Abstract
Purpose: Piscidin-1 is an effective antimicrobial peptide (AMP) against a variety of microbes. However, its toxicity has been reported as a limitation for its potential therapeutic applications. The toxicity of piscidin-1 may be related to the long nonpolar face of this AMP. Here, we investigated different piscidin-1 analogs to reach a peptide with the reduced toxicity.
Material and methods: In vitro and in vivo antibacterial activity and toxicity of piscidin-1 analogs generated by replacement of isoleucine at the border (I9) or the center (I16) of the nonpolar face of piscidin-1 by alanine or lysine were investigated.
Results: The results indicated that among all peptides, piscidin-1 with the highest HPLC retention time (RT) and I16K-piscidin-1 with the lowest RT had the highest and lowest cytotoxicity, respectively. Although I16K-piscidin-1 possessed the same MIC value as the parent peptide (piscidin-1) and other analogs, I16K-piscidin-1 exhibited a higher rapidity of bactericidal action at 5×MIC. The β-galactosidase leakage and propidium iodide staining assays indicated a higher pore-forming capacity of I16K-piscidin-1 relative to the parent peptide (piscidin-1). Taken together, RT is suggested to have a direct association with the toxicity and an inverse association with the rapidity of bactericidal action and pore-forming capacity. After infection of mice with clinical colistin-resistant Acinetobacter baumannii or clinical methicillin-resistant Staphylococcus aureus strains, treatment with I16K-piscidin-1, but not piscidin-1 and other analogs, resulted in a significantly stronger bactericidal potency. Furthermore, I16K-piscidin-1 exhibited the lowest in vivo toxicity.
Conclusion: Overall, in vitro and in vivo comparison of piscidin-1 and its analogs together documented that replacement of isoleucine at the center of the nonpolar face of piscidin-1(I16) by lysine leads to not only a decrease in toxicity potential but also an increase in bactericidal potential.
Keywords: antimicrobial peptide, piscidin-1, toxicity activity, Staphylococcus aureus, Acinetobacter baumannii
Introduction
The increasing prevalence of multidrug-resistant bacteria increases the demand for the discovery of new promising classes of antimicrobial agents.1 Among potentially attractive antibacterial candidates, antimicrobial peptides (AMPs) are increasingly of interest as promising alternatives for conventional antibiotics.2 Piscidins are first and well-known AMP family isolated from fish. These peptides are produced by mast cells, and play a key role in the innate immune system of fish.3,4 Among all identified piscidins, piscidin-1 exhibits the highest activity against a broad spectrum of microbes, and is the first AMP isolated from the fish hybrid striped bass.5 The remarkable activity of piscidin-1 against microbes has led it to attract attention as a promising alternative for classic antibiotics, encouraging many research efforts to elucidate the structure and antimicrobial mechanism of this AMP in recent years.6,7 Despite having a potent activity against microorganisms, the nonselective activity of piscidin-1 against mammalian cells, as a considerable obstacle, limits its potential therapeutic applications. To overcome this problem, several studies attempted to design a piscidin-1-analog lacking toxicity while retaining or even increasing its antibacterial activity through amino acid substitutions.8,9 The negatively charged cell membranes of bacteria enhance the selective activity of AMPs against them, while the cytoplasmic membranes of mammalian cells, which have a neutral net charge, protect mammalian cells against AMPs. However, AMPs possessing highly hydrophobic properties can interact with cell membrane through hydrophobic interactions, subsequently leading to mammalian cell lysis.10 Piscidin-1 has an amphipathic alpha-helical structure in which the hydrophobic nonpolar face, and the long heptad repeat sequence, exist on one side of the helix and the cationic face is located on the other side.9 Although the segregated amphipathic structure has been known as a requirement for antimicrobial activity, recent studies have revealed that disruption of this segregated amphipathic structure through scrambling of the nonpolar face can improve the antibacterial activity of AMPs as well as can reduce their cytotoxicity.11,12 Accordingly, manipulation of the nonpolar face of highly hydrophobic AMPs could be employed to disrupt their continuous nonpolar face and consequently to reduce their toxicity.13,14 In the present study, we designed derivatives from piscidin-1 by substitution of isoleucine 16 or 9 with alanine or lysine and compared in vitro and in vivo antibacterial activity and toxicity of analogs. This study was conducted to address whether substitution of isoleucine 9 at the border or isoleucine 16 at the center of the nonpolar face of piscidin-1 with either a less hydrophobic residue (ie, alanine) or a hydrophilic (positively charged) one (ie, lysine) would lead to an effective reduction in its toxicity while retaining or improving antibacterial activity.
Materials and methods
Solid-phase synthesis, purification, and identification of peptides
All peptides listed in Table 1 were synthesized by a solid-phase synthesis method using N-(9-fluorenyl) methoxycarbonyl (Fmoc) chemistry (Pepmic, Suzhou, China), while the amine group was added at the C-terminal of them. Subsequently, peptides were purified by reversed-phase HPLC (RP-HPLC) using an Inertsil ODS-SP (4.6 mm × 250 mm × 5 μm) column (GL Sciences, Tokyo, Japan) and eluted using 0–100% H2O/acetonitrile gradient containing 0.1% trifluoroacetic acid (TFA) for 30 min. The peptide purity was evaluated by analytical HPLC using the Inertsil ODS-SP column; a purity of 95% was obtained for every peptide.
Table 1.
Physicochemical properties of piscidin-1 and its derivatives
| Peptide | Sequence | Molecular weight | Nonpolar face hydrophobicity | Hydrophobic moment <µH> | Net charge (pH7)/pI | Retention time (min) |
|---|---|---|---|---|---|---|
| Piscidin-1 | FFHHIFRGIVHVGKTIHRLVTG | 2,572 | 1.49 | 0.57 | +3/12.13 | 22.85 |
| I9A-piscidin- 1 | FFHHIFRGAVHVGKTIHRLVTG | 2,530 | 1.34 | 0.49 | +3/12.13 | 21.58 |
| I16A-piscidin-1 | FFHHIFRGIVHVGKTAHRLVTG | 2,530 | 1.34 | 0.49 | +3/12.13 | 19.86 |
| I9K-piscidin-1 | FFHHIFRGKVHVGKTIHRLVTG | 2,587 | 1.21 | 0.43 | +4/12.16 | 18.66 |
| I16K-piscidin-1 | FFHHIFRGIVHVGKTKHRLVTG | 2,587 | 1.21 | 0.43 | +4/12.16 | 17.47 |
Mass spectrometry (MS) was performed to confirm the correct atomic mass of purified peptides. Physico-chemical properties of peptides were determined using the Protparam program from the ExPASy server (http://www.expasy.ch/tools/protparam.html) (Table 1).
In order to determine the helical wheel and hydrophobic/hydrophilic interface on the secondary structure of peptides, the amino acid sequence of peptides was submitted in helical wheel projection (http://rzlab.ucr.edu/scripts/wheel/wheel.cgi).
Retention time of peptides
RP-HPLC was used to measure retention time (RT) of the peptides. The peptides individually were injected into the RP-HPLC system equipped with the analytical Inertsil ODS-SP column and eluted according to the following conditions: a linear gradient of 0–100% acetonitrile, supplemented with 0.1% trifluoroacetic acid and flow rate of 1 mL/min for 30 min (Figures S1–S5).
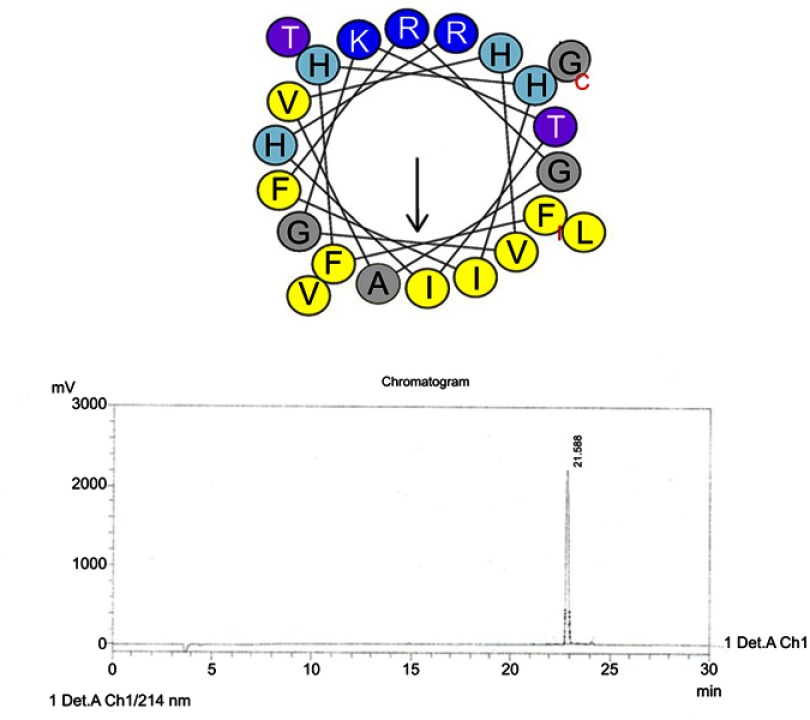
Figure S1.
Helical wheel projections and retention time for piscidin-1. Upper picture: Residues are numbered starting from the N-terminus. Hydrophobic and positive charge residues are defined with yellow and blue color, respectively. Lower picture: Chromatogram for piscidin-1, dominant peak is synthetic piscidin-1.
Abbreviation: Pis-1, piscidin-1.
Figure S5.
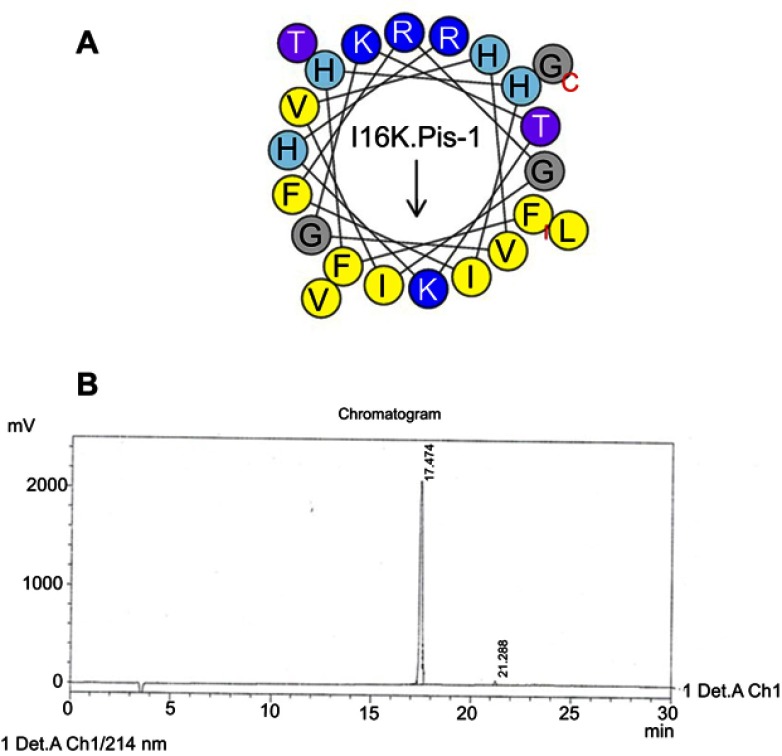
Helical wheel projections and retention time for I16K-piscidin-1. (A) Residues are numbered starting from the N-terminus. Hydrophobic and positive charge residues are defined with yellow and blue color, respectively. (B) Chromatogram for piscidin-1, dominant peak is synthetic I16K-piscidin-1.
Abbreviation: I16K.Pis-1, I16K-piscidin-1.
Bacterial strains
We determined antimicrobial activity of peptides against two clinical and four standard bacterial strains including three strains of Gram-negative bacteria, [Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 10662), and clinical strain of colistin-resistant Acinetobacter baumannii], and three strains of Gram-positive bacteria [Staphylococcus aureus (ATCC 25923), Staphylococcus epidermidis (ATCC1435), and clinical strain of methicillin-resistant Staphylococcus aureus (MRSA)]. Standard strains were provided from Iranian Pasture Institute, Tehran, Iran. The clinical strain of MRSA was provided from Persian Gulf Martyrs Educational Hospital affiliated to Bushehr University of Medical Sciences, Bushehr, Iran. In addition, a clinical strain of colistin-resistant A. baumannii that had been investigated in our previous study was used.15
Minimum inhibitory concentration
Minimum inhibitory concentration (MIC) of each peptide against all bacteria was evaluated as described previously.16 Briefly, bacteria were cultured in brain heart infusion (BHI) medium until mid-logarithmic phase and then diluted in BHI medium to give a density of 1×106 cell/mL. Subsequently, 50 µL of twofold dilutions of each peptide generated using 0.01% acid acetic and 0.2% BSA (bovine serum albumin) was added to wells containing 50 µL of the diluted bacteria to provide final concentrations of 100, 50, 25, 12.5, 6.25, 3.12 and 1.56 μg/mL. After incubation at 37°C for 18 h, the lowest peptide concentration lacking observable growth was considered as MIC. Finally, the MIC mean of three experiments was calculated for each peptide.
The lowest concentration of peptides causing a reduction of at least 99.9% (equal to a ≥3-log10 decrease) in the initial inoculum was considered as minimum bactericidal concentration (MBC).
Time-kill kinetics test
Time-kill kinetics assay was performed to determine the rapidity of bactericidal activity of peptides and also to investigate whether the peptidoglycan layer of the bacterial cell wall acts as a barrier to prevent access of AMPs to the cytoplasmic membrane. Bactericidal activity of peptides alone or in combination with lysozyme was examined against clinical MRSA (as the representative of Gram-positive bacteria) and clinical colistin-resistant A. baumannii (as the representative of Gram-negative ones) for various incubation periods. Bacterial strains (1×107 cells/mL) were exposed to different concentrations (1×MIC and 5×MIC) of peptides alone or in combination with lysozyme (0.5 mg/mL) in the 96-well microplates (Sigma-Aldrich Co., St Louis, MO, USA). After incubation for 1, 2, 3, 4 and 5 h, 10 µL of samples were taken from each well and viable bacteria were counted by plating of the 100-fold diluted samples on LB agar. The lowest limit of detection for time-kill assays was 2 log10 CFU/mL.
β-galactosidase leakage assay
The β-galactosidase leakage assay was implemented to investigate membrane damage in the presence of peptides alone and in combination with lysozyme as described previously.17 The JM101 E. coli containing plasmid PUC 19 was cultured in LB medium until the OD600 reached 0.6 (logarithmic phase). Bacterial cultures were treated with 1 mM isopropyl β-D-1-thiogalactopyranoside to induce expression of β-galactosidase. One hour after induction, bacterial cultures were centrifuged and the cell pellets were resuspended in fresh LB medium and exposed to different concentrations (1×MIC and 5×MIC) of peptides alone or in combination with lysozyme (0.5 mg/mL). After incubation at 37°C for 1 h, the supernatant was isolated from the cell pellets by centrifugation (4400 rpm for 10 min). Subsequently, ortho-nitrophenyl-β-galactoside (ONPG), a colorimetric and spectrophotometric substrate for detection of β-galactosidase activity, was added to the supernatant at a final concentration of 0.8 mg/mL. Finally, the absorbance of wells was determined at 405 nm with a microplate reader (BioTek Synergy 4, BioTek, Winooski, VT, USA).
Propidium iodide staining
The integrity of bacterial cell membrane treated with peptides alone or in combination with lysozyme was evaluated by propidium iodide (PI) uptake assay, as explained previously.18 Briefly, overnight bacterial cultures were centrifuged at 2,000 rpm for 10 min and the cell pellets were resuspended in PBS to reach a density of 1×108 cell/mL. Thereafter, 100 µl of the resuspended bacteria were incubated in the presence of increasing concentrations of peptides (2.5, 5, 10 μg/mL) alone or in combination with lysozyme (0.5 mg/mL) at 30°C for 1 h. The cells were then stained with PI solution (20 mg/mL) for 45 min in dark conditions. Following staining with PI, the cell suspensions were centrifuged and the cell pellets were washed with PBS. Finally, the percentage of PI-stained cells were measured with a flow cytometry system.
Agar diffusion assay
To further evaluate the antimicrobial activity of peptides, an agar diffusion assay was performed. was examined against MRSA by using an agar diffusion assay. To do this, overnight bacterial culture was diluted in fresh LB broth and grown to mid-logarithmic phase, and then added to an LB agar plate. After solidification of LB agar medium, 5 μL of the peptides (500 μg/mL) was added to each punctured well generated by sterile sampler tip and plate was incubated at 37°C for 18 h. Bactericidal activity of peptides was determined according to the diameter of clear zones around the wells.
Hemolysis assay
Hemolytic activity of peptides was determined against human red blood cells (hRBCs). For this test, the hRBC pellets isolated by centrifugation were resuspended in PBS to reach a concentration of 4% v/v and incubated with twofold dilutions of peptides (100, 50, 25, 12.5, 6.25 3.12 and 1.56 μg/mL) at 37°C for 1 h. After incubation, the cells were pelleted using centrifugation (2,000 rpm for 10 min) and supernatant of each dilution (100 μL) was transferred to the corresponding well in a sterile 96-well plate. Finally, the absorbance of wells was measured at 405 nm using microplate reader. The RBCs treated with Triton-x100 (0.1%) and untreated were used as positive and negative control, respectively. The percentage of hemolysis activity for peptides was calculated according to the following equation:19
Peptide concentration exhibiting 50% hemolytic activity (HC50) was also determined for each peptide.
Cytotoxicity assay
Cytotoxic activity of peptides against human embryonic kidney cells (HEK-293) was assessed by a standard colorimetric assay using thiazolyl blue tetrazolium bromide (MTT, Sigma-Aldrich Co.) as described previously.20,21 Briefly, the fresh HEK-293 cells in wells of a 96-well microplate (1×105 cell/well) were exposed to twofold serial dilutions of peptides (1.56, 3.12, 6.25, 12.50, 25, 50 μg/mL), and incubated under 5% CO2 and 95% air condition at 37°C for 48 h. To determine the percentage of viable cells, 10 μL of MTT (5 mg/mL) was added to wells containing HEK-293 cells and then microplates were incubated for 4 h. Subsequently, formazan crystals were dissolved by the addition of 100% DMSO (100 μL) to wells. Finally, the absorbance of wells was determined at 575 nm using a microplate reader.
Evaluation of binding affinity of AMPs to HEK-293 cells and E. coli
We optimized the method to compare the binding affinity of peptides to HEK-293 cells and E. coli. The affinity of peptides for RBCs was evaluated by a fluorometric-based method. The HEK-293 cells and E. coli were centrifuged and the cell pellets were washed three times with PBS, resuspended in PBS buffer and aliquoted to small portions (100 μL) in polypropylene microtubes (1.5 mL) (Eppendorf, Hamburg, Germany). Twofold dilutions of FAM-labeled peptides (2.5, 5 and 10 μg/mL) were then added into wells containing HEK-293 cells (1×105 cell/well) or E. coli cells (12.5×106 cell/well), and into wells lacking cells as a negative control. Following incubation at 37°C for 30 min, in order to remove non-binding AMPs, the cells were pelleted by centrifugation at 2,000 rpm for 10 min and washed three times with PBS buffer. To measure cell-binding AMPs, cells were suspended in 100 PBS, added to corresponding wells, and fluorescence intensity of wells were determined by a fluorescence microplate reader at Ex/Em=490/560 nm and measured according to the following equation: FIC– FIwc, where FIC is fluorescence intensity (Ex/Em =490/560 nm) of wells containing cells, while FIwc represents fluorescence intensity (Ex/Em =490/560 nm) from wells without cells.
Mouse models of sepsis
Male Balb/c mice (22.25±1.18 g) were used in the development of mouse models of sepsis. All laboratory mouse handling techniques were in accordance with the laboratory animal ethics committee of Bushehr University of Medical Sciences. To develop sepsis, mice were intraperitoneally inoculated with clinical MRSA or clinical colistin-resistant A. baumannii strains (106 CFU/mouse). Fifteen minutes after injection of bacteria, mice were treated with piscidin-1 and its analogs (100 μg/mouse) through intraperitoneal injection. The survival rate was monitored every 24 h for up to 192 h.
In a separate set of experiments to examine time-dependent effects, mice were treated with the peptides (100 μg/mouse) through intraperitoneal injection at 15, 60 or 180 min after infection with clinical MRSA or clinical A. baumannii strains (106 CFU/mouse). Survival rate and mouse status were recorded every 24 h for up to 192 h.
To examine bacterial dissemination and killing rate, mice were infected with clinical MRSA or clinical A. baumannii strains (106 CFU/mouse) and then treated the peptides 15 min after infection. After incubation for 72 h, mice were sacrificed for investigation of bacterial dissemination and killing rate. Control group was infected with bacteria, but untreated with the peptides. Bacterial numbers in blood, liver, and mesenteric lymph nodes were determined. Colony counts from the diluted bacterial solutions were expressed relative to those at the initial bacterial inoculum.
In vivo toxicity testing
To examine the toxicity of piscidin-1 and its analogs, these peptides were dissolved in phosphate-buffered saline (PBS; pH 7.4) and delivered through intramuscular bolus injection in the left thigh (0.5, 1, or 2 mg/mouse). Mice were monitored for signs of systemic toxicity.
For biochemistry analysis, mice were administered with PBS (control group) or the peptides (50 or 100 μg/mouse) through ip injection (100 μL/mouse). Blood samples (0.2 mL) were collected 48 h after the injection and used to quantify the serum levels of glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT), total bilirubin (TBIL), and uric acid (UA).
Statistical analysis
The experiments were done in triplicate and repeated three times. Each in vivo experimental group consisted of five mice. Data were expressed as the mean (± standard deviation, SD) of three independent repeats. The value of p<0.05 was considered statistically significant. Data from peptides were compared with each other using analysis of variance (ANOVA) with SPSS 20.0 statistical software (IBM Corporation, Armonk, NY, USA).
Results
Piscidin-1 and its analogs exhibited comparable potency for inhibition of bacterial growth
Comparison of antibacterial activities of piscidin-1 and its derivatives using the broth microdilution method demonstrated no difference in antibacterial activity between piscidin-1 derivatives and parent peptide, except for S. aureus. P. aeruginosa and S. epidermidis (ATCC 1435) which exhibited the lowest and highest susceptibility to peptides, respectively (Table 2). Actual mean of hydrophobicity of peptides determined by RT was used to compare hydrophobic features of the peptides. As expected, piscidin-1 has the highest RT score relative to its derivatives, and I16K-piscidin-1 represented the lowest RT. In fact, I16K-piscidin-1 generated by substitution of lysine for isoleucine at the central site of the nonpolar face of piscidin-1 reduced RT more than other piscidin-1 analogs including I9K-piscidin-1, I9A-piscidin-1, and I16A-piscidin-1. We did not observe a significant association between MIC value and hydrophobic property of peptides represented by RT against the studied bacteria (Table 1), except for S. aureus; there was an inverse association between the RT and MIC values of peptides against S. aureus.
Table 2.
Minimum inhibitory concentration of piscidin-1 and its analogs against bacterial strains
| Bacterial strains | Minimum inhibitory concentration (μg/mL) | ||||
|---|---|---|---|---|---|
| Piscidin-1 | I9A-piscidin-1 | I16A-piscidin-1 | I9K-piscidin-1 | I16K-piscidin-1 | |
| Escherichia coli | 3.1 | 3.1 | 3.1 | 3.1 | 3.1 |
| Pseudomonas aeruginosa | 25 | 25 | 25 | 25 | 25 |
| Clinical strain of colistin-resistant A. baumannii | 3.1 | 3.1 | 3.1 | 3.1 | 3.1 |
| Staphylococcus aureus | 3.1 | 3.1 | 3.1 | 3.1 | 6.2 |
| Staphylococcus epidermidis | 1.5 | 1.5 | 1.5 | 3.1 | 3.1 |
| Clinical strain of methicillin resistant Staphylococcus aureus | 3.1 | 3.1 | 3.1 | 6.2 | 6.2 |
I16K-piscidin-1 exhibited faster bactericidal activity compared to piscidin-1 and other piscidin-1 analogs at 5×MIC
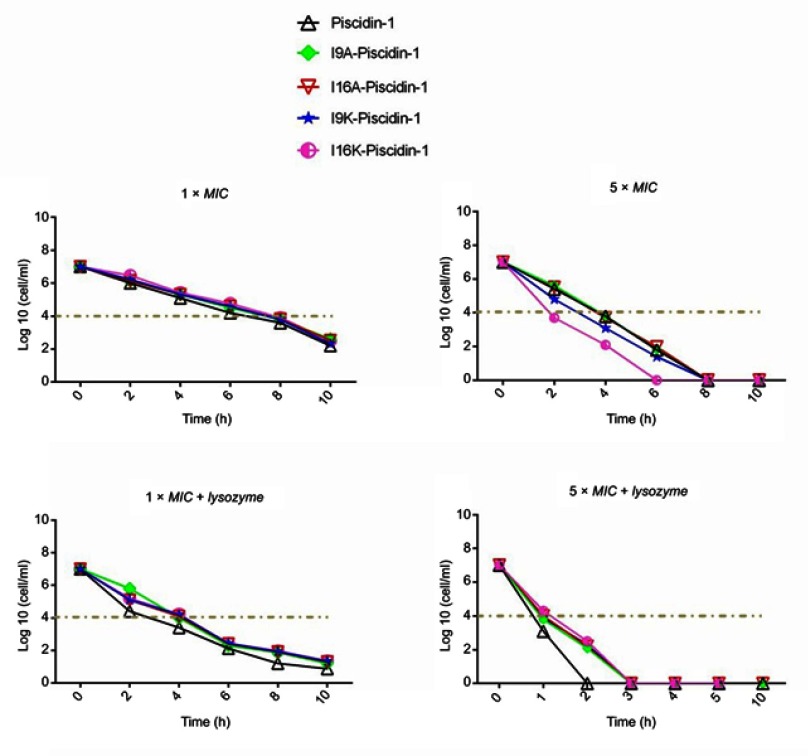
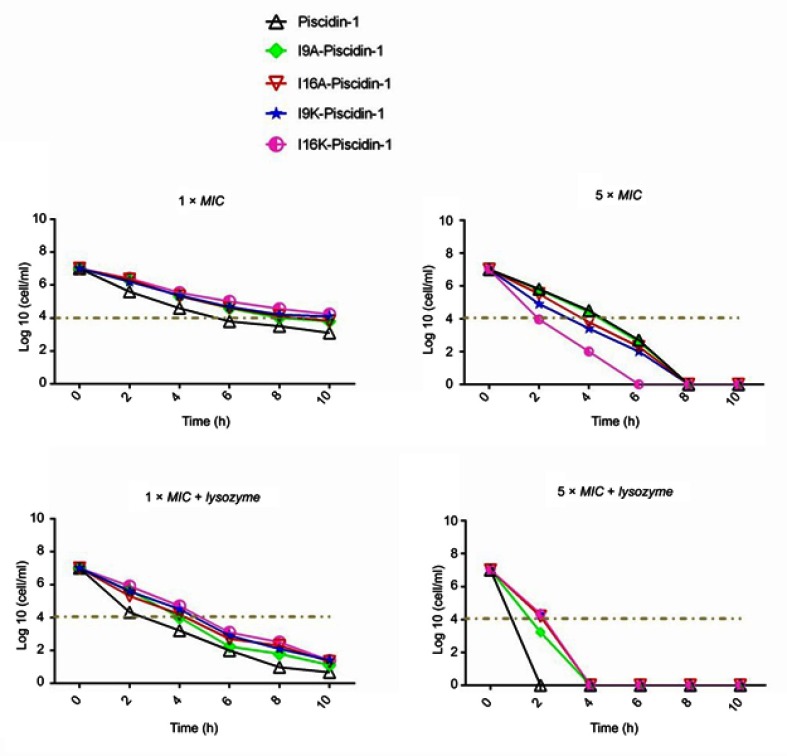
Time-killing kinetics test was performed to examine the rapidity of bactericidal activity of peptides against Gram-positive (clinical MRSA) and Gram-negative (clinical A. baumannii) bacteria. The results showed that all peptides at 1×MIC had bactericidal activity and required at least 8 h to cause a ≥3-log10 reduction in initial inoculum of bacteria, but none of the peptides were capable to eradicate all bacteria at 1×MIC. Overall, a time-dependent growth inhibition was observed for all peptides at 1×MIC (Figures 1 and 2). Increasing the peptide concentration to 5×MIC completely eliminated all initial inoculum of bacteria 8–10 h after exposure. Surprisingly, at a higher concentration of peptides (5×MIC), I16K-piscidin-1 showed the most rapid bactericidal activity, followed by I9K-piscidin-1. The I16K-piscidin-1 peptide caused a ≥3-log10 reduction in initial inoculum of A. baumannii and S. aureus only after incubation for 2 h, whereas piscdin-1 exhibited such activity after 4 h. Furthermore, piscidin-1 at a concentration of 5×MIC completely eliminated A. baumannii and S. aureus after 8 and 10 h, respectively, whereas I16K-piscidin-1 did so after 6 h at 5×MIC (Figures 1 and 2). To examine whether the bacterial peptidoglycan layer of the cell wall acts as a barrier to prevent access of AMPs to the cytoplasmic membrane, we determined the killing kinetics of peptides in combination with lysozyme. The result showed that lysozyme can improve the bactericidal activity of all peptides. The highest improvement in the rapidity of bactericidal activity was observed regarding the killing time of piscidin-1 followed by I9A-piscidin. Piscidin-1 alone requires 8 or 10 h to completely eradicate the initial inoculum of A. baumannii or S. aureus at 5×MIC, respectively, whereas the killing time of piscidin-1 against both bacteria was reduced to 2 h in combination with lysozyme. In summary, peptidoglycan degradation increased the susceptibility of bacteria to AMPs, especially in Gram-positive bacteria.
Figure 1.
Time-kill kinetics data for piscidin-1 and its derivatives against A. baumannii. Bactericidal activity of peptides against A. baumannii (clinical isolate) was determined alone or in combination with lysozyme (0.5 mg/mL) at 1×MIC and 5×MIC. All data represent the mean of two independent experiments.
Abbreviations: A. baumannii, Acinetobacter baumannii; MIC, minimum inhibitory concentration.
Figure 2.
Time-kill kinetics data for piscidin-1 and its derivatives against MRSA. Bactericidal activity of peptides against MRSA (clinical isolate) was determined alone or in combination with lysozyme (0.5 mg/mL) at 1×MIC and 5×MIC. All data represent the mean of two independent experiments.
Abbreviations: MRSA, methicillin resistant Staphylococcus aureas; MIC, minimum inhibitory concentration.
Agar diffusion assay
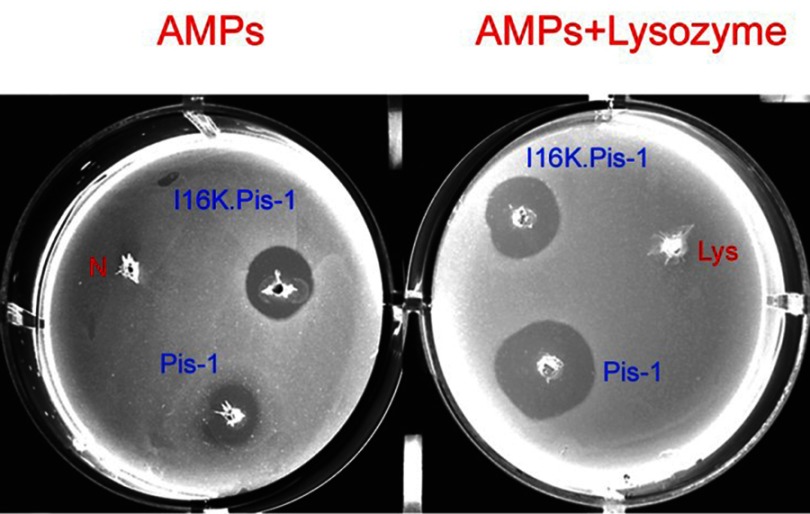
The I16K-piscidin-1 and piscidin-1 peptides were selected to evaluate their bactericidal activity due to significant differences in their rapidity of bactericidal against MRSA at 5×MIC. As can be seen in Figure 3, a larger clear zone appeared around the punched wells containing I16K-piscidin-1 compared to those containing piscidin-1. However, in contrast, the combination of piscidin-1 with lysozyme showed greater bactericidal activity than the combination of I16K-piscidin-1 with lysozyme.
Figure 3.
Assessment of bactericidal activity of piscidin-1 and I16K-piscidin-1 against MRSA through agar diffusion test at 5×MIC. The clear zone around well represents the antimicrobial potency of peptides. N: negative control (without an antimicrobial agent); Lys: lysozyme (0.5 mg/mL).
Abbreviations: AMPS, antimicrobial peptide; MRSA, methicillin resistant Staphylococcus aureus.
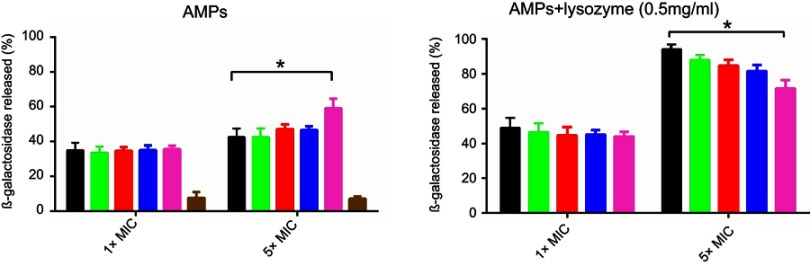
I16K-piscidin-1 induced greater β-galactosidase leakage compared to other peptides at 5×MIC
Antimicrobial peptides are supposed to exert antimicrobial activity through pore formation in the bacterial cell wall.22 For this purpose, we evaluated pore-forming capacity of peptides in bacteria cell walls via measuring the amount of β-galactosidase leakage from bacteria expressing β-galactosidase. The results indicated that in the presence of high concentration of I16K-piscidin-1 peptides (5×MIC), bacteria released considerably more β-galactosidase compared to with other peptides, providing evidence for high rapidity of bactericidal activity of I16K-piscidin-1 at 5×MIC, whereas in the presence of low concentration of peptides (1×MIC) bacteria released nearly same level of β-galactosidase. Evaluation of combined effect of peptides and lysozyme on β-galactosidase leakage revealed that lysozyme enhanced the pore-forming capacity of peptides. In fact, the damage to bacterial peptidoglycan caused by lysozyme facilitates accessibility of cell membrane to peptides. Piscidin-1 was capable of inducing greater amounts of β-galactosidase leakage relative to other peptides at 5×MIC when used in combination with lysozyme (Figure 4).
Figure 4.
Β-galactosidase leakage assay. E. coli was exposed to peptides (1×MIC or 5×MIC) alone or in combination with lysozyme (0.5 mg/mL).
Note: *p-value ≤0.05.
Abbreviations: AMPs, antimicrobial peptides; E.coli, Escherichia coli; MIC, minimum inhibitory concentration.
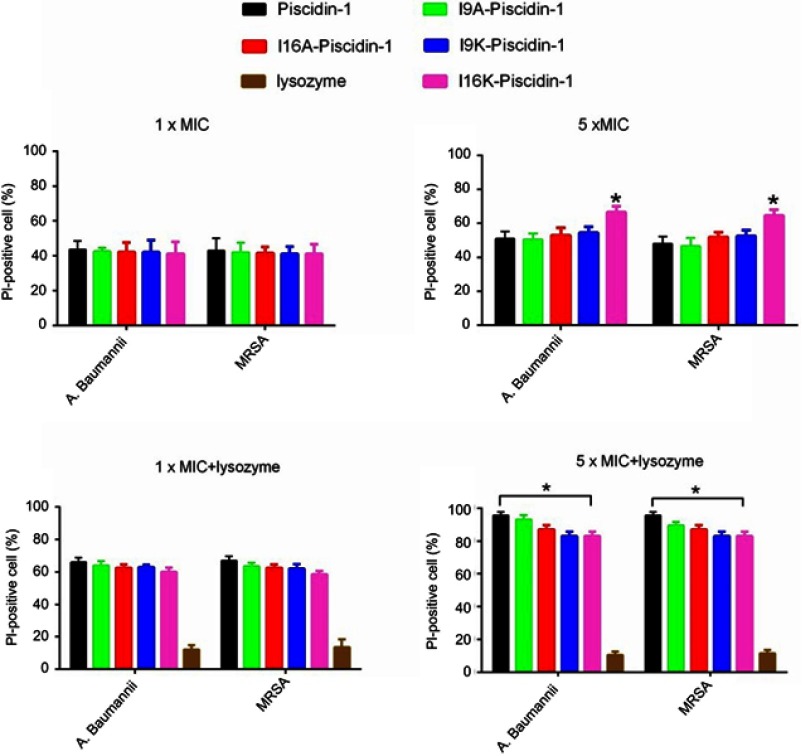
I16K-piscidin-1 reduced the membrane integrity more than other peptides in 5×MIC
PI uptake assay was used to evaluate the effect of peptides on the reduction of membrane integrity. As can be seen in Figure 5, a similar percentage of PI-stained A.baumannii was detected in the presence of low concentration of peptides (1×MIC). However, a more percentage of PI-stained MRSA was detected only in the presence of piscidin-1 at 1×MIC. Like the results of β-galactosidase leakage assay, at high concentration of peptides (5×MIC) I16K-piscidin-1 reduced membrane integrity more than other peptides; in contract to low concentration (1×MIC), high concentration (5×MIC) of piscidin-1 led to the lowest percentages of PI-stained MRSA. A direct association existed between peptide concentration and the percentage of PI-stained cells. All peptides increased the percentage of PI-stained bacteria, especially the percentage of PI-stained A. baumanii (Figure 5). The combination of peptides with lysozyme showed a synergism effect to increase membrane permeability, particularly in case of bacteria exposed to piscidin-1. This result suggested that lysozyme improves the antibacterial activity of AMPs by removing the cell wall and subsequently facilitates easy accessibility of peptides to the cell membrane.
Figure 5.
Effect of peptides alone or in combination with lysozyme (0.5 mg/mL) on the integrity of the E. coli cell membrane. The percentage of propidium iodide (PI)-stained cells was determined by flow cytometry.
Note: *p-value ≤0.05.
Abbreviations: A. baumannii, Acinetobacter baumannii; AMPs, antimicrobial peptides; E. coli, Escherichia coli; MIC, minimum inhibitory concentration; MRSA, methicillin resistant Staphylococcus aureus.
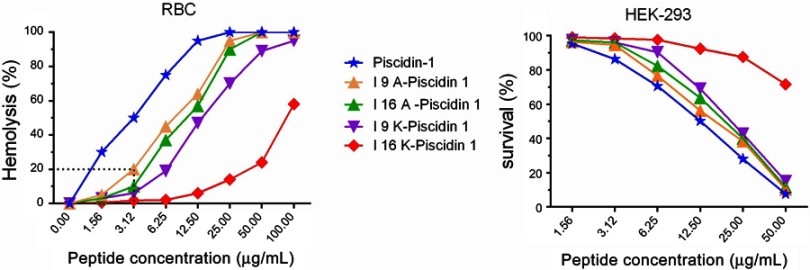
I16K-piscidin-1 exhibited the lowest hemolytic and cytotoxic activity compared to the parent peptide piscidin-1 and other peptide derivatives
The cytotoxicity of AMPs is considered as the most important challenge for their clinical application;23 therefore, the hemolytic activity of piscidin-1 and its derivatives were evaluated against hRBCs. As shown in Figure 6, I16K-Piscidin-1 significantly exhibited the weakest hemolytic activity relative to other peptides. Piscidin-1 was found to display the hemolytic effect in a dose-dependent manner. In addition to hemolytic potency, the cytotoxic activity of peptides was investigated against HEK-293. In accordance with the results from the hemolysis assay, I16K-piscidin-1 represented the lowest activity against HEK-293 cells compared to other peptides (Figure 6).
Figure 6.
Activity of peptides against mammalian cells. Cytotoxic activity of peptides against human RBCs and HEK-293 was estimated at increasing concentrations of peptides.
Abbreviations: RBC, red blood cells; HEK, human embryonic kidney cells.
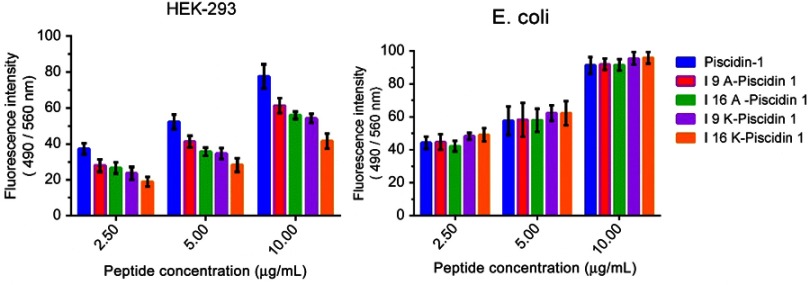
Binding affinity of AMPs to HEK-293 and bacterial cells
The binding affinity of peptides to HEK-293 and bacterial cells was evaluated by a fluorometric-based method. Results showed a direct correlation between the binding affinity of the peptide to HEK-293 and cytotoxic activity. Piscidin-1, with the highest cytotoxic activity, exhibited the highest binding affinity to HEK-293 cells, whereas I16K-piscidin-1 with the weakest cytotoxic potency showed the lowest binding affinity to HEK-293 cells. The evaluation of peptide binding affinity to bacteria demonstrated no significant difference between peptides (Figure 7).
Figure 7.
Binding affinity of peptides to bacteria and HEK-293 cells. Binding affinity of peptides to E. coli and HEK-293 cells were measured with a fluorometric-based method. Fluorescence intensity (FI) of wells was measured on a microplate reader at excitation/emission (Ex/Em) =490/560 nm. Binding affinity scores represent mean ±SD of three independent experiments.
Abbreviation: E. coli, Escherichia coli; HEK, human embryonic kidney cells.
I16K-piscidin-1 exhibited more potent in vivo antibacterial activity against clinical MRSA and clinical colistin-resistant A. baumannii strains compared to piscidin-1 and other analogs
To compare in vivo bactericidal potency of piscidin-1 and its analogs, we monitored their effect on the survival of mice infected with clinical colistin-resistant A. baumannii or clinical MRSA strains. The survival rate on day 8 after infection with clinical MRSA was 0%, 73.3%, 94.6%, 80%, 66.6%, and 73.3% for mice treated with PBS (control), piscidin-1, I16K-piscidin-1, I9K-piscidin-1, I9A-piscidin-1 and I16A-piscidin-1, respectively, 15 min after infection. On the other hand, the survival rate on day 8 after infection with clinical A. baumannii was 0%, 78.3%, 100%, 86.6%, 73.3%, and 73.3% for mice treated with PBS (control), piscidin-1, I16K-piscidin-1, I9K-piscidin-1, I9A-piscidin-1 and I16A-piscidin-1, respectively. The survival rate of mice treated with I16K-piscidin-1 after infection with clinical A. baumannii or clinical MRSA strains was significantly higher than those treated with the parent peptide (piscidin-1) (p<0.05) (Table 3). However, the survival rate of mice treated with other analogs exhibited no statistically significant difference with the parent peptide piscidin-1.
Table 3.
Survival rate of mice infected with clinical strains of methicillin-resistant S. aureus and colistin-resistant A. baumannii treated with piscidin-1 and its analogs 15 min after infection
| Treatment | Survived rate (%) of mice infected with clinical strain of methicillin-resistant S. aureus (MRSA) | Survived rate (%) of mice infected with clinical strain of colistin-resistant A. baumannii |
|---|---|---|
| Piscidin-1 | 73.3±14.4 | 78.3±14.21 |
| I16K-piscidin-1 | 93.3±13.5 | 100 |
| I9K-piscidin-1 | 80±26.6 | 86.6±16.4 |
| I9A-piscidin-1 | 66.6±34.8 | 73.3±28.2 |
| I16A-piscidin-1 | 73.3±22.5 | 73.3±38.6 |
| PBS (control) | 0 | 0 |
Notes: Survival rate is related to 168 h after the treatment. The survival rate of mice treated with I16K-piscidin-1 after infection with clinical strains of A. baumannii or MRSA was significantly higher than those treated with the parent peptide (piscidin-1) (p<0.05). Data are presented as mean±SD.
Abbreviations: A. baumannii; Acinetobacter baumannii; S. aureus, Staphylococcus aureus.
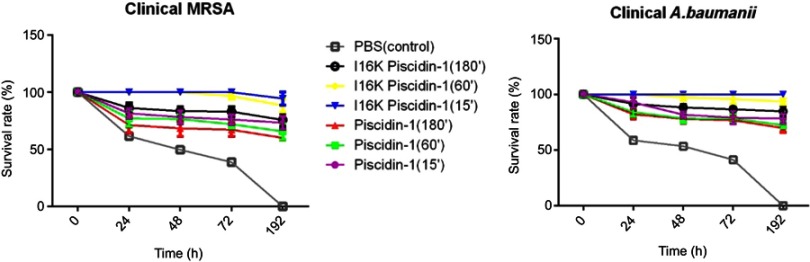
To investigate the curative potential of I16K-piscidin-1 in comparison with the parent peptide (piscidin-1), mice were first infected with clinical A. baumannii or clinical MRSA strains, and then treated with the peptides 15, 60, or 180 min after infection. The results showed a higher survival rate in the mice treated with I16K-piscidin-1 and piscidin-1 15 min after infection (Figure 8). These data indicate that the immediate application of I16K-piscidin-1 is essential to prevent severe infection in mice.
Figure 8.
The survival rate of mice injected with piscidin-1 and its analogs after infection. Injection of peptides was performed at the represented times (15, 60, 180 min) after infection with a clinical strain of MRSA and colistin-resistant A. baumannii. The survival rate was monitored every day up to 192 h. Data show the means, and error bars show standard deviations.
Abbreviations: A. baumannii; Acinetobacter baumannii; S.aureus, Staphylococcus aureus.
We next compared in vivo bactericidal properties of piscidin-1 and its analogs. Examination of bacterial load in various mouse organs after infection with clinical MRSA or clinical A. baumannii and treatment with the peptides revealed that I16K-piscidin-1 significantly exhibited stronger in vivo bactericidal potency compared to the parent peptide piscidin-1. However, treatment with other piscidin-1 analogs showed no statistically significant difference with the parent peptide piscidin-1. Compared to piscidin-1, treatment with I16K-piscidin-1 resulted in a greater reduction in the bacterial load in blood, liver, and mesenteric lymph nodes (p<0.05) (Table 4). These results indicate that I16K-piscidin-1 can significantly control the proliferation of MRSA and A. baumannii in these organs of mice.
Table 4.
Effects of treatment with piscidin-1 and its analogs on bacterial counts in blood, liver and mesenteric lymph nodes of mice infected with a clinical strain of MRSA and colistin-resistant A. baumannii
| Peptides | Bacterial count of MRSA (CFU/mL) | Bacterial count of A. baumannii (CFU/mL) | ||||
|---|---|---|---|---|---|---|
| Blood | Liver | Mesenteric lymph nodes | Blood | Liver | Mesenteric lymph nodes | |
| Piscidin-1 | 4.5×105±0.7×105a | 9.7×106±2.1×107a | 2.6×106±0.7×106a | 5.8×106±0.4×106a | 8.2×106±0.9×106a | 3.8×107±0.7×107a |
| I16K--piscidin-1 | 5.1×104±0.5×104b | 1.1×106±0.1×106b | 3.5×105±0.3×105b | 4.4×105±0.1×105b | 7.5×105±1.2×106b | 4.3×106±0.6×106b |
| I9K-piscidin-1 | 3.5×106±0.9×105c | 1.6×107±0.6×107a | 4.2×106±1.2×107a | 2.6×106±1.2×107a | 9.3×107±1.6×108c | 7.6×107±1.7×108a |
| I9A-piscidin-1 | 7.4×105±1.2×106a | 1.3×107±0.9×107a | 5.3×106±1.8×107a | 6.2×106±0.8×106a | 1.2×107±0.2×107a | 6.2×107±1.4×108a |
| I16A--piscidin-1 | 5.7×106±1.4×106c | 9.6×107±1.7×108c | 2.6×107±0.2×107c | 4.8×107±1.8×108c | 9.8×107±2.6×108c | 4.2×108±0.8×108c |
| PBS (control) | 6.8×106±0.7×107c | 9.8×107±1.5×108c | 4.7×107±0.8×107c | 7.4×107±1.3×108c | 2.6×108±0.9×108c | 6.7×108±1.2×109c |
Notes: Data are represented as the mean±SD. Statistically significant differences between groups in the same column are shown by different superscripted letters (p<0.05). The most significant change in bacterial count occurred after injection with I16K-piscidin-1.
Abbreviations: A. baumannii; Acinetobacter baumannii; S. aureus, Staphylococcus aureus.
I16K-piscidin-1 exerted no toxic effects in mice as compared to the parent peptide piscidin-1 and other peptide derivatives
We examined in vivo toxicity of the peptides by administering them through intramuscular injection and then monitoring signs of systemic toxicity. Grading of toxicity was performed based on the signs as follows: level 1 (narrowing of eyes), level 2 (crouching and huddling), or no toxicity. The results showed that mice injected with I16K-piscidin-1 did not exert toxic effects, even at the highest tested dose (2 mg/mouse), whereas mice injected with the parent peptide piscidin-1 and other analogs at a dose of 2 mg/mouse were found to exhibit level 1 or/and level 2 toxicity. The lower doses (0.5 and 1 mg/mouse) of all peptides were found to have no toxicity potential following intramuscular injection (Table 5).
Table 5.
Grading of toxicity in mice (n =7 per group) intramuscularly injected with piscidin-1 and its analogs
| Dose (mg/mouse) | Toxicity grades | ||||
|---|---|---|---|---|---|
| Piscidin-1 | I16K-piscidin-1 | I9K-piscidin-1 | I9A-piscidin-1 | I16A-piscidin-1 | |
| 0.5 | NT | NT | NT | NT | NT |
| 1 | NT | NT | NT | NT | NT |
| 2 | 1: NT 3: level 1 3: level 2 |
NT | 2: NT 3: level 1 2: level 2 |
1: NT 3: level 1 3: level 2 |
2: NT 2: level 1 3: level 2 |
Notes: Toxicity grades: level 1, narrowing of eyes; level 2, crouching and huddling.
Abbreviation: NT, no toxicity.
Furthermore, assessment of biochemical factors in the blood of mice treated with a low dose (50 μg/mouse) of the peptides through intraperitoneal injection demonstrated that piscidin-1 and its analogs did not cause any significant change in the serum level of the biochemical factors (Table 6). However, intraperitoneal injection of a higher dose (100 μg/mouse) of piscidin-1 and its analogs, but not I16K-piscidin-1, resulted in significant changes in the quantity of glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), total bilirubin (TBIL), or uric acid (UA). Intraperitoneal injection of I16K-piscidin-1 with a dose of 100 μg/mouse did not lead to any significant change in the amount of the biochemical factors (Table 6).
Table 6.
The serum level of the biochemical factors in mice intraperitoneally injected with different doses of piscidin-1 and its analogs
| Peptides | The serum level (the mean ± standard deviation) of the biochemical factors | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment with 50 μg/mouse | Treatment with 100 μg/mouse | |||||||
| GOT (U/L) | GPT (U/L) | UA (mg/dL) | TBIL (mg/dL) | GOT (U/L) | GPT (U/L) | UA (mg/dL) | TBIL (mg/dL) | |
| PBS (control) | 9.15±0.93 | 6.12±0.57 | 0.43±0.08 | 0.19±0.05 | 8.27±0.85 | 4.73±0.78 | 0.51±0.10 | 0.25±0.08 |
| Piscidin-1 | 9.48±1.06 | 5.83±0.52 | 0.45±0.06 | 0.19±0.04 | 10.64±0.97 | 4.62±0.42 | 0.53±0.08 | 0.27±0.07 |
| I16K-piscidin-1 | 8.88±0.86 | 6.43±0.86 | 0.41±0.11 | 0.20±0.05 | 8.53±1.15 | 6.55±0.66 | 0.79±0.13 | 0.46±0.09 |
| I9K-piscidin-1 | 9.27±0.78 | 6.26±0.22 | 0.43±0.05 | 0.18±0.07 | 10.32±0.88 | 6.86±0.82 | 0.81±0.07 | 0.48±0.10 |
| I9A-piscidin-1 | 8.96±1.12 | 6.67±0.47 | 0.42±0.07 | 0.21±0.06 | 10.86±1.07 | 7.17±0.37 | 0.87±0.09 | 0.51±0.09 |
| I16A-piscidin-1 | 9.22±0.90 | 5.92±1.10 | 0.39±0.12 | 0.20±0.04 | 11.08±0.94 | 7.33±1.10 | 0.77±0.14 | 0.53±0.08 |
Abbreviations: GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; UA, uric acid; TBIL, total bilirubin.
Accordingly, in vivo toxicity testing indicated no toxicity potential for I16K-piscidin-1, even at doses as high as 100 μg/mouse.
Discussion
Unfortunately, S. aureus and A. baumannii are increasingly developing resistance to classic antibiotics, necessitating the discovery of novel antimicrobial agents. AMPs were considered as promising candidates for developing antimicrobial drugs. Piscidin as an AMP with broad-spectrum antimicrobial potency possesses toxicity against mammalian cells, which can potentially limit its clinical application. Despite the existence of the potential limitation in its clinical application, the potent antimicrobial activity of piscidin-1 encourages researchers to generate analogs with minimum toxicity.24 It has been found that disruption of the nonpolar face of AMPs reduces their toxicity. In addition, such a disruption may lead to improved antimicrobial activity of AMPs. However, such studies reported in vitro data, but not in vivo ones.8,9,11,13 Accordingly, we evaluated in vitro and in vivo antibacterial and toxicity of piscidin-1-analogs generated by substitution of alanine or lysine for isoleucine at the border16 or center (I16) of the nonpolar face of piscidin-1. The measurement of MIC revealed that there was no difference in antibacterial activity between peptides, except for S. aureus against which I16K-piscidin-1 had the highest antibacterial activity compared to piscidin-1 and other analogs.
It is important to note that a direct association between RT of peptides and their toxicity was observed. However, the analogs possessing the same predicted hydrophobicity and hydrophobic movement (eg, I9K-piscidin-1 and I16K-piscidin-1) exhibited different cytotoxic activity. Accordingly, RT could be suggested as a more reliable criterion to estimate the toxicity of peptides based on hydrophobicity. Similarly, RT was reported as a significant scale to determine the toxicity of peptides by previous studies.25–27
The I16K-piscidin-1 peptide possessed the lowest toxicity, which may be due to its lower binding affinity to mammalian cells compared to other peptides. Consistent with our result, other studies reported that analog with weaker toxicity exhibited the lower activity against S. aureus relative to the parent peptide.6,11
A time-killing kinetics test was performed to examine the rapidity of bactericidal activity of peptides against Gram-positive (clinical MRSA) and Gram-negative (clinical A. baumannii) bacteria. The results showed that all peptides at 1×MIC had bactericidal activity and required at least 8 h to cause a ≥3-log10 reduction in initial inoculum of bacteria, but none of the peptides were capable of eradicating all bacteria at 1×MIC. Tncreasing the peptide concentration to 5×MIC completely eliminated all initial inocula of bacteria 8–10 h after exposure. At a higher concentration of peptides (5×MIC), I16K-piscidin-1 showed the most rapid bactericidal activity. The I16K-piscidin-1 peptide caused a ≥3-log10 reduction in initial inoculum of A. baumannii and S. aureus only after incubation for 2 h, whereas piscidin-1 exhibited such activity after 4 h. Furthermore, piscidin-1 completely eliminated A. baumanniiand S. aureus after 8 and 10 h, respectively, whereas I16K-piscidin-1 did it after 6 h at 5×MIC (Figures 1 and 2). So I16K-piscidin-1 may be beneficial against bacterial infection through fast eradication of bacteria in the first time of bacterial colonization. The most likely explanation for the inverse correlation between bactericidal activity and retention time at 5×MIC is that the increase of the concentration of AMPs containing high hydrophobic nonpolar face may cause an increase in their oligomerization capacity. Oligomerization could happen through hydrophobic nonpolar face interactions of peptides in aqueous solution. Previous studies demonstrated that self-associated structures reduce the diffusing capacity of peptides through cell wall due to the large size of oligomeric structure, resulting in a decrease in rapidity of bactericidal activity of peptides and an increase in toxicity.13,14,28,29 To find out more about the inhibitory action of a thick cell wall on the bactericidal activity of peptides at high concentrations, the rapidity of bactericidal activity was assessed by time-killing kinetics assay in combination with lysozyme.30 The results showed a dramatically enhanced rapidity of bactericidal activity of the peptides, especially in piscidin-1 with the highest RT, suggesting an inhibitory function of the cell wall preventing the accessibility of peptides to the cell membrane. Our findings are consistent with results of other studies that demonstrated the synergistic effects of AMPs in combination with lysozyme against bacteria.30,31 The Β-galactosidase leakage and PI staining tests revealed that accessibility of peptides to the cell membrane and the subsequent pore-forming capacity of peptides at 5×MIC has an inverse association with their RT. These results explained the higher rapidity of bactericidal activity of I16K-piscidin-1 (with lower RT) relative to parent peptide (with higher RT). In contrast, piscidin-1 rendered higher cell membrane leakage after damage to cell wall caused by lysozyme, suggesting a possible explanation for high bactericidal activity of piscidin-1 in combination with lysozyme and a protective role of the bacterial cell wall against AMPs. In the current study, we compared some antimicrobial properties of piscidin-1 and its derivatives, while some characteristics are yet to be investigated. For example, the lack of data on the self-association ability of peptides is considered as a limitation of the present study.
Evaluation of in vivo bactericidal potential indicates that I16K-piscidin-1 can significantly control the proliferation of MRSA and A. baumannii in some organs of mice. Importantly, in vivo behavioral and biochemical analysis revealed that I16K-piscidin-1 exhibited no acute toxic effect at doses of up to 2 mg/mouse. However, further research is needed to investigate adverse effects on various organs with a greater sensitivity using biomarkers. In addition, it is necessary that such studies are performed for a longer time period (eg, daily injections of peptides for one month). These points could be considered as other limitations of our study.
Together, this study provided in vitro and in vivo descriptive information about the designed piscidin-1 derivatives and revealed that disruption of continuity of nonpolar face in piscidin-1 through replacement of isoleucine 16, a hydrophobic amino acid, by lysine, a hydrophilic amino acid, caused an increase in the rapidity of bactericidal activity and a reduction in toxicity. Furthermore, this substitution increased the rapidity of bactericidal activity; on the other hand, earlier treatment with I16K-piscidin-1 led to the higher survival rate for mice infected with clinical S. aureus and A. baumannii strains, providing evidence for I16K-piscidin-1 to be beneficial against bacterial infections through fast eradication of bacteria in the first time of bacterial colonization; besides, these results showed that the immediate application of I16K-piscidin-1 are essential to prevent severe infection.
Acknowledgment
This work was supported by Bushehr University of Medical Sciences [grant No 416].
Disclosure
The authors report no conflict of interest in this work.
Supplementary materials
Figure S2.
Helical wheel projections and retention time for I9A-piscidin-1. Upper picture: Residues are numbered starting from the N-terminus. Hydrophobic and positive charge residues are defined with yellow and blue color, respectively. Chromatogram for piscidin-1, dominant peak is synthetic I9A-piscidin-1.
Figure S3.
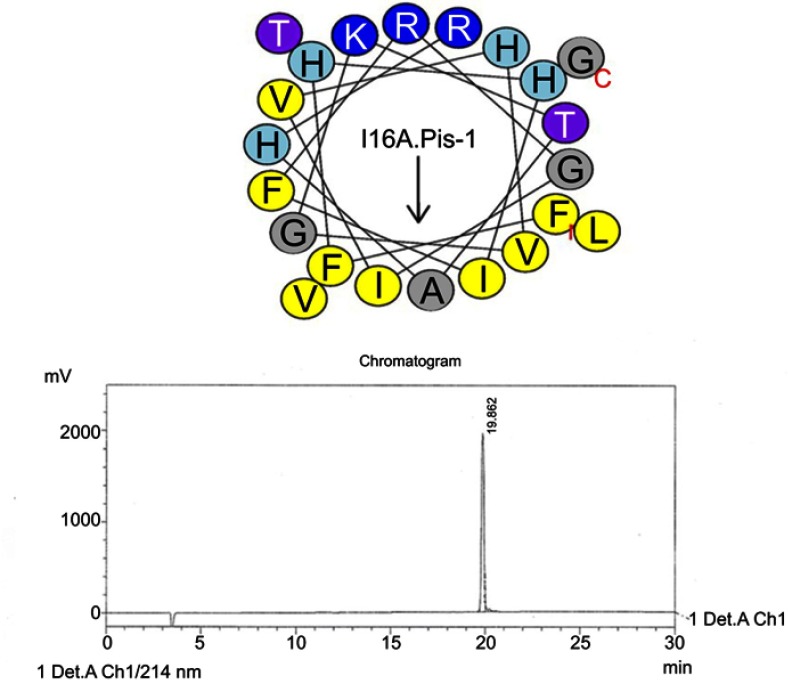
Helical wheel projections and retention time for I16A-piscidin-1.Upper picture: Residues are numbered starting from the N-terminus. Hydrophobic and positive charge residues are defined with yellow and blue color, respectively. Chromatogram for piscidin-1, dominant peak is synthetic I16A-piscidin-1.
Abbreviation: I16A.Pis-1, I16A-piscidin-1.
Figure S4.
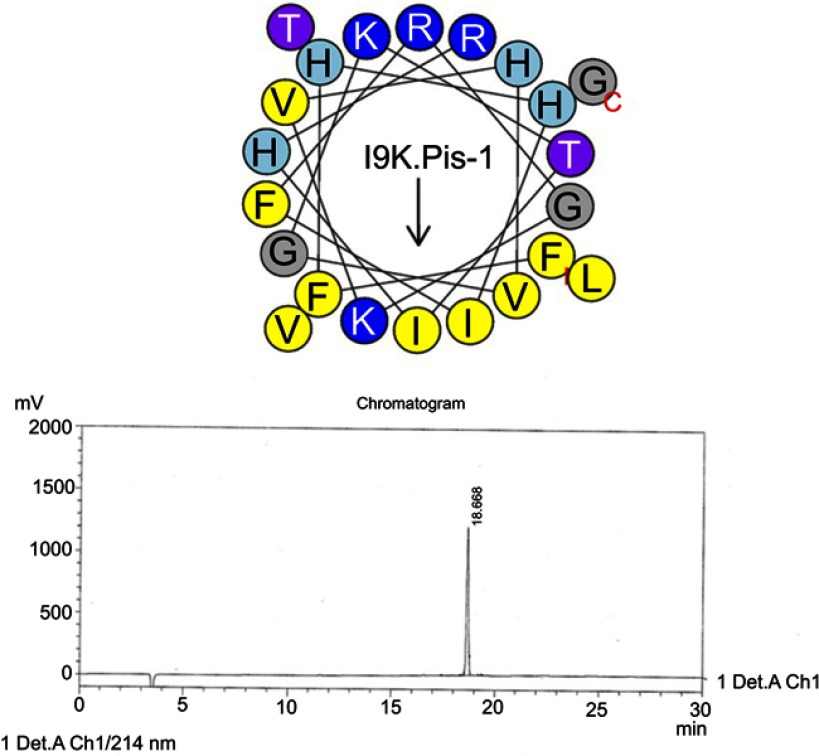
Helical wheel projections and retention time for I9K-piscidin-1. Upper picture: Residues are numbered starting from the N-terminus. Hydrophobic and positive charge residues are defined with yellow and blue color, respectively. Chromatogram for piscidin-1, dominant peak is synthetic I9K-piscidin-1.
Abbreviation: I9K.Pis-1, I9K-piscidin-1.
References
- 1.Taheri B, Mohammadi M, Nabipour I, Momenzadeh N, Roozbehani M. Identification of novel antimicrobial peptide from Asian sea bass (Lates calcarifer) by in silico and activity characterization. PLoS One. 2019;14(2):e0212759. doi: 10.1371/journal.pone.0212759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farshadzadeh Z, Modaresi MH, Taheri B, Rahimi S, Bahador A. In vitro antimicrobial activity of dermcidin-1L against extensively-drug-resistant and pandrug-resistant acinetobacter baumannii. Jundishapur J Microbiol. 2017;10(5):e13201. doi: 10.5812/jjm [DOI] [Google Scholar]
- 3.Silphaduang U, Noga EJ. Antimicrobials: peptide antibiotics in mast cells of fish. Nature. 2001;414(6861):268. doi: 10.1038/35104690 [DOI] [PubMed] [Google Scholar]
- 4.Lee S-A, Kim YK, Lim SS, et al. Solution structure and cell selectivity of piscidin 1 and its analogues. Biochemistry. 2007;46(12):3653–3663. doi: 10.1021/bi062233u [DOI] [PubMed] [Google Scholar]
- 5.Sun B, Xie H, Song Y, Nie P. Gene structure of an antimicrobial peptide from mandarin fish, Siniperca chuatsi (Basilewsky), suggests that moronecidins and pleurocidins belong in one family: the piscidins. J Fish Dis. 2007;30(6):335–343. doi: 10.1111/j.1365-2761.2007.00789.x [DOI] [PubMed] [Google Scholar]
- 6.Zhang S-K, Song J-W, Gong F, et al. Design of an α-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity. Sci Rep. 2016;6:27394. doi: 10.1038/srep27394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hancock RE, Sahl H-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24(12):1551. doi: 10.1038/nbt1267 [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Tripathi AK, Kathuria M, et al. Single amino acid substitutions at specific positions of the heptad repeat sequence of piscidin-1 yielded novel analogs that show low cytotoxicity and in vitro and in vivo antiendotoxin activity. Antimicrob Agents Chemother. 2016;60(6):3687–3699. doi: 10.1128/AAC.02341-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JK, Lee SA, Shin S, Lee JY, Jeong KW, Kim Y. Structural flexibility and the positive charges are the key factors in bacterial cell selectivity and membrane penetration of peptoid-substituted analog of Piscidin. Biochim Biophys Acta. 2010;1798:1913–1925. doi: 10.1016/j.bbamem.2010.06.026 [DOI] [PubMed] [Google Scholar]
- 10.Mahlapuu M, Håkansson J, Ringstad L, Björn C. Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol. 2016;6:194. doi: 10.3389/fcimb.2016.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Son M, Lee Y, Hwang H, Hyun S, Yu J. Disruption of interactions between hydrophobic residues on nonpolar faces is a key determinant in decreasing hemolysis and increasing antimicrobial activities of α‐helical amphipathic peptides. ChemMedChem. 2013;8(10):1638–1642. doi: 10.1002/cmdc.201300264 [DOI] [PubMed] [Google Scholar]
- 12.Schmitt MA, Choi SH, Guzei IA, Gellman SH. Residue requirements for helical folding in short α/β-peptides: crystallographic characterization of the 11-helix in an optimized sequence. J Am Chem Soc. 2005;127(38):13130–13131. doi: 10.1021/ja0536163 [DOI] [PubMed] [Google Scholar]
- 13.Edwards IA, Elliott AG, Kavanagh AM, Zuegg J, Blaskovich MAT, Cooper MA. Contribution of amphipathicity and hydrophobicity to the antimicrobial activity and cytotoxicity of β-hairpin peptides. ACS Infect Dis. 2016;2(6):442–450. doi: 10.1021/acsinfecdis.6b00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glukhov E, Burrows LL, Deber CM. Membrane interactions of designed cationic antimicrobial peptides: the two thresholds. Biopolymers. 2008;89(5):360–371. doi: 10.1002/bip.20917 [DOI] [PubMed] [Google Scholar]
- 15.Farshadzadeh Z, Taheri B, Rahimi S, et al. Growth rate and biofilm formation ability of clinical and laboratory-evolved colistin-resistant strains of acinetobacter baumannii. Front Microbiol. 2018;9:153. doi: 10.3389/fmicb.2018.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Lei M, Du X, Cui P, Zhang S. Identification of a novel antimicrobial peptide from amphioxus Branchiostoma japonicum by in silico and functional analyses. Sci Rep. 2015;5:18355. doi: 10.1038/srep18355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juliano SA, Pierce S, DeMayo JA, Balunas MJ, Angeles-Boza AM. Exploration of the innate immune system of Styela clava: zn2+ binding enhances the antimicrobial activity of the tunicate peptide clavanin A. Biochemistry. 2017;56(10):1403–1414. doi: 10.1021/acs.biochem.6b01046 [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, M-F L, Sun L, Fischer U. NKLP27: a teleost NK-lysin peptide that modulates immune response, induces degradation of bacterial DNA, and inhibits bacterial and viral infection. PLoS One. 2014;9(9):e106543. doi: 10.1371/journal.pone.0106543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao W, Xing L, Qu P, et al. Identification of a novel cathelicidin antimicrobial peptide from ducks and determination of its functional activity and antibacterial mechanism. Sci Rep. 2015;5:17260. doi: 10.1038/srep17260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva O, De La Fuente-Núñez C, Haney E, et al. An anti-infective synthetic peptide with dual antimicrobial and immunomodulatory activities. Sci Rep. 2016;6:35465. doi: 10.1038/srep35465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammadi M, Taheri B, Momenzadeh N, et al. Identification and characterization of novel antimicrobial peptide from hippocampus comes by In Silico and experimental studies. Mar Biotechnol. 2018;20(6)1–11. [DOI] [PubMed] [Google Scholar]
- 22.Bennett WD, Hong CK, Wang Y, Tieleman DP. Antimicrobial peptide simulations and the influence of force field on the free energy for pore formation in lipid bilayers. J Chem Theory Comput. 2016;12(9):4524–4533. doi: 10.1021/acs.jctc.6b00265 [DOI] [PubMed] [Google Scholar]
- 23.Forde E, Schutte A, Reeves E, et al. Differential in vitro and in vivo toxicities of antimicrobial peptide prodrugs for potential use in cystic fibrosis. Antimicrob Agents Chemother. 2016;60(5):2813–2821. doi: 10.1128/AAC.00157-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin SC, Ahn IH, Ahn DH, et al. Characterization of two antimicrobial peptides from Antarctic fishes (Notothenia coriiceps and Parachaenichthys charcoti). PLoS One. 2017;12(1):e0170821. doi: 10.1371/journal.pone.0170821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian X, Sun F, Zhou XR, Luo SZ, Chen L. Role of peptide self‐assembly in antimicrobial peptides. J Pept Sci. 2015;21(7):530–539. doi: 10.1002/psc.2788 [DOI] [PubMed] [Google Scholar]
- 26.Mohammadi M, Taheri B, Momenzadeh N, et al. Identification and characterization of novel antimicrobial peptide from hippocampus comes by In Silico and experimental studies. Mar Biotechnol. 2018;20(6):718–728. doi: 10.1007/s10126-018-9843-3 [DOI] [PubMed] [Google Scholar]
- 27.Aoki W, Ueda M. Characterization of antimicrobial peptides toward the development of novel antibiotics. Pharmaceuticals. 2013;6(8):1055–1081. doi: 10.3390/ph6081055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandal D, Shirazi AN, Parang K. Self-assembly of peptides to nanostructures. Org Biomol Chem. 2014;12(22):3544–3561. doi: 10.1039/C4OB00447G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangoni ML, Shai Y. Temporins and their synergism against Gram-negative bacteria and in lipopolysaccharide detoxification. Biochimica Et Biophysica Acta (Bba)-Biomembr. 2009;1788(8):1610–1619. doi: 10.1016/j.bbamem.2009.04.021 [DOI] [PubMed] [Google Scholar]
- 30.Cabrefiga J, Montesinos E. Lysozyme enhances the bactericidal effect of BP100 peptide against Erwinia amylovora, the causal agent of fire blight of rosaceous plants. BMC Microbiol. 2017;17(1):39. doi: 10.1186/s12866-017-0957-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Niyonsaba F, Ushio H, et al. Synergistic effect of antibacterial agents human β-defensins, cathelicidin LL-37 and lysozyme against Staphylococcus aureus and Escherichia coli. J Dermatol Sci. 2005;40(2):123–132. doi: 10.1016/j.jdermsci.2005.03.014 [DOI] [PubMed] [Google Scholar]