Abstract
Background: In conjunction with the methionine adenosyltransferase 2A (MAT2A), MAT2B protein catalyses the formation of methyl donor S-adenosylmethionine to mediate cell metabolism, including proliferation and apoptosis. In this study, we investigated the functional and molecular mechanisms by which MAT2B influences triple-negative breast cancer (TNBC).
Methods: The mRNA level of MAT2B in three human TNBC cell lines and 40 TNBC tissue samples was analysed using quantitative reverse transcription polymerase chain reaction. The relationship between MAT2B expression and the clinicopathological characteristics of TNBC patients was also analysed. Further, MAT2B function was investigated using a series of in vitro and in vivo assays with cells in which MAT2B was inhibited using RNAi.
Results: We found that the mRNA levels of MAT2B were upregulated in all human TNBC cell lines tested. Moreover, positive expression of MAT2B was significantly correlated with higher T classification and M-stage. We also found that a higher level of MAT2B was correlated with worse relapse-free survival (RFS) according to a log-rank test. Next, we showed that the direct inhibition, using RNAi, of MAT2B in MDA-MB-231 and MDA-MB-468 cells inhibited cell growth and migration and induced apoptosis. Knockdown of MAT2B in MDA-MB-231 cells also repressed the expression of phosphorylated AKT and phosphorylated extracellular regulated protein kinases 1/2 (ERK1/2). Both phosphorylated AKT and ERK1/2 inhibitors reduced cell growth and migration, and induced apoptosis in MDA-MB-231 cells. As expected, knockdown of MAT2B in MDA-MB-231 cells significantly decreased the rate of tumour growth in vivo.
Conclusion: Our results demonstrated that targeting MAT2B could suppress cell growth and migration and induce apoptosis by inhibiting the AKT and ERK pathways in TNBC. Thus, targeting MAT2B requires further investigation as a therapeutic intervention for TNBC.
Keywords: triple-negative breast cancers; methionine adenosyltransferase; prognosis; apoptosis, AKT; ERK
Background
Breast cancer (BC) is one of the largest causes of morbidity and mortality among women globally.1 A particularly serious situation has been attributed to specific patient subgroups, including triple-negative breast cancer (TNBC).2 This is a heterogenous group of tumours that lacks immunohistochemical staining or overexpression of the oestrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), and thus presents diagnostic difficulties. Compared with ER-positive or HER2-positive BC, TNBC is generally aggressive, and associated with a high histologic grade, frequent distant metastasis, and high mortality rate.3 Moreover, it lacks targeted therapies. Although it has been intensively studied, the mechanism underlying the poor prognosis of TNBC remains to be elucidated. Biologically, it represents a heterogeneous disease that exhibits various degrees of response to chemotherapy. Therefore, the identification of new molecular targets is needed for more effective treatments.
Originally identified as a non-catalytic subunit of the MAT enzyme, MAT2B is responsible for regulating intracellular S-adenosylmethionine (SAM) content.4,5 A study of MAT1A knockout mice revealed that 50% of animals developed hepatocellular carcinoma by 18 months of age, indicating a critical role for SAM in normal liver function.6–8 It has been shown in hepatoma cells in vitro that this role involves a proapoptotic effect.9 Another function of MAT2B is to regulate MATII activity by lowering its Km for L-methionine.10 Increasing evidence suggests that MAT2B plays a significant role in tumourigenesis, particularly in melanoma, and gastric, colon and prostate cancers.11–13 In a notable example, one study showed an association between high MAT2B expression and proliferative advantage in hepatoma cells via its interaction with MATIIα2, and downregulation of SAM expression.11 Furthermore, it has recently been shown that interaction between MAT2B and GIT1 serves as a scaffold to regulate multiple steps of the Ras/Raf/MEK/extracellular regulated protein kinase (ERK) pathway, emphasizing the importance of the MAT2B/GIT1 interaction in cancer growth.14 Expression of MAT2B has been seen in most, though not all human tissues,15 but the expression and role of MAT2B in TNBC have not been investigated in detail.
In this study, we aimed to gain new insights into the role of MAT2B during BC progression by evaluating MAT2B gene expression in three TNBC cell lines, and examining the relationship between MAT2B levels and prognosis in patients with TNBC. We also investigated the effect of MAT2B knockdown in two TNBC cell lines.
Materials and methods
Patients and TNBC samples
All patients signed a general informed consent to agree to the use of BC tissues for clinical research and this was conducted in accordance with the Declaration of Helsinki. The study was approved by Ethics Committee of Shengjing Hospital. The original data of patients were reviewed in the context of clinicopathologic and follow-up information. We collected 40 TNBC fresh tumour samples and 40 Non-TNBC fresh samples (28 ER-positive BC, and 12 HER2-positive BC) from patients who underwent surgery between September 2010 and October 2014. Meanwhile, clinical pathology data (age, tumour size, T-stage and N-stage) were obtained from 80 BC cases. Of these, follow-up data were obtained for 30 cases (15 TNBC cases and 15 non-TNBC cases), for time periods ranging from 42 to 87 months. All tumour tissues were diagnosed by at least two pathologists.
Cell lines and inhibitors
Human breast cancer and normal cell lines used in this study were purchased from the Cell Biology Institute of Shanghai, Chinese Academy of Science. The TNBC cell lines MDA-MB-468, MDA-MB-231, and SUM149, and the non-TNBC cell lines MCF-10A, MCF-7, and SKBR3 were used. Cells were cultured as described previously (8, 9) in 5% CO2 saturated humidity at 37 °C. The phosphorylated AKT and phosphorylated ERK inhibitors, MK2206 and UO126, respectively, were purchased from Selleckchem (Houston, TX, USA).
Generation of a MAT2B short hairpin RNA (shRNA) lentivirus
We seeded MDA-MB-231 or MDA-MB-468 cells (5×105) in six-well plates (Nest Biotechnology, Hong Kong, China). At 70–80% confluence, the cells were infected with a lentivirus encoding MAT2B shRNA as previously described.16 The shRNA sequence was as follows: GCAGTTCATCACATCATTCAT. The lentivirus was introduced into cells by transient co-transfection of three plasmids, including 20 µg of the transfer vector pGCSIL-GFP-shMAT2B (shMAT2B) or negative control shRNA (shCtrl), 15 µg of the packaging vector pHelper 1.0, and 10 µg of the virus envelope glycoprotein (VSVG) expression plasmid pHelper 2.0. After 72 h of transfection, reverse transcription polymerase chain reaction (RT-PCR) and western blotting were performed to determine the transfection efficiency.
Gene expression analysis by quantitative RT-PCR
Quantitative RT-PCR (qPCR) was conducted to measure the mRNA level of MAT2B in human breast cell lines and tissues. The efficiency of transfection in MDA-MB-231 and MDA-MB-468 cells was also determined by qPCR. Total RNA was extracted from tissues and cells with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). After assessing the purity of RNA samples by ultraviolet spectrophotometry, qPCR was performed on the 7,500 Fast Real-Time PCR system. The expression levels of MAT2B were analysed using the 2−ΔΔCt method. The relative level of MAT2B mRNA was calculated by subtracting CT values of GAPDH from the CT values of MAT2B. The sequences of the forward (F) and reverse (R) primers were as follows: MAT2B (F) ACAGAGAGGAAGACATACCAG, (R) GTTCATTGCCAGACCAGTG, with an amplified PCR product of 324 base pairs (bp); GAPDH (F) GAGTCAACGGATTTGGTCGT, (R) ATCCACAGTCTTCTGGGTGG, with an amplified PCR product of 121 bp. Experiments were performed in triplicate independently.
Cell proliferation assay
Three methods were used to investigate the effect of MAT2B downregulation on proliferation of TNBC cells. Firstly, MDA-MB-231 and MDA-MB-468 cells were infected with sh Ctrl or sh MAT2B lentivirus for 72 h and collected. A Cellomics ArrayScan VTI HCS Reader (Thermo Scientific, Waltham, MA, USA) was used to measure cell growth by counting viable cells. Briefly, 2,000 cells/100 μl were added to each well of a 96-well plate. Viable cells expressing the lentivirus generated a green fluorescence signal, which could be detected with the Cellomics ArrayScan VTI HCS Reader. The cells were counted at 1, 2, 3, 4, 5, 6, and 7 days after plating. The growth curve was generated with the graphing software Sigma Plot (San Jose, CA, USA). Proliferation, following 72 h transfection with shCtrl or shMAT2B, was also assessed using the BrdU incorporation enzyme-linked immunosorbent assay (ELISA) (Roche Diagnostics, Milan, Italy), and MTT assay (Roche Diagnosis GmbH, Germany), according to the manufacturer’s instructions. To explore whether the AKT and ERK signalling pathways were associated with inhibition of cell proliferation by MAT2B knockdown in the MDA-MB-231 cell line, cells were divided into four groups: shCtrl, shMAT2B, UO126 and MK2206. The MTT assay was performed to test proliferation in each group. In the UO126 and MK2206 groups, cells were grown in 10% foetal bovine serum (FBS) for 24 h, then the media were changed prior to treatment with UO126 (10 μmol/l) and/or MK2206 (10 μmol/l) for another 24 h. Experiments were performed in triplicate independently.
Formation of colonies
We infected MDA-MB-231 and MDA-MB-468 cells with shMAT2B or shCtrl for 72 h, seeded them in six-well plates, and cultured them for 14 days. The medium was changed every 3 days. After the medium was aspirated, the cells were washed with phosphate-buffered saline (PBS). Following the addition of Giemsa stain, they were then rinsed in distilled water and incubated for 20 min. Colonies were then counted in triplicate. To explore whether the AKT and ERK signalling pathways are associated with inhibition of colony formation by MAT2B knockdown in MDA-MB-231, cells were divided into four groups as described above. Experiments were performed in triplicate independently.
Apoptosis assays
The effect of MAT2B downregulation on apoptosis of TNBC cells in vitro was evaluated using the Annexin V-APC Apoptosis Detection Kit (Roche, Indianapolis, IN, USA). Briefly, MDA-MB-231 and MDA-MB-468 cells were infected with shMAT2B or shCtrl for 72 h, 5×105 cells were collected by centrifugation, and 5 μl of Annexin V-APC was added for 5 min at 20 °C in the dark. A minimum of 10,000 cell events per assay were acquired by FACScan. Other apoptosis assays, including DNA-end labelling (TUNEL) and caspase-3 activity assays, were performed as previously described.4 To explore whether the AKT and ERK signalling pathways were associated with the induction of apoptosis by MAT2B knockdown in TNBC, MDA-MB-231 cells were divided into four groups as described above. Activity assays for TUNEL and caspase-3 were conducted for each group as previously described.17 Experiments were performed in triplicate independently.
Wound healing assay
Wound healing assays were carried out on MDA-MB-231 and MDA-MB-468 cells infected with shMAT2B or shCtrl for 72 h and seeded in six-well plates. When confluency was reached, a pipette tip was used to scrape a straight line on each monolayer. Medium with or without the above-mentioned compounds was replaced after three washes with PBS to remove detached cells. To explore whether the AKT and ERK signalling pathways are associated with inhibition of migration by MAT2B knockdown in TNBC, MDA-MB-231 cells were divided into four groups as described above. Wound widths at five different positions were measured at the beginning and the end of the assay. Results were expressed as a migration index, which is the distance covered by migrated cells in the knockdown group in comparison with the distance covered by migrated cells in the control group. Experiments were performed in triplicate independently.
Western blot analysis
To measure the protein level of MAT2B in human breast cell lines and explore whether AKT and ERK signalling pathways were associated with MAT2B knockdown in TNBC, we performed western blot analysis. We divided MDA-MB-231 cells into four groups as described above. Proteins were extracted, loaded, separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis, and transferred onto a polyvinylidene difluoride membrane. The membrane was blocked, washed, and incubated overnight with primary antibodies against MAT2B (1:500 dilution;ab109484;Abcam), phosphorylated AKT (p-AKT; 1:1,000 dilution; ab38449; Abcam), AKT (1:1,000 dilution; ab8805; Abcam), phosphorylated ERK1/2 (p-ERK1/2; 1:1,000 dilution; ab223500; Abcam), ERK1/2 (1:1,000 dilution; ab17942; Abcam), and actin (1:2,000 dilution; sc70319;Santa Cruz). The membrane was then washed, incubated with secondary antibodies (Santa Cruz Biotechnology), and visualised by enhanced chemiluminescence. Band intensity was quantified with ImageJ software and protein expression was standardised to actin.
Tumour growth experiment in vivo
Animal studies were performed with the approval of the Ethics Committee of China Medical University Shengjing Hospital and conducted according to the institutional and national guidelines. For our in vivo tumour model using transplanted TNBC cells, twenty 6-week-old female mice were divided into two groups (N=10 per group). Briefly, MDA-MB-231 cells were infected with shMAT2B or shCtrl for 72 h, and then 1×105 cells were collected by centrifugation. The cell suspension (100 μl) was subcutaneously injected into the nude mice. Tumour volumes were measured regularly using the formula V=0.5×L×W, where L represents the longest diameter, and W the shortest diameter. Five weeks later, animals were sacrificed, and then their tumours were isolated.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) with SPSS 19.0 software. Data are presented as the mean ± standard deviation. Relapse-free curves were plotted according to the Kaplan–Meier method, and the log-rank test was used for comparison. All P-values were analysed from at least three independent experiments. Significant differences were inferred where p<0.05.
Results
MAT2B expression is significantly elevated in human TNBC tissues and also associated with prognosis
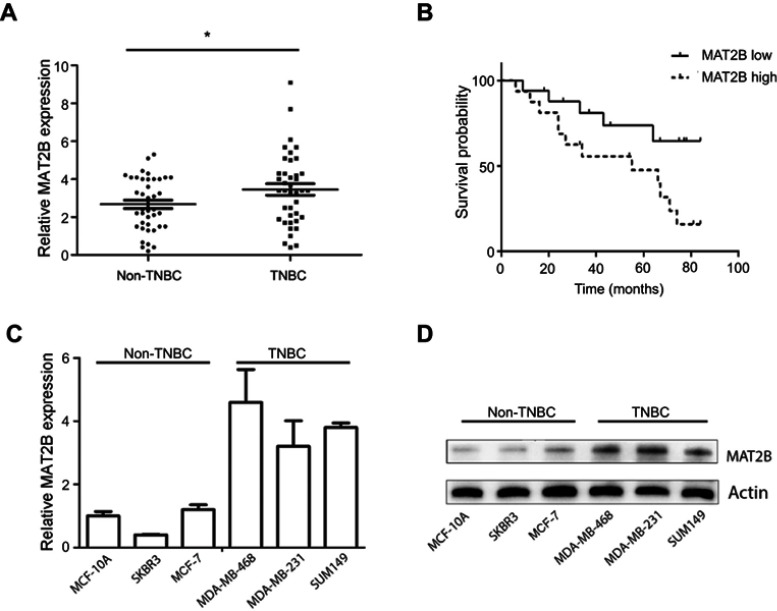
To evaluate MAT2B expression in human TNBC tissues, the level of MAT2B was detected in 40 TNBC fresh tissues and 40 Non-TNBC tissues by qPCR. The data showed that MAT2B was upregulated in human TNBC tissues compared with non-TNBC tissues (p<0.05; Figure 1A). Next, we explored the correlation between expression of MAT2B and clinical pathological characteristics in 40 patients with TNBC. Stage of BC was classified according to TNM classification.18 As shown in Table 1, high expression level of MAT2B (MAT2B levels in cancer/adjacent tissues >2) was associated with higher T (p<0.01) and M classifications (p<0.01) compared to patients with low expression levels of MAT2B (MAT2B levels in cancer/adjacent tissues <2). Furthermore, we used the Kaplan–Meier method to calculate RFS curves. As shown in Figure 1B, the survival analysis showed a significantly higher RFS rate for patients with low MAT2B expression compared with patients with high expression (p<0.05 by log rank test).
Figure 1.
Expression of MAT2B mRNA in TNBC and the relationship between MAT2B expression and TNBC relapse-free survival (RFS). (A) The expression of MAT2B mRNA was significantly upregulated in 40 TNBC cancer tissues and 40 non-TNBC cancer tissues (control group) from 80 BC patients. (B) High levels of MAT2B expression were correlated with worse RFS prognosis in patients with TNBC. The expression of MAT2B mRNA (C) and protein (D) was upregulated in all three TNBC cell lines. *p<0.05.
Table 1.
Correlation between MAT2B expression and clinicopathological characteristics in patients with TNBC
| Variables | n | MAT2B | p | |
|---|---|---|---|---|
| Low | High | |||
| Total | 40 | 20 | 20 | |
| Age, years | ||||
| ≤52 | 28 | 12 | 16 | 0.168 |
| >52 | 12 | 8 | 4 | |
| Tumour size (CM) | ||||
| ≤2.5 | 20 | 12 | 8 | 0.206 |
| >2.5 | 20 | 8 | 12 | |
| T-stage | ||||
| Tis/T1/T2 | 18 | 14 | 4 | 0.001** |
| T3/T4 | 22 | 6 | 16 | |
| N-stage | ||||
| N0 | 24 | 15 | 9 | 0.053 |
| N1 | 16 | 5 | 11 | |
| M-stage (TNM) | ||||
| M0 | 26 | 17 | 9 | 0.008** |
| M1 | 14 | 3 | 11 | |
Note: Significant values are in bold. **p<0.01.
Abbreviation: NM, tumour node metastasis.
We also measured the levels of MAT2B mRNA and protein in three human TNBC cell lines, and found that MAT2B was upregulated in all three cell lines compared with non-TNBC cells (Figure 1C and D).
MAT2B knockdown inhibits growth and migration of TNBC cells
To explore the role of MAT2B in the growth of MDA-MB-231 cells, the shRNA sequence was introduced to MDA-MB-231 and MDA-MB-468 cells. As shown in Figure 2A, shMAT2B effectively knocked down the expression of MAT2B in both cell lines. Simple cell counting assay results, depicted in Figure 2B, showed that MAT2B knockdown significantly inhibited cell proliferation. Incorporation of BrdU is a well-utilised marker of cell proliferation. The results shown in Figure 2C confirmed that MAT2B knockdown significantly decreased BrdU optical density (OD) in cells, confirming its anti-proliferative activity. To test cell viability, a routine MTT assay was performed. Knockdown of MAT2B largely inhibited survival (“MTT OD”) of cells (Figure 2D). Additionally, the number of viable cell colonies was also significantly decreased following MAT2B knockdown (Figure 2E). Compared with control cells (Figure 2F), the wound-healing assay revealed a consistent reduction of cell migration with MAT2B knockdown. This effect was reproducible and was observed in more than three independent infection experiments.
Figure 2.
MAT2B knockdown inhibits growth and migration of TNBC cells. (A) Cells were transfected with control shRNA or MAT2B shRNA, then analysed for MAT2B mRNA expression. (B–D) Cells were transfected with control shRNA or MAT2B shRNA, then analysed for cell proliferation with a Cellomics ArrayScan VTI HCS Reader, and BrdU incorporation ELISA and MTT assays. (E) Cells were transfected with control shRNA or MAT2B shRNA and subjected to a colony formation assay. The pictures show a consistent reduction in colony number when cells were infected with shMAT2B. (F) Cells were transfected with control shRNA or MAT2B shRNA, then subjected to a cell migration assay. Data show percent inhibition of migration after MAT2B knockdown. *p<0.05, **p<0.01.
MAT2B knockdown activates apoptosis in TNBC cells
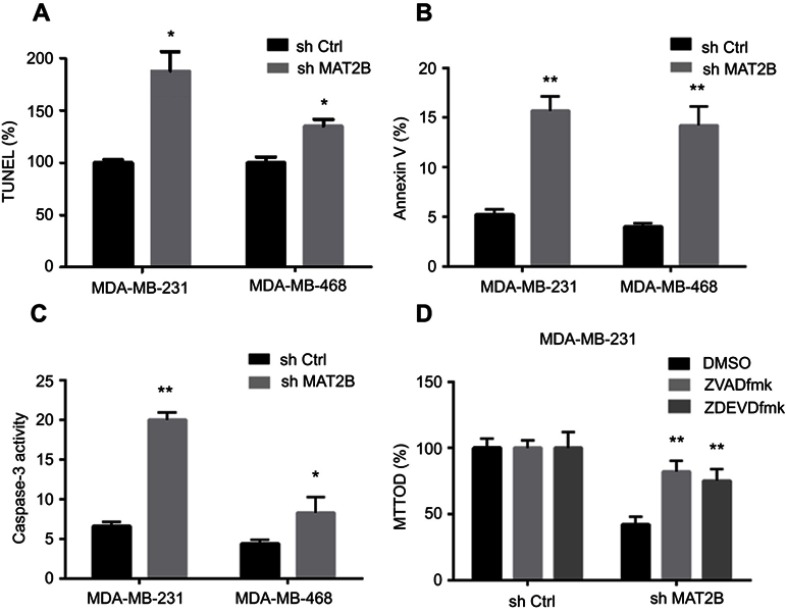
We next studied the effect of MAT2B on apoptosis. Various apoptosis assays were performed, including TUNEL, Annexin V fluorescence-activated cell sorting (FACS), and caspase-3 activity assays. Knockdown of MAT2B significantly increased the percentage of TUNEL-stained cells (Figure 3A), the Annexin V ratio (Figure 3B), and caspase-3 activity (Figure 3C). These results indicated profound activation of apoptosis in MAT2B knockdown cells (Figure 3A–C). To block apoptosis, caspase-based inhibitors were employed. Co-treatment with the caspase-3 or pan caspase inhibitors, zDEVDfmk or zVADfmk, respectively, largely attenuated the MAT2B knockdown-induced reduction in cell viability (MTT OD; Figure 3D). The two caspase inhibitors almost completely blocked MAT2B knockdown-induced activation of apoptosis (data not shown).
Figure 3.
MAT2B knockdown activates apoptosis in TNBC cells. Cells were transfected with control shRNA or MAT2B shRNA, then harvested for apoptosis analyses. TNBC cells were examined using the TUNEL (A), FACScan (B), and caspase-3 activity (C) assays. In (D), cells were co-treated with 50 μM of the caspase-3 inhibitor zDEVDfmk or the pan caspase inhibitor zVADfmk, and cell viability was tested with the MTT assay. *p<0.05, **p<0.01.
AKT and ERK signalling pathways are associated with MAT2B knockdown in TNBC cells
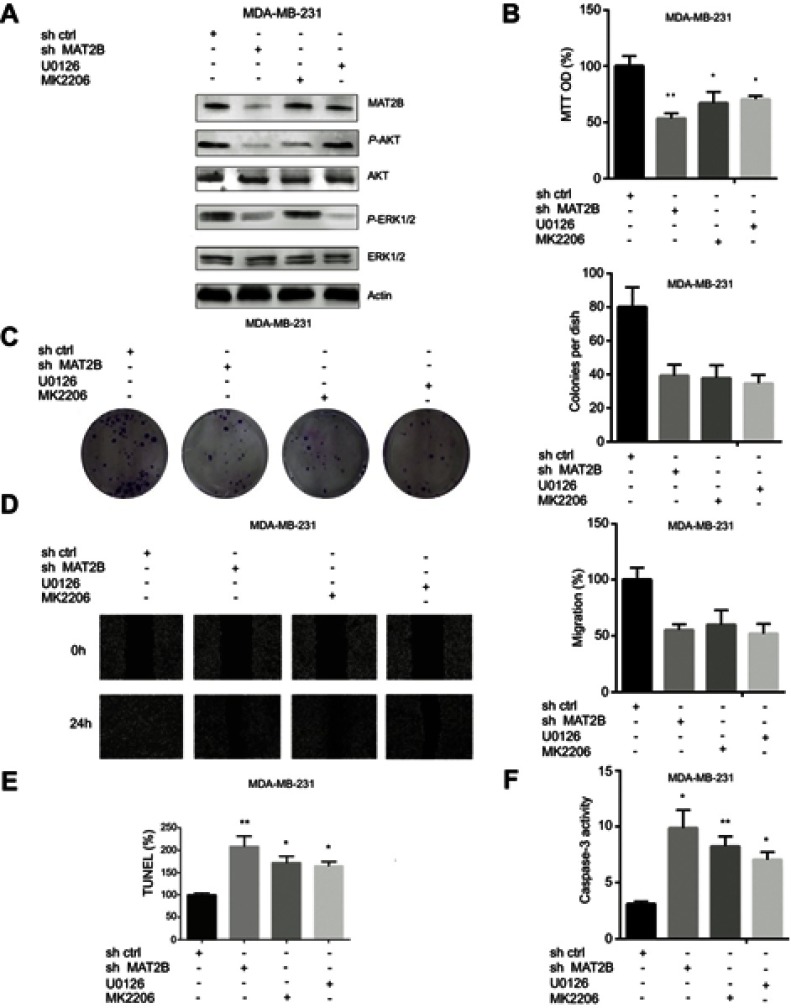
We further investigated intracellular signalling pathways using western blot analysis. Knockdown of MAT2B reduced the expression of p-ERK1/2 and p-AKT (Figure 4A). However, MAT2B knockdown did not affect the expression of AKT or ERK1/2 (Figure 4A). We were able to induce downregulation of p-AKT and p-ERK1/2 in MDA-MB-231 cells using the p-AKT inhibitor MK-2206 and the p-ERK1/2 inhibitor U0126 (Figure 4A). Additionally, MTT, colony formation, and wound healing assays showed that both MK-2206 and U0126 partially inhibited the proliferation and migration of MDA-MB-231 cells (Figure 4B–D). Our TUNEL and caspase-3 activity assays showed that both MK-2206 and U0126 partially induced apoptosis of MDA-MB-231 cells (Figure 4E and F).
Figure 4.
AKT and ERK signalling pathways are associated with MAT2B knockdown in TNBC cells. (A) MDA-MB-231 cells were treated with control shRNA, MAT2B shRNA, U0126, or MK2206, then harvested for western blots analyses. The data are from densitometric experiments illustrating P-AKT, AKT, P-ERK, and ERK protein expression in cells. (B) Cells were treated with control shRNA, MAT2B shRNA, U0126, or MK2206, then subjected to cell proliferation analyses. (C) Cells were treated with control shRNA, MAT2B shRNA, U0126, or MK2206, then subjected to a colony formation assay. (D) Cells were treated with control shRNA, MAT2B shRNA, U0126, or MK2206, then subjected to a wound-healing assay. (E, F) Cells were treated with control shRNA, MAT2B, shRNA, U0126, or MK2206, then subjected to TUNEL and caspase-3 activity assays. *p<0.05, **p<0.01.
MAT2B downregulation delays tumour growth in vivo
To investigate whether MAT2B is involved in tumour growth in vivo, a transplanted tumour model was established by subcutaneously injecting MDA-MB-231, infected by shCtrl or shMAT2B lentivirus, into nude mice. Tumours were identified in each group and used for further analysis. The shMAT2B group formed smaller and lighter tumours than the shCtrl group (Figure 5A and B). Tumour growth curves are shown in Figure 5A.
Figure 5.
MAT2B knockdown inhibits TNBC cell growth in vivo. (A) Effect of MAT2B knockdown on tumour volume. Mice were injected with MDA-MB-231 cells in which MAT2B was downregulated. The growth curve shows that tumour growth was significantly inhibited after 4 weeks compared with the control mice (n=10). (B) Effect of MAT2B knockdown on tumour weight. Mice were injected with MDA-MB-231 cells in which MAT2B was downregulated. Five weeks later, tumour weight was significantly decreased compared with the control mice (n=10). *p<0.05.
Discussion
In recent years, there has been an increasing number of studies focused on understanding the molecular and biological characteristics of TNBC.19 Members of the MAT gene family are reported to play essential roles in carcinogenesis and cancer progression. Increased expression of the MATII genes, including MAT2B, was first reported in hepatocellular carcinoma ten years ago.11 More recent studies have also shown upregulation of MAT2B expression in tissues of hepatocellular carcinoma, and also liver cirrhosis, and several researchers have reported evidence that SAM deficiency could lead to malignant degeneration.4,20,21 Further, inhibition of cell growth and induction of apoptosis in melanoma and hepatocellular carcinoma via targeting of MAT2B by shRNA interference has also been shown previously.12 However, neither abnormal expression of MAT2B in TNBC, nor investigation of the association between MAT2B and the development or prognosis of TNBC have been previously reported.
In this study, we investigated the expression of MAT2B in TNBC and explored the effects of its depletion in TNBC cell lines. Our qPCR experiments showed significant upregulation of MAT2B in TNBC tissues and cell lines. We also found that high levels of MAT2B correlated with poor prognosis in 30 TNBC patients, suggesting that MAT2B may act as a tumour promoter gene in TNBC carcinogenesis. Our in vitro experiments supported the hypothesis of a direct antitumour effect of MAT2B in TNBC, and we also found that inhibition of MAT2B expression markedly suppressed tumour growth in mouse models.
The other principal aim of this study was to explore the molecular mechanism by which MAT2B acts in TNBC progression. Downregulating the expression of MAT2B in TNBC cells using shRNA resulted in reduced cell proliferation and migration, and induced apoptosis in MDA-MB-231 and MDA-MB-468 cells. We also showed that expression levels of p-AKT and p-ERK1/2, the inhibition of which may decrease proliferation and induce apoptosis,22–24 were suppressed following treatment with shMAT2B. The mechanism underlying this interesting dual effect of MAT2B on AKT and ERK signalling requires further study. This work is particularly relevant because investigation of these pathways is an important direction towards increasing understanding of the molecular biology of TNBC in general.
Lentivirus-mediated shRNAs produce sustained silencing by integrating into the host genome.25–27 Use of the lentivirus vector is safe in humans. It has been reported that lentivirus vector-mediated antisense targeting of the HIV envelope sequence has been used for HIV treatment in clinical trials.28–30 In addition, the use of a lentivirus vector containing the beta-globin gene has been approved for use in phase I/II clinical trials for human beta thalassemia and sickle cell anaemia gene therapy.31 Thus, MAT2B gene silencing via lentivirus could be used as a potential therapy in BC. Also suggestive was a study by Peng et al, who reported that an interaction between MAT2B and GIT1 activates the MEK1/ERK1/2 to promote growth in human liver and colon cancer.14 This novel MAT2B-GIT1 complex may provide a candidate target for TNBC therapy in the future.
Conclusions
To our knowledge, this is the first report to demonstrate altered MAT2B expression in human TNBC cell lines and tissues. Our results show potential tumour-promoting effects of MAT2B, and a positive correlation of its expression level with malignancy status. Our data also show that MAT2B gene silencing via lentivirus inhibits malignant progression of TNBC by inhibiting the AKT and ERK pathways. While the mechanism underlying the role of MAT2B in TNBC still requires further elucidation, these findings suggest that MAT2B knockdown could be efficient for inhibiting TNBC cell proliferation through simultaneous suppression of these survival signals, supporting its potential as a therapeutic strategy. We also suggest that MAT2B deserves further investigation as a novel potential marker for TNBC prognosis.
Acknowledgments
The authors thank Feiran Wei (School of Medicine, Southeast University) for the technical advice. This work was supported by funds from the National Natural Science Foundation of Liaoning (20170541044); Program of Liaoning province department of education (LK201602).
Abbreviation list
MAT, Methionine adenosyltransferase; SAM, S-adenosylmethionine; BC, Breast cancer; RFS, relapse-free survival; TNBC, triple-negative breast cancer.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of China Medical University, and each subject provided signed informed consent.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Golubnitschaja O, Debald M, Yeghiazaryan K, et al. Breast cancer epidemic in the early twenty-first century: evaluation of risk factors, cumulative questionnaires and recommendations for preventive measures. Tumour Biol. 2016;37:12941–12957. doi: 10.1007/s13277-016-5168-x [DOI] [PubMed] [Google Scholar]
- 2.Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, et al. Adjuvant capecitabine in combination with docetaxel, epirubicin, and cyclophosphamide for early breast cancer: the randomized clinical FinXX trial. JAMA Oncol. 2017;3:793–800. doi: 10.1001/jamaoncol.2016.6120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gershuni V, Li YR, Williams AD, et al. Breast cancer subtype distribution is different in normal weight, overweight, and obese women. Breast Cancer Res Treat. 2017;163:375–381. doi: 10.1007/s10549-017-4192-x [DOI] [PubMed] [Google Scholar]
- 4.Lo TF, Tsai WC, Chen ST. MicroRNA-21-3p, a berberine-induced miRNA, directly down-regulates human methionine adenosyltransferases 2A and 2B and inhibits hepatoma cell growth. PLoS One. 2013;8:883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang Q, Yin J, Li F, Zhang J, Watford M. Characterization of methionine adenosyltransferase 2β, gene expression in skeletal muscle and subcutaneous adipose tissue from obese and lean pigs. Mol Biol Rep. 2010;37:2517–2524. doi: 10.1007/s11033-009-9767-0 [DOI] [PubMed] [Google Scholar]
- 6.Lu SC, Alvarez L, Huang ZZ, et al. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci U S A. 2001;98:5560–5565. doi: 10.1073/pnas.091016398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mato JM, Corrales FJ, Lu SC, Avila MA. S-Adenosylmethionine: a control switch that regulates liver function. FASEB J. 2002;16:15–26. doi: 10.1096/fj.01-0401rev [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Chantar ML, Corrales FJ, Martínez-Cruz LA, et al. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;16:1292–1294. doi: 10.1096/fj.02-0078fje [DOI] [PubMed] [Google Scholar]
- 9.Ansorena, E, García-Trevijano ER, Martínez-Chantar ML, et al. S-adenosylmethionine and methylthioadenosine are antiapoptotic in cultured rat hepatocytes but proapoptotic in human hepatoma cells. Hepatology. 2002;35:274–280. doi: 10.1053/jhep.2002.30419 [DOI] [PubMed] [Google Scholar]
- 10.Lu SC, Mato JM. Role of methionine adenosyltransferase and S-adenosylmethionine in alcohol-associated liver cancer. Alcohol. 2005;35(3):227–234. doi: 10.1016/j.alcohol.2005.03.011 [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Liu QY, Liu ZS, et al. Inhibition of hepatocelluar carcinoma MAT2A and MAT2beta gene expressions by single and dual small interfering RNA. J Exp Clin Cancer Res. 2008;27:1–9. doi: 10.1186/1756-9966-27-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lei Y, Zhang B, Zhang Y, et al. Lentivirus-mediated downregulation of MAT2B inhibits cell proliferation and induces apoptosis in melanoma. Int J Oncol. 2016;49:981–990. doi: 10.3892/ijo.2016.3603 [DOI] [PubMed] [Google Scholar]
- 13.Maldonado LY, Arsene D, Mato JM, Lu SC. Methionine adenosyltransferases in cancers: mechanisms of dysregulation and implications for therapy. Exp Biol Med (Maywood). 2018;243:107–117. doi: 10.1177/1535370217740860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng H, Li TWH, Yang H, et al. Methionine adenosyltransferase 2B–GIT1 complex serves as a scaffold to regulate Ras/Raf/MEK1/2 activity in human liver and colon cancer cells. Am J Pathol. 2015;243:1135–1144. doi: 10.1016/j.ajpath.2014.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nordgren KK, Peng Y, Pelleymounter LL, et al. Methionine adenosyltransferase 2A/2B and methylation: gene sequence variation and functional genomics. Drug Metab Dispos. 2011;39:2135–2147. doi: 10.1124/dmd.111.040857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bin Z, Keyi L, Weifeng Z, et al. Downregulation of KLF8 expression by shRNA induces inhibition of cell proliferation in CAL27 human oral cancer cells. Med Oral Patol Oral Cir Bucal. 2013;18:2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu PH, Chen MB, Ji C, et al. Aqueous Oldenlandia diffusa extracts inhibits colorectal cancer cells via activating AMP-activated protein kinase signalings. Oncotarget. 2016;19:45889–45900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah R, Rosso K, Nathanson SD. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World J Clin Oncol. 2014;5(3):283–298. doi: 10.5306/wjco.v5.i3.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ademuyiwa FO, Tao Y, Luo J, Weilbaecher K, Ma CX. Differences in the mutational landscape of triple-negative breast cancer in African Americans and Caucasians. Breast Cancer Res Treat. 2017;161:491–499. doi: 10.1007/s10549-016-4062-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Liu QY, Liu ZS, et al. Lentivirus mediated shRNA interference targeting MAT2B induces growth-inhibition and apoptosis in hepatocelluar carcinoma. World J Gastroenterol. 2008;14:4633–4642. doi: 10.3748/wjg.14.4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Magilnick N, Noureddin M, Mato JM, Lu SC. Effect of hepatocyte growth factor on methionine adenosyltransferase genes and growth is cell density-dependent in HepG2 cells. J Cell Physiol. 2007;210:766–773. doi: 10.1002/(ISSN)1097-4652 [DOI] [PubMed] [Google Scholar]
- 22.Wu YM, Chen ZJ, Jiang GM, et al. Inverse agonist of estrogen-related receptor α suppresses the growth of triple negative breast cancer cells through ROS generation and interaction with multiple cell signaling pathways. Oncotarget. 2016;15:12568–12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huo X, Liu S, Shao T, et al. GSK3 protein positively regulates type I insulin-like growth factor receptor through forkhead transcription factors FOXO1/3/4. J Biol Chem. 2014;289:24759–24770. doi: 10.1074/jbc.M114.580738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng L, Wang X, Luo W, et al. Brucine, an effective natural compound derived from nux-vomica, induces G1 phase arrest and apoptosis in LoVo cells. Food Chem Toxicol. 2013;58:332–339. doi: 10.1016/j.fct.2013.04.018 [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Choi SB, Jin M, et al. Euglycemia in diabetic rats leads to reduced liver weight via increased autophagy and apoptosis through increased AMPK and caspase-3 and decreased mTOR activities. J Diabetes Res. 2015;2015:1–12. doi: 10.1155/2015/815839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao B, Yang C, Yang S, Gao Y, Wang J. Construction of conditional lentivirus-mediated shRNA vector targeting the human Mirk gene and identification of RNAi efficiency in rhabdomyosarcoma RD cells. Int J Oncol. 2013;43:1253–1259. doi: 10.3892/ijo.2013.2048 [DOI] [PubMed] [Google Scholar]
- 27.Gong Y, Wang H, Xia H. Stable transfection into rat bone marrow mesenchymal stem cells by lentivirus-mediated NT-3. Mol Biol Rep. 2015;11:367–373. doi: 10.3892/mmr.2014.2727 [DOI] [PubMed] [Google Scholar]
- 28.Hill AL, Rosenbloom DIS, Nowak MA. Evolutionary dynamics of HIV at multiple spatial and temporal scales. J Mol Med. 2012;90:543–561. doi: 10.1007/s00109-012-0892-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham BS. Advances in antiviral vaccine development. Immunol Rev. 2013;255:230–242. doi: 10.1111/imr.2013.255.issue-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilaparty SP, Agarwal R, Singh P, Kannan K, Ali N. Endoplasmic reticulum stress-induced apoptosis accompanies enhanced expression of multiple inositol polyphosphate phosphatase 1 (Minpp1): a possible role for Minpp1 in cellular stress response. Cell Stress Chaperones. 2016;21:1–16. doi: 10.1007/s12192-016-0684-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bank A, Dorazio R, Leboulch P. A phase I/II clinical trial of beta-globin gene therapy for beta-thalassemia. Ann N Y Acad Sci. 2005;1054:308–316. doi: 10.1196/annals.1345.007 [DOI] [PubMed] [Google Scholar]