Abstract
Background:
Cisplatin is one of the most effective chemotherapy antineoplastic drugs. Panax ginseng is a well-known medicinal herb and has a long history of medicinal use as a tonic to promote health.
Aim:
This work aimed to study the effect of ginseng on the liver damage induced by cisplatin in rats. It included biochemical and histological investigations.
Materials and Methods:
Twenty adult rats were divided into four equal groups. Group I served as control. Group II received ginseng orally (100 mg/kg/day) for 4 weeks. Group III animals were injected intraperitoneally with cisplatin in three equal doses (each 3.3 mg/kg) daily for 3 consecutive days. Group IV animals received ginseng together with cisplatin by the same previously mentioned methods and doses. Rats were sacrificed after 4 weeks, and blood samples and liver tissues were collected for biochemical and histological examinations.
Results:
Cisplatin-induced liver damage manifested biochemically by an increase in serum alanine aminotransferase and aspartate aminotransferase. Histologically, hepatocytes appeared with vacuolated cytoplasm and small dark-stained nuclei with dilatation of blood sinusoids as well as marked accumulation of collagen fibers around enlarged portal tracts. Administration of ginseng together with cisplatin improved the hepatic dysfunctions and damage caused by cisplatin.
Conclusion:
Ginseng has a protective role in the amelioration of cisplatin-induced hepatotoxicity.
Keywords: Chemotherapy, ginseng, hepatic toxicity, oxidative stress
INTRODUCTION
Chemotherapy includes chemical agents that are used for stopping the growth of tumor cells as well as for eradicating it. It does not differentiate between a tumor and rapidly growing normal cells in the body.[1] Cisplatin is one of the most effective chemotherapeutic antineoplastic drugs, which has been used extensively for the treatment of a variety of tumors such as breast, ovarian, and lung cancers.[2] However, its clinical utility is associated with serious side effects in many organs, including liver and kidneys. Recent studies suggested that hepatotoxicity is a major dose-limiting side effect of cisplatin-based chemotherapy, leading to restriction in its use.[3,4]
Cisplatin-induced toxicity is most likely mediated by diverse oxidative damage to the cells mainly through generation of free reactive oxygen species (ROS) exceeding the antioxidant capability of the cell, resulting in imbalance of the pro-oxidant and antioxidant in the cells. This process is called oxidative stress, which eventually induces cell death.[5]
The liver is one of the major organs in the body responsible for numerous functions; one of the most important is drugs conversion into products that are more easily excreted. The central role of the liver in drug metabolism predisposes it to toxic injury.[6] Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activates are used as the indicators of hepatotoxicity that are only found in the serum in significant amount when there is damage to the liver.[7]
Recently, attention has been drawn to natural products for its safety. Panax ginseng (Araliaceae) is a well-known medicinal herb in traditional Asian medicine and has a long history of medicinal use as a tonic to promote health. Ginseng and its constituents have been thought to possess antineoplastic, antioxidant, anti-inflammatory, and anti-aging activities.[8,9]
Cancer is a growing health problem around the world. The World Health Organization reported that cancer is the second leading cause of death in 2018, accounting for an estimated 9.6 million deaths worldwide.[10] More than half of cancer patients use chemotherapeutic drugs.[11] The use of chemotherapy to prevent cancer cells growth or cause them to die can damage healthy cells in the body and produce adverse effects in many organs including the liver.[1]
There is considerable information suggesting that a combination of chemotherapy and phytochemicals with antioxidant action can increase the efficiency of chemotherapeutic agents and attenuate the hepatotoxicity.[12] ROS are the leading factors in toxicity of cisplatin.[5] Mechanisms that can control oxidative stress may decrease its toxicity by means of antioxidant.
Aim of the work
Based on these facts, we investigated in this study the possible protective effect of Panax ginseng on the liver damage induced by cisplatin in rats. Biochemical and histological study was done.
MATERIALS AND METHODS
Chemicals
Cisplatin was obtained from Merck (Rodalben, Germany); it was provided as 50 mg/50 ml saline. Ginseng was purchased from Mulini Pharmaceutical, Saudi Arabia, and was present in the form of syrup containing the dried roots of the Panax ginseng; each 1 ml contains 10 mg.
Animals
Twenty adult male albino rats, with body weights of 150–200 g at the beginning of the experiment, were used in the current study. The animals were fed on standard laboratory food and water. They were kept under standard cases of humidity and temperature. Ethical animal treatment protocols were followed.
Experimental design
The animals were randomly classified into four equal groups (five rats per group), as follows:
Group I (control group): Administered distilled water
Group II (ginseng group): Animals received ginseng (100 mg/kg/day) once/day orally by intragastric tube for 4 weeks[13]
Group III (cisplatin-treated group): Animals were injected intraperitoneally with three equal doses of cisplatin (each containing 3.3 mg/kg) daily for 3 consecutive days as previously reported[14]
Group IV (cisplatin/ginseng-treated group): The animals were concomitantly treated by both cisplatin and ginseng in which cisplatin treatment was begun 2 h before ginseng treatment at the same previous routes, doses, and duration.
The experiment lasted for 4 weeks, and then, all rats were weighed and sacrificed by ether anesthesia. Blood samples and small pieces of the right lobe of the liver tissues were taken.
Biochemical study
Venous blood samples from all animals were collected in heparinized tubes and centrifuged at 3000 rpm for 15 min to separate plasma used for the determination of serum activity of AST and ALT. These enzymes used as markers of liver functions and were assayed as previously reported using commercially available diagnostic kits.[15]
Histological study
Small pieces of the right lobe of the liver were fixed in 10% buffered formalin, dehydrated in a gradual series of ethyl alcohol, and then embedded in paraffin wax after clearance by xylol. Five-micron sections were obtained and stained with hematoxylin and eosin (H and E) to study histological features and Masson's trichrome for the detection of collagen fibers.[16] Finally, the sections were examined and photographed using an Olympus light microscope Japan at histological units.
Statistics
Biochemical and histological statistical analysis was performed using SPSS program, version 17 (IBM Corporation, Somers, New York, USA) in the form of mean ± standard deviation. A one-way analysis of variance was used for calculation of differences between groups’ means. The results were considered statistically significant when P < 0.05 against the control group.
RESULTS
Body weights of the rats
There was no significant difference in the body weights in rats treated with ginseng alone (Group II) comparing with the control one. After administration of cisplatin (Group III), significant loss of body weights was observed compared to control (P < 0.001).
Administration of ginseng along with cisplatin (Group IV) significantly ameliorated this change and showed a trend of recovery to control level [Table 1 and Histogram 1].
Table 1.
Body weights of control and experimental rats
| Groups | Body weight (g) |
|---|---|
| Group I | 204±2.6a |
| Group II | 198±3.1a |
| Group III | 145±3.3b |
| Group IV | 187±3.9a |
Values are mean±SD (n=5). Means in the same column with different letters differ significantly (P<0.001). SD: Standard deviation
Histogram 1.
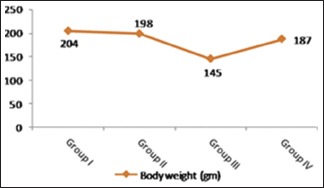
Level of body weights in control and experimental groups. A significant decrease in body weight was observed in Group III in comparison with the other groups. Note the significant return of body weight to control level in Group IV
Biochemical results
As shown in Table 2 and Histograms 2, 3, cisplatin injection (Group III) resulted in a significant increase in serum levels of AST and ALT compared with the control group (P < 0.001). However, ginseng administration with cisplatin (Group IV) was able to significantly decrease these parameters when compared with Group III. Nonsignificant difference (P > 0.05) was observed in serum levels of AST and ALT of the control and ginseng groups (Groups I and II, respectively).
Table 2.
Serum aspartate aminotransferase and alanine aminotransferase of control and experimental rats
| Groups | ALT (IU/L) | AST (IU/L) |
|---|---|---|
| Group I | 22.32±1.2a | 27.91±4.1a |
| Group II | 21.26±2.6a | 26.87±2.7a |
| Group III | 47.76±1.9b | 48.76±1.2a |
| Group IV | 29.75±4.3a | 28.74±5.9b |
Values are mean±SD (n=5). Means in the same column with different letters differ significantly (P<0.001). AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, SD: Standard deviation
Histogram 2.
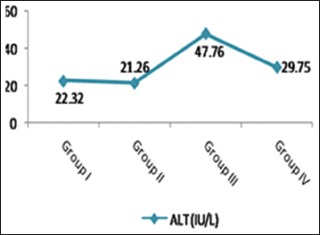
A significant increase of ALT level in Group III when compared with the control group. Note the significant return of ALT to control level in Group IV. ALT: Alanine aminotransferase
Histogram 3.
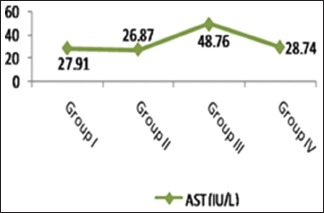
A significant increase of the AST level in Group III when compared with the control group. Note the significant return of AST to control level in Group IV. AST: Aspartate aminotransferase
Histological results
Control and ginseng groups (Groups I and II)
Hematoxylin and eosin stain
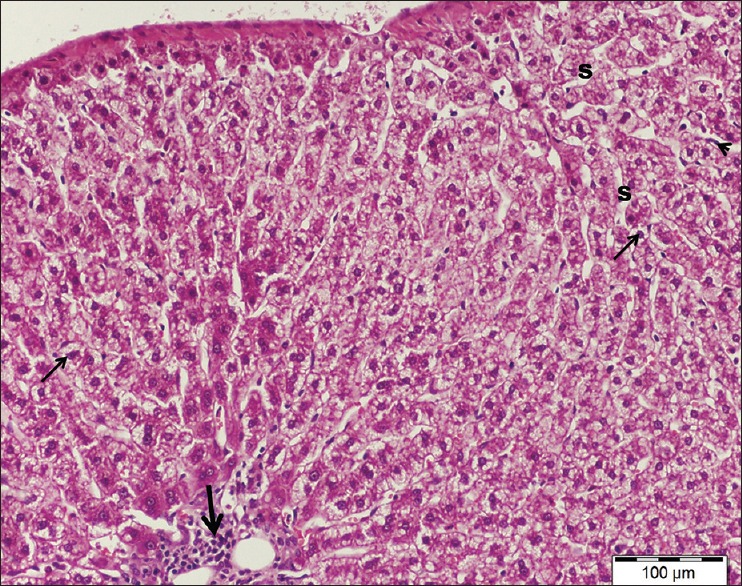
Examination of liver sections stained by H and E of control and ginseng treated rats showed the normal structure of the liver that consists of ill-defined hepatic lobules had cords of hepatocytes radiating from the central veins to the periphery. The polyhedral hepatocytes contained prominent round vesicular nuclei and finely granular acidophilic cytoplasm. Some hepatocytes appeared binucleated. At the periphery of the lobules, there were portal tracts contained branches of the portal vein, hepatic artery, and bile duct. Hepatic sinusoids appeared in between hepatic cords as narrow spaces lined with Kupffer cells [Figure 1].
Figure 1.
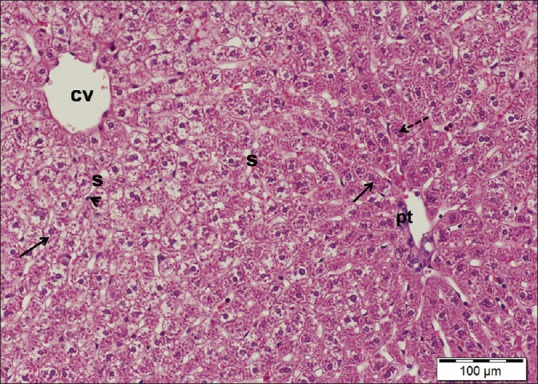
A liver section of control rat showing hepatocytes (arrows) with a granular cytoplasm and vesicular nuclei and the central vein (cv). Note a portal tract (pt) and blood sinusoids (s) lining with Kupffer cells (arrowhead). Dotted arrow points to binucleated hepatocyte. H and E stain, scale bar 100 μm
Masson's trichrome
Examination of liver sections stained by Masson's trichrome of these groups revealed fine few collagen fibers (stained blue) around the portal tracts as well as around the central veins [Figure 2].
Figure 2.
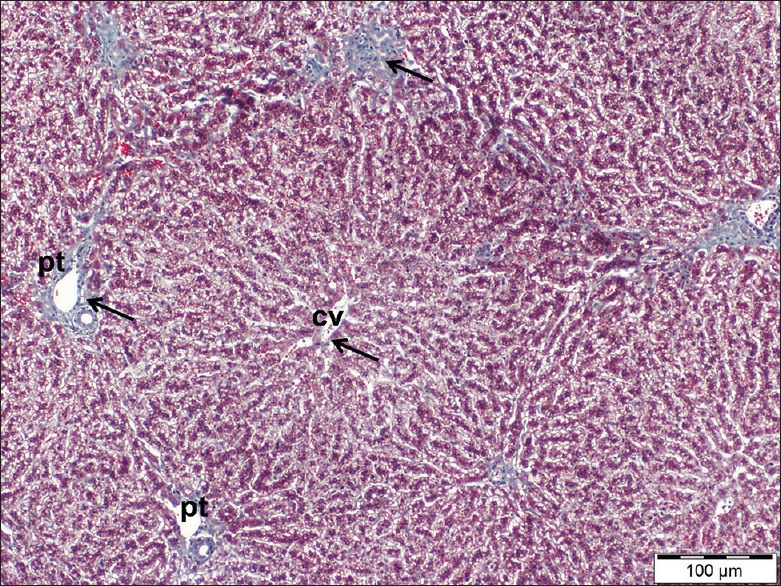
A liver section of control rat showing few fine collagen fibers (arrows) around a central vein (cv) as well as portal tracts (pt). Masson's trichrome, scale bar 100 μm
Cisplatin-treated group (Group III)
Hematoxylin and eosin stain
Marked histological changes were observed after administration of cisplatin. The hepatic architecture was disturbed characterized by diffuse affection of the hepatic lobules. Most hepatocytes appeared swollen with small darkly stained nuclei and vacuolated cytoplasm. Other cells were fused forming deeply acidophilic areas associated with dissolutions of hepatic cords. Central veins as well as blood sinusoids were markedly dilated. Enlargement of portal tracts with dilatation of branches of portal veins and proliferation of bile ductules was observed. Periportal mononuclear inflammatory cell infiltrations were also noted [Figures 3–5].
Figure 3.
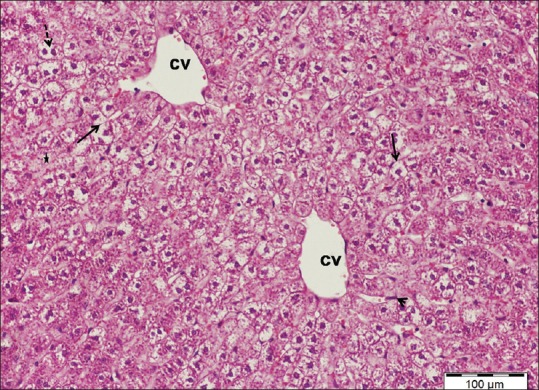
A liver section of cisplatin-treated group showing swollen hepatocytes with vacuolated cytoplasm and small, dense nuclei (arrows), dilated central veins (cv), and areas of hepatocytes dissolutions (star). Note hypertrophic Kupffer cells (arrowhead) and vacuolated binucleated hepatocytes (dotted arrow). H and E stain, scale bar 100 μm
Figure 5.
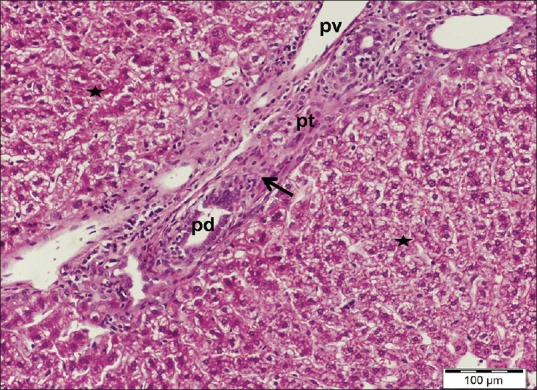
A liver section of cisplatin-treated group showing enlargement of a portal tract (pt) with dilated portal vein (pv) and proliferating bile ductules (pd) as well as periportal mononuclear cellular infiltrations (arrow). Note the loss of cellular cords arrangement with area of hepatocytes dissolutions (stars). H and E stain, scale bar 100 μm
Figure 4.
A liver section of cisplatin-treated group showing disturbance of the normal architecture of liver with small deeply stained nuclei of most hepatocytes (thin arrows), dilated blood sinusoids(s) lining with hypertrophic Kupffer cells (arrowhead). Note mononuclear cell infiltrations (thick arrow). H and E stain, scale bar 100 μm
Masson's trichrome
Examination of liver sections stained by Masson's trichrome of cisplatin-treated rats showed marked accumulations of collagen fibers around the portal tracts [Figure 6].
Figure 6.
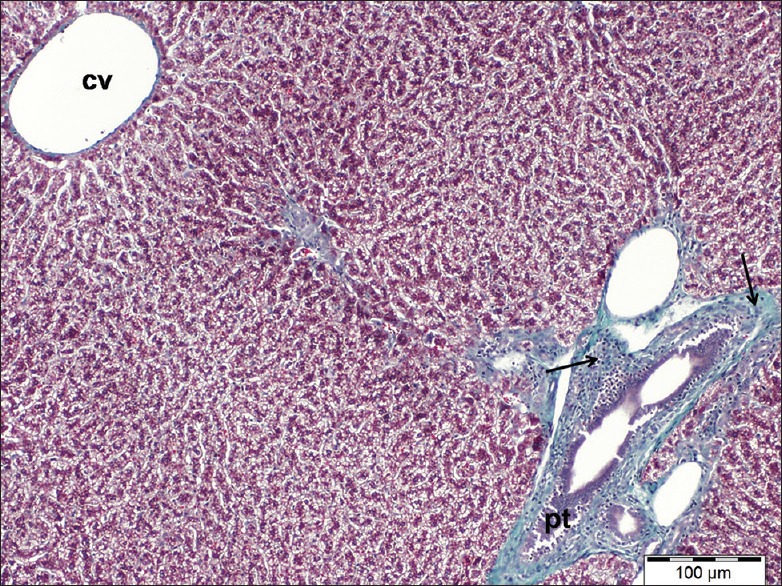
A liver section of cisplatin-treated group showing marked collagen fibers accumulation (arrows) around enlarged a portal tract (pt). Note dilated central vein (cv). Masson's trichrome, scale bar 100 μm
Cisplatin/ginseng-treated group (Group IV)
Hematoxylin and eosin stain
Examination of liver sections stained by H and E of cisplatin/ginseng-treated group showed partial improvement in histological structure of the liver tissue. Most hepatocytes appeared more or less normal and had round vesicular nuclei and pale acidophilic cytoplasm [Figure 7]. However, some cells still affected with deeply stained nuclei and vacuolated cytoplasm. Dilated central veins and blood sinusoids were observed [Figure 8].
Figure 7.
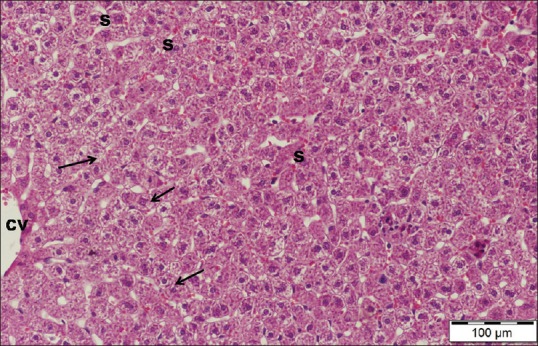
A liver section of cisplatin/ginseng-treated group showing more or less normal appearance of hepatic tissue and most hepatocytes have granular cytoplasm and vesicular nuclei (arrows). Note dilated congested blood sinusoids (s) and part of a central vein (cv). H and E stain, scale bar 100 μm
Figure 8.
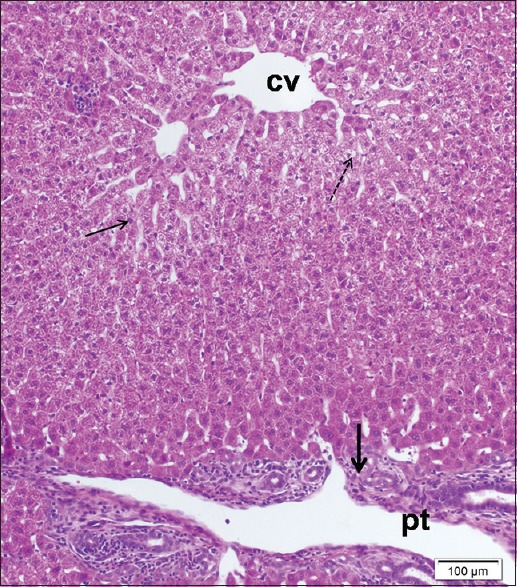
A liver section of cisplatin/ginseng-treated group showing some hepatocytes still affected, have small dense nuclei (thin arrow) and others with vacuolated cytoplasm (dotted arrow). Note enlargement of a portal tract (pt) with cellular infiltrations (thick arrow). H and E stain, scale bar 100 μm
Masson's trichrome
Collagen fibers around the central veins as well as the portal tracts appeared more or less similar to control one as only a few fibers appeared around them [Figure 9].
Figure 9.
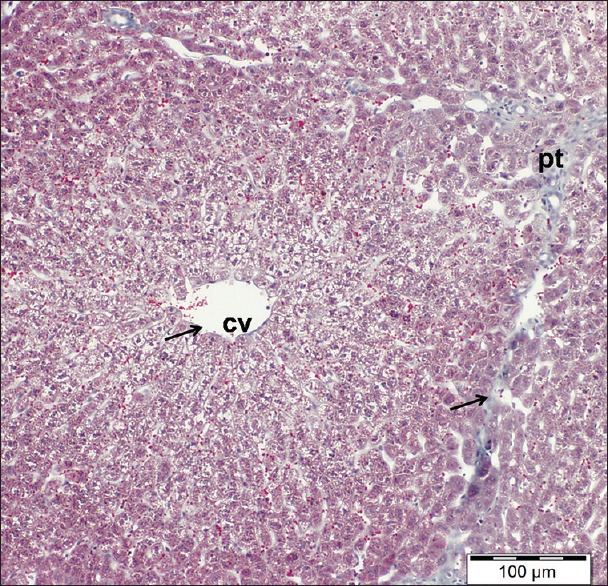
A liver section of cisplatin/ginseng-treated group showing few normal distributions of collagen fibers (arrows). cv: central vein, pt: portal tract. Masson's trichrome, scale bar 100 μm
DISCUSSION
Detoxification and metabolism of chemicals take place in the liver, making it the target organ for the toxicity.[17] Cancer is one of the most common causes of death in the world and affects >6 million people every year. Treatment of cancer with chemotherapy is not as safe as it has many side effects on healthy organs.[18] Cisplatin, one of the chemotherapeutic agents, is used for the treatment of a wide spectrum of tumors.[2]
In the current study, all results of ginseng group (Group II) were similar to the control one indicating that intake of ginseng is safe and nontoxic. These observations were in agreement with results of previous researchers.[13] In addition, another study in united states reported the results of the National Toxicology Program chronic toxicity and tumorigenicity bioassay and showed that Panax ginseng is not toxic or a tumor agent.[19]
Treatment of rats with cisplatin (Group III) produced a significant loss of body weight in consistence with results of previous work which attributed that to a loss of adipose tissue as well as skeletal muscles.[20] Loss of body weight is also accompanied by decreased food intake noted during the present experiment.
In this study, cisplatin-induced hepatotoxicity in animals of Group III manifested by a significant increase of serum levels of AST and ALT as compared to the control group. These data were in agreement with other investigators.[21,22] Hepatic enzymes AST and ALT are used as markers of liver functions, and the observed increase of these enzymes in the blood indicated impairment of liver function and hepatic lesion.[23] In accordance with our results, previous study reported marked elevation of hepatic enzymes AST and ALT in rats after single injection of 2.5 mg/kg cisplatin and attributed that to damage to hepatocytes with subsequent leakage of enzymes into the circulation.[24] In addition, liver toxicity caused by cisplatin has been investigated clinically by increasing levels of hepatic enzymes in the blood and jaundice appearance.[25]
Biochemical changes came hand in hand with histological results which showed marked disturbance of hepatic tissue in the cisplatin-treated group. There were vacuolated hepatocytes with pyknotic nuclei and dissolution of hepatic cords as well as dilated congested blood vessels and collagen fibers accumulation around the blood vessels. These observations were in agreement with previous reports,[26] which states that treatment with chemotherapy as cisplatin and doxorubicin causes toxicity to the liver manifested by hepatic necrosis and periportal fibrosis. Furthermore, another study reported that cisplatin chemotherapy has induced severe disturbance in functions of liver and kidney with tissue necrosis and fibrosis in rats.[24]
The liver is the main site of cisplatin metabolism. Cisplatin-induced hepatotoxicity is attributed to drug metabolism and ROS generation with decreasing levels of antioxidant enzymes, which propagate direct cytotoxicity of hepatocytes resulting in oxidative stress, tissue damage, and ultimately cell death. Moreover, the ability of cisplatin to form adducts with DNA as a part of its activity against the tumor, causing inhibition of cell divisions and its death.[22,24,26]
The observed cytoplasmic and nuclear changes in hepatocytes of cisplatin-treated animals could be the result of induction of oxidative stress and damage of DNA that causes degeneration and death of hepatocytes. Furthermore, the picture of portal fibrogenesis that was observed in the cisplatin-treated group in the form of marked accumulation of collagen fibers and proliferating bile ducts are the resultant of ROS generation.[27]
In the present study, the observed cellular infiltration of portal tract in animals treated with cisplatin was in accordance with other works.[28] These infiltrating cells, leukocytes, are a prominent response of body tissues facing any injurious impacts. Moreover, the observed dilatation of blood sinusoids might be reflected impairment of substance exchange and liver dysfunction as the integrity of sinusoidal structure is very important for the continuous normal exchange of metabolites and fluids.[20]
Comparing with cisplatin-treated animals (Group III), administration of ginseng along with cisplatin (Group IV) showed a significance increase of body weight as well as a significant lowering in serum levels of AST and ALT. These findings were in consistence with the observations of other investigators who reported that ginseng had a protective effect to liver by decreasing the levels of serum transaminases following aflatoxin B1 toxicity in rats.[29] The reduction of liver enzymes (AST and ALT) to normal values indicated that ginseng effectively improved hepatic function impairments induced by cisplatin.
These results have been supported by light microscopic findings that revealed the effectiveness of Panax ginseng in protecting the liver from the damaging effect of cisplatin as most of the histological changes induced by cisplatin were greatly improved. The hepatic tissue has restored its normal structure and the liver cells appeared more or less normal with normal distribution of collagen fibers. Results of other investigators reported that ginseng was effective against hepatic toxicity caused by oxidative stress after cadmium intoxication.[30]
The balance between oxidative and antioxidative systems prevented tissue damage and cells death and any disturbance in this balance led to many pathological conditions. Previous studies reported that antioxidants prevent the adriamycin chemotherapy-induced toxicity in experimental animals as well as in human.[20]
The hepatoprotective effects of Panax ginseng are closely related to its antioxidant properties as it enhanced the antioxidant defense mechanism and has a potential role in scavenging free radicals and consequently alleviating the oxidative stress.[31]
CONCLUSION
The results of this study postulate that cisplatin induces hepatotoxicity and ginseng has a protective role in amelioration of this toxicity. Panax ginseng possesses the ability to decrease the higher serum levels of AST and ALT and partially restore the histological changes of the liver induced by cisplatin in the rats.
Recommendations
Since it has been proven that Panax ginseng has a hepatoprotective effect on liver damage induced by cisplatin, it is advised to give ginseng to those exposed to chemotherapeutic agents
Further studies must be carried out to evaluate the proper dose and the best time for administration (before, with the chemotherapy or both) to obtain the most beneficial results for treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sundaram MK, Sangavai R. Tissue processing effects of doxorubicin and 5-fluorouracil (5-FU) on the hepatocyte region of albino rats. Adv Biotech. 2009:23–5. [Google Scholar]
- 2.Lin CC, Hsu CH, Huang CY, Cheng AL, Chen J, Vogelzang NJ, et al. Weekly cisplatin plus infusional high-dose 5-fluorouracil and leucovorin (P-HDFL) for metastatic urothelial carcinoma: An effective regimen with low toxicity. Cancer. 2006;106:1269–75. doi: 10.1002/cncr.21738. [DOI] [PubMed] [Google Scholar]
- 3.Ajani JA. Optimizing docetaxel chemotherapy in patients with cancer of the gastric and gastroesophageal junction: Evolution of the docetaxel, cisplatin, and 5-fluorouracil regimen. Cancer. 2008;113:945–55. doi: 10.1002/cncr.23661. [DOI] [PubMed] [Google Scholar]
- 4.Yuan JN, Chao Y, Lee WP, Li CP, Lee RC, Chang FY, et al. Chemotherapy with etoposide, doxorubicin, cisplatin, 5-fluorouracil, and leucovorin for patients with advanced hepatocellular carcinoma. Med Oncol. 2008;25:201–6. doi: 10.1007/s12032-007-9013-3. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Cederbaum AI. Cisplatin-induced hepatotoxicity is enhanced by elevated expression of cytochrome P450 2E1. Toxicol Sci. 2006;89:515–23. doi: 10.1093/toxsci/kfj031. [DOI] [PubMed] [Google Scholar]
- 6.Manov I, Motanis H, Frumin I, Iancu TC. Hepatotoxicity of anti-inflammatory and analgesic drugs: Ultrastructural aspects. Acta Pharmacol Sin. 2006;27:259–72. doi: 10.1111/j.1745-7254.2006.00278.x. [DOI] [PubMed] [Google Scholar]
- 7.Agbai EO, Nwanegwo CO. Hepatoprotective effect of hibiscus sabdariffa in alloxanized mice. JMR. 2013;5:169–75. [Google Scholar]
- 8.Kiefer D, Pantuso T. Panax ginseng. Am Fam Physician. 2003;68:1539–42. [PubMed] [Google Scholar]
- 9.Lee TK, Johnke RM, Allison RR, O’Brien KF, Dobbs LJ., Jr Radioprotective potential of ginseng. Mutagenesis. 2005;20:237–43. doi: 10.1093/mutage/gei041. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Cancer. 2018. [Last accessed on 2018 Feb 01]. Available from: http://www.who.int/news-room/fact-sheets/detail/cancer .
- 11.Alwan A, Maclean DR, Riley LM, d’Espaignet ET, Mathers CD, Stevens GA, et al. Monitoring and surveillance of chronic non-communicable diseases: Progress and capacity in high-burden countries. Lancet. 2010;376:1861–8. doi: 10.1016/S0140-6736(10)61853-3. [DOI] [PubMed] [Google Scholar]
- 12.Russo M, Spagnuolo C, Tedesco I, Russo GL. Phytochemicals in cancer prevention and therapy: Truth or dare? Toxins (Basel) 2010;2:517–51. doi: 10.3390/toxins2040517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gadkariem EA, Al-Ashban RM, Babikir LB, Al-Joher HI. Toxicity study of Korean ginseng herbal medicine. Res J Pharmacol. 2010;4:86–90. [Google Scholar]
- 14.Pace A, Savarese A, Picardo M, Maresca V, Pacetti U, Del Monte G, et al. Neuroprotective effect of Vitamin E supplementation in patients treated with cisplatin chemotherapy. J Clin Oncol. 2003;21:927–31. doi: 10.1200/JCO.2003.05.139. [DOI] [PubMed] [Google Scholar]
- 15.Juma A, Eliud NM, Joseph JN. Estimation of reference values for liver function tests for adult population in north-rift Valley, Kenya. AJMS. 2011;3:243–9. [Google Scholar]
- 16.Bancroft JD, Layton C. The hematoxylin and eosin. In: Suvarna SK, Layton C, Bancroft JD, editors. Bancroft's Theory & Practice of Histological Techniques. 7th ed. Ch. 10. Philadelphia: Churchill Livingstone of Elsevier; 2013. pp. 172–86. [Google Scholar]
- 17.Watkins PB, Seeff LB. Drug-induced liver injury: Summary of a single topic clinical research conference. Hepatology. 2006;43:618–31. doi: 10.1002/hep.21095. [DOI] [PubMed] [Google Scholar]
- 18.Shivakumar P, Rani MU, Reddy AG, Anjaneyulu Y. A study on the toxic effects of doxorubicin on the histology of certain organs. Toxicol Int. 2012;19:241–4. doi: 10.4103/0971-6580.103656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan PC, Peckham JC, Malarkey DE, Kissling GE, Travlos GS, Fu PP. Two-year toxicity and carcinogenicity studies of Panax moringe in Fischer 344 Rats and B6C3F1 Mice. Am J Chin Med. 2011;39:779–86. doi: 10.1142/S0192415X11009184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed MA. The protective effect of ginger (Zingiber officinale) against adriamycin-induced hepatotoxicity in rats: Histological study. Life Sci J. 2013;10:1412–22. [Google Scholar]
- 21.Mansour HH, Hafez HF, Fahmy NM. Silymarin modulates cisplatin-induced oxidative stress and hepatotoxicity in rats. J Biochem Mol Biol. 2006;39:656–61. doi: 10.5483/bmbrep.2006.39.6.656. [DOI] [PubMed] [Google Scholar]
- 22.Kadikoylu G, Bolaman Z, Demir S, Balkaya M, Akalin N, Enli Y, et al. The effects of desferrioxamine on cisplatin-induced lipid peroxidation and the activities of antioxidant enzymes in rat kidneys. Hum Exp Toxicol. 2004;23:29–34. doi: 10.1191/0960327104ht413oa. [DOI] [PubMed] [Google Scholar]
- 23.Mahesh A, Shaheetha J, Thangadurai D, Rao DM. Protective effect of Indian honey on acetaminophen induced oxidative stress and liver toxicity in rat. Biologia. 2009;64:1225–31. [Google Scholar]
- 24.Işeri S, Ercan F, Gedik N, Yüksel M, Alican I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology. 2007;230:256–64. doi: 10.1016/j.tox.2006.11.073. [DOI] [PubMed] [Google Scholar]
- 25.Moriya A, Hyodo I, Nishina T, Imaoka H, Imagawa A, Doi T, et al. Extensive liver metastasis of gastric cancer effectively treated by hepatic arterial infusion of 5-fluorouracil/cisplatin. Gastric Cancer. 2000;3:110–5. doi: 10.1007/pl00011695. [DOI] [PubMed] [Google Scholar]
- 26.El-Sayyad HI, Ismail MF, Shalaby FM, Abou-El-Magd RF, Gaur RL, Fernando A, et al. Histopathological effects of cisplatin, doxorubicin and 5-fluorouracil (5-FU) on the liver of male albino rats. Int J Biol Sci. 2009;5:466–73. doi: 10.7150/ijbs.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He YJ, Shu JC, Lü X, Fang L, Sheng Y. Prophylactic effect of curcumin on hepatic fibrosis and its relationship with activated hepatic stellate cells. Zhonghua Gan Zang Bing Za Zhi. 2006;14:337–40. [PubMed] [Google Scholar]
- 28.El-Naggar AE, Hussein SH. Protective and therapeutic effects of fucoidan, brown algae extract against diclofenac sodium hepatonephrotoxicity in rat. Egypt J Comp Pathol Clin Pathol. 2010;23:154–73. [Google Scholar]
- 29.Kim YS, Kim YH, Noh JR, Cho ES, Park JH, Son HY, et al. Protective effect of Korean red ginseng against aflatoxin B1-induced hepatotoxicity in rat. J Ginseng Res. 2011;35:243–9. doi: 10.5142/jgr.2011.35.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shukla R, Kumar M. Role of Panax ginseng as an antioxidant after cadmium-induced hepatic injuries. Food Chem Toxicol. 2009;47:769–73. doi: 10.1016/j.fct.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Huu Tung N, Uto T, Morinaga O, Kim YH, Shoyama Y. Pharmacological effects of ginseng on liver functions and diseases: A minireview. Evid Based Complement Alternat Med. 2012;2012:173297. doi: 10.1155/2012/173297. [DOI] [PMC free article] [PubMed] [Google Scholar]


