Abstract
Background:
The relationship between intensive volume removal in ADHF patients with pre-existing WRF and renal tubular injury, post-discharge renal function, and clinical outcomes is unknown.
Methods and Results:
We used data from the multicenter CARRESS-HF trial that randomized patients with ADHF and pre-existing WRF to intensive volume removal with stepped pharmacologic therapy (SPT) or fixed rate ultrafiltration (UF). Patients in the urinary renal tubular injury biomarker sub-study (NAG, KIM-1, and NGAL) were evaluated (N=105). The severity of pre-randomization WRF was unrelated to baseline renal tubular injury biomarkers (r=0.14, P=0.17). During randomized intensive volume removal, creatinine further worsened in 53% of patients. Despite a small to moderate magnitude increase in creatinine in most of these patients, post-randomization WRF was strongly associated with worsening in renal tubular injury biomarkers (OR=12.6, P=0.004). This observation did not differ by mode of volume removal (SPT vs. UF, Pinteraction=0.46). Increase in renal tubular injury biomarkers was associated with a higher incidence of hemoconcentration (OR=3.1, P=0.015), and paradoxically, better recovery of creatinine at 60 days (P=0.01).
Conclusions:
In ADHF patients with pre-existing WRF, intensive volume removal resulted in a further worsening of creatinine approximately half of the time, a finding associated with a rise in tubular injury biomarkers. However, decongestion and renal function recovery at 60 days was superior in patients with increased tubular injury markers. These data suggest that the benefits of decongestion may outweigh any modest or transient increases in serum creatinine or tubular injury markers that occur during intensive volume removal.
Keywords: Acute Heart Failure, Renal Failure, Kidney Injury, Ultrafiltration, Biomarkers
Introduction
It is generally assumed that worsening renal function (WRF) during the treatment of acute decompensated heart failure (ADHF) is causally related to adverse outcomes and should be avoided.1–3 Consequently, diuretics are often reduced or discontinued in the setting of rises in creatinine, despite the strong association between incomplete decongestion and worsened short and long-term outcomes in HF. In some cases, these patients may even be given intravenous fluids.4, 5
Even though WRF affects therapeutic decisions and is frequently employed as an endpoint in clinical trials, accumulating evidence indicates it may be a poor surrogate for adverse clinical outcomes. Indeed, its association with adverse events is highly dependent on the inciting event: for example, WRF following initiation of renin-angiotensin-aldosterone inhibition appears to be benign.6–9 Furthermore, small to moderate increases in creatinine commonly encountered during aggressive diuresis appear unrelated to significant renal injury and have even been associated with improved outcomes.7, 10
Nonetheless, ongoing concerns about the safety of diuresis-induced WRF in the clinical arena remain; physicians are likely to halt attempts at aggressive diuresis when WRF occurs in an effort to limit the perceived severity of kidney injury.5 We recently demonstrated that small to moderate increases in creatinine in the above setting were not primarily driven by renal tubular injury.7 However, a primary concern remains that further diuresis of a patient with WRF will transition the mechanism from functional/hemodynamic to true renal injury, which could in turn negatively impact long term renal and clinical outcomes. However, this hypothesis has not yet been addressed.
The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial helps us to explore this question. In this study, ADHF patients were enrolled only after they had already experienced WRF but remained congested.11 In this setting, they underwent protocol directed aggressive volume removal using either stepped pharmacologic therapy (SPT) or fixed rate ultrafiltration (UF) with renal function and clinical outcomes queried at 60 days. As part of a prospective ancillary study, urine levels of 3 key renal tubular injury biomarkers [neutrophil gelatinase-associated lipocalin (NGAL), N-acetyl-b-D-glucosaminidase (NAG), and kidney injury molecule-1 (KIM-1)] were assessed. These biomarkers provide discrimination for histopathologic severity of renal tubular injury, whether induced by nephrotoxins or ischemia-reperfusion injury.12
Our goal was to use the experimental platform described above to understand the impact of intensive volume removal in ADHF patients with pre-existing WRF on biomarkers of kidney injury, kidney filtration (creatinine), and clinical outcomes.
Methods
Patient Population and Study Protocol
The rationale and design of the NHLBI funded CARRESS-HF trial has been previously described in detail.11 Briefly, the overall study comprised 188 patients who were hospitalized for ADHF and had pre-enrollment WRF (defined as an increase in creatinine concentration of ≥0.3 mg/dL) in addition to persistent congestion. All patients were required to have at least 2 of the following parameters of congestion at the time of randomization: ≥2+ peripheral edema, jugular venous pressure>10 cm of water, or pulmonary edema or pleural effusion on chest radiography. Those with a creatinine level >3.5 mg/dL at the time of admission and those receiving intravenous vasodilators or inotropic agents were excluded. Patients were then randomized 1:1 to SPT or UF. The SPT was managed according to a recommended treatment algorithm developed by the Heart Failure Network (HFN) that provided treating physicians with guidelines for the intensification of diuretic therapy with early addition of a thiazide type diuretic. Patients in the UF group underwent continuous ultrafiltration at a fixed rate of 200 ml/hour using the Aquadex System 100 (CHF Solutions) according to the manufacture’s specifications. In both groups, the assigned treatment strategy was to be continued until the signs and symptoms of congestion in the patient were reduced to the best extent possible as determined by the treating clinician. The primary end-point of the trial was the bivariate change from baseline in the creatinine level and body weight, assessed 4 days after randomization. There was no difference in terms of weight loss however in subjects randomized to UF a greater increase in creatinine at 4 days was observed. The study protocol was approved by the Institutional Review Boards at each participating site and written informed consent was obtained from all patients prior to randomization.
Data Availability Statement
This manuscript was prepared using CARRESS-HF research materials obtained directly from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC).13 The data, analytic methods, and study materials are available to other researchers for purposes of reproducing the results via BioLINCC.
Measurement of Biomarkers
As previously described, patients had creatinine and NT-proBNP levels determined at a core lab (Heart Failure Clinical Research Network Core Biomarker Laboratory, University of Vermont).7, 10, 14 Urinary biomarkers were measured at study entry and 4 days after study entry from clean voided urine or collected from a Foley catheter. These samples were tested for neutrophil gelatinase associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), and N-Acetyl-β-D-glucosaminidase (NAG) at Dr. Joseph Bonventre’s laboratory (Brigham and Women’s Hospital, Harvard Medical School).7 NGAL was determined by ELISA (capture and detection antibodies from Enzo Lifesciences). KIM-1 using microbeads (R&D systems) quantified using Bio-Plex 200 system (Bio-Rad). Urinary NAG was measured using the N-acetyl-β-D-glucosaminidase kit per manufacturer’s (Roche Diagnostics) instructions. Since patients were undergoing varying strategies for decongestion and differences in urine dilution is expected to be substantial, all urine biomarker levels were indexed to urine creatinine. Core laboratory values for urine creatinine were used when available and local site laboratory values utilized when core lab values were not available. If the 60-day post randomization follow up urine creatinine values were not available, the 30-day visit creatinine values was carried forward (N=11). Supplementary Figure 1 shows a consort diagram of the current study.
Definitions and Calculations
Several different creatinine levels were examined to describe longitudinal changes in renal function over the CARRESS-HF study timeline: 1) Nadir creatinine— this was the primary creatinine considered by the CARRESS-HF investigators and used as the patient’s “true baseline” creatinine. The median time from the nadir creatinine to the qualifying creatinine (see below) could have been determined up to 12 weeks prior to the index hospitalization; 2) Qualifying creatinine— this was the creatinine utilized by the CARRESS-HF investigators to meet the inclusion criteria of a 0.3 mg/dL increase in creatinine above the patient’s nadir creatinine. The CARRESS-HF qualifying creatinine (which could be different from the creatinine at time of randomization) occurred on the same day of randomization in 80% of patients and within 2 days of randomization in 97.2%. This qualifying creatinine was obtained in the hospital in 93.3% of patients; 3) Randomization creatinine— this was the creatinine level on the day of randomization; 4) Day 4 creatinine— this was the creatinine 4 days post randomization; 5) Day 60 creatinine— this was the creatinine 60 days after randomization. Estimated GFR was calculated using the CKD-EPI formula.15 To maintain consistency with our prior publications, WRF was defined as a ≥20% reduction in eGFR.7, 16 Hemoconcentration was defined as an increase in hemoglobin on day 4 relative to the admission value.
Renal tubular injury biomarker score:
We utilized our previously described renal tubular injury biomarker score as the primary analytic parameter in this analysis for several reasons7: The sample size of the CARRESS-HF biomarker sub-study is small and the signal to noise ratio of the individual renal tubular injury biomarkers is low. In principle these markers are capturing the same signal (renal tubular injury), thus combining these markers into a renal tubular injury biomarker score should improve the signal to noise ratio. As these biomarkers are known to be not normally distributed, have many extreme outliers, thus a rank-based approach was taken. To accomplish this, each biomarker level in the population was ranked from highest to lowest. Next the average of the sum of the three biomarker ranks was taken for each patient. A biomarker score was calculated at baseline (randomization) and again at day 4. The change in renal tubular injury biomarker score was calculated as the difference between the two scores. Values above the median for the change in biomarker were designated as ↑injury.
Statistical Analysis
Baseline characteristics are presented as median (quartile1-quartile3) or percentiles. Student’s t test the Mann-Whitney test or the Wilcoxon Rank Sum test were used to compare continuous variables. Paired samples were analyzed using a paired samples t test. The χ2 test was used to evaluate associations between categorical variables. Correlations reported throughout the paper are Spearman’s ρ. Longitudinal changes in creatinine across the CARRESS-HF trial study periods were examined using mixed effect model with compound symmetry variance-covariance matrix of correlations within subjects. The difference in changed creatinine between groups by increase and decrease in tubular injury biomarker score were tested with the estimates and standard errors obtained from mixed effect model. Statistical analysis was performed with IBM SPSS Statistics version 23 (IBM Corporation, Armonk, NY) and SAS version 9.4 for mixed effect model (PROC MIXED), and statistical significance was defined as 2-tailed P<0.05 for all analyses, except for interaction testing, where P<0.01 was considered significant.
Results
Of the 188 patients enrolled in the CARRESS-HF trial, 105 patients participated in the urine biomarker sub-study and had urinary levels of NGAL, NAG, and KIM-1 assessed. The baseline characteristics of these patients were similar to those of the overall trial population11 (Table 1). Randomization remained balanced in this subset as 52 of these patients were randomized to the SPT and 53 to UF (P=0.92). For the analysis, we partitioned the study into 3 distinct phases that we frame in terms of impact on the kidneys: (1) Pre-randomization WRF, (required per protocol for enrollment), (2) Intensive volume removal during the (up to) 4-day period of randomized intervention, and (3) the Renal Recovery Period, with creatinine assessed 60 days post-randomization.
Table 1:
Baseline characteristics grouped by mode of decongestion and change in tubular injury biomarker score from randomization to day 4
| Characteristics | SPT (N=52) | UF (N=53) | P | ↓ Injury (N=45*) | ↑Injury (N=46*) | P |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, years | 67±13 | 69±12 | 0.45 | 68±13 | 69±11 | 0.59 |
| Male sex, % | 75 | 81 | 0.45 | 80 | 78 | 0.84 |
| White race, % | 73 | 72 | 0.99 | 73 | 78 | 0.84 |
| Comorbidities | ||||||
| Diabetes, % | 71 | 57 | 0.12 | 64 | 65 | 0.94 |
| AF/AFL, % | 46 | 66 | 0.04 | 51 | 61 | 0.35 |
| Ischemic Cardiomyopathy, % | 48 | 67 | 0.04 | 53 | 67 | 0.17 |
| Hospitalization for Heart Failure in Previous Year, % | 74 | 74 | 0.96 | 75 | 64 | 0.44 |
| Physical examination | ||||||
| Weight, lb | 223±57 | 217±68 | 0.14 | 220±67 | 224±60 | 0.79 |
| SBP, mmHg | 112 (102-127) | 113 (104-124) | 0.82 | 113 (107-128) | 112 (104-125) | 0.56 |
| JVP>8cm water% | 96.1 | 96 | 0.98 | 93 | 100 | 0.09 |
| Severe Peripheral Edema, % | 44 | 32 | 0.20 | 36 | 39 | 0.73 |
| Heart Failure Variables | ||||||
| LVEF, % | 38±17 | 36±18 | 0.56 | 41±17 | 34±16 | 0.04 |
| NYHA class | ||||||
| III | 69 | 58 | 53.3 | 69 | 0.13 | |
| IV | 31 | 42 | 0.27 | 46.7 | 31 | |
| Medications | ||||||
| ACE-I/ARB, % | 54 | 57 | 0.78 | 49 | 54 | 0.60 |
| β-blocker, % | 75 | 83 | 0.31 | 75.6 | 82.6 | 0.41 |
| Hydralazine, % | 19 | 13 | 0.40 | 11.1 | 15.2 | 0.56 |
| Nitrates, % | 39 | 19 | 0.03 | 24.4 | 32.6 | 0.39 |
| Aldosterone Antagonist, % | 17 | 23 | 0.50 | 64.4 | 76.1 | 0.22 |
| Digoxin, % | 14 | 23 | 0.22 | 24.4 | 8.7 | 0.05 |
| Loop diuretic dose (Furosemide-equivalents mg/day) | 100 (80-160) | 120 (45-240) | 0.94 | 100 (80-160) | 110 (48-170) | 0.94 |
| Laboratories | ||||||
| Sodium, mmol/L | 138 (135-140) | 137 (133-140) | 0.37 | 137 (134-140) | 137 (134-140) | 0.50 |
| Hemoglobin, g/dL | 11.0±1.6 | 10.9±1.7 | 0.66 | 10.9±1.6 | 10.8±1.7 | 0.74 |
| BUN, mmol/L | 50 (40-66) | 47 (34-65) | 0.80 | 53 (39-65) | 49 (38-68) | 0.98 |
| Creatinine, mg/dL | 2.2±0.6 | 2.0±0.7 | 0.21 | 2.1±0.6 | 2.0±0.7 | 0.26 |
| eGFR, ml/min/1.73m2 | 33 (26-43) | 40 (28-51) | 0.12 | 34 (26-45) | 35 (26-48) | 0.39 |
| NT-proBNP, pg/ml | 4838 (1194-9676) | 4620 (2854-9596) | 0.99 | 6366 (2888-12034) | 3847 (1479-7851) | 0.50 |
| NGAL (ng/mg.uCR) | 18.2 (1.9-140.6) | 8 (.6-36.3) | 0.33 | 21.8 (1.7-287.01) | .7 (7.4-34.9) | 0.04 |
| NAG (mU/mg.uCR) | 4.8 (2.9-13.2) | 5.1 (1.7-9.6) | 0.26 | 6.6 (2.7-14.9) | 4.08 (2.3-8.3) | 0.02 |
| KIM-1 (pg/mg.uCR) | 1333.7(635.6-2553.8) | 836.5 (399.1-2698.1) | 0.55 | 1549 (562-3284) | 945 (392-1843) | 0.38 |
SPT; Stepped pharmacologic therapy. UF: ultrafiltration.
Injury: decrease in tubular injury score.
Injury: Increase in tubular injury score. AF, atrial fibrillation; AFL, atrial flutter; SBP, systolic blood pressure; JVP; jugular venous pressure; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; ACE-I, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; NT-proBNP, N-terminal pro b-type natriuretic peptide.
N=14 participants did not have paired biomarker samples.
Pre-Randomization WRF and Renal Tubular Injury Biomarkers
In line with the inclusion criteria of the study (≥0.3 mg/dL increase in from nadir to qualifying creatinine), the average increase in creatinine at the time of randomization was a 0.39 ± 0.35 mg/dL (median 0.31 mg/dL) above the patient’s nadir creatinine. We found no evidence of an association between the severity of the nadir to randomization change in creatinine and the renal tubular injury biomarker score (r=0.14, P=0.17) or with the individual biomarker levels (P≥0.31, for all). Correspondingly, we noted no differences in either the tubular injury biomarker scores (51.1 ± 25.8 vs. 50.1 ± 22.3; P=0.84) or levels of the component biomarkers (Figure 1) when comparing those with and without WRF (≥ 20% reduction in eGFR nadir to randomization, 60.6% of the population). We found a similar lack of association between WRF from nadir to qualifying creatinine (incidence ≥ 20% reduction in eGFR, 76.0%) and baseline renal tubular injury biomarker score or the individual components (P>0.48 for all).
Figure 1:

Renal tubular injury biomarkers the day of randomization in patients with and without a nadir to randomization day decline in eGFR of ≥20%. eGFR= Estimated glomerular filtration rate. NGAL=Neutrophil Gelatinase-Associated Lipocalin; NAG=N-acetyl-beta-D-glucosaminidase; KIM-1=Kidney Injury Molecule-1
Post-Randomization Intensive Volume Removal, WRF, and Tubular Injury Biomarkers
In the biomarker sub-study, the mean weight loss over the intervention period was 11.1 lbs ± 9.8 lbs and fluid loss 7.1L ± 4.1L. Despite this protocol driven intensive volume removal in the setting of pre-existing WRF, on average no significant change in creatinine from randomization (2.1 ± 0.6 mg/dL) to 4 days (2.1± 0.9 mg/dL, P=0.32) was observed. Similarly, there was no overall change in renal tubular injury biomarkers during this period (n=91 patients with paired biomarker data, P>0.1 for all biomarkers, Figure 2). However, there was a high degree of variability on an individual patient level, with roughly half of patients experiencing some worsening of tubular injury markers or creatinine, and half improving during intensive volume removal (Figure 3 and Supplementary Figure 2). Importantly, the increases in creatinine were of mild to moderate magnitude with only 15% (N=15) having a >0.5mg/dL increase and only 7% (N=7) having a >1mg/dL increase. Only 6 patients had a 50% increase and 2 patients had a doubling of creatinine from randomization to day 4. Supplementary Figure 3 shows the changes in individual renal tubular injury biomarkers grouped according to increase vs decreased injury. Comparing patients with an increase versus decrease in renal tubular injury biomarkers score, we found few differences in baseline characteristics between groups (Table 1). However, following the 4-day randomized period of intensive volume removal, several parameters related to decongestion tended to be greater in patients with an increase in the renal tubular injury biomarker score (Table 2). Notably, both hemoconcentration (P=0.015) and the absence of severe edema (P=0.03) at day 4 were significantly more likely in patients with an increase in renal tubular injury score. The increase in the renal tubular injury biomarker score correlated with the increase in hemoglobin (r=0.21, P=0.011). We noted a strong association between change in creatinine during this period of intensive volume removal and changes in renal tubular injury biomarkers (Figure 4). The change in renal tubular injury biomarker score correlated positively with the change in creatinine (r=0.25, P=0.02). This relationship was strongest for the change in KIM-1 (r=0.34, P=0.001) and NAG (r=0.33, P=0.001) but not statistically significant for NGAL (P=0.3). Patients with an increase in biomarker score had a substantially increased risk of WRF during the intensive volume removal period (OR=12.6, P=0.004). We noted similar observations within the components of the biomarker score, with a higher risk for WRF during this period with increases in NGAL (OR=5.7, P=0.02), NAG (OR=5.4, P=0.02), but this did not reach significance for KIM-1 (P=0.69). In line with renal tubular injury rather than a “pre-renal” etiology for the increase in creatinine, blood urea nitrogen to creatinine ratio did not increase in patients with worsening renal tubular injury biomarkers (paired sample P=0.27, Table 2) whereas it increased significantly in patients without worsening in tubular injury markers (paired sample P<0.0001, Table 2). We also looked at association of CRP with renal tubular injury biomarkers. No strong associations were noted between day 4 CRP and the day 4 or change in renal tubular injury biomarkers. There was, however, a weak association between KIM-1 and inflammation, which was absent with NGAL and NAG, possibly explaining why slightly different findings were observed with KIM-1 as compared to the other biomarkers (Supplementary Table 1).
Figure 2:
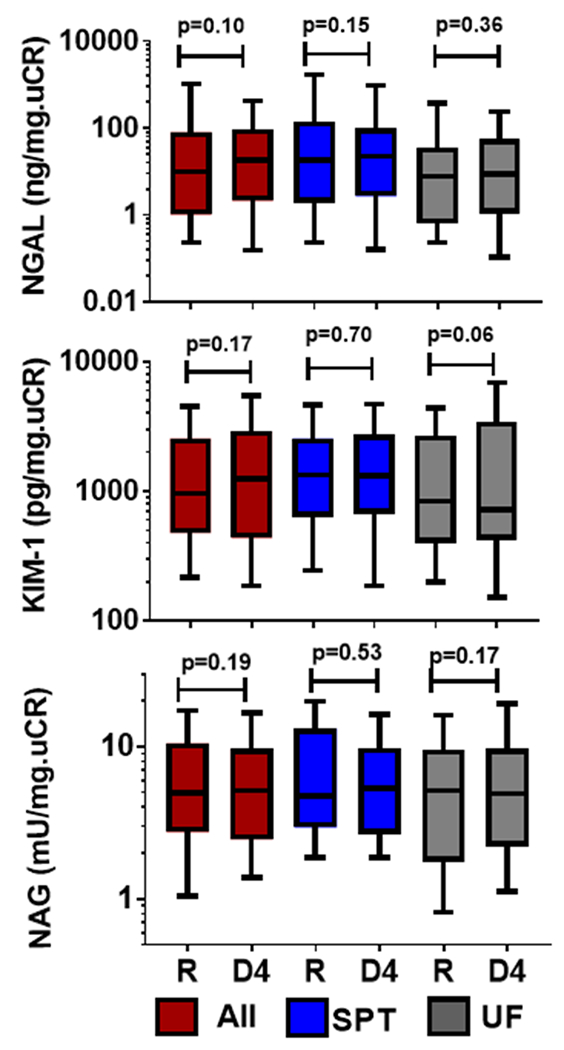
Renal tubular injury markers the day of randomization and day 4. Overall cohort (red bars), STP (blue bars) and UF (grey bars). NGAL=Neutrophil Gelatinase-Associated Lipocalin; NAG=N-acetyl-beta-D-glucosaminidase; KIM-1=Kidney Injury Molecule-1; R= day of randomization; D4=Day 4 after randomization. SPT= Stepped Pharmacologic Therapy; UF= Ultrafiltration.
Figure 3:
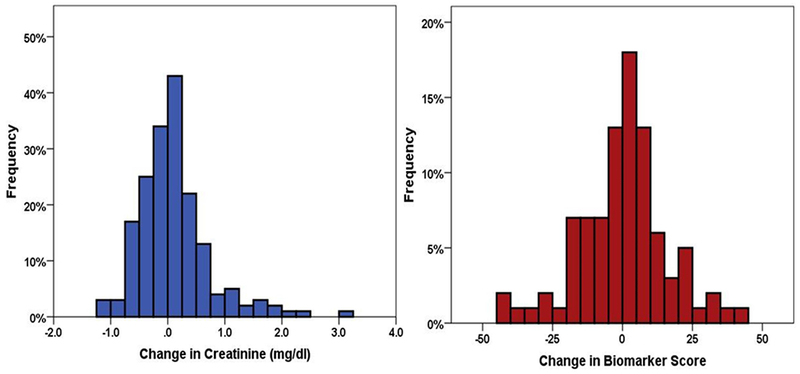
Change in creatinine and renal tubular injury biomarker score from the day of randomization to day 4.
Table 2:
Metrics of decongestion grouped by change in renal tubular injury score from randomization to day 4.
| Decongestion Parameter | ↓ Injury (n=45) | ↑Injury (n=46) | P |
|---|---|---|---|
| Δ Weight (lbs) | −11±11 | −13 ±8 | 0.55 |
| Patient free of Congestion at day 4, (%) | 3 | 12 | 0.10 |
| JVP>8cm water at day 4, % | 41 | 26 | 0.18 |
| Severe Peripheral Edema at day 4, % | 17 | 2 | 0.03* |
| Δ NT-proBNP, pg/mL | −610 (−2592, −5) | −287 (−1358, −154) | 0.07 |
| Δ Sodium, mmol/L | −0.4±4.3 | −1.5±3.6 | 0.23 |
| Δ Creatinine, mg/dL | −0.1±0.5 | 0.2±0.7 | 0.03* |
| Δ Hemoglobin, g/dL | −0.1±0.8 | 0.2±0.7 | 0.08 |
| Hemoconcentration at day 4, % | 40 | 68 | 0.02* |
| Δ BUN, mg/dL | 12±27 | 8±14 | 0.40 |
| Δ BUN/Creatinine | 7.0±8.5 | 1.3±7.4 | <0.01* |
| SPT, % | 56 | 44 | 0.25 |
| UF, % | 44 | 57 | |
| Δ SBP, mmHg | 2±16 | −4±22 | 0.18 |
| Δ Microalbumin, mg/ mg.uCR | −6.4 (−7.4, 31.9) | −.8 (−10.8, 43.8) | 0.08 |
Change in parameters is from randomization to day 4. Static measures (i.e.,JVP, Edema, freedom from congestion) are day 4 values. JVP; jugular venous pressure; BUN, blood urea nitrogen; NT-proBNP, N-terminal pro b-type natriuretic peptide.
Figure 4:
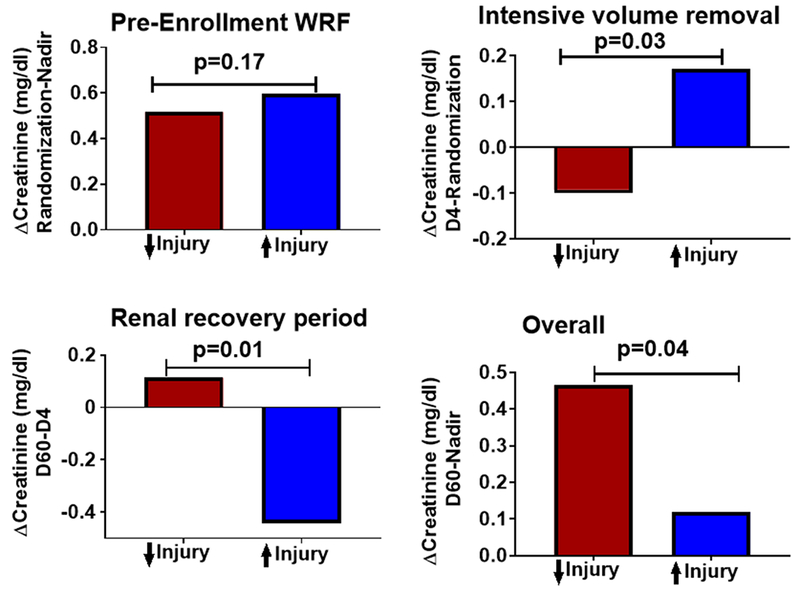
Longitudinal change in creatinine across the CARRESS-HF trial study periods grouped by increase or decrease in tubular injury biomarker score. Change in creatinine from the patients’ pre-enrollment “nadir” creatinine to day of randomization (Pre-Enrollment WRF), from randomization to day 4 (Intensive volume removal), from day 4 to day 60 (Renal recovery period) and from the patients pre-enrollment “nadir” to day 60 (Overall) grouped by increase (↑Injury) or decrease (↓Injury) of renal tubular injury biomarker score from randomization to day 4.
Renal Recovery Period and Overall Impact on Renal Function
Patients with an increase in renal tubular injury biomarker score from randomization to day 4 experienced substantially greater improvement in creatinine from day 4 to 60 days compared to patients without an increase (Figure 4). Worsening in the tubular injury biomarker score from randomization to day 4 correlated with subsequent improvements in creatinine from day 4 to 60 days (r=0.34, P=0.002). Similarly, the odds for a 20% improvement in eGFR from day 4 to day 60 was greater in patients with an increase in renal tubular injury biomarker score from randomization to day 4 (OR=3.6, P=0.007). These associations did not appear to simply reflect that patients with greater worsening in creatinine subsequently experienced greater improvement. The association persisted after adjustment for the change in creatinine during 1) pre-enrollment WRF (partial correlation r=0.28, P=0.015) 2) intensive volume removal (partial correlation r=0.25, P=0.027) and 3) the changes during both periods (partial correlation r=0.31, P=0.007). Furthermore, at 60 days, patients with an increase in tubular injury biomarker score achieved a creatinine closer to their pre-enrollment nadir creatinine (Figure 4). We further examined these results using a mixed effect model, and found similar results (Supplementary Table 2). The increase in hemoglobin from randomization to day 4 also correlated with the improvement in creatinine from day 4 to 60 days (r=0.30, P=0.011). After adjustment for both the change in creatinine and change in hemoglobin from randomization to day 4, the association between an increase in biomarker score and improved creatinine at 60 days was attenuated (partial correlation r=0.19, P=0.15), suggesting superior decongestion may have partially mediated the improved creatinine at 60 days. Of the component biomarkers, NGAL (r=0.32, P =0.004) correlated the strongest with subsequent improvement in creatinine, with NAG (r=0.12, P =0.3) and KIM-1 (r=0.13. P =0.26) not significantly associated with the improvement in creatinine from randomization to day 60. Similarly, the odds of a 20% improvement in eGFR from day 4 to day 60 was strongest with an increase in NGAL (OR=6.3, P <0.001) but not statistically significant for NAG (OR=1.3, P =0.5) or KIM-1 (OR=1.8, P =0.2).
Changes in Injury Biomarkers and Mode of Decongestion
Although limited by statistical power, we noted no significant differences in changes to renal tubular injury biomarkers between patients randomized to SPT versus UF (Figure 2). The odds of worsened tubular injury biomarker score with UF was 1.6 (95% CI 0.7-3.7) but this did not reach statistical significance (P=0.25). Among patients in whom WRF occurred, the incidence of an increase in tubular injury biomarkers was not different between patients randomized to SPT vs. UF (Pinteraction=0.46). We noted linear trends suggesting that UF patients with an increase in biomarker score experienced the greatest worsening of creatinine from randomization to day 4 and the greatest improvement from day 4 to 60 days (Figure 5). However, a comparison of patients with increased injury biomarker score that received STP versus UF was not statistically significantly different for either time period (P≥0.17).
Figure 5:

Change in creatinine stratified by randomized decongestive therapy and change in renal tubular injury biomarker score during both the randomized period of intensive volume removal and during renal recovery out to 60 days. Change in creatinine from randomization to day 4 (Intensive volume removal) and from day 4 to day 60 (Renal recovery period) grouped by increase (↑Inj) or decrease (↓Inj) of renal tubular injury biomarker score and SPT (Stepped Pharmacologic Therapy) or UF (Ultrafiltration) from randomization to day 4.
Discussion
In this analysis of the CARRESS-HF trial, we assessed the impact of intensive volume removal on parameters of renal filtration and injury in patients with pre-existing WRF. Our key findings are as follows. First, when patients with pre-existing WRF underwent protocol driven intensive volume removal, further worsening in creatinine was only noted in approximately half of patients. Importantly, in those patients where creatinine worsened, it was of mild to moderate severity. Second, in contrast to the null association between pre-enrollment worsening in creatinine and renal tubular injury biomarkers, further worsening in creatinine with intensive volume removal was strongly associated with an increase in renal tubular injury biomarkers. Third, patients with an increased tubular injury biomarker score paradoxically experienced the largest recovery of renal function at 60 days, with near restoration of their creatinine to pre-study nadir or true baseline levels. Fourth, this increase in renal tubular injury biomarker score was associated with a greater incidence of hemoconcentration and trends toward other metrics of superior decongestion, offering a potential mediator of these findings. Finally, neither WRF nor worsening renal tubular injury biomarkers was associated with an increased risk of post-discharge death or rehospitalization. This constellation of findings reinforces the notion that adequacy of decongestion in ADHF might offset any short-term insults to the kidney, both in terms of renal function and clinical outcomes, in the months following hospital discharge.
Although the CARRESS-HF trial was designed to compare UF to SPT, one of the most unique aspects of the study was exposure to patients with pre-existing WRF to intensive volume removal. Notably, we have previously demonstrated that the mild to moderate increases in creatinine that occur during standard ADHF therapy are not associated with increases in renal tubular injury biomarkers (NAG, NGAL, KIM-1). We validated these observations in the current analysis showing that individually or as a biomarker score these markers were not associated with the severity of pre-randomization WRF in the setting of usual HF care. However, the reassuring safety signals related to WRF seen in usual care were in clinical settings where physicians likely decreased or stopped decongestive therapies when WRF occurred, as to avoid further deterioration in renal function. For example, in the DOSE trial we observed that an increase in creatinine was associated with improved outcomes, but many patients had early de-escalation of therapy in response to the rise in creatinine.17, 18 As such, the observational literature did not inform what would happen if diuresis is continued or even escalated in congested patients after the occurrence of mild WRF. Importantly, one of the primarily concerns is that further diuresis of a patient with WRF transitions the mechanism from functional/hemodynamic to true renal injury, which would negatively impact long term renal and clinical outcomes.
In the context of the above arguments, our study has two critical findings. First, in ADHF patients with residual congestion and WRF who were exposed to protocol driven intensive volume removal, we did not see universal worsening of creatinine; in fact, many patients had improvements in creatinine and in those where it worsened, it was rarely severe. Second, even if creatinine worsened and tubular injury biomarkers increased, renal function usually recovered by 60 days. These data can provide us with confidence that renal outcomes are likely to be acceptable even if decongestion is continued when mild WRF is noted. However, these data should not be interpreted to imply that we ignore changes in renal function during diuresis as there is almost assuredly a degree of renal impairment that will result in adverse outcomes. However, they do provide further reassurance that judicious continued diuresis in the setting of WRF of mild to moderate severity may be an acceptable option from the renal standpoint. Further research will be needed to understand the threshold change in filtration or tubular injury where the benefit from additional decongestion diminish.
Our data, when considered alongside the significant body of evidence showing treatment induced WRF to be benign, should also discourage its future use as a surrogate endpoint in HF trials. Importantly, WRF has been a ubiquitous outcome measure in recent ADHF trials and potentially effective therapies may have been deemed hazardous or ineffective based on their impact (or lack of impact) on renal function.11, 19–23 Whereas this surrogate endpoint might be convenient as it allows for smaller clinical trials, the lack of a clear relationship between acute changes in renal function and “hard” clinical or even intermediate renal outcomes makes it an unacceptable surrogate outcome. Additionally, current nephrology practice guidelines have lumped all increases in creatinine into a syndrome called “Acute Kidney Injury (AKI)” and stress the importance of changing therapeutic strategies when this occurs.24–26 Our data, along with significant supportive evidence, argues against this approach and suggests that it is the context by which a change in creatinine develops rather than its mere presence that determines adverse outcomes.26–32
A key implication of the above assertion involves the conclusions of CARRESS-HF, which was considered a negative trial based on a safety signal for renal harm with UF.33 Its publication resulted in substantially reduced enthusiasm for and use of UF as a decongestion strategy despite contradictory signals from studies such as AVOID-HF and UNLOAD.34, 35 When the trial results are reconsidered in light of the current findings, the fact emerges that there was no evidence of inferior decongestion or worse post-discharge clinical outcomes in CARRESS-HF, just a concern for an increased rate of renal injury with UF, as defined by creatinine. Our findings suggest that the signals for change in creatinine noted in CARRESS-HF should not be the major factor to dissuade the study or use of UF in other patient populations where a therapeutic advantage may exist.
Limitations:
Several limitations of our study should be considered. First, CARRESS-HF was not specifically designed to determine if escalation of decongestion is safe in patients with WRF as there was no control group receiving standard care. Therefore, we do not know if renal outcomes would have been inferior or superior had further aggressive decongestion not been attempted. Second, given the small size of the study, we were unable to definitively examine the impact of UF versus SPT on cardiorenal parameters. Third, we did not have data on renal function beyond 60 days, nor did we have an understanding if recovery from injury may have set up adverse and potentially self-perpetuating renal pathophysiology such as renal fibrosis. As a result, the long-term impact of renal tubular injury is not addressed by this analysis and additional study will be required. Furthermore, not all patients had data on creatinine levels at 60 days, which required extrapolation from their 30-day data (N=11). Additionally, the severity of WRF in the CARRESS-HF trial was largely in the mild to moderate range, yet patients rarely were fully decongested. This implies the investigators still de-escalated therapy early. As such, the question of continued decongestion in the setting of larger changes in creatinine remains unanswered. Fourth, there is no single agreed upon gold standard biomarker for renal tubular injury in humans, with known limited correlation between individual markers. As such we chose to combine three biomarkers into a renal tubular injury biomarker score to improve signal to noise ratio, as we have reported previously.7 However, this approach has not been validated against histology and it is unclear if this approach outperforms individual biomarker levels in reflecting true tubular injury. Furthermore, while each marker individually accurately predicts renal tubular injury, our analysis presupposes they provide similar information when analyzed collectively. Notably, there were differences observed between individual biomarkers post-hoc. This study does not have the power to determine if these findings reflect true biological differences intrinsic to the biomarker or its simply noise in the data. Lastly, investigators adjusted heart failure therapies during the study, some in response to changes in renal function, inserting an unmeasured confounder into our analysis. Therefore, these data should not imply that all changes in renal function during diuresis or UF should be ignored. Rather, they can provide reassurance that judicious removal of excess volume in the setting of ADHF and mild-moderate increases in creatinine might be an acceptable option from a renal standpoint. Given these limitations, we would not recommend assessing biomarkers to guide clinical decision-making at this juncture. Further research will be needed to understand what degree of change in renal filtration or renal tubular injury offsets the benefit from additional decongestion.
Conclusions
We found that escalation of decongestive therapies in patients with pre-existing WRF did not universally result in a continued decline in renal function, and rarely resulted in a severe decline. However, when creatinine did worsen, it was associated with increases in renal tubular injury biomarkers, regardless of the mode of decongestion. Paradoxically, an increase in renal tubular injury biomarker score in this setting was associated with superior in-hospital decongestion and recovery of renal function at 60 days. These data suggest that the benefits of decongestion may outweigh any modest or transient increases in serum creatinine or tubular injury markers that occur during intensive volume removal.
Supplementary Material
What is new?
In a post-hoc analysis of the CARRESS-HF trial, we demonstrate that in patients hospitalized with heart failure and pre-existing worsening renal function, initiation of aggressive volume removal with either ultrafiltration or steeped pharmacologic care did not cause further deterioration in renal function for in the majority of patients.
In those patients with further decline in renal function during aggressive volume removal, this was associated with increases in renal tubular injury biomarkers. However, these patients actually had better renal recovery at 60 days than patients without increase in renal tubular injury biomarkers.
What are the clinical implications?
Not all patients hospitalized with heart failure and worsening renal function will experience a further rise in creatinine with continued aggressive volume removal.
From a renal perspective, the benefits of decongestion may outweigh short-term harm of tubular damage that occurs in the setting of aggressive diuresis or ultrafiltration.
Acknowledgments
This manuscript was prepared using CARRESS-HF research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the study investigators or the NHLBI.
Funding Sources
This work was supported by NIH Grants, K23HL114868, L30HL115790, R01HL139629, R01HL128973 (JMT), and K23DK097201, R01DK113191 (FPW), and K23HL128933 (MAB). JVB was supported in part by R37 DK39773 and RO1 DK072381. TA was supported by a grant from the HFSA. The funding sources had no role in study design, data collection, analysis or interpretation.
Footnotes
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT00608491
Conflict of Interest Disclosures
JVB appears as co-inventor on KIM-1 patents which have been licensed by Partners Healthcare to several companies. He has received royalty income from Partners Healthcare.
References
- 1.Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB and Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. [DOI] [PubMed] [Google Scholar]
- 2.Butler J, Forman DE, Abraham WT, Gottlieb SS, Loh E, Massie BM, O’Connor CM, Rich MW, Stevenson LW, Wang Y, Young JB and Krumholz HM. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J. 2004;147:331–8. [DOI] [PubMed] [Google Scholar]
- 3.Krumholz HM, Chen YT, Vaccarino V, Wang Y, Radford MJ, Bradford WD and Horwitz RI. Correlates and impact on outcomes of worsening renal function in patients > or =65 years of age with heart failure. Am J Cardiol. 2000;85:1110–3. [DOI] [PubMed] [Google Scholar]
- 4.Konstam MA. Renal function and heart failure treatment: when is a loss really a gain? Circulation Heart failure. 2011;4:677–9. [DOI] [PubMed] [Google Scholar]
- 5.Bikdeli B, Strait KM, Dharmarajan K, Li SX, Mody P, Partovian C, Coca SG, Kim N, Horwitz LI, Testani JM and Krumholz HM. Intravenous fluids in acute decompensated heart failure. JACC Heart Fail. 2015;3:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Testani JM and Ter Maaten JM. Decongestion in Acute Heart Failure: Does the End Justify the Means? JACC Heart Fail. 2016;4:589–590. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad T, Jackson K, Rao VS, Tang WHW, Brisco-Bacik MA, Chen HH, Felker GM, Hernandez AF, O’Connor CM, Sabbisetti VS, Bonventre JV, Wilson FP, Coca SG and Testani JM. Worsening Renal Function in Patients With Acute Heart Failure Undergoing Aggressive Diuresis Is Not Associated With Tubular Injury. Circulation. 2018;137:2016–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Testani JM, McCauley BD, Chen J, Coca SG, Cappola TP and Kimmel SE. Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. J Card Fail. 2011;17:993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Testani JM and Brisco-Bacik MA. Worsening Renal Function and Mortality in Heart Failure: Causality or Confounding? Circulation Heart failure. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O’Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O’Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Davila-Roman VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM and Network NHFCR. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310:2533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E and Heart Failure Clinical Research N. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parikh CR and Mansour SG. Perspective on Clinical Application of Biomarkers in AKI. J Am Soc Nephrol. 2017;28:1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giffen CA, Wagner EL, Adams JT, Hitchcock DM, Welniak LA, Brennan SP and Carroll LE. Providing researchers with online access to NHLBI biospecimen collections: The results of the first six years of the NHLBI BioLINCC program. PLoS One. 2017;12:e0178141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM and Braunwald E. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J and Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Testani JM, McCauley BD, Chen J, Shumski M and Shannon RP. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio-renal interactions. Cardiology. 2010;116:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM and Network NHFCR. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, Tang WH and Testani JM. Relevance of Changes in Serum Creatinine During a Heart Failure Trial of Decongestive Strategies: Insights From the DOSE Trial. J Card Fail. 2016;22:753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parikh CR, Moledina DG, Coca SG, Thiessen-Philbrook HR and Garg AX. Application of new acute kidney injury biomarkers in human randomized controlled trials. Kidney Int. 2016;89:1372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voors AA, Dittrich HC, Massie BM, DeLucca P, Mansoor GA, Metra M, Cotter G, Weatherley BD, Ponikowski P, Teerlink JR, Cleland JG, O’Connor CM and Givertz MM. Effects of the adenosine A1 receptor antagonist rolofylline on renal function in patients with acute heart failure and renal dysfunction: results from PROTECT (Placebo-Controlled Randomized Study of the Selective Adenosine A1 Receptor Antagonist Rolofylline for Patients Hospitalized with Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function). J Am Coll Cardiol. 2011;57:1899–907. [DOI] [PubMed] [Google Scholar]
- 21.Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JG, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC, Investigators P and Committees. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363:1419–28. [DOI] [PubMed] [Google Scholar]
- 22.Sackner-Bernstein JD, Skopicki HA and Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–91. [DOI] [PubMed] [Google Scholar]
- 23.Felker GM, Mentz RJ, Cole RT, Adams KF, Egnaczyk GF, Fiuzat M, Patel CB, Echols M, Khouri MG, Tauras JM, Gupta D, Monds P, Roberts R and O’Connor CM. Efficacy and Safety of Tolvaptan in Patients Hospitalized With Acute Heart Failure. J Am Coll Cardiol. 2017;69:1399–1406. [DOI] [PubMed] [Google Scholar]
- 24.Barasch J, Zager R and Bonventre JV. Acute kidney injury: a problem of definition. Lancet. 2017;389:779–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Guideline C. KDIGO clinical practice guideline for acute kidney injury. 2012. https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf
- 26.Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, Feldman HI, Fernandez H, Gitelman Y, Lin J, Negoianu D, Parikh CR, Reese PP, Urbani R and Fuchs B. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet. 2015;385:1966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verbrugge FH, Dupont M, Shao Z, Shrestha K, Singh D, Finucan M, Mullens W and Tang WH. Novel urinary biomarkers in detecting acute kidney injury, persistent renal impairment, and all-cause mortality following decongestive therapy in acute decompensated heart failure. J Card Fail. 2013;19:621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felker GM, O’Connor CM, Braunwald E and Heart Failure Clinical Research Network I. Loop diuretics in acute decompensated heart failure: necessary? Evil? A necessary evil? Circulation Heart failure. 2009;2:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Testani JM, Kimmel SE, Dries DL and Coca SG. Prognostic importance of early worsening renal function after initiation of Angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circulation Heart failure. 2011;4:685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Testani JM, Brisco MA, Chen J, McCauley BD, Parikh CR and Tang WH. Timing of hemoconcentration during treatment of acute decompensated heart failure and subsequent survival: importance of sustained decongestion. J Am Coll Cardiol. 2013;62:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metra M, Nodari S, Parrinello G, Bordonali T, Bugatti S, Danesi R, Fontanella B, Lombardi C, Milani P, Verzura G, Cotter G, Dittrich H, Massie BM and Dei Cas L. Worsening renal function in patients hospitalised for acute heart failure: clinical implications and prognostic significance. Eur J Heart Fail. 2008;10:188–195. [DOI] [PubMed] [Google Scholar]
- 32.Testani JM, Damman K, Brisco MA, Chen S, Laur O, Kula AJ, Tang WH and Parikh C. A combined-biomarker approach to clinical phenotyping renal dysfunction in heart failure. J Card Fail. 2014;20:912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang WH. Reconsidering ultrafiltration in the acute cardiorenal syndrome. N Engl J Med. 2012;367:2351–2. [DOI] [PubMed] [Google Scholar]
- 34.Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA and Investigators UT. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. [DOI] [PubMed] [Google Scholar]
- 35.Costanzo MR, Negoianu D, Jaski BE, Bart BA, Heywood JT, Anand IS, Smelser JM, Kaneshige AM, Chomsky DB, Adler ED, Haas GJ, Watts JA, Nabut JL, Schollmeyer MP and Fonarow GC. Aquapheresis Versus Intravenous Diuretics and Hospitalizations for Heart Failure. JACC Heart Fail. 2016;4:95–105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This manuscript was prepared using CARRESS-HF research materials obtained directly from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC).13 The data, analytic methods, and study materials are available to other researchers for purposes of reproducing the results via BioLINCC.


