Abstract
Objective
the objective of the study was to determine whether preoperative [18F]fludeoxyglucose (FDG)-PET asymmetry in temporal lobe metabolism predicts neuropsychological and seizure outcomes after temporal lobectomy (TL).
Methods
An archival sample of 47 adults with unilateral temporal lobe epilepsy who underwent TL of their language-dominant (29 left, 1 right-) or non-dominant (17 right) hemisphere were administered neuropsychological measures pre- and post-operatively. Post-TL seizure outcomes were measured at 1-year. Regional FDG uptake values were defined by an automated technique, and a quantitative asymmetry index (AI) was calculated to represent the relative difference in the FDG uptake in the epileptic relative to the nonepileptic temporal lobe for four regions of interest: medial anterior temporal (MAT), lateral anterior (LAT), medial posterior (MPT), and lateral posterior temporal (LPT) cortices.
Results
In language-dominant TL, naming outcomes were predicted by FDG uptake asymmetry in the MAT (r=−0.38) and LPT (r=−0.45) regions. For all patients, visual search and motor speed outcomes were predicted by FDG uptake asymmetry in all temporal regions (MPT, r=0.42; MAT, r=0.34; LPT, r=0.47; LAT, r=0.51). Seizure outcomes were predicted by FDG uptake asymmetry in the MAT (r=0.36) and MPT (r = 0.30) regions. In all of these significant associations, greater hypometabolism in regions of the epileptic temporal lobe was associated with better postoperative outcomes.
Conclusions
Our results support the conclusion that FDG uptake asymmetry is a useful clinical tool in assessing risk for cognitive changes in patients being considered for TL.
Keywords: epilepsy, verbal memory, naming, epilepsy surgery, positron emission tomography
1. Introduction
Temporal lobectomy (TL) is an effective treatment for seizures in patients with medically intractable temporal lobe epilepsy [1]. While seizure outcomes are good, post-TL cognitive decline is frequently reported [2, 3]. In their meta-analysis of neuropsychologic outcomes following TL, Sherman and colleagues [3] found significant postoperative declines in verbal memory were present in up to 63% of left- and 34% of right-TL patients. Postoperative decline in naming was present in up to 54% of patients following left-sided resections. Despite the relatively common incidence of post-TL cognitive change, only a small fraction of patients (about 9%) perceive a decline following epilepsy surgery, and nearly twice as many report improvement in cognition[3]. For most patients, cognitive decline may go unnoticed in their routine daily activities, as these tasks are unlikely to overtax commonly affected abilities, such as memory and naming skills[4]. However, decline may be more conspicuous to those hoping to pursue more cognitively demanding activities after TL, such as attending school or obtaining employment in skilled professional settings [5]. Thus, while many patients do not perceive cognitive changes following TL, accurately assessing the likelihood of decline is critical for making informed clinical decisions on an individual level.
Predicting postoperative outcomes is a complex process, as certain factors confer more or less risk based on the outcome being predicted. For example, factors such as low preoperative IQ, left hemisphere resection, and older age at the time of surgery are all associated with increased likelihood for cognitive decline, but not poorer seizure outcomes [6]. Structural abnormalities consistent with mesial temporal sclerosis (MTS) on magnetic resonance imaging (MRI) is a positive prognostic indicator for both cognitive and seizure outcomes [6–8], but some 20–30% of patients with temporal lobe epilepsy may have no MRI evidence of MTS [9, 10]. [18F]Fludeoxyglucose PET (FDG-PET) may be informative in predicting seizure outcomes for patients with normal preoperative MRIs [11]. However, the relationship between preoperative PET and post-TL cognitive change remains uncertain, as there are less than a handful of relevant published studies, and findings have been inconsistent [12, 13].
Griffith et al[12] studied the relationship between pre-operative FDG uptake and post-TL memory outcomes in 27 left- and 22 right-TL patients by visually analyzing the difference in FDG uptake between the right and left temporal lobes and grouping patients by “none/mild” or “moderate/severe” uptake asymmetry. They found that left-TL patients with no or mild asymmetries had greater postoperative verbal memory decline than those with moderate to severe asymmetries. Leeman, Leveroni, and Johnson [13] conducted a similar study, but used an automated technique for identifying anatomical structures, which allowed for quantification of the tracer uptake in temporal lobe regions. In their pilot study of 14 left-TL patients, FDG uptake asymmetry within the hippocampi (their region of interest) was not found to be a statistically significant predictor of postoperative changes in verbal memory. However, the relationship between temporal lobe asymmetry and verbal memory outcomes was close to statistical significance (p=0.06), hinting at similar findings to those of Griffith et al [12].
This report provides an analysis of temporal lobe asymmetries in FDG uptake and post-TL outcomes, using semi-quantitative tools for PET image analysis and empirically based methods for quantifying the degree of pre- to postoperative cognitive change. Using automated methods for quantification of FDG uptake avoids reliance on descriptors such as “mild” or “severe” asymmetry, which—although useful starting points—do not capture the bidirectional nature of asymmetry. For instance, “mild asymmetry” could result from mild hypermetabolism or mild hypometabolism in the epileptic temporal lobe. In the present study, the terms “relative hypometabolism” or “relative hypermetabolism” are used to specify the relationship between baseline FDG-uptake in the epileptic compared to the non-epileptic temporal regions. We tested the following predictions: 1) A greater degree of relative hypometabolism in the medial temporal region would be associated with better verbal memory and naming outcomes for patients who underwent language-dominant TL; 2) A greater degree of relative hypometabolism in the medial temporal region would be associated with better seizure outcomes for all patients. Lastly, we explored FDG correlates of outcomes of simple visual search and motor speed, executive functioning, and verbal fluency; although, since only a few studies have evaluated such outcomes following TL, no specific predictions were made for these measures [3].
2. Methods
2.1. Subjects
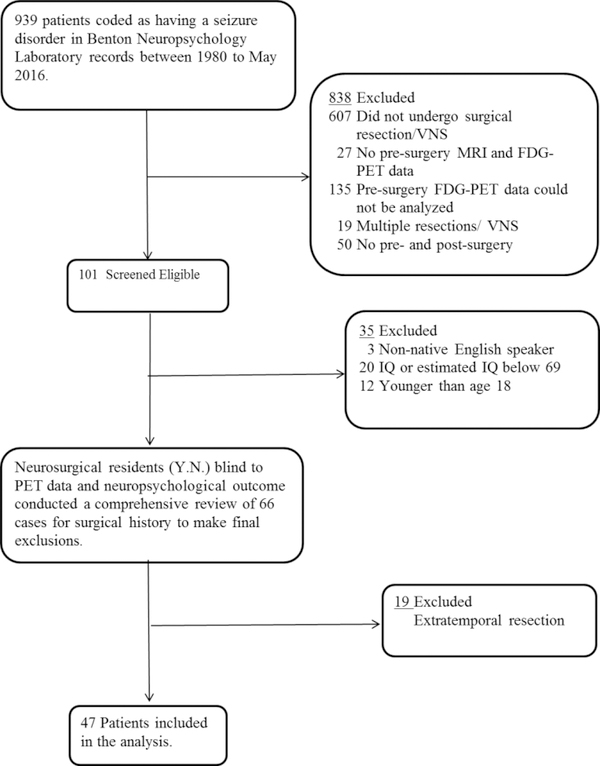
An archival sample of 47 patients with unilateral temporal lobe epilepsy who underwent surgical resection of the language-dominant (29 left-TL, 1 right-TL) or non-dominant (17 right-TL) temporal lobe from the years 2004 to 2016 were selected from the Patient Registry of the Division of Neuropsychology and Cognitive Neuroscience at the University of Iowa. Figure 1 presents a flow chart of case ascertainment, inclusion/exclusion, and information regarding patients examined for eligibility, confirmed eligible, and ultimately included in the study. All met the following criteria: unilateral temporal lobe epilepsy; no MRI abnormalities other than atrophy consistent with MTS; age18 years or older; and an IQ or an estimated IQ (based on the reading subtest of the Wide Range Achievement Test; N=5) of 69 or greater. For patients (N=7) in which the preoperative evaluations were not consistently concordant with unilateral temporal lobe epilepsy (or suggested temporal-plus epilepsy), an evaluation was conducted using intracranial electrodes to confirm the localization of the epileptogenic zone. All patients had video-EEG monitoring, high-resolution MRI, FDG-PET, and pre/post-TL neuropsychological testing. Language dominance was determined by intracarotid amobarbital testing in all but five patients (2 left- and 3 right-TL). Left hemisphere language dominance was assumed for these individuals, all of whom were right-handed. One patient (who underwent right-TL) demonstrated right hemisphere dominance for language; accordingly, this patient was placed in the “language-dominant TL” group.
Figure 1.
Flow chart of participant selection
2.2. Surgery
All patients underwent cortico-amygdalohippocampectomy (CAH), involving resection or disconnection of the temporal pole and temporal neocortex with resection of the amygdala and hippocampus. All surgeries were performed by one of two neurosurgeons experienced in epilepsy surgeries. The extent of the lateral neocortical disconnection/resection, measured from the anterior tip of the temporal pole, ranged from approximately 4 cm in the language dominant hemisphere to 6 cm in the non-dominant hemisphere. For CAH of the dominant hemisphere, language areas were mapped with a standard electrical stimulation protocol [14]. The superior temporal gyrus was preserved in some of the dominant hemisphere cases. The extent of hippocampal resection was tailored based on intraoperative electrocorticography and ranged from approximately 1 to 4 cm measured from the head of the hippocampus. The extent of the lateral and mesial resection or disconnection was also tailored by findings from intraoperative electrocorticography in all cases.
2.3. Neuropsychological Assessment
A comprehensive neuropsychological exam was administered 10 months prior to surgery and 1-year postoperatively, on average. Measures of verbal memory, language, executive functioning, and visual search and motor speed were selected for outcome analysis.
2.3.1. Verbal Memory
The Rey Auditory Verbal Learning Test (AVLT) is a list learning task that assesses episodic verbal learning and memory. The outcome of interest was the number of words recalled following a 30-minute delay.
2.3.2. Language
The Boston Naming Test (BNT) is a 60-item visual naming task. Number of items correctly named was the outcome of interest. Controlled Oral Word Association Test (COWAT)measures speeded word generation, and number of words generated across three, 1-minute trials was the outcome of interest.
2.3.3. Executive functioning, visual search and motor speed
Trail Making Test, parts A and B of the trail making test (TMT A and TMT B) were used to assess visual search and motor speed [15] and executive functioning, respectively. Time in seconds to complete each part was the outcome of interest.
2.4. Seizure Outcome
Seizure outcome at a 1-year postoperative interval was based on electronic chart review, using criteria outlined by International League Against Epilepsy (ILAE) [16].
2.5. PET Imaging Methods and Analysis
Patients were administrated 5 ± 10% mCi of FDG under fasting conditions (minimum = 6 hours, blood glucose ≤ 200 mg/dL) in a quiet, darkened room with eyes open and ears unplugged. Imaging was from 50 – 60 minutes post-administration. Images were analyzed using a standardized region-of-interest (ROI) approach, as implemented by the commercially-available program, NeuroQ (version 3.7, Syntermed, Inc.). By comparing the patient’s regional FDG uptake to the metabolic pattern exhibited by uptake in the whole-brain, standardized difference scores were derived for each of the following regions (bilaterally): medial anterior temporal (MAT), medial posterior temporal (MPT), lateral anterior temporal (LAT), and lateral posterior temporal (LPT) regions. These standardized difference scores were used to calculate asymmetry indices (AIs), by dividing the score from a given region within the epileptic temporal lobe by the corresponding contralateral score.
2.6. Statistical Analysis
A standardized regression method (SRB) was used to determine if meaningful post-surgical cognitive change occurred [17]. This method corrects for practice effects, retest reliability, and variability in post-surgical scores [18]. Briefly, published data [19, 20] from unoperated epilepsy comparisons (tested at a baseline and follow-up) was used to derive predicted cognitive change scores for the neuropsychological outcomes of interest (AVLT, BNT, COWAT, TMT A, and TMT B). This predicted score was then applied to our clinical sample. The resulting SRB change score is the standardized difference (i.e. Z score) between the expected follow-up score and the observed follow-up score for the individual patient within our sample.
To address potential effects of language lateralization on outcome analysis, verbal memory and language outcomes (AVLT, BNT, and COWAT) were analyzed by grouping patients by language-dominant and non-dominant TL. The following outcomes were analyzed without grouping: seizure (ILAE classification post-TL), visual search and motor speed (TMT A), and executive functioning (TMT B).
Univariate linear regressions analyses examined the relationship between each of the four temporal asymmetry indices (MAT AI; MPT AI; LAT AI; LPT AI) and the standardized neuropsychological outcome scores. Analyses of covariates were performed for any significant univariate analysis. Covariates tested included: years of education, full scale IQ, MRI findings consistent with MTS, age at surgery, years of seizure duration, number of post-surgical medications, time from surgical intervention to postoperative neuropsychological evaluation, and ILAE seizure outcome classification.
3. Results
3.1. Subjects
Table 1 presents descriptive statistics and patient characteristics. There were 30 patients in the language-dominant group; of that group, 29 of 30 underwent left hemisphere TL. One patient showed right hemisphere language dominance on intracarotid amobarbital testing and underwent right hemisphere TL. All patients in the non-dominant group underwent right hemisphere TL.
Table 1.
Patient characteristics for all patients (N=47)
| Sex (# of M/F) | 25/22 |
| Handedness (# of R/L) | 38/8 |
| Lansuase dominance (# of R/L) | 18/29 |
| IQ | 89.07(12.59) |
| Age at surgery (years) | 38.03 (13.62) |
| Age at sz onset (years) | 21.25(14.57) |
| Sz duration (years) | 17.64(16.48) |
| Education (years) | 13.13(1.80) |
| # of preoperative AEDs | 1.30(0.51) |
| # of postoperative AEDs | 1.28(0.62) |
| Time from preoperative neuropsychological examination to surgery (years) | 0.82(0.54) |
| Time from surgery to postoperative neuropsychological examination (years) | 1.09(0.85) |
| # of participants employed (presurgery/postsurgery) | 27/31 |
| MTS +/− | 31/16 |
| Pre-operative Psychological Co-morbidities (number of total participants): | |
| Anxiety disorder (# of total participants) | 8 |
| Depressive disorder (# of total participants) | 24 |
| Psychotic disorder (# of total participants) | 3 |
Sz=seizure; R=right; L=Left; AED=antiepileptic drug; MTS=mesial temporal sclerosis
3.1.1. Verbal memory and language in language-dominant TL
Figure 2 shows scatterplots for each regional AI and the standardized outcome scores on the BNT and the delayed recall trial of the AVLT for language-dominant TL. In language-dominant TL, uptake asymmetry in the medial anterior temporal region (MAT AI) significantly predicted AVLT outcome scores (r = −0.46, p = 0.02, n = 27), suggesting that a greater degree of relative hypometabolism was associated with better postoperative memory performance. When MTS was added as a covariate, it trended toward significance (p = 0.07, ΔR2 = 0.10) and MAT AI became non-significant as a predictor (p = 0.13). An additional analysis was performed to examine if MTS had a differential effect on MAT AI prediction of AVLT outcome scores. MTS individually predicted AVLT outcome scores (r = 0.49). Bivariate correlations between MAT AI and AVLT outcome scores in MTS+ and MTS- groups showed an attenuated relationship in the MTS + group (r= −0.29, p =0.25, n = 18) compared to the MTS- group (r= −0.29, p =0.25, n = 18). When MTS was held constant (i.e., 0), MAT AI maintained a negative relationship ( i.e., greater hypometabolism = better AVLT outcomes) and had the lowest p-value (p =0.14) and largest effect compared to MTS (p=0.32) and the interaction term (MTS*MAT AI; p = 0.29). The interaction term attenuated the negative relationship between MAT and AVLT, suggesting the relationship between MAT and AVLT is stronger in participants without MTS. No other significant relationships were observed between the AVLT outcome scores and the AIs from the other temporal regions.
Figure 2.
Scatterplots of the standardized outcome score on Boston Naming Test (BNT) and Rey Auditory Verbal Learning Test (AVLT) for the asymmetry indices of each temporal lobe region in language dominant-TL patients.
Note: Statistically significant relationships are denoted with an asterisk (*). An Asymmetry Index value of 1.0 indicates FDG uptake within the epileptic temporal lobe was id,entical to that of uptake in the corresponding region of the non-epileptic temporal lobe. A value < 1.0 indicates decreased uptake (referred to as relative hypometabolism), and a value > 1.0 indicates increased uptake (referred to as relative hypermetabolism). The SRB Z-score refers to the degree of pre- to post-operative gain (positive scores) or loss (negative scores).
BNT outcomes scores were predicted by asymmetry in the MAT (r=−0.38 p = 0.04, n = 30) and LPT (r = −0.45, p = 0.01, n = 30) regions in language-dominant TL, showing that better postoperative naming performance was associated with a greater degree of relative hypometabolism in these regions. Time from surgery to follow-up examination was a significant covariate (p = 0.01, n = 30, ΔR2 = 0.19), while the MAT AI remained a significant predictor. No significant relationships were observed between the BNT outcome scores and the AIs from the other temporal regions (MPT, LAT).
No significant relationships were evident between any of the four temporal AIs and COWAT outcomes scores in the language-dominant TL group.
3.1.2. Verbal memory and language in non-dominant TL
In the non-dominant TL group, no significant relationships or near significant trends were evident between any of the AIs from the four temporal regions and the standardized outcome scores for memory and language measures.
3.1.3. Visual Search and motor speed, and executive functioning
Across all patients (n=47), TMT A outcome scores were predicted by all four of the AIs: MAT (r= 0.34, p = 0.02); MPT (r= 0.42, p < 0.01); LAT (r= 0.51, p < 0.01); and LPT (r= 0.47, p < 0.01), showing that a greater degree of relative hypometabolism was associated with faster postoperative visual search and motor speed performance (see Figure 3). No significant relationships were evident between any of the AIs from the four regions of interest and the outcome scores for the measure of executive functioning (TMT B).
Figure 3.
Scatterplots of standardized outcome score (SRB) for Trail Making Test part A (TMT A) and the asymmetry indices of each temporal lobe region in all patients.
Note: Statistically significant relationships are denoted with an asterisk (*). An Asymmetry Index value of 1.0 indicates FDG uptake within the epileptic temporal lobe was identical to that of uptake in the corresponding region of the non-epileptic temporal lobe. A value < 1.0 indicates decreased uptake (referred to as relative hypometabolism), and a value > 1.0 indicates increased uptake (referred to as relative hypermetabolism). SRB Z-score refers to the degree of pre- to post-operative change, with positive scores indicating postoperative performance on the measure of visual search and motor speed was a slower (i.e. more time spent), and negative scores indicating a faster postoperative performance (i.e. less time spent).
3.1.4. Seizure Outcomes
Seizure outcomes by ILAE classification are presented in Table 2. Across all patients, seizure outcome at 1-year was predicted by FDG uptake asymmetry in the MAT (r = 0.36, p = 0.01, n = 45) and MPT (r = 0.30, p = 0.04, n = 45) regions, indicating that the greater the relative hypometabolism the more likely a patient would experience a good outcome (i.e., lower AI score was associated with lower ILAE classification). No other significant or near significant relationships were observed.
Table 2.
ILAE Classification
| Year 0 (preoperative baseline) (n=47) | Year 1 (n=45) |
|---|---|
| Class 1: Completely seizure free since surgery; no auras | n=29 |
| Class 2: Only auras; no other seizures | n=3 |
| Class 3: 1–3 seizure days per year; ± auras | n=7 |
| Class 4: 4 seizure days per year to 50% reduction of baseline seizure days; ± auras | n=2 |
| Class 5: <50% reduction of baseline seizure days to 100% increase | n=1 |
| Class 6: More than 100% increase of baseline seizure days; ± auras | n=0 |
4. Discussion
Our results support the hypothesis that metabolic asymmetry between the epileptic and nonepileptic temporal lobe on preoperative FDG-PET is predictive of cognitive and seizure outcomes following TL. For patients undergoing language-dominant TL, FDG uptake asymmetry in parts of the medial and lateral sectors of the epileptic temporal lobe predicted cognitive change scores on measures visual naming ability, indicating that a greater degree of relative hypometabolism in the epileptic lobe was associated with reduced likelihood of postoperative decline. For all patients, asymmetry in the medial anterior and medial posterior temporal regions predicted seizure outcomes; and, FDG uptake AI’s from all four temporal lobe regions predicted postoperative visual search and motor speed outcomes, with faster postsurgical performance associated with a greater degree of relative hypometabolism in the epileptic temporal lobe.
Lastly, for patients undergoing language-dominant TL, there was a significant relationship between FDG uptake asymmetry in the medial anterior region and verbal memory. When MTS was added as a predictor, neither variable was found to be a statistically significant predictor of the verbal memory outcome. When MTS+ patients were removed, we observed the expected relationship between the asymmetry in the medial anterior region and verbal memory outcomes with effects sizes similar to the other effects found in this study; however, it was not at a level that reached statistical significance. It is unlikely that the number of participants in our sample without MTS undergoing language-dominant TL (n=9) was large enough to produce a statistically significant finding (CITE owen) despite evidence for a meaningful relationship. Overall, our analysis suggested the relationship between asymmetry in medial anterior region and verbal memory outcome is stronger in participants without MTS. This is consistent with research supporting the use of FDG-PET for predicting seizure outcomes for patients with normal preoperative MRIs [11], and may explain the conflicting results produced by prior FDG-PET studies of memory outcomes in which the frequency of patients without MTS was unclear [12] or underrepresented [13] within their sample.
A parsimonious interpretation of the findings from this study is that temporal asymmetry in FDG uptake reflects the functional status of the brain tissue. On this view, hypometabolic tissue is contributing less to the cognitive function, and removal of the dysfunctional tissue has less adverse impact on cognitive outcomes. There is a certain degree of anatomical specificity in our findings that supports this interpretation. For example, the relation between the medial anterior temporal region and verbal memory performance fits an established pattern [21]; likewise, the relation between the lateral posterior temporal area and word-retrieval (naming) is consistent with findings from both lesion and functional imaging studies [22].
The retrospective design of this study presents certain methodological limitations, including the use of referral populations and lack of control group. Several steps were taken to address design limitations, including the use of automated tools for PET analysis and empirically based statistical methods for quantifying the degree of postoperative cognitive change. Further, our sample characteristics suggest a representative sample with respect to seizure outcomes and imaging findings. The majority of our patients (65%) were seizure and aura free at a 1-year follow-up, and 34% of patients showed no evidence of MTS on preoperative MRI. This can be compared to the unclear status of MTS in the similar study by Griffith et al [12], and 14% (2 of 14) reported by Leeman et al[13]. Lastly, comprehensive clinical data was collected to evaluate for multiple possible covariates (see section 2.6). A larger sample size would allow for multivariate analysis which could improve our understanding of these findings, and likely offer a more comprehensive understanding of the interplay of factors that lead to better cognitive outcomes.
5. Conclusion
We have shown that in patients who underwent TL for epilepsy treatment, FDG-PET asymmetries do predict neuropsychological and seizure outcomes. The current findings support work by Griffith et al [12] and suggest that measurement of FDG uptake asymmetry can be a useful tool in assessing risk for cognitive changes in patients being considered for TL. Future research should examine the predictive ability of FDG uptake asymmetry on verbal memory outcomes in patients with normal preoperative MRIs.
Acknowledgments
Funding
This work was supported by the Conte Center (NIH# 2P50MH094258-06), James S. McDonnell Foundation (#220020387), and the Spastic Paralysis Research Foundation.
Footnotes
Disclosure of Conflict of Interest
None of the authors have any conflict of interest to disclose.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- [1].Weibe B, Warren J, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8 [DOI] [PubMed] [Google Scholar]
- [2].Ives-Deliperi VL, Butler JT. Naming outcomes of anterior temporal lobectomy in epilepsy patients: A systematic review of the literature. Epilepsy Behav. 2012;24:194–198. [DOI] [PubMed] [Google Scholar]
- [3].Sherman E, Wiebe S, Fay-Mcclymont T, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Hader W, Jette N. Neuropsychological outcomes after epilepsy surgery: Systematic review and pooled estimates. Epilepsia; 2011. p. 857–869. [DOI] [PubMed] [Google Scholar]
- [4].Sawrie SM, Martin RC, Kuzniecky R, Faught E, Morawetz R, Jamil F, Viikinsalo MG, F. Subjective versus objective memory change after temporal lobe epilepsy surgery. Neurology 1999;53:1511. [DOI] [PubMed] [Google Scholar]
- [5].Dikmen FS, Morgan FS. Neuropsychological Factors Related to Employability and Occupational Status in Persons with Epilepsy. J Nerv Ment Dis. 1980;168: 236–240. [DOI] [PubMed] [Google Scholar]
- [6].Jobst BC, Cascino GD. Resective Epilepsy Surgery for Drug-Resistant Focal Epilepsy: A Review. JAMA 2015;313: 285–293. [DOI] [PubMed] [Google Scholar]
- [7].Seidenberg M, Hermann B, Wyler AR, Davies K, Dohan FC, Leveroni C. Neuropsychological outcome following anterior temporal lobectomy in patients with and without the syndrome of mesial temporal lobe epilepsy. Neuropsychology 1998;12: 303–316. [DOI] [PubMed] [Google Scholar]
- [8].Trenerry M, Jack R, Ivnik ea. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology 1993;43: 1800–1805. [DOI] [PubMed] [Google Scholar]
- [9].Carne RP, Brien TJ, Kilpatrick CJ, MacGregor LR, Hicks RJ, Murphy MA, Bowden SC, Kaye AH, Cook MJ. MRI- negative PET- positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain 2004;127: 2276–2285. [DOI] [PubMed] [Google Scholar]
- [10].Kuzniecky RI, Bilir E, Gilliam F, Faught E, Palmer C, Morawetz R, Jackson G. Multimodality MRI in mesial temporal sclerosis: relative sensitivity and specificity. Neurology 1997;49: 774. [DOI] [PubMed] [Google Scholar]
- [11].Jones AL, Cascino GD. Evidence on Use of Neuroimaging for Surgical Treatment of Temporal Lobe Epilepsy: A Systematic Review. JAMA Neurology 2016;73: 464–470. [DOI] [PubMed] [Google Scholar]
- [12].Griffith HR, PS B, Woodard AR, Rutecki PA, Jones JC, Ramirez LF, DeLaPena R, Seidenberg M, Hermann BP. Preoperative FDG- PET temporal lobe hypometabolism and verbal memory after temporal lobectomy. Neurology 2000;54: 1161–1165. [DOI] [PubMed] [Google Scholar]
- [13].Leeman BA, Leveroni CL, Johnson KA. Does hippocampal FDG- PET asymmetry predict verbal memory dysfunction after left temporal lobectomy? Epilepsy Behav. 2009;16: 274–280. [DOI] [PubMed] [Google Scholar]
- [14].Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. 1989. J Neurosurg. 2008;108: 411. [DOI] [PubMed] [Google Scholar]
- [15].Crowe SF. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the trail making test. J Clinical Psychol. 1998;54: 585–591. [DOI] [PubMed] [Google Scholar]
- [16].Wieser H, Blume D, Fish D, Goldensohn E, Hufnagel A, King D, et al. ILAE commissionreport proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia 2001;42:282–6. [PubMed] [Google Scholar]
- [17].Crawford JR, Garthwaite PH. Comparison of a single case to a control or normative sample in neuropsychology: Development of a Bayesian approach. Cogn Neuropsychol. 2007;24: 343–372. [DOI] [PubMed] [Google Scholar]
- [18].Duff K Evidence- Based Indicators of Neuropsychological Change in the Individual Patient: Relevant Concepts and Methods. Arch Clin Neuropsychol. 2012;27: 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hermann BP, Seidenberg M, Schoenfeld J, Peterson J, Leveroni C, Wyler AR. Empirical Techniques for Determining the Reliability, Magnitude, and Pattern of Neuropsychological Change After Epilepsy Surgery. Epilepsia 1996;37: 942–950. [DOI] [PubMed] [Google Scholar]
- [20].Sawrie SM, Chelune GJ, Naugle RI, Lüders HO. Empirical methods for assessing meaningful neuropsychological change following epilepsy surgery. J Int. Neuropsychol Soc. 1996;2: 556. [DOI] [PubMed] [Google Scholar]
- [21].Wolk D, Dickerson B. Fractionating Verbal Episodic Memory in Alzheimer’s Disease. NeuroImage 2011;52: 1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hanna D, Thomas JG, Daniel T, Richard DH, Antonio RD. A neural basis for lexical retrieval. Nature 1996;380: 499. [DOI] [PubMed] [Google Scholar]