Abstract
Background:
The immune system is the body's defense against foreign organisms and harmful chemicals. Cyclosporine A (CsA) is an immunosuppressant drug widely used. Echinacea purpurea root (EPR) extract is used as an immunostimulant plant.
Aim of the Work:
The present study aimed at evaluation of the EPR effects against the CsA immunosuppressive rat model.
Material and Methods:
Thirty-two male Wistar albino rats were randomly divided into control, CsA (immunosuppressive models), CsA + EPR (100 mg/kg/day orally), and CsA + EPR (200 mg/kg/day orally). The biological parameters regarding the food consumption were assessed including feed intake (FI), feed efficiency ratio (FER), and body weights (BW). In addition, the splenic specimens were assessed histopathology. The blood was collected for measuring the blood parameters. All the measured parameters were collected and statistically analyzed. The biological results indicated a significant decrease in BW, FI, and FER in rats treated orally with low and high EPR doses as compared to the control group.
Results:
The results displayed that the CsA induced a significant decrease in red blood cells (RBCs) and white blood cells (WBCs) count. Histopathologically, CsA induced a marked decrease in the cellularity of the white pulp with congested blood sinusoids of the red pulp together with significant depletion of periarteriolar lymphoid sheath. Both the high and low doses of EPR significantly reversed the altered RBCs and WBCs counts. Histopathologically, both the low and high doses of EPR displayed apparently increase in the periarteriolar area together with the persistence of the congestion of the red pulp blood sinusoids compared to CsA group, indicating partial amelioration of the structural changes.
Conclusion:
In a nutshell, the current findings revealed that EPR extract ameliorated the hematological changes. However, there was a partial correction of the CsA-induced microscopic changes of the rat spleen.
Keywords: Cyclosporine, Echinacea purpurea roots, immunosuppression, rat, spleen
INTRODUCTION
Echinacea purpurea L. is a medicinal plant that belongs to the family Asteraceae, commonly known as the purple coneflower.[1] The most commonly used preparation is a liquid extract made from the root of E. purpurea.[2] It contains various biologically active compounds including polysaccharides, polyacetylenes, flavonoids, alkamides, caffeic acid derivatives, and cichoric acid, which is the major active compound.[3]
E. purpurea has been used in the prevention and treatment of different pathological conditions.[4] It has wound-healing properties[5] and anti-inflammatory[6] and antioxidant activities.[7] Echinacea potentiates the immune system through activation of the macrophages, polymorphonuclear leukocytes, and natural killer cells. It was used as prevention or treatment of some diseases including influenza and common cold.[8]
The effect of E. purpurea against gamma radiation-induced oxidative stress and immune responses in male rats was investigated. The results showed that total white cell, lymphocytes, neutrophils, and monocytes were significantly lower in irradiated rats, compared with their corresponding levels in the control group. E. purpurea supplementation has significantly ameliorated these decreases. Thus, E. purpurea is shown to have a radioprotective effect against gamma irradiation by preventing oxidative stress in the spleen tissues and modulation of immune responses.[9]
Cyclosporine (CsA) is an immunosuppressant drug widely used in organ transplantation to prevent rejection in people with organ transplants. In spite of its therapeutic effects, it has many adverse effects, such as anemia, thrombocytopenia, and increased liability to various infections.[10] It reduces the immune system activity by interfering with the activity and growth of T-cell and decreasing the production of lymphocytes.
Immunosuppressive drugs play an important role in anemia. Reduction in the white blood cells (WBCs), the red blood cell counts, and the hemoglobin (Hb) concentration, with an increase in the platelet counts, were observed in rats treated with CsA at a daily oral dose of 7.5 mg/kg.[11] In addition, administration of CsA (30 mg/kg) and ketoconazole (10 mg/kg) drugs induced significantly decrease in total WBC and lymphocyte counts compared to control animal.[12] Another study revealed that the treatment with cyclosporine daily doses (low, 2 mg/kg; medium 5 mg/kg, and high 10 mg/kg) for 31 days suppressed white cells count and lymphocytes and inhibited cell-mediated immunity in dose-dependent manner compared with untreated group.[13]
In addition, in another study, rats were daily treated with CsA (5 mg/kg body weight [BW]), and the blood hematology was evaluated after 7 weeks of treatment. CsA-treated rats showed that a noticeable reduction in red blood cells (RBCs), hematocrit, Hb concentration, mean platelet volume, and platelet distribution width were significantly observed in both groups under immunosuppressive treatment compared with the control group.[14] Female rats received CsA (5 mg/kg/day by oral gavage) for 2 weeks showed that many subpopulations of suppressed lymphocytes. Moreover, qualitative, quantitative, and morphological changes of the immune system in pharmacologically immunosuppressed females have been observed.[15]
In the way of finding a natural dietary supplement trying to decrease the side effects of immunosuppressive drugs before and after their therapeutic use. The present study aimed at evaluation of the impact of the low and high doses of EPR on CsA-induced immunosuppression on the blood parameters and microscopic changes of the spleen in the male rats.
MATERIALS AND METHODS
Drugs and plant
E. purpurea root (EPR) liquid extract (USDA Organic) was purchased from iHerb.com, Herb Pharm, Saudi Arabia. The country of origin of goods was the USA.
Cyclosporine (CsA) 50 mg/ml (Sandimmune-Novartis) was obtained from King Abdul-Aziz University Hospital. The content of the capsules was dissolved in glycol and freshly prepared for subcutaneous (SC) injection depending on the weight of each animal.
Experimental animals
Wistar albino male rats (n = 32 rats) ranged from 170 to 210 g were obtained from the animal experimental unit of King Fahd Center for Medical Research (KFMRC), King Abdul-Aziz University (KAU). All animals were allowed to acclimatize for 1 week in animal housing conditions before being used for the study. The rats were housed in standard laboratory conditions at a temperature of (22°C ± 3°C), relative humidity (50%–55%) and a 12-h light/dark cycle (4 rats/cage). All animals were fed on the standard balanced diet according to Reeves et al.[16] and drinking water ad libitum, following the protocols, that were approved by the Ethical Committee for Animal Care of the KAU, Jeddah, Saudi Arabia, which are in accordance with the guidelines of the Canadian Council on Animal Care.
Diet formula
Standard balanced diet was obtained from KFMRC, KAU, Jeddah, Saudi Arabia. The basic diet consists of the following ingredients: crude protein (casein) 20%; crude fat 4.0%; sucrose 10%; corn oil 4%; choline chloride 0.2%; vitamin mixture 1%; salt mixture 3.5%; fibers (cellulose) 5%; and the remainder being corn starch up to 100%. The diet manufactured by Grain Silos and Flour Mills Organization, KSA.
Methods
Induction of immunosuppression by cyclosporine
Immunosuppression was induced by CsA. Rats were injected with CsA SC at a dose level of (15 mg/kg) for 21 days.[17]
Experimental design
The rats were randomly divided into the following four groups (8 each): Group I (control): The animals were injected with the glycol (the solvent used for dilution of cyclosporine) SC daily for the 21 days. Group II (immunosuppressive models): The rats were injected with a daily single SC dose of CsA as previously mentioned. Group III and IV (CsA + EPR) (protected groups): The animals were ingested EPR extract of 100 or 200 mg/kg, orally, daily, respectively. The chosen dose (100 mg/kg, orally, daily) was according to a previous study.[18] Then, the rats of both groups were injected SC with the daily single dose of CsA as previously mentioned.
Biological evaluation
Daily feed intake (FI) per group was calculated throughout the experimental period (21 days). The feed efficiency ratio (FER) was calculated using the following equation according to the method of Chapman et al. FER = Gain in body weight (g)/food consumed (g).
At the end of the experimental period, all rats were fasted overnight and then sacrificed. The final BW was recorded and used for calculation of the body weight gain percent (BWG%). BWG% was calculated at the end of the experimental period according to the following equations.[19]
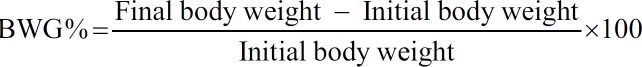
Hematological study
Blood samples were immediately collected from the retro-orbital plexus of veins into the sterile heparin-coated tubes using the capillary tubes under mild ether anesthesia. The RBC parameters included total RBCs count, Hb, and mean corpuscular hemoglobin (MCH). The WBC parameters included the total WBCs and lymphocyte differential counts. The analysis was done at AL-Borg Medical Laboratory (Jeddah, Saudi Arabia) using rat detection kit by the auto-hematology analyzer ROCHE (Sysmex XS-500i).
Histopathological studies
By the end of the experiment, the spleen was dissected and washed with a cold saline solution and dried between two filter papers and then weighed. The organs were trimmed and preserved in phosphate-buffered formalin for the tissue processing and embedding in the paraffin. The sections stained with hematoxylin and eosin (H and E) to assess the microscopic changes.
Statistical analysis
Results were expressed as a mean ± standard error. Data were analyzed statistically by one-way analysis of variance followed by LSD test being used for determining the significances among different groups at a level of <0.05. A DELL computer with a software system SPSS version 16 (SPSS Inc., Chicago, IL, U.S.A.) (installed from the Saudi Digital Library) was used for these calculations.
RESULTS
Biological evaluation
The changes in the BW, BWG%, FI, and FER in different groups are summarized in Table 1 and Histogram 1. The results revealed that there was no significant difference in initial BW between all the experimental groups. There was a significant decrease (−9.88%) (P < 0.05) in the final BW and the BWG% in CsA group compared to the control. After 21 days, CsA + EPR (100 mg/kg/BW) and CsA + EPR (200 mg/kg/BW) groups showed a significant (P ≤ 0.001) reduction in final BW and BWG% as compared to the control group. On the other hand, there was a significant difference in final BW and BWG% between CsA group and both CsA + EPR at low and high doses.
Table 1.
Effect of Echinacea purpurea root (100 and 200 mg/kg body weight) on the biological evaluation parameters among different groups
| Parameters | Experimental groups | |||
|---|---|---|---|---|
| Group I (control) | Group II (CsA) | Group III (CsA + EPR) (100 mg/kg BW) | Group IV (CsA + EPR) (200 mg/kg BW) | |
| Initial BW (g) | 194.75±1.03 | 193.88±1.72 | 193.25±1.20 | 196.63±0.96 |
| Final BW (g) | 222.75±7.84 | 200.75±4.73a,* | 182.75±5.51a,*** | 189.75±7.23a,*** |
| BWG (%) | 14.39±4.55 | 3.16±1.48a,* | −5.44±2.59a,*** | −3.50±2.39a,*** |
| FI (g/rat/day) | 16.83±1.49 | 14.67±1.39 | 12.18±0.93a,* | 11.25±1.08a,** |
| FER | 0.08±0.02 | 0.02±0.01a,* | −0.04±0.02a,***,b,* | −0.03±0.01a,***,b,* |
Data are mean±SE (n=8). aSignificant difference from control, bSignificant difference from CsA, cSignificant difference between CsA + EPR (100 mg/kg BW) and CsA + EPR (200 mg/kg BW), *P<0.05, **P<0.01 and ***P<0.001. BWG%: Body weight gain percent, FI: Feed intake, FER: Feed efficiency ratio, BW: Body weight, EPR: Echinacea purpurea root, CsA: Cyclosporine, SE: Standard error
Histogram 1.
Demonstration of the effect of EPR (100 and 200 mg/kg BW) on the biological evaluation parameters among different groups. Data are mean ± standard error (n = 8). aSignificant difference from control. bSignificant difference from CsA. cSignificant difference between CsA + EPR (100 mg/kg BW) and CsA + EPR (200 mg/kg BW). (*P < 0.05, **P < 0.01, and ***P < 0.001). EPR: Echinacea purpurea root, CsA: Cyclosporine, BW: Body weight
Hematological study
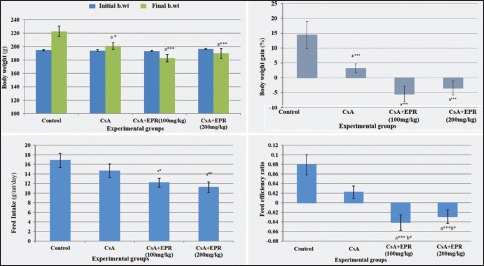
The statistical analysis of the hematological parameters is summarized in Table 2 and Histogram 2. The results revealed that, in the CsA group, Hb level was significantly lower (P < 0.001) than in the control group (9.85 ± 0.82 and 14.97 ± 0.77 g/dl, respectively). On the other hand, there was a significant increase (P < 0.001) in Hb level in EPR-treated groups (100 and 200 mg/kg) when compared with CsA group. The level of Hb returned to nearly normal range in treated groups. Concerning RBCs, CsA rats showed a significant decrease (P < 0.05) in RBCs count as compared with the control group; whereas treated rats with low and high dose of EPR displayed insignificant elevation when compared with CsA group (mean values 8.40 ± 0.22 and 8.24 ± 0.17 vs. 7.86 ± 0.20, respectively).
Table 2.
Effect of Echinacea purpurea root (100 and 200 mg/kg) on some hematological parameters in different groups
| Parameters | Experimental groups | |||
|---|---|---|---|---|
| Group I (control) | Group II (CsA) | Group III (CsA + EPR) (100 mg/kg BW) | Group IV (CsA + EPR) (200 mg/kg BW) | |
| Hb (g/dl) | 14.97±0.77 | 9.85±0.82a,*** | 13.83±0.48b,*** | 14.29±0.56b,*** |
| RBCs (×1012/L) | 8.80±0.26 | 7.86±0.20a,* | 8.40±0.22 | 8.24±0.17 |
| MCH (pg) | 18.28±0.54 | 14.07±0.39a,*** | 17.57±0.49b,*** | 18.58±0.44b,*** |
| WBCs (×109/L) | 6.17±0.53 | 1.94±0.14a,*** | 6.89±0.51b,*** | 6.33±0.65b,*** |
| Lymphocytes (×109/L) | 2.30±0.17 | 1.76±0.08a,* | 2.08±0.1 | 2.40±0.17b,** |
Data are mean±SE (n=8). aSignificant difference from control, bSignificant difference from CsA, cSignificant difference between CsA + EPR (100 mg/kg BW) and CsA + EPR (200 mg/kg BW), *P<0.05, **P<0.01 and ***P<0.001(. SE: Standard error, BW: Body weight, EPR: Echinacea purpurea root, CsA: Cyclosporine, Hb: Hemoglobin, RBCs: Red blood cell count, MCH: Mean corpuscular hemoglobin, WBCs: White blood cell count
Histogram 2.
Demonstration of the effect of EPR on the measured blood parameters in different groups. Data are mean ± standard error (n = 8). aSignificant difference from control. bSignificant difference from CsA. cSignificant difference between CsA + EPR (100 mg/kg BW) and CsA + EPR (200 mg/kg BW). (*P < 0.05, **P < 0.01, and ***P < 0.001). EPR: Echinacea purpurea root, CsA: Cyclosporine, BW: Body weight
Furthermore, data revealed that CsA group caused a significant reduction (23.03%) (P < 0.001) in MCH as compared with control group. Oral administration of EPR induced significant (P < 0.001) amelioration in treated groups as compared with CsA group.
Concerning the level of WBCs, there was significantly decreased (P < 0.001) in CsA group with a mean value of 1.94 ± 0.14 when compared to the control group with a mean value of 6.17 ± 0.53. Oral administration of EPR with low and high doses to CsA-injected rats induced significant (P < 0.001) elevation in WBCs as compared with CsA group.
At the same time, there was a significant decrease (P < 0.05) in lymphocytes when compared between control group and CsA group, with the mean values being 2.30 ± 0.17 vs. 1.76 ± 0.08 for control and CsA group, respectively. The improvement in high dose of EPR (200 mg/kg) had a significant difference (P < 0.01) when compared with CsA group. Further, there was no significant difference between low doses of EPR (100 mg/kg) and CsA group. As seen in this experiment, treatment with EPR had positive effects on rat's lymphocytes count.
Microscopic examination
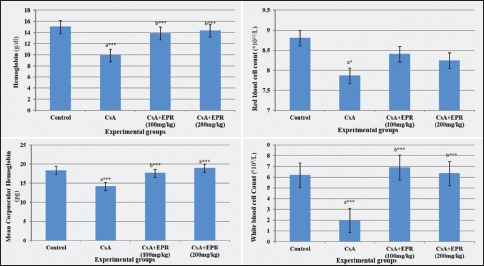
Examination of the control spleen sections stained by H and E showed the embedded follicular lymphatic tissues (white pulp) in the highly vascular (red pulp). The white pulp was subdivided into periarterial lymphatic sheath (PALS) around the central artery, follicular areas, and the marginal zones between white and red pulp. The PALS could be demarcated as the area around the central artery. The red pulp displayed a reticular network of splenic cords and in between the venous blood sinusoids [Figure 1a]. The spleen sections from CsA group revealed a marked decrease in the cellularity of PALS. The congested blood sinusoids in both the white and red pulp were the prominent feature in most of the examined sections together with thickened trabeculae [Figure 1b]. In the CsA groups treated with low and high dose of EPR, the PALS area had an apparent increase compared to the cyclosporine-treated group (CsA) together with the persistence of the congestion of the red pulp blood sinusoids. The higher magnification revealed the prominence of the medium and large lymphocytes on the expense of the small lymphocytes, especially in the marginal zone [Figure 1c and d].
Figure 1.
Photomicrographs of the rat spleen. (a) A control one showing the parenchyma with aggregation of lymphocytes around the arteriole (A) in the white pulp, marginal zone (M) around it and the different cell series in the red pulp (R). (b). a rat spleen from the CsA group, showing marked decrease in the lymphocytes in the periarteriolar area of the white pulp (w). Notice the marked increase in dilated sinusoids with difficult demarcation between different zones. (c) A rat spleen from CsA + Echinacea purpurea root (100 mg/kg BW) group, showing the active germinal center (GC) of the white pulp with predominance of the cell nest appearance of the macrophages (→) and increased number of large macrophages (▸) in the marginal zone (M). (d) a rat spleen from CsA + Echinacea purpurea root (200 mg/kg BW) group, showing the increased number of activated lymphocytes in the marginal zone (M). Arteriole (A) and white pulp (W). Bar 50 μ (H and E stain)
DISCUSSION
Cyclosporine A (CsA) is considered one of the common worldwide immunosuppressive drugs that are used to prevent the allograft rejection in organ transplantation. It has been given for the treatment of autoimmune diseases such as psoriasis and rheumatoid arthritis. Their side effects have been attributed to the increase production of reactive oxygen species and the altered antioxidant status. Treatment with CsA inhibits the expression and activity of antioxidant enzymes. Thus, administration of the antioxidant supplementation with CsA treatment might be beneficial to prevent severe side effects.
One of the observations in the current study was the significant decrease of BW after ERP intake. This can be explained by the change of one of its metabolites (alkamides) to caffeine during its metabolism and this may be a cause of appetite decrease. In the present study, administration of EPR- to CsA-injected rats induced a significant decrease in final BW, BWG%, and FI compared with the control group. The obtained results were in agreement with previous authors.[20] These results may be attributed to the synergistic effect of EPR-induced anorexia with the toxicity of CsA, resulting in further reduction in the FI. In contrast, other studies reported that Echinacea has a positive effect on BW gain after 4 weeks of treatment. The difference could be attributed to study design including the dose and the duration of experiment.[21,22]
The current reduction of the total WBCs and differential lymphocytic counts together with a decrease in RBCs count and Hb concentration were consistent with the study of Lekhooa.[13] This reduction was previously explained by the reduced erythropoietin production, resulting in the decreased stimulation of bone marrow erythropoiesis.[23] Meanwhile, administration of EPR- to CsA-injected rats presented a significant correction in all hematological parameters, showing a significant difference between the low and high dose-treated groups. These findings were in accordance with those reported previously by many authors[9,21,24,25] This improvement attributed to the contents of E. purpurea as cichoric acid and echinacin, where each stimulates bone marrow and hematopoietic stem cells as mentioned in a previous study.[26]
The increase in the leukocyte count was a similar finding by other studies.[22,27] This result could be explained by the ability of polysaccharides and echinacocide to increase the number of leukocytes and the ability of cichoric acid and echinacin to activate macrophages and stimulate bone marrow. In addition, it was found that Echinacea-induced changes in the percentage of lymphocyte subpopulations indicated that Echinacea might modulate both innate and adaptive immune functions.[5]
Oxidative stress-induced tissues damage can be ameliorated by lower oxidative stress status. Antioxidants act as radical scavenger and inhibit lipid peroxidation and other free radicals-mediated processes, thereby protecting the human body from various diseases.[28] In the present study, the protective effect of EPR involves in the maintenance of antioxidant capacity for protecting the tissues against oxidative stress. This positive improvement may be attributed to the mechanism of antioxidant activity of EPR-included free radicals scavenging and transition metal chelating.[29] The superoxide dismutase activity in the blood was increased because of antioxidants compounds such as echinacocide and caffeine acid in E. purpurea which eliminate superoxide by free radicals scavenging effect as explained in a previous study.[30] Furthermore, analysis of E. purpurea extracts illuminated that it contains a series of active compounds as caffeic acid and polyphenolics including cichoric acid and glycosylated flavonoids and polysaccharides, which is responsible for certain anti-inflammatory and antioxidant activities.[31]
However, other studies mentioned opposite findings compared to the current one;[5,32,33] this could be attributed to the drugs used in the research, duration of the experiment, and extraction methods.
Finally, the current findings revealed that EPR extract ameliorated the hematological changes. However, there was a partial correction of the CsA-induced microscopic changes of the rat spleen. Biologically, it may be a BW decreasing agent, which carries a chance for medicinal pharmacists searching for new drug discovery.
Ethics approval
The animal protocol of the study was approved by the local committee for animal care (KACST, Jeddah, Saudi Arabia; Protocol No. AT-37-1943) and was in accordance with the National Institutes of Health guidelines for the use of live animals (NIH publication No. 85-23, revised 1996). Further, the fund body had approved the proposal for this study.
Financial support and sponsorship
We acknowledge with thanks the support of KACST for their financial and logistical support and for providing necessary guidance concerning projects implementation of the Research No. AT-37-1943.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We acknowledge with thanks the support of King Abdulaziz City for Science and Technology (KACST) for their financial and logistical support and for providing necessary guidance concerning projects implementation of the Research No. AT-37-1943.
REFERENCES
- 1.Kumar K, Ramaiah S. Pharmacological importance of Echinacea purpurea. Int J Pharm Biol Sci. 2011;2:304–14. [Google Scholar]
- 2.Kligler B. Echinacea. Am Fam Physician. 2003;67:77–80. [PubMed] [Google Scholar]
- 3.Bauer Ra, Wagner H. Echinacea species as potential immunostimulatory drugs. 1991 [Google Scholar]
- 4.Mistrikova I, Vaverkova S. Echinacea- chemical composition, immunostimulatory activities and uses. Thaiszia J Bot. 2006;16:11–26. [Google Scholar]
- 5.Zhai Z. Ph.D. Thesis. Ames, Iowa: Iowa State University; 2008. Immunomodulatory, Anti-Inflammatory and Wound Healing Properties of Echinacea Species. [Google Scholar]
- 6.Yu D, Yuan Y, Jiang L, Tai Y, Yang X, Hu F, et al. Anti-inflammatory effects of essential oil in Echinacea purpurea L. Pak J Pharm Sci. 2013;26:403–8. [PubMed] [Google Scholar]
- 7.Jukić H, Habeš S, Aldžić A, Durgo K, Kosalec I. Antioxidant and prooxidant activities of phenolic compounds of the extracts of Echinacea purpurea (L.) Bull Chem Technol Bosn Herzegovina. 2015;44:43–52. [Google Scholar]
- 8.Barrett B. Medicinal properties of Echinacea: A critical review. Phytomedicine. 2003;10:66–86. doi: 10.1078/094471103321648692. [DOI] [PubMed] [Google Scholar]
- 9.Ezz M. The ameliorative effect of Echinacea purpurea against gamma radiation induced oxidative stress and immune responses in male rats. Aust J Basic Appl Sci. 2011;5:506–12. [Google Scholar]
- 10.BC Renal Agency Cyclosporine (Neoral®) Medication Info Sheet. 2013. [Last accessed on 2013 Jan]. Available from: http://www.bcrenalagency.ca/resourcegallery/Documents/Cyclosporine%20(Neoral).pdf .
- 11.Pally C, Tanner M, Rizvi H, Papageorgiou C, Schuurman HJ. Tolerability profile of sodium mycophenolate (ERL080) and mycophenolate mofetil with and without cyclosporine (Neoral) in the rat. Toxicology. 2001;157:207–15. doi: 10.1016/s0300-483x(00)00334-6. [DOI] [PubMed] [Google Scholar]
- 12.Jivrajani M, Shaikh MV, Shrivastava N, Nivsarkar M. An improved and versatile immunosuppression protocol for the development of tumor xenograft in mice. Anticancer Res. 2014;34:7177–83. [PubMed] [Google Scholar]
- 13.Lekhooa M. Development of a Model to Characterize the Effect of Phela on Selected Immune Markers in Immune-Suppressed rats. University of the Free State, Pharmacology; 2015. [Google Scholar]
- 14.Reis F, Parada B, Teixeira de Lemos E, Garrido P, Dias A, Piloto N, et al. Hypertension induced by immunosuppressive drugs: A comparative analysis between sirolimus and cyclosporine. Transplant Proc. 2009;41:868–73. doi: 10.1016/j.transproceed.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Kabat-Koperska J, Kolasa-Wołosiuk A, Wojciuk B, Wojciechowska-Koszko I, Roszkowska P, Krasnodębska-Szponder B, et al. Changes in the immune system of female wistar rats after exposure to immunosuppressive treatment during pregnancy. Scand J Immunol. 2016;83:418–26. doi: 10.1111/sji.12434. [DOI] [PubMed] [Google Scholar]
- 16.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad Hoc Writing Committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 17.Rezzani R, Rodella L, Bianchi R. Melatonin antagonises the cyclosporine A immunosuppressive effects in rat thymuses. Int Immunopharmacol. 2001;1:1615–9. doi: 10.1016/s1567-5769(01)00077-7. [DOI] [PubMed] [Google Scholar]
- 18.Rezaie A, Fazlara A, Haghi Karamolah M, Shahriari A, Najaf Zadeh H, Pashmforosh M, et al. Effects of Echinacea purpurea on hepatic and renal toxicity induced by diethylnitrosamine in rats. Jundishapur J Nat Pharm Prod. 2013;8:60–4. [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman DG, Castillo R, Campbell JA. Evaluation of protein in foods. I. A method for the determination of protein efficiency ratios. Can J Biochem Physiol. 1959;37:679–86. [PubMed] [Google Scholar]
- 20.Nematalla KH, Arafa SA, Yousef GM, Zainb A. Effect of Echinacea as antioxidant on markers of aging. Aust J Basic Appl Sci. 2011;5:18–26. [Google Scholar]
- 21.Skaudickas D, Kondrotas AJ, Baltrusaitis K, Vaitiekaitis G. Effect of Echinacea (Echinacea purpurea L.Moench) preparations on experimental prostate gland. Medicina (Kaunas) 2003;39:761–6. [PubMed] [Google Scholar]
- 22.Ali EH. Protective effects of Echinacea on cyproterone acetate induced liver damage in male rats. Pak J Biol Sci. 2008;11:2464–71. doi: 10.3923/pjbs.2008.2464.2471. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen FT, Jensen BL, Marcussen N, Skøtt O, Bie P. Inhibition of mineralocorticoid receptors with eplerenone alleviates short-term cyclosporin a nephrotoxicity in conscious rats. Nephrol Dial Transplant. 2008;23:2777–83. doi: 10.1093/ndt/gfn204. [DOI] [PubMed] [Google Scholar]
- 24.Chaves F, Chacón M, Badilla B, Arévalo C. Effect of Echinacea purpurea (Asteraceae) aqueous extract on antibody response to bothrops asper venom and immune cell response. Rev Biol Trop. 2007;55:113–9. doi: 10.15517/rbt.v55i1.6061. [DOI] [PubMed] [Google Scholar]
- 25.Dehkordi SH, Fallah V. Enhancement of broiler performance and immune response by Echinacea purpurea supplemented in diet. Afr J Biotechnol. 2011;10:11280–6. [Google Scholar]
- 26.Goel V, Chang C, Slama JV, Barton R, Bauer R, Gahler R, et al. Alkylamides of Echinacea purpurea stimulate alveolar macrophage function in normal rats. Int Immunopharmacol. 2002;2:381–7. doi: 10.1016/s1567-5769(01)00163-1. [DOI] [PubMed] [Google Scholar]
- 27.Agnew LL, Guffogg SP, Matthias A, Lehmann RP, Bone KM, Watson K, et al. Echinacea intake induces an immune response through altered expression of leucocyte hsp70, increased white cell counts and improved erythrocyte antioxidant defences. J Clin Pharm Ther. 2005;30:363–9. doi: 10.1111/j.1365-2710.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 28.Marsoul RD, Abbood RM, Abbas MT. Effect of garlic oil on cyclosporine induced renal toxicity in rats. Hum J. 2016;5:209–21. [Google Scholar]
- 29.Hu C, Kitts DD. Studies on the antioxidant activity of Echinacea root extract. J Agric Food Chem. 2000;48:1466–72. doi: 10.1021/jf990677+. [DOI] [PubMed] [Google Scholar]
- 30.Mishima S, Saito K, Maruyama H, Inoue M, Yamashita T, Ishida T, et al. Antioxidant and immuno-enhancing effects of Echinacea purpurea. Biol Pharm Bull. 2004;27:1004–9. doi: 10.1248/bpb.27.1004. [DOI] [PubMed] [Google Scholar]
- 31.Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi JD. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. N Engl J Med. 2005;353:341–8. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- 32.Sasagawa M, Cech NB, Gray DE, Elmer GW, Wenner CA. Echinacea alkylamides inhibit interleukin-2 production by Jurkat T cells. Int Immunopharmacol. 2006;6:1214–21. doi: 10.1016/j.intimp.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 33.Matthias A, Banbury L, Bone KM, Leach DN, Lehmann RP. Echinacea alkylamides modulate induced immune responses in T-cells. Fitoterapia. 2008;79:53–8. doi: 10.1016/j.fitote.2007.07.012. [DOI] [PubMed] [Google Scholar]


