Wang Y, Shi H, Tomisato W, Li X, Xia Y, Beutler B (2012) iRhom2 is required for the secretion of mouse TNFalpha. Blood 119, 5769–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye BD and McGovern DP (2016) Genetic variation in IBD: progress, clues to pathogenesis and possible clinical utility. Expert Rev Clin Immunol 12, 1091–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Cohen A, Colombel JF, Cottone M, Stronati L, Denson T, De Vos M, D’Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van Gossum A, Guthery SL, Halfvarson J, Verspaget HW, Hugot JP, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panes J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PC, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D’Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet 42, 1118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D, Sina C, Onnie CM, Weersma RK, Stokkers PC, Wijmenga C, Gazouli M, Strachan D, McArdle WL, Vermeire S, Rutgeerts P, Rosenstiel P, Krawczak M, Vatn MH, group, I. s., Mathew CG, Schreiber S (2008) Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet 40, 1319–23. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–74. [DOI] [PubMed] [Google Scholar]
- 27.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M (2014) Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol 104, Unit 15 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moolenbeek C and Ruitenberg EJ (1981) The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim 15, 57–9. [DOI] [PubMed] [Google Scholar]
- 29.Meyerholz DK and Beck AP (2018) Principles and approaches for reproducible scoring of tissue stains in research. Lab Invest [DOI] [PubMed]
- 30.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF (2007) Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc 2, 2307–11. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S, Yang B, Strong R, Xi X, Brenneman M, Grotta JC, Aronowski J, Savitz SI (2010) Bone marrow mononuclear cells protect neurons and modulate microglia in cell culture models of ischemic stroke. J Neurosci Res 88, 2869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Jiao Y, Hou S, Tian T, Yuan Q, Hao H, Wu Z, Bao X (2017) S100A4 contributes to colitis development by increasing the adherence of Citrobacter rodentium in intestinal epithelial cells. Sci Rep 7, 12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majowicz A, van der Marel S, te Velde AA, Meijer SL, Petry H, van Deventer SJ, Ferreira V (2012) Murine CD4(+)CD25(−) cells activated in vitro with PMA/ionomycin and anti-CD3 acquire regulatory function and ameliorate experimental colitis in vivo. BMC Gastroenterol 12, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang BW, Li M, Ma LC, Wei FW (2006) A widely applicable protocol for DNA isolation from fecal samples. Biochem Genet 44, 503–12. [DOI] [PubMed] [Google Scholar]
- 35.Ritchie LE, Burke KF, Garcia-Mazcorro JF, Steiner JM, Suchodolski JS (2010) Characterization of fecal microbiota in cats using universal 16S rRNA gene and group-specific primers for Lactobacillus and Bifidobacterium spp. Vet Microbiol 144, 140–6. [DOI] [PubMed] [Google Scholar]
- 36.Malinen E, Rinttila T, Kajander K, Matto J, Kassinen A, Krogius L, Saarela M, Korpela R, Palva A (2005) Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol 100, 373–82. [DOI] [PubMed] [Google Scholar]
- 37.Pils MC, Bleich A, Prinz I, Fasnacht N, Bollati-Fogolin M, Schippers A, Rozell B, Muller W (2011) Commensal gut flora reduces susceptibility to experimentally induced colitis via T-cell-derived interleukin-10. Inflamm Bowel Dis 17, 2038–46. [DOI] [PubMed] [Google Scholar]
- 38.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C (2012) Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13, R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seiffart V, Zoeller J, Klopfleisch R, Wadwa M, Hansen W, Buer J, Riedel C, Westendorf AM (2015) IL10-Deficiency in CD4(+) T Cells Exacerbates the IFNgamma and IL17 Response During Bacteria Induced Colitis. Cell Physiol Biochem 36, 1259–73. [DOI] [PubMed] [Google Scholar]
- 40.Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol M, Bjarnason I (2000) A simple method for assessing intestinal inflammation in Crohn’s disease. Gut 47, 506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D and Dubois RN (2010) The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 29, 781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zupancic T, Stojan J, Lane EB, Komel R, Bedina-Zavec A, Liovic M (2014) Intestinal cell barrier function in vitro is severely compromised by keratin 8 and 18 mutations identified in patients with inflammatory bowel disease. PLoS One 9, e99398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heimerl S, Moehle C, Zahn A, Boettcher A, Stremmel W, Langmann T, Schmitz G (2006) Alterations in intestinal fatty acid metabolism in inflammatory bowel disease. Biochim Biophys Acta 1762, 341–50. [DOI] [PubMed] [Google Scholar]
- 44.Mohapatra SK, Guri AJ, Climent M, Vives C, Carbo A, Horne WT, Hontecillas R, Bassaganya-Riera J (2010) Immunoregulatory actions of epithelial cell PPAR gamma at the colonic mucosa of mice with experimental inflammatory bowel disease. PLoS One 5, e10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Argenio G, Biancone L, Cosenza V, Della Valle N, D’Armiento FP, Boirivant M, Pallone F, Mazzacca G (1995) Transglutaminases in Crohn’s disease. Gut 37, 690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolf C, Qian Y, Brooke MA, Kelsell DP, Franzke CW (2016) ADAM17/EGFR axis promotes transglutaminase-dependent skin barrier formation through phosholipase C gamma1 and protein kinase C pathways. Sci Rep 6, 39780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultz G, Rotatori DS, Clark W (1991) EGF and TGF-alpha in wound healing and repair. J Cell Biochem 45, 346–52. [DOI] [PubMed] [Google Scholar]
- 48.Rowland KJ, Choi PM, Warner BW (2013) The role of growth factors in intestinal regeneration and repair in necrotizing enterocolitis. Semin Pediatr Surg 22, 101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matthews AL, Noy PJ, Reyat JS, Tomlinson MG (2017) Regulation of A disintegrin and metalloproteinase (ADAM) family sheddases ADAM10 and ADAM17: The emerging role of tetraspanins and rhomboids. Platelets 28, 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunbar AJ and Goddard C (2000) Structure-function and biological role of betacellulin. Int J Biochem Cell Biol 32, 805–15. [DOI] [PubMed] [Google Scholar]
- 51.Zganiacz A, Santosuosso M, Wang J, Yang T, Chen L, Anzulovic M, Alexander S, Gicquel B, Wan Y, Bramson J, Inman M, Xing Z (2004) TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J Clin Invest 113, 401–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clay H, Volkman HE, Ramakrishnan L (2008) Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity 29, 283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalliolias GD and Ivashkiv LB (2016) TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol 12, 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP (1997) A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385, 729–33. [DOI] [PubMed] [Google Scholar]
- 55.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA (1998) An essential role for ectodomain shedding in mammalian development. Science 282, 1281–4. [DOI] [PubMed] [Google Scholar]
- 56.Jones-Hall YL and Nakatsu CH (2016) The Intersection of TNF, IBD and the Microbiome. Gut Microbes 7, 58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alhagamhmad MH, Day AS, Lemberg DA, Leach ST (2016) An overview of the bacterial contribution to Crohn disease pathogenesis. J Med Microbiol 65, 1049–1059. [DOI] [PubMed] [Google Scholar]
- 58.Becker C, Neurath MF, Wirtz S (2015) The Intestinal Microbiota in Inflammatory Bowel Disease. ILAR J 56, 192–204. [DOI] [PubMed] [Google Scholar]
- 59.Berry D, Stecher B, Schintlmeister A, Reichert J, Brugiroux S, Wild B, Wanek W, Richter A, Rauch I, Decker T, Loy A, Wagner M (2013) Host-compound foraging by intestinal microbiota revealed by single-cell stable isotope probing. Proc Natl Acad Sci U S A 110, 4720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheinin T, Butler DM, Salway F, Scallon B, Feldmann M (2003) Validation of the interleukin-10 knockout mouse model of colitis: antitumour necrosis factor-antibodies suppress the progression of colitis. Clin Exp Immunol 133, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hale LP and Greer PK (2012) A novel murine model of inflammatory bowel disease and inflammation-associated colon cancer with ulcerative colitis-like features. PLoS One 7, e41797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Round JL and Mazmanian SK (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9, 313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sartor RB (2008) Therapeutic correction of bacterial dysbiosis discovered by molecular techniques. Proc Natl Acad Sci U S A 105, 16413–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kefaloyianni E, Muthu ML, Kaeppler J, Sun X, Sabbisetti V, Chalaris A, Rose-John S, Wong E, Sagi I, Waikar SS, Rennke H, Humphreys BD, Bonventre JV, Herrlich A (2016) ADAM17 substrate release in proximal tubule drives kidney fibrosis. JCI Insight 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blaydon DC, Biancheri P, Di WL, Plagnol V, Cabral RM, Brooke MA, van Heel DA, Ruschendorf F, Toynbee M, Walne A, O’Toole EA, Martin JE, Lindley K, Vulliamy T, Abrams DJ, MacDonald TT, Harper JI, Kelsell DP (2011) Inflammatory skin and bowel disease linked to ADAM17 deletion. N Engl J Med 365, 1502–8. [DOI] [PubMed] [Google Scholar]