Abstract
Purpose:
General surgical procedures are among the most commonly performed operations in the United States. Despite advances in surgical and anesthetic techniques and peri-operative care, complications after general surgery in older adults remain a significant cause of increased morbidity, mortality and healthcare costs. Frailty, a geriatric syndrome characterized by multisystem physiologic decline and increased vulnerability to stressors and adverse clinical outcomes, has emerged as a plausible predictor of adverse outcomes after surgery in older patients. Thus, the goal of this topical review is to evaluate the evidence on the association between pre-operative frailty and clinical outcomes after general surgery and whether frailty evaluation may have a role in surgical risk-stratification in vulnerable older patients.
Methods:
A PubMed database search was conducted between September and October 2018 to identify relevant studies evaluating the association between frailty and clinical outcomes after general surgery. Key words (frailty and surgery) and Medical Subject Heading term (general surgery) were used and specific inclusion and exclusion criteria were applied.
Findings:
The available evidence from meta-analyses and cohort studies suggest that pre-operative frailty is significantly associated with adverse clinical outcomes after emergent or non-emergent general surgery in older patients. Although these studies are limited by a high degree of heterogeneity of frailty assessments, types of surgery and primary outcomes, baseline frailty appears to increase risk for post-operative complications and morbidity, hospital length of stay, 30-day mortality and long-term mortality after general surgical procedures in older adults.
Implications:
There is evidence to support the further development of pre-operative frailty evaluation as a risk-stratification tool in older adults undergoing general surgery. Research is urgently needed to quantify and differentiate the predictive ability of validated frailty instruments in the context of different general surgical procedures and medical acuity and in conjunction with existing surgical risk indices widely used in clinical practice. Practical applicability of frailty instrument as well as geriatrics-centered outcomes need to be incorporated in future studies in this line of research. Furthermore, clinical care pathways that integrate frailty assessment, geriatric medicine focused peri- and post-operative management, and patient-centered interdisciplinary care models should be investigated as a comprehensive intervention approach in older adults undergoing general surgery. Finally, early implementation of palliative care should occur at the outset of hospital encounter in frail older patients who present with indications for emergent general surgery.
Keywords: Frailty, general surgery, mortality, post-operative complications
INTRODUCTION
The population in the United States is rapidly aging. While the number of Americans aged 65 years or older was 43 million in 2012, this number is expected to be more than doubled by 2060.1 Interestingly, this rapid rate of population aging has been outpaced by an increase in number of older patients needing surgical intervention as a main modality of treatment.2,3 It is estimated that more than half of the surgeries performed in the United States are provided to those over 65 years of age. Moreover, it is estimated that a surgeon’s average caseload had increased by 14 to 47% from year 2000 to 2010 in all surgical fields due to an aging population.2 Thus, the growth in demand for surgical services in older patients poses a new challenge for surgeons.
Post-operative complications in older adults remain a significant cause of increased costs, hospital length of stay (LOS), and patient distress and suffering despite major advances in surgical and anesthetic techniques and peri-operative care.3 By one estimate, 7% of patients who underwent non-cardiac surgery experienced at least 1 post-operative complication that led to a 78% increased hospital cost and 114% increased LOS.4 These complications after non-cardiac surgery frequently occurred within 30 days of procedure.5 Moreover, post-operative complications were more common with increasing age, with 20% of those ≥80 years old experiencing 1 or more complications compared with 12.1% in those <80 years of age.6,7 The high frequency of these surgery-induced complications are particularly alarming as they were more important determinants of mortality after major non-cardiac surgery than either pre-operative or intra-operative risk factors.6 Given that post-operative complications are common and have significant medical and financial consequences, and that surgical interventions are exceedingly common in an older population that is vulnerable to surgery-associated adverse sequelae, there is an urgent need to validate tools that accurately and reliably identify older adults at increased risk for surgical complications. Such validated tools would guide clinicians in risk-stratifying older patients prior to surgery with a goal to reduce surgery-induced morbidity and mortality and improve quality of care.
The risk assessment of surgical outcomes in older adults has historically focused on age and pre-existing medical co-morbidities.3,6,8 Examples of surgery risk-stratification tools used by clinicians include the American Society of Anesthesiologists (ASA) Physical Status Classification System, Acute Physiology and Chronic Health Evaluation (APACHE-II), Physiologic and Severity Score for the Enumeration of Mortality and Morbidity (POSSUM), and Goldman Cardiac Risk Index.9 However, the predictive accuracy of these tools is quite variable among different patient populations, surgery indication and procedure performed, and age groups. A likely explanation for the limitation of these risk-stratification strategies may be their inability to capture physiologic compromise unique to older adults. For instance, these tools typically assess physiologic compromise of a few select end-organs (i.e., heart failure, renal failure); however, specific physiologic compromise of these organs likely do not capture a more global physiologic decline pertinent to the decrease in resilience to stress in older patients.10,11 Thus, the ability to better quantitate physiologic reserve in older patients may be key to improving the inexact science of pre-operative risk assessment.
Frailty is a geriatric syndrome characterized by multisystem physiologic decline and increased vulnerability to stressors and adverse clinical outcomes.12,13 For example, frailty in the non-surgical population has been shown to be independently predictive of incident falls, worsening mobility or disability, hospitalization, morbidity and mortality in a large number of studies.14-16 While frailty as a medical syndrome and its application as a measure of decreased physiologic reserve are well recognized, there is no gold standard definition of frailty that is universally used in the clinical and research setting. Buta and colleagues recently systematically categorized the different purposes and contexts of use for frailty instruments frequently cited in the research literature and identified 67 such instruments.17 The Physical Frailty Phenotype (“phenotype”) was the most used instrument followed by the Deficit Accumulation Index (“Frailty Index”). In the phenotype model described by Fried and colleagues, frailty is characterized by 5 clinical features – decline in lean body mass, grip strength, endurance, walking speed and physical activity.15 Patients who display ≥3 features are deemed frail, 1-2 features are pre-frail, and 0 are non-frail. In the Frailty Index (FI) model developed by Rockwood and colleagues in the Canadian Study of Health and Aging (CSHA), frailty is conceptualized as a multidimensional risk state quantifiable by the number of deficit accumulation.18 Regardless of the definition, frailty has emerged as an important assessment tool for determining physiologic reserve and vulnerability in the geriatric population and has recently gained attention as a potential risk-stratification tool in surgical patients.
The relationship between frailty and post-operative outcomes in various surgical specialties has been a “hot topic” over the past few years.3 In a recent systematic review, Lin and colleagues evaluated 23 studies that spanned cardiac, oncological, general, vascular and hip fracture surgeries in patients 75 to 87 years old.19 Twenty-one different instruments were used to measure frailty in these studies. Regardless of how frailty was measured, strong evidence was noted in the association of frailty with increased 30-day, 90-day and 1-year mortality, post-operative complications and LOS. Similar findings from other studies strongly suggest that frailty assessment in the peri-operative period would be helpful in identifying those patients who are more susceptible to adverse surgical outcomes, and that this line of investigation is warranted to better define these relationships and further develop frailty instruments into pre-operative risk stratification tools.20-22
General surgical procedures are among the most commonly performed operations in the United States. By one estimate, 32.9% of all surgeries completed in patients aged 80 and older were general surgical procedures.6 Among these procedures, colectomy (18.2%) and cholecystectomy (10.5%) comprised over a quarter of general surgeries performed. Furthermore, older patients represented 28.8% of all major emergency general surgery cases, with partial colectomy, small-bowel resection, cholecystectomy, operative management of peptic ulcer disease, lysis of peritoneal adhesions, appendectomy and laparotomy collectively accounting for 80% of all procedures, 80.3% of deaths, 78.9% of complications, and 80.2% of inpatient costs.23,24 Given the importance of general surgery as a common and main therapeutic intervention in older patients, its associated risk for complications and other adverse clinical outcomes, it is paramount to develop reliable risk-stratification tools to aptly guide clinicians and patients in medical decision making when general surgery is offered as a treatment. A critical first step to achieving this goal is to determine whether frailty, a measure of physiologic reserve and vulnerability in older patients, is predictive of adverse clinical outcomes after general surgery. Thus, the goal of this narrative review is to summarize and evaluate recently published studies that investigated the association between pre-operative frailty and clinical outcomes including surgical complications, mortality, and hospital length of stay after general surgery in older patients.
MATERIALS AND METHODS
PubMed database was searched to identify relevant studies evaluating the association between frailty and clinical outcomes after general surgery using key words (including frailty and surgery) and Medical Subject Heading term (general surgery). The search was conducted between September and October 2018. Filters were applied to limit results to the English language and human research. No date restrictions were employed. The criteria for inclusion in this topical review were: (1) the patient population underwent a surgical procedure; (2) frailty was assessed using a validated method that included more than one domain of health deficit consistent with current conceptual idea of frailty;15,25 (3) frailty was a main variable of interest in the study; and (4) the association between frailty and post-operative clinical outcomes was evaluated. Exclusion criteria included conference abstracts, studies that assessed frailty as a single measure (e.g., gait speed only), and studies that used large scale database analysis to assess frailty and surgery outcomes. Large scale database analysis studies were excluded because of issues such as incomplete data capture inherent to this type of study design. Search results and reference lists of identified articles were manually reviewed to further identify pertinent articles.
RESULTS
The search strategy described above identified a number of systematic reviews and meta-analyses and prospective cohort studies. In total, 1,257 records were identified through database search and screened. Full-text articles of 38 of these records were assessed for eligibility. Twenty-nine of these full-text articles were excluded according to selection criteria described above (i.e., frailty was not assessed using a validated method (n = 10); frailty was not a main variable of interest (n = 10); association between frailty and post-operative outcomes was not evaluated (n = 3); large scale database analysis was used (n = 6)). No randomized controlled trials were identified. The major findings of these studies (i.e., systematic review and meta-analysis (n = 2) and observational studies (n = 7)) are reported in this narrative review.
Systematic Review and Meta-analysis
The association between frailty and clinical outcomes after general surgery has been examined by Hewitt and colleagues in a recently published systematic review and meta-analysis.26 In this study, 9 prospective observational studies published from 2010 to 2017 were included in analysis. All studies used a validated method of frailty assessment (i.e., Physical Frailty Phenotype, Deficit Accumulation Index, Groningen Frailty Indicator/GFI, 7-point clinical frailty score).17 In total, 2,281 patients (49.3% men) with mean age ranging from 61 to 77 years old were analyzed. Surgery types included elective and emergent upper and lower abdominal, colorectal cancer and gastric cancer surgeries among others. The prevalence estimates for baseline frailty ranged 10.4% to 37.0%, and pre-frailty ranged 31.3% to 45.8%. Patients who were frail and pre-frail had higher 30-day mortality (8%; 95% CI 4-12%; I2 = 0%) compared to those who were non-frail (1%; 95% CI 0-2%; I2 = 75%). Moreover, post-operative complications, assessed using the Accordion Severity Classification and the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) definitions,27 were more common in patients who were frail (24%; 95% CI 20-31%; I2 = 92%) then those who were pre-frail (9%; 95% CI 5-14%; I2 = 82%) or non-frail (5%; 95% CI 3-7%; I2 = 70%). Lastly, the mean LOS was longer in frail patients (9.6 days; 95% CI 6.2-12.9) than those who were non-frail (6.4 days; 95% CI 4.9-7.9). The authors concluded that frailty in patients who underwent general surgery was associated with poorer post-operative clinical outcomes including surgical complications, mortality and hospital length of stay.26
A second systematic review and meta-analysis on this topic focused on pre-operative frailty as a predictor of morbidity and mortality after major elective abdominal surgery.28 In addition to the assessment of clinical outcomes after abdominal surgery, the authors also evaluated the performance of different frailty metrics given that a multitude of frailty definitions and scoring systems have been used in this type of studies. Therefore, all possible definitions of frailty were considered including studies that used measures other than a validated method of frailty assessment.17 In total, 12 different definitions of frailty that incorporated 1 to 70 domains in different combinations were included. Moreover, studies that used large scale database analysis to assess frailty and surgery outcomes were included in addition to prospective cohort studies. In total, 35 studies with 1,153,684 patients with mean age ranging from 58 to 85 years old and a frailty prevalence ranging 0.5% to 67.2% were analyzed. Major abdominal surgery was defined as all gastrointestinal (colorectal, gastric, small bowel, hepatic, pancreatic resection), urological (nephrectomy, cystectomy, prostatectomy) and gynecological (uterus and ovary resection, pelvic floor reconstruction) operations, undertaken for any indication. The primary outcomes were 30-day major morbidity, defined by the Clavien–Dindo classification,29 NSQIP,27 or the Veterans Affairs Surgical Quality Improvement Program (VASQIP) classification30; short-term mortality, defined as death within 90 days after operation; and long-term mortality, defined as any death occurring before 1 year after surgery. Using random-effects meta-analysis, frailty was associated with increased risk of post-operative major morbidity (OR 2.56; 95% CI 2.08-3.16), short-term mortality (OR 5.77; 95% CI 4.41-7.55) and long-term mortality (HR 2.71; 95% CI 1.63-4.49). Moreover, all domains of frailty measurements were associated with the occurrence of post-operative major morbidity (OR 1.09; 95% CI 1.00-1.18) and no moderator effect was observed according to the number of frailty components. Given these results, the authors of this study concluded that baseline frailty was significantly associated with an increased risk of post-operative morbidity and mortality after major abdominal surgery regardless of the frailty definition.28 Furthermore, data from this analysis did not support the superiority of one frailty definition or any particular domain of frailty instruments.
The analyses presented thus far have provided evidence that pre-operative frailty is significantly associated with increased poor clinical outcomes after general surgery. While these analyses were limited by a high degree of heterogeneity of frailty assessments, types of surgery and primary outcomes, baseline frailty appears to increase risk for post-operative complications and morbidity, hospital length of stay, 30-day mortality and long-term mortality after general surgical procedure in older adults. Given an absence of data from randomized trials, these meta-analyses have provided the most meaningful results to-date in this area of research.
Observational Studies
A number of prospective studies have investigated the association of frailty and post-operative clinical outcomes.31-37 Because of a lack of data from randomized trials, well-conducted prospective studies exploring these associations may yield better insight into this clinically important question in geriatric medicine. Moreover, given the heterogeneity in the type of surgeries performed and clinical outcomes measured in studies of this type, high-quality observational studies can provide evidence specific to the acuity and severity of the surgical intervention performed. In the following section, we will summarize findings from these studies grouped by those that focused on emergent vs. non-emergent surgeries. Key findings from these studies are summarized in Table 1.
Table 1.
Cohort studies that evaluated association between pre-operative frailty and post-operative adverse outcome
| Association with adverse outcomes: |
||||||||
|---|---|---|---|---|---|---|---|---|
| Author | Study population | Type of surgery | Frailty measure | Post-operative complications |
Hospital length of stay |
Discharge to facility |
30-day mortality |
90-day or long- term mortality |
| Emergent surgery | ||||||||
| Joseph et al. (Ref. 31) | N = 220 Age: 75.5 ± 7.7 Men: 56% |
Abdominal | 50-variable Rockwood Preadmission FI |
+ | ND | ND | ND | ND |
| Kenig et al. (Ref. 32) | N = 184 Age: 76.9 ± 5.8 Men: 47% |
Abdominal | VES-13 Geriatric-8 GFI Balducci |
+ | ND | ND | + | ND |
| Non-emergent surgery | ||||||||
| Makary et al. (Ref. 33) | N = 594 Age: 71.3 (65-94) Men: 40% |
General | Fried phenotype | + | + | + | ND | ND |
| Hewitt et al. (Ref. 34) | N = 325 Age: 77.3 ± 8.2 Men: 43% |
General | CSHA 7-point scale | ND | + | ND | + | + |
| Robinson et al. (Ref. 35) | N = 201 Age: 74 ± 6 Men: 98% |
Abdominal | 7-domain based score | + | + | ND | ND | ND |
| Saxton et al. (Ref. 36) | N = 226 Age: 61 ± 13 Men: 47% |
General | CSHA 70-point scale | + | ND | ND | – | ND |
| Tegels et al. (Ref. 37) | N = 180 Age: 69.8 (73-88) Men: 59% |
Abdominal | GFI | + | – | ND | + | – |
+, p < 0.05; –, p is not significant
Abbreviations: FI = Frailty Index; VES-13 = Vulnerable Elderly Survey; GFI = Groningen Frailty Index; CSHA = Canadian Study of Health and Ageing; ND = not done
Emergent surgery
Emergent general surgery leads to significant morbidity and mortality in older patients. While clinical outcomes after elective operations are similar in younger and older patients, the latter has higher rates of complications and mortality after emergency surgery.7 Abdominal surgical procedures such as colectomy, small-bowel resection, cholecystectomy, appendectomy and others constitute ~80% of all emergent surgeries performed in older patients.23,24 The association of frailty and in-hospital complications after emergent general surgery in older patients was examined in a study published by Joseph and colleagues.31 In this prospective study, 220 consecutive patients ≥65 years old who presented to a single acute care surgery trauma center were enrolled. The participants averaged 75.5 ± 7.7 years of age (56% men) and 37% were frail as determined by a modified 50-variable Rockwood Preadmission Frailty Index (i.e., FI score ≥0.25). Surgeries that were performed included appendectomy, cholecystectomy, hernia repair and bowel resection. The in-hospital post-operative complications were categorized using NSQIP definitions to include major (e.g., sepsis, intra-abdominal abscess, enterocutaneous fistula, cholangitis, delirium, pneumonia, pulmonary emboli, deep venous thrombosis, hemorrhage/ischemia, acute kidney injury, deep surgical site infection) and minor (e.g., urinary tract infection, superficial surgical site infection, gastroenteritis) complications. In this cohort, 35% of patients had post-operative complications and 19% had major complications. Using multivariate regression analysis, the FI independently predicted the development of in-hospital complications (OR 2.13; 95% CI 1.09-4.16; p = 0.02) and major complications (OR 3.87; 95% CI 1.69-8.84; p = 0.001) with 80% sensitivity and 72% specificity. Moreover, age and American Society of Anesthesiologists score were not predictive of post-operative and major complications. Taken together, this study provided evidence that the use of frailty measurements may be superior to chronological age in predicting outcomes and provide added insight to post-operative hospital course in older patients who undergo emergent general surgery.31
The diagnostic accuracy of screening instruments for frailty in emergent general surgery is largely unknown. Kenig and colleagues explored the predictive value of 6 screening instruments in geriatrics, several of which were validated frailty assessment tools, in a cohort of 184 consecutive older patients who underwent emergent abdominal surgery within 24 hours after admission to a tertiary referral hospital.32 In this study, the patients’ mean age was 76.9 ± 5.8 years (47% men). The prevalence of frailty as assessed by the following instruments were: Vulnerable Elderly Survey/VES-13 (70.7%),38 Triage Risk Screening Tool/TRST (63.6%),39 Geriatric-8/G8 (79.9%),40 Groningen Frailty Index/GFI (54.3%),41 Rockwood FI (50%)42 and Balducci (50%).43 The most common surgical indications were cholecystitis (27%), ileus (adhesions, incarcerated hernia; 20%), appendicitis (13%) and ulcer perforation (9%). The primary outcomes were 30-day mortality (24.5%) and 30-day morbidity (58.7%; 21.7%with minor complications and 37% with major complications) as defined by the Clavien~Dindo complications scale.29 Multivariate regression analyses showed that all screening instruments, except TRST and Rockwood FI, were independent predictors of 30-day mortality and morbidity (OR 1.4-2.4 and 1.5-2.4, respectively) in the frail compared with non-frail patients. Moreover, the sensitivity and negative predictive values in post-operative mortality ranged 60-91% (Rockwood-VES-13) and 30-93% (GFI-VES13), respectively. In case of post-operative morbidity, these values were 52-85% (Rockwood-VES-13 and G8) and 44-70% (Rockwood-VES-13), respectively. Drawing from these results, the authors concluded that the VES-13 was the best screening instrument for adverse post-operative outcomes after emergent abdominal surgery in older adults.32
The results of the studies described in the preceding paragraphs suggested that frailty assessment in the setting of emergent abdominal surgery in older patients may help to predict in-hospital complications and 30-day mortality. Moreover, pre-operative frailty status may be a better predictor than chronologic age in this surgical setting. This finding is consistent with a large database analysis of >35,000 older Americans who underwent emergent general surgery in which a modified CSHA FI was deemed to be a superior predictor of 30-day mortality than ASA grading.44 However, it is also evident that the discrepant findings in FI from the Joseph and Kenig studies that much more research is needed to determine which frailty instrument is best suited for risk-stratification in emergent general surgeries.
Non-emergent surgery
The first study to investigate frailty as a predictor of surgical complications and whether it enhances known peri-operative risk models was conducted by Makary and colleagues.33 This study prospectively followed 594 patients ≥65 years old who presented to a university hospital for elective surgery categorized as major procedure, intra-abdominal procedure, open procedure or procedure for cancer. Pre-operative frailty status was assessed using the Fried phenotype determined by 5 measures (i.e., weakness, weight loss, exhaustion, low physical activity, slowed walking speed) and the patients were categorized as frail (10.4%), pre-frail (31.3%) or non-frail (58.3%).15 The primary outcome measures were 30-day surgical complications as defined by NSQIP, LOS and discharge disposition from acute hospitalization. Using regression analyses and after adjusting for known surgical risk indices, pre-operative frailty was found to be an independent predictor of these adverse outcomes. Specifically, frailty was associated with increased post-operative complications (OR 2.54; 95% CI 1.12-5.77), LOS (incidence rate ratio/IRR 1.69; 95% CI 1.28-2.23) and discharge to a skilled or assisted-living facility (OR 20.48; 95% CI 5.54-75.68). Pre-frailty was similarly associated with post-operative complications (OR 2.06; 95% CI 1.18-3.60), LOS (IRR 1.49; 95% CI 1.24-1.80) and discharge to a skilled or assisted-living facility (OR 3.16; 95% CI 1.0-9.99). In addition, pre-operative Fried phenotype improved predictive ability of known surgical risk indices including the ASA, Lee and Eagle scores (p < 0.01). These findings led the authors to conclude that frailty status before surgery can independently predict adverse outcomes in older surgical patients and may enhance conventional risk-stratification models.33
Clinical outcomes such as 30-day and 90-day mortality and hospital readmission, in addition to LOS in frail older patients after general surgery were investigated in a United Kingdom study.34 The authors of this prospective study assessed 325 consecutive patients averaging 77.3 ± 8.2 years of age (43% men) who were admitted to 3 acute surgical admission units with diagnoses in keeping with acute general surgical conditions. Frailty status measured using a 7-point clinical frailty score derived from CSHA was severe (6%), moderate (13%) or mild (8%).18 The patients underwent general surgery procedures that were categorized as minor, intermediate, major or complex major operations. Compared to the non-frail group, frail patients had higher mortality at 30 days (OR 4.0; 95% CI 1.1-15.2; p = 0.04) and 90 days (OR 3.0; 95% CI 1.3-7.4; p = 0.02). The LOS in the frail group was longer than those who were non-frail (11.1 days; 95% CI 7.2-15.0 vs. 7.6 days; 95% CI 6.1-9.2; p = 0.03), consistent with that reported by Makary and colleagues. Hospital readmission between frail and non-frail groups did not differ (OR 1.1; 95% CI 0.5-2.3). These findings added evidence that morbidity and mortality after acute general surgery are increased in older adults who are frail.34
The association of baseline frailty and clinical outcomes after elective colorectal surgery in older adults was examined in a study published by Robinson and colleagues.35 This prospective cohort study conducted at the Denver Veterans Affairs Medical Center included 201 patients aged 74 ± 6 years old (98% were men), 72 of whom underwent colorectal operations. Surgical procedures performed for the colorectal group included right colectomies (36%), left colectomies (22%), sigmoid colectomies (31%) and colostomy takedowns (11%). Pre-operative frailty status was ascertained using 7 baseline traits, which were Katz Score ≤5, Timed Up-and-Go ≥15 seconds, Charlson Index ≥3, anemia defined as hematocrit <35%, Mini-Cog score ≤3, albumin <3.4 gm/dL and ≥1 fall within past six-months. A 7-domain based score was then calculated as the total number of positive traits – frail (≥4 traits), pre-frail (2-3 traits) and non-frail (0–1 trait). The VASQIP definitions were used to determine post-operative complications.30 Categories of complications included were cardiac (cardiac arrest, myocardial infarction), respiratory (pneumonia, pulmonary embolism, reintubation), renal insufficiency, neurologic (cerebral vascular accident, coma), post-operative infection (deep or superficial surgical site wound infection, urinary tract infection, sepsis), deep vein thrombosis, and re-operation. In this cohort, pre-operative frailty was associated with higher rates of complications following colorectal surgery (frail 58%, pre-frail 40%, non-frail 21%; p = 0.016) independent of age. Compared with the non-frail group, frail patients were 13.36 times more likely to have a complication (95% CI 2.56-69.81; p = 0.002) and pre-frail patients were 3.4 times more likely to have a complication (95% CI 0.77-14.97; p = 0.106). Moreover, the receiver operating characteristic curves examining frailty’s ability to forecast complications was 0.7 (95% CI 0.58-0.83; p = 0.004) for colorectal surgery. In addition to post-operative complications, patients who were frail also had longer hospital stays (frail, 14 ± 11.0 days; non-frail, 6 ± 3.6 days; p < 0.001) and higher 30-day readmission rates (frail, 29%; non-frail, 6%; p = 0.046). Thus, these findings added to the rationale that pre-operative frailty evaluation may be a plausible risk-stratification tool in older adults subjected to general surgery.33,35
The association between pre-operative frailty and complications after elective general surgery of different complexity was also explored in a study reported by Saxton and colleagues.36 In this prospective study, 226 patients aged 61 ± 13 years old (47% men) admitted to hospital for general surgical operations were enrolled. Pre-operative frailty was determined by CSHA Frailty Index and patients with a FI score >0.12 was defined as frail.18 Surgical procedures the patients underwent were divided into 3 categories: (1) 31% had superficial or laparoscopic procedures for benign disease (e.g., hernia repair, cholecystectomy, appendectomy); (2) 17% had open or intra-abdominal procedures for benign disease (e.g., pancreatectomy, enterostomy); and (3) 52 % had open or laparoscopic procedures for malignant disease (e.g., esophagectomy, gastrectomy, colectomy, Whipple). Primary outcomes ascertained were occurrence and number of 30-day complications and severity of complications determined by the Clavien-Dindo classification (i.e., none or minor (Clavien class I) vs. severe (Clavien class II–V) complications).29 In this cohort, patients who had 1 or more post-operative complications had higher median pre-operative FI than those without complications [0.075 (IQR 0.046-0.118) vs. 0.059 (IQR 0.045-0.089); P = 0.007]. Moreover, multiple logistic regression analysis showed that FI >0.12 was associated with increased risk of post-operative complications (OR 2.71; 95% CI 1.08-6.78; p = 0.03) but not mortality (OR 0.42; 95% CI 0.04–4.20; p = 0.5). Hence, results from this study suggested that pre-operative frailty status as measured by CSHA FI may help identify patients at higher risk for post-operative complications.
Tegels and colleagues published a study specifically evaluating the relationship between gastric cancer surgical complications and mortality and pre-operative assessment of frailty.37 In this study, 180 patients (58.9% men), aged 69.8 (73-88) years old with histologically proven gastric adenocarcinoma underwent surgical treatment in a community teaching hospital that was a referral center for surgical treatment of gastric malignancy. Frailty was assessed using Groningen Frailty Indicator (GFI), a 15-item (scored 0 or 1 point) questionnaire that evaluates mobility, self-perceived physical fitness, nourishment (i.e., weight loss), morbidity (i.e., polypharmacy) and cognition.45 Surgical procedures performed were total gastrectomy (23.9%), subtotal gastrectomy (57.8%), gastroenterostomy (9.4%) and explorative laparotomy (8.9%) with an overall in-hospital mortality of 8.3%. Patients who were more frail (i.e., GFI ≥3) had a higher mortality rate of 23.3% compared with 5.2% in patients with GFI <3 (OR 4.0; 95% CI 1.1-14.1; p = 0.03). In addition, patients with GFI ≥3 had increased risk of serious adverse events (Clavien-Dindo grade ≥3a) compared to those with GFI <3 (OR 3.62; 95% CI 1.53-8.58; p = 0.04) independent of age, type of surgery, tumor stage, ASA classification and neoadjuvant chemotherapy in multivariate analysis.29 Higher GFI score was not associated with 6-month mortality or hospital LOS. In a subset of 125 patients who underwent surgery with curative intent, in-hospital mortality was 27.3% in those with GFI ≥3 compared with 5.7% in those with GFI <3 (OR 4.6; 95% CI 1.0-20.9; p = 0.05). Thus, these findings suggested that frailty assessment using GFI can help to identify individuals at risk for higher adverse events and in-hospital mortality after gastric cancer surgery.37
Although these studies were limited by heterogeneity of surgical interventions and frailty measures and outcome assessments, they added to the evidence base that frailty in older patients may influence clinical outcomes after non-emergent general surgery. The finding that pre-operative Fried phenotype improved predictive ability of known surgical risk indices further suggested that frailty instruments have a role in improving the clinicians’ ability to perform risk assessment before non-emergent general surgery in older patients.
Recommendations for Future Directions
In summary, the studies presented in this topical review strongly suggest that pre-operative frailty is associated with increased poor clinical outcomes after emergent or non-emergent general surgery in older adults. The outcomes that may be adversely influenced by baseline frailty include post-operative complications and morbidity, hospital length of stay, 30-day mortality and long-term mortality. These findings have several implications for future research and clinical care as outlined below.
There remain considerable gaps in knowledge regarding frailty as a predictive indicator for outcomes after general surgery.19,28 First, it is unclear if one frailty instrument is superior to another in this setting given that different types of surgical procedures (e.g., degree of invasiveness, duration of surgery) likely would induce variable extent of stress responses. Moreover, the high acuity nature of emergent general surgery, frequently complicated by associated infection, bleeding and cardiovascular decline, likely would further compromise the limited physiologic reserve in vulnerable older patients. Thus, while the identification of a single unified frailty assessment tool in the setting of general surgery would greatly enhance its adaptability and ease of use, this may not be a feasible or pragmatic approach. Future research will be needed to better quantify and differentiate the predictive ability of existing frailty instruments in the context of different surgical procedures and medical acuity in older patients undergoing general surgery. A potential approach to this type of investigation is to simultaneously administer multiple candidate frailty instruments based on best available evidence, in large cohorts of older patients who undergo specific categories of surgical procedures (e.g., open vs. laparoscopic) and medical acuity (e.g., emergency vs. elective). Second, considerations should be given to the selection of frailty instruments for future investigations depending on practical applications. For instance, a short questionnaire-based instrument may be better suited for pre-operative assessment prior to emergent surgery given the feasibility and rapidity of its administration. Moreover, a phenotype-based instrument may not be an optimal tool in older patients who are severely deconditioned or physically incapacitated due to their inability to participate in performance-based assessment (e.g., walking speed). In contrast, a frailty instrument grounded in Deficit Accumulation Index, if robust and valid, may be well suited for elective general surgery evaluation administered in a pre-operative evaluation clinic. Third, the predictive values of frailty instruments must be investigated in conjunction with existing surgical risk indices widely used in clinical practice (e.g., ASA, Goldman Cardiac Risk Index). This study design will help to determine if pre-operative frailty assessment enhances known conventional risk-stratification models and whether it should be an added component, or a stand-alone tool, in pre-operative risk assessment in older adults. Last, while post-operative complications, hospital length of stay and mortality are important factors to consider in the care of older adults, other key aspects of geriatric care including post-operative functional and cognitive decline and quality of life are issues that should not be overlooked. Thus, these geriatrics-centered outcomes need to be included in future studies in this line of research.
The utility of frailty assessment should extend beyond its role in pre-operative risk-stratification. In fact, the positive identification of frailty in an older surgical patient should trigger the initiation of a set of interventions that may reduce morbidity and enhance functional recovery after surgery.28 Potential types interventions to be considered include: (1) the Enhanced Recovery After Surgery (ERAS) pathways, an integrated clinical care delivery program recently shown to improve clinical and functional outcomes in older patients after colorectal surgery;46,47 (2) prehabilitation programs that aim to optimize physical function and comorbidity pre-operatively;48 and (3) geriatric interdisciplinary assessment and treatment models that have been demonstrated to improve clinical outcomes of frail older adults.12 While further investigation is needed, clinical care pathways that integrate frailty assessment, geriatric medicine focused peri- and post-operative management, and patient-centered interdisciplinary care models should be delivered as a comprehensive intervention in frail older adults undergoing surgery.
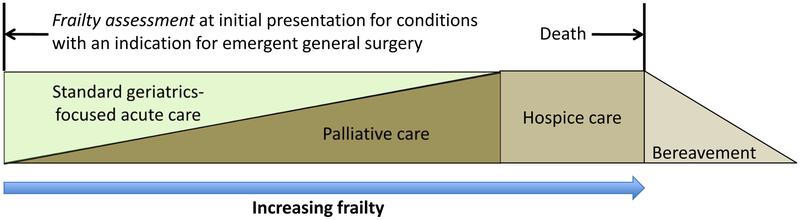
A fundamental principle in geriatric medicine is that standard indications for medical and surgical interventions may not be generalizable to older adults because age-associated changes in physiologic reserve and associated multi-morbidity could alter risk to benefit ratio. Frailty, as presented in this review, has shown promise as an important assessment tool to determine physiologic reserve and vulnerability in older surgical patients. Thus, the use of a validated frailty instrument that accurately predicts adverse outcomes after general surgery can provide key information to help guide medical decision making for patients and their families. Emergent general surgery, given its high morbidity and mortality in older patients, is a clinical scenario where risk to benefit ratio of surgical intervention could be significantly influenced by early assessment and diagnosis of frailty. Here, we propose a model of care for emergent general surgery in frail older patients that incorporates early frailty assessment and palliative care. (Figure 1) Palliative care is an interdisciplinary specialty that focuses on improving quality of life for patients by providing an added layer of support (e.g., pain and symptom management, goals of care discussions, care coordination) in the setting of serious illness and is provided concurrently with other disease-directed or curative treatments. In this model, frailty assessment with a validated tool is to be completed when an older patient presents to the emergency room for a condition that requires emergent general surgical intervention as the primary means of treatment. If the patient is found to be frail and the risk to benefit ratio of an emergent surgery increases, the medical and surgical team should introduce and include palliative care in the medical decision discussions with the patient and his or her proxy and integrate palliative care in the patient’s management. Thus, palliative care is implemented at the outset of emergency room presentation and can be scaled up in those who are highly frail. Finally, hospice care needs to be considered and administered if appropriate in patients who are severity frail and present with other advanced co-morbidity and acute multi-system decompensation.
Figure 1.
A model of care for emergent general surgery in frail older patients
CONCLUSIONS
There is growing evidence that pre-operative frailty is associated with increased adverse clinical outcomes after emergent or non-emergent general surgery in older adults. While more research is critically needed in this field, frailty assessment holds promise as a pre-operative risk-stratification tool in geriatric and peri-operative medicine. The validation of frailty instruments in the surgical setting will likely improve patient-centered, comprehensive intervention in older adults undergoing general surgery across the spectrum of frailty syndrome.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institute on Aging (K08 AG050808, PI Ko; P30 AG028741, PI Siu). The funding sources do not create any conflict of interest for the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The author has indicated no conflicts of interest with regard to the content of this article.
REFERECES
- 1.Mather M, Jacobsen LA, Pollard KM Aging in the United States. (2015). [Google Scholar]
- 2.Etzioni DA, Liu JH, Maggard MA & Ko CY The aging population and its impact on the surgery workforce. Ann Surg 238, 170–177, doi: 10.1097/01.SLA.0000081085.98792.3d (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Partridge JS, Harari D & Dhesi JK Frailty in the older surgical patient: a review. Age Ageing 41, 142–147, doi: 10.1093/ageing/afr182 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Khan NA et al. Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J Gen Intern Med 21, 177–180, doi: 10.1111/j.1525-1497.2006.00319.x (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamgbade OA, Rutter TW, Nafiu OO & Dorje P Postoperative complications in obese and nonobese patients. World J Surg 31, 556–560; discussion 561, doi: 10.1007/s00268-006-0305-0 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Hamel MB, Henderson WG, Khuri SF & Daley J Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc 53, 424–429, doi: 10.1111/j.1532-5415.2005.53159.x (2005). [DOI] [PubMed] [Google Scholar]
- 7.Pofahl WE & Pories WJ Current status and future directions of geriatric general surgery. J Am Geriatr Soc 51, S351–354 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Polanczyk CA et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med 134, 637–643 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Chand M, Armstrong T, Britton G & Nash GF How and why do we measure surgical risk? J R Soc Med 100, 508–512, doi: 10.1177/014107680710001113 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleisher LA et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy--a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Anesth Analg 104, 15–26, doi: 10.1213/01.ane.0000243335.31748.22 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Davenport DL, Bowe EA, Henderson WG, Khuri SF & Mentzer RM Jr. National Surgical Quality Improvement Program (NSQIP) risk factors can be used to validate American Society of Anesthesiologists Physical Status Classification (ASA PS) levels. Ann Surg 243, 636–641; discussion 641-634, doi: 10.1097/01.sla.0000216508.95556.cc (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko FC The clinical care of frail, older adults. Clin Geriatr Med 27, 89–100, doi: 10.1016/j.cger.2010.08.007 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Walston J Frailty--the search for underlying causes. Sci Aging Knowledge Environ 2004, pe4, doi: 10.1126/sageke.2004.4.pe4 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Hogan DB et al. A Scoping Review of Frailty and Acute Care in Middle-Aged and Older Individuals with Recommendations for Future Research. Can Geriatr J 20, 22–37, doi: 10.5770/cgj.20.240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried LP et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56, M146–156 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Bandeen-Roche K et al. Phenotype of frailty: characterization in the women's health and aging studies. J Gerontol A Biol Sci Med Sci 61, 262–266 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Buta BJ et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 26, 53–61, doi: 10.1016/j.arr.2015.12.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rockwood K et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 173, 489–495, doi: 10.1503/cmaj.050051 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin HS, Watts JN, Peel NM & Hubbard RE Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr 16, 157, doi: 10.1186/s12877-016-0329-8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DH, Kim CA, Placide S, Lipsitz LA & Marcantonio ER Preoperative Frailty Assessment and Outcomes at 6 Months or Later in Older Adults Undergoing Cardiac Surgical Procedures: A Systematic Review. Ann Intern Med 165, 650–660, doi: 10.7326/M16-0652 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sepehri A et al. The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg 148, 3110–3117, doi: 10.1016/j.jtcvs.2014.07.087 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Lin HS, Peel NM & Hubbard RE Baseline Vulnerability and Inpatient Frailty Status in Relation to Adverse Outcomes in a Surgical Cohort. J Frailty Aging 5, 180–182 (2016). [PubMed] [Google Scholar]
- 23.Scott JW et al. Use of National Burden to Define Operative Emergency General Surgery. JAMA Surg 151, e160480, doi: 10.1001/jamasurg.2016.0480 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Vilches-Moraga A & Fox J Geriatricians and the older emergency general surgical patient: proactive assessment and patient centred interventions. Salford-POP-GS. Aging Clin Exp Res 30, 277–282, doi: 10.1007/s40520-017-0886-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones DM, Song X & Rockwood K Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc 52, 1929–1933, doi: 10.1111/j.1532-5415.2004.52521.x (2004). [DOI] [PubMed] [Google Scholar]
- 26.Hewitt J et al. The prevalence of frailty and its association with clinical outcomes in general surgery: a systematic review and meta-analysis. Age Ageing, doi: 10.1093/ageing/afy110 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Fink AS et al. The National Surgical Quality Improvement Program in non-veterans administration hospitals: initial demonstration of feasibility. Ann Surg 236, 344–353; discussion 353-344, doi: 10.1097/01.SLA.0000027082.79556.55 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandini M et al. Systematic review and meta-analysis of frailty as a predictor of morbidity and mortality after major abdominal surgery. BJS Open 1, 128–137, doi: 10.1002/bjs5.22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clavien PA et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250, 187–196, doi: 10.1097/SLA.0b013e3181b13ca2 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Khuri SF et al. The Department of Veterans Affairs' NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg 228, 491–507 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joseph B et al. Emergency General Surgery in the Elderly: Too Old or Too Frail? J Am Coll Surg 222, 805–813, doi: 10.1016/j.jamcollsurg.2016.01.063 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Kenig J, Zychiewicz B, Olszewska U, Barczynski M & Nowak W Six screening instruments for frailty in older patients qualified for emergency abdominal surgery. Arch Gerontol Geriatr 61, 437–442, doi: 10.1016/j.archger.2015.06.018 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Makary MA et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 210, 901–908, doi: 10.1016/j.jamcollsurg.2010.01.028 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Hewitt J et al. Prevalence of frailty and its association with mortality in general surgery. Am J Surg 209, 254–259, doi: 10.1016/j.amjsurg.2014.05.022 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Robinson TN et al. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg 206, 544–550, doi: 10.1016/j.amjsurg.2013.03.012 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saxton A & Velanovich V Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg 253, 1223–1229, doi: 10.1097/SLA.0b013e318214bce7 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Tegels JJ, de Maat MF, Hulsewe KW, Hoofwijk AG & Stoot JH Value of geriatric frailty and nutritional status assessment in predicting postoperative mortality in gastric cancer surgery. J Gastrointest Surg 18, 439–445; discussion 445-436, doi: 10.1007/s11605-013-2443-7 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Saliba D et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc 49, 1691–1699 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Meldon SW et al. A brief risk-stratification tool to predict repeat emergency department visits and hospitalizations in older patients discharged from the emergency department. Acad Emerg Med 10, 224–232 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Bellera CA, Artaud F, Rainfray M, Soubeyran PL & Mathoulin-Pelissier S Modeling individual and relative accuracy of screening tools in geriatric oncology. Ann Oncol 28, 1152–1157, doi: 10.1093/annonc/mdx068 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slaets JP Vulnerability in the elderly: frailty. Med Clin North Am 90, 593–601, doi: 10.1016/j.mcna.2006.05.008 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Rockwood K et al. A brief clinical instrument to classify frailty in elderly people. Lancet 353, 205–206, doi: 10.1016/S0140-6736(98)04402-X (1999). [DOI] [PubMed] [Google Scholar]
- 43.Balducci L & Beghe C The application of the principles of geriatrics to the management of the older person with cancer. Crit Rev Oncol Hematol 35, 147–154 (2000). [DOI] [PubMed] [Google Scholar]
- 44.Farhat JS et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg 72, 1526–1530; discussion 1530-1521, doi: 10.1097/TA.0b013e3182542fab (2012). [DOI] [PubMed] [Google Scholar]
- 45.Bielderman A et al. Multidimensional structure of the Groningen Frailty Indicator in community-dwelling older people. BMC Geriatr 13, 86, doi: 10.1186/1471-2318-13-86 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braga M et al. Enhanced Recovery Program in High-Risk Patients Undergoing Colorectal Surgery: Results from the PeriOperative Italian Society Registry. World J Surg 41, 860–867, doi: 10.1007/s00268-016-3766-9 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Slieker J et al. Enhanced recovery ERAS for elderly: a safe and beneficial pathway in colorectal surgery. Int J Colorectal Dis 32, 215–221, doi: 10.1007/s00384-016-2691-6 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Li C et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc 27, 1072–1082, doi: 10.1007/s00464-012-2560-5 (2013). [DOI] [PubMed] [Google Scholar]