Cyanobacteria are oxygenic photosynthetic bacteria that are found in a wide variety of ecological environments, where they are important contributors to global carbon and nitrogen cycles. Genetic manipulation systems have been developed in a number of cyanobacterial strains, allowing both the interruption of endogenous genes and the introduction of new genes and entire pathways. However, unicellular diazotrophic cyanobacteria have been generally recalcitrant to genetic transformation. These cyanobacteria are becoming important model systems to study diurnally regulated processes. Strains of the Cyanothece genus have been characterized as displaying robust growth and high rates of nitrogen fixation. The significance of our study is in the establishment of a genetic modification system in a unicellular diazotrophic cyanobacterium, the demonstration of the interruption of the glgX gene in Cyanothece sp. strain ATCC 51142, and the characterization of the increased nitrogen-fixing ability of this strain.
KEYWORDS: conjugation, cyanobacteria, glycogen, nitrogen fixation, photosynthesis
ABSTRACT
Cyanobacteria are oxygenic photosynthetic prokaryotes with important roles in the global carbon and nitrogen cycles. Unicellular nitrogen-fixing cyanobacteria are known to be ubiquitous, contributing to the nitrogen budget in diverse ecosystems. In the unicellular cyanobacterium Cyanothece sp. strain ATCC 51142, carbon assimilation and carbohydrate storage are crucial processes that occur as part of a robust diurnal cycle of photosynthesis and nitrogen fixation. During the light period, cells accumulate fixed carbon in glycogen granules to use as stored energy to power nitrogen fixation in the dark. These processes have not been thoroughly investigated, due to the lack of a genetic modification system in this organism. In bacterial glycogen metabolism, the glgX gene encodes a debranching enzyme that functions in storage polysaccharide catabolism. To probe the consequences of modifying the cycle of glycogen accumulation and subsequent mobilization, we engineered a strain of Cyanothece 51142 in which the glgX gene was genetically disrupted. We found that the ΔglgX strain exhibited a higher growth rate than the wild-type strain and displayed a higher rate of nitrogen fixation. Glycogen accumulated to higher levels at the end of the light period in the ΔglgX strain, compared to the wild-type strain. These data suggest that the larger glycogen pool maintained by the ΔglgX mutant is able to fuel greater growth and nitrogen fixation ability.
IMPORTANCE Cyanobacteria are oxygenic photosynthetic bacteria that are found in a wide variety of ecological environments, where they are important contributors to global carbon and nitrogen cycles. Genetic manipulation systems have been developed in a number of cyanobacterial strains, allowing both the interruption of endogenous genes and the introduction of new genes and entire pathways. However, unicellular diazotrophic cyanobacteria have been generally recalcitrant to genetic transformation. These cyanobacteria are becoming important model systems to study diurnally regulated processes. Strains of the Cyanothece genus have been characterized as displaying robust growth and high rates of nitrogen fixation. The significance of our study is in the establishment of a genetic modification system in a unicellular diazotrophic cyanobacterium, the demonstration of the interruption of the glgX gene in Cyanothece sp. strain ATCC 51142, and the characterization of the increased nitrogen-fixing ability of this strain.
INTRODUCTION
Cyanobacteria are photosynthetic microbes that are widely studied for their contributions to global carbon sequestration and nitrogen fixation (1). The cyanobacterial strains that are able to fix atmospheric N2 to bioavailable forms have evolved strategies to separate the two incompatible processes. In some filamentous cyanobacteria, nitrogen fixation occurs in specialized cells called heterocysts, whereas photosynthesis is performed in the vegetative cells (2). In contrast, nitrogen-fixing unicellular cyanobacteria separate the incompatible processes of photosynthesis and nitrogen fixation temporally within the same cell (3). In these cells, light is harvested during the day and used by photosynthesis to fix atmospheric CO2 and in turn to generate energy and reducing equivalents. Fixed CO2 is stored in glycogen granules, which are used by respiration during the night to fuel energy-demanding processes like nitrogen fixation. The products of nitrogen fixation are stored in cyanophycin bodies, which are nonribosomally synthesized polymers of arginine and aspartic acid (4). Single-cell nitrogen-fixing cyanobacteria have been recognized as crucial players in all ecosystems, contributing significantly to carbon and nitrogen levels.
Of the unicellular nitrogen-fixing cyanobacteria, Cyanothece constitutes an important genus, with members exhibiting robust growth and high rates of nitrogen fixation (5). Cyanothece strains are known to occur worldwide in diverse ecosystems and have been studied extensively (6–9). One Cyanothece strain, Cyanothece sp. strain ATCC 51142, has been comprehensively studied at the genomic, transcriptomic, proteomic, ultrastructural, and physiological levels (10–13). This strain was found to have several novel attributes, including high levels of nitrogen fixation and hydrogen production (14–16). Despite these insights, until now genetic manipulation and development of a reliable system of genetic engineering have remained elusive in Cyanothece 51142, handicapping the potential to develop this strain further as a model system (17).
Carbon metabolism is central to cyanobacterial energy management, and carbon synthesis and degradation pathways have been shown to have significant effects on cellular processes (18, 19). The ability to store metabolic products in inclusion bodies for later mobilization is particularly crucial for unicellular diazotrophic cyanobacteria that maintain an active metabolic profile throughout the diel cycle. For example, mobilization of energy stored in glycogen granules is essential to drive the energy-expensive nitrogenase reaction at night.
Storage polysaccharides are found in bacteria, algae, and green plants, where they are key to survival (20). Soluble glycogen is found in most strains of bacteria, including cyanobacteria, whereas starch is synthesized by algae and plants as insoluble granules. After endosymbiosis of an ancestor of present-day cyanobacteria by a eukaryotic cell, starch metabolism in the host resulted from the merging of the pathways of storage polysaccharide production in the two organisms (21). In fact, probing these pathways has provided insights into the polysaccharide metabolism in the endosymbiont before endosymbiosis, the acquisition of starch metabolism in cyanobacteria, and the important role of the debranching enzyme (20, 22). While starch and glycogen are both constructed from α-1,4 linkages and α-1,6 branches, these two polymers differ greatly in appearance and characteristics. Unlike glycogen, which is limited in size to a maximum of ∼40 nm, starch size is unlimited (20).
Interestingly, within the Cyanothece genus, two different types of polysaccharide storage bodies have been described (23, 24). In Cyanothece 51142, storage bodies formed as large granules (∼50 nm by ∼150 nm) between the thylakoid membranes (13) are commonly referred to as glycogen granules (13, 25). In contrast, Cyanothece sp. strain PCC 7822 forms much smaller glycogen granules (26). The structures of the different types of granules were found to be semiamylopectin in Cyanothece 51142 and β-granules of glycogen in Cyanothece 7822 (26). Thus, members of the Cyanothece genus offer opportunities to dissect the carbon storage and degradation pathways and to elucidate their implications in the diel cycle.
Here we report the development and application of a reproducible genetic modification system in Cyanothece 51142 and the disruption of the glycogen-debranching gene glgX in this organism. In order to determine the effects of interference in the polysaccharide accumulation and degradation system during the diurnal cycle in this strain, we inactivated the glgX gene by targeted mutagenesis. We found that the ΔglgX strain exhibited a higher growth rate, compared to the wild-type (WT) strain, when grown under nitrogen-replete conditions and displayed a higher rate of nitrogen fixation under nitrogen-depleted conditions. Glycogen accumulated to higher levels at the end of the light period in the ΔglgX strain, compared to the WT strain. These data suggest that the larger glycogen pool maintained by the ΔglgX mutant is able to fuel greater growth and nitrogen fixation ability.
RESULTS
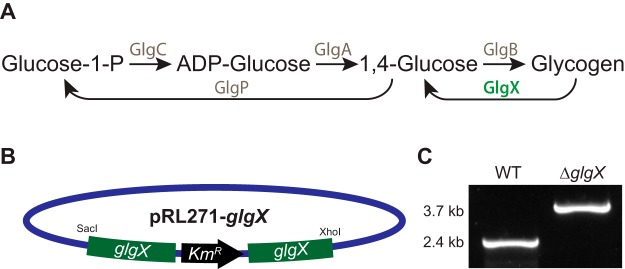
In bacteria, including cyanobacteria, glycogen synthesis is performed by glycogen synthase (GlgA), ADP-glucose pyrophosphorylase (GlgC), and the branching enzyme (GlgB) (Fig. 1A). The process begins with the addition of ADP to glucose to form ADP-glucose, which is then polymerized to the end of an α-1,4-linked glucan chain. The branching enzyme GlyB then links the chains together at the α-1,6 position. During degradation, the phosphorylases (GlgP) degrade the α-1,4 linkages but are unable to break the α-1,6 branch, so that degradation requires a specific debranching enzyme, GlgX (Fig. 1A). In Cyanothece 51142, there are two copies each of glgA (cce_3396 and cce_0890), glgB (cce_2248 and cce_4595), and glgC (cce_0987 and cce_2658). The annotated degradation genes are glgP (cce_1629, cce_5186, and cce_1603) and glgX (cce_3465).
FIG 1.
(A) Metabolic pathway of glycogen synthesis and degradation in Cyanothece 51142. The GlgX enzyme is shown in green. (B) Plasmid construct used for interruption of the glgX gene, showing restriction enzyme sites used for cloning. (C) PCR results showing the kanamycin resistance gene inserted into glgX, yielding a larger product than in the WT strain.
Earlier attempts to genetically transform Cyanothece 51142 cells were unsuccessful (17), despite the application of a variety of methods by different research groups. We employed a conjugation strategy to genetically transform Cyanothece 51142 cells that used modified media and plating methods. Cyanothece 51142 was isolated from the intertidal zones and was found to grow optimally in saltwater-based medium (ASP2 medium) (5). The strain was also found to be fastidious about growth on plates, forming colonies only on certain specific solidifying agents. The strain of Cyanothece 51142 that we used grew well in BG11 medium, although ASP2 medium continued to be the medium of choice for optimal growth. Comparison of conjugation results indicated that the use of BG11 medium favored the conjugation process and BG11 plates allowed cells to grow at low titer and to form colonies more readily, compared to growth on ASP2 plates.
For conjugation experiments, we engineered a version of the pRL271 plasmid by introducing a kanamycin resistance cassette into the AgeI site within the glgX open reading frame (Fig. 1B) and then we used a modified version of the conjugation protocol developed for Anabaena sp. strain PCC 7120 to introduce this plasmid into Cyanothece 51142 (27). PCR was used to verify that the resistance cassette was inserted into the gene (Fig. 1C).
We grew the WT and ΔglgX strains under different conditions, with and without added nitrogen and in continuous light and alternating 12-h light/dark cycles. For these experiments, ASP2 medium with and without added sodium nitrate (NaNO3) was used. In the absence of NaNO3, Cyanothece 51142 cells transition to nitrogen fixation. The mutant strain grown in ASP2 medium under nitrogen-fixing conditions with a 12-h light/dark regimen was consistently more yellow-green in color, which was reflected in changes in the absorption spectrum for phycocyanin at 625 nm (Fig. 2A). We calculated the chlorophyll and phycobilin contents in these two strains (28) and found that the ΔglgX mutant exhibited a 12% lower chlorophyll content, compared to the WT strain. A much greater difference was noted for the phycocyanin content, with the ΔglgX mutant having a 2-fold decrease in the phycocyanin content, compared to the WT strain.
FIG 2.
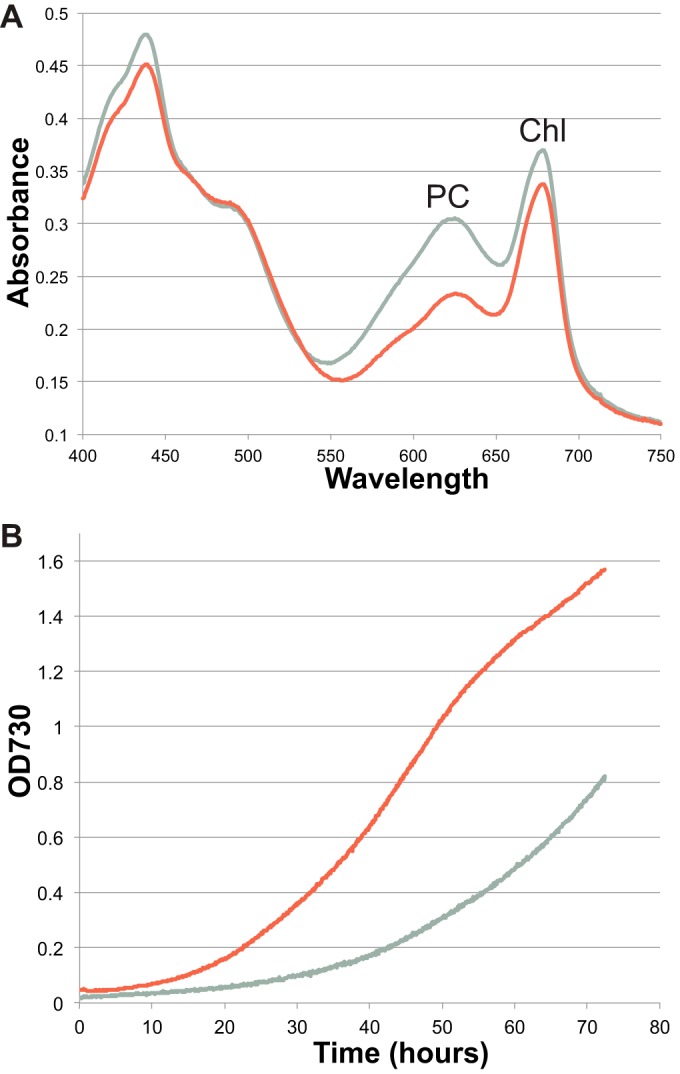
(A) Absorption spectra of the WT (gray line) and ΔglgX (red line) strains grown under nitrogen-fixing conditions. The absorbance for both chlorophyll (Chl) at 680 nm and phycocyanin (PC) at 625 nm was lower in the ΔglgX strain than in the WT strain, with the difference being much greater for phycocyanin. (B) Growth of the WT and ΔglgX strains with added nitrate at 38°C and 3% CO2, showing the faster growth of the mutant strain.
Growth of the WT and ΔglgX strains with added nitrogen was compared under different conditions, including temperatures of 30°C and 38°C and with and without additional CO2. Under all conditions assessed, the ΔglgX strain showed faster growth than the WT strain. In Fig. 2B, the fastest growth conditions are shown, i.e., 38°C and 3% CO2; under these conditions, the ΔglgX mutant was able to grow considerably faster than the WT strain.
We compared, by transmission electron microscopy (TEM), the cellular ultrastructure of the WT and mutant strains grown under nitrogen-fixing conditions during both the light and dark periods (Fig. 3). In WT cells at the end of the light period (peak of glycogen accumulation), granules were observed as mostly uniformly shaped electron-opaque structures of 110 to 220 nm, measured along the long axis (mean, 167 nm; standard deviation [SD], 31 nm [n = 23]) (Fig. 3A). In comparison, in the ΔglgX strain at the same time point, glycogen granules were less uniform in size and shape and could be observed as larger, less regular structures ranging from 123 to 347 nm in length (mean, 227 nm; SD, 56 nm [n = 34]) (Fig. 3B). Late in the dark period (expected minimum of glycogen content), the WT cells showed almost no glycogen granules (Fig. 3C), whereas most of the ΔglgX cells showed remaining glycogen (Fig. 3D). As a further determination of glycogen size, we measured the average area of the granules in cells in electron micrographs at these time points. We found that the area of granules in the mutant was 57% larger than that in the WT strain at the end of the light period (ΔglgX: mean, 0.025 µm2; SD, 0.012 µm2 [n = 55]; WT: mean, 0.014 µm2; SD, 0.004 µm2 [n = 53]). In the dark, few granules were present for measurement in the WT strain, but the remaining granules were similar to those seen in the light (mean, 0.010 µm2; SD, 0.003 µm2 [n = 23]); in the ΔglgX strain, the granules remained larger and more numerous than those in the WT strain (mean, 0.018 µm2; SD, 0.005 µm2 [n = 58]).
FIG 3.
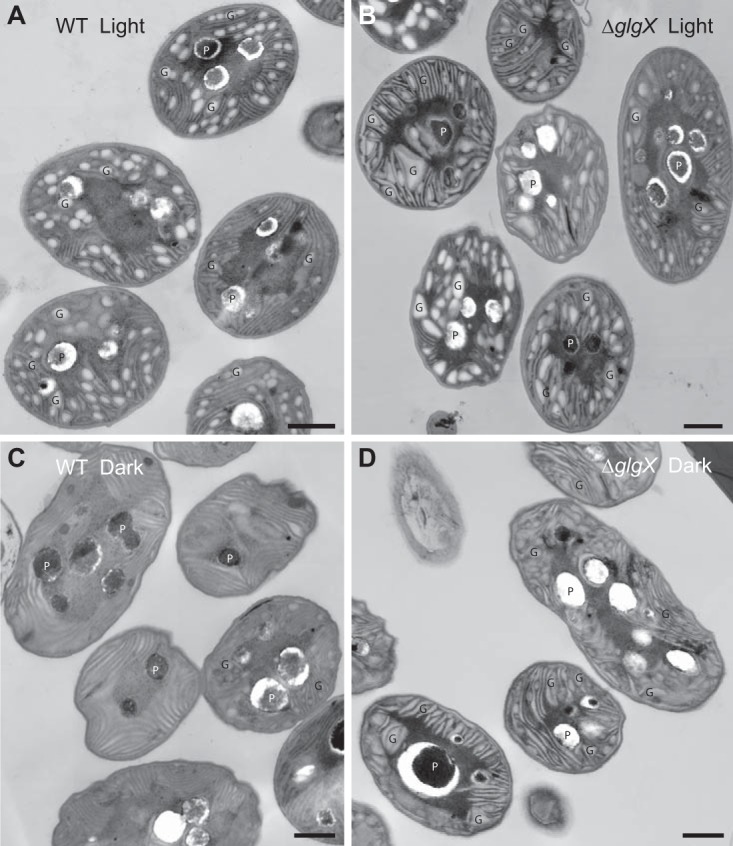
Electron microscopy images of Cyanothece 51142 WT (A and C) and ΔglgX (B and D) cells grown under nitrogen-fixing conditions and harvested at the end of the light (A and B) and dark (C and D) periods. Glycogen granules (G) and polyphosphate bodies (P) are labeled. Bar = 500 nm.
Measurements of glycogen contents (Fig. 4) showed that the ΔglgX strain maintained overall higher levels of glycogen throughout the diurnal cycle, compared to the WT strain. In both the WT and ΔglgX strains, glycogen levels decreased during the dark period and increased throughout the light period. Interestingly, the peak was earlier in the ΔglgX strain than in the WT strain.
FIG 4.
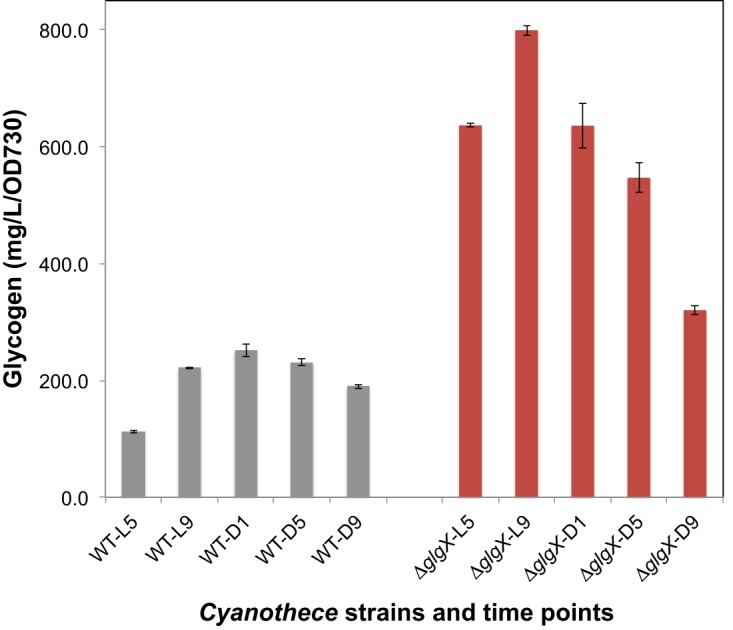
Glycogen contents measured in Cyanothece 51142 WT (gray) and ΔglgX (red) cells grown under nitrogen-fixing conditions and harvested at different time points during the light (L)/dark (D) cycle. Error bars indicate the SDs from the averages of technical replicates. Representative data are shown, from a total of three biological replicates.
Rates of nitrogen fixation, as assayed by reduction of acetylene to ethylene, were measured to determine whether the disruption of glgX would affect the nitrogen-fixing ability (Fig. 5). The ΔglgX strain displayed considerably higher rates of nitrogen fixation under both aerobic and anaerobic (argon-spurged) conditions. When glycerol was added to the medium as a carbon source, the enhanced nitrogen-fixing ability of the ΔglgX strain was no longer observed.
FIG 5.
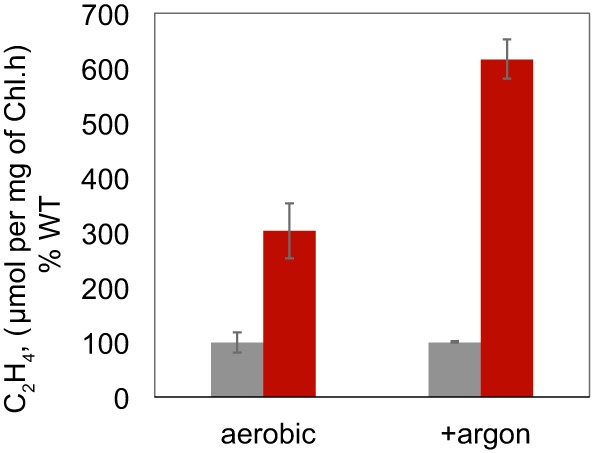
Nitrogenase activity measured in Cyanothece 51142 WT (gray) and ΔglgX (red) cells grown under nitrogen-fixing conditions and incubated under aerobic or anaerobic conditions. Representative data are shown as the averages of three biological replicates, and error bars show the SDs from the averages.
DISCUSSION
Despite intense interest and attempts over decades, a genetic transformation system in Cyanothece 51142 has remained elusive (17). Due to the lack of genetic tools in this organism, important questions regarding the consequences of mutagenesis of genes involved in critical processes, including carbon storage and nitrogen fixation, have gone unanswered until now. In fact, among the sequenced Cyanothece strains (3), successful genetic manipulation has been reported only for Cyanothece sp. strain PCC 7822. In that strain, a ΔnifK mutant was generated by transformation with single-stranded DNA and was shown to be unable to grow without added nitrate (17). Also in Cyanothece 7822, a ΔhupL strain was constructed by the same method, and the mutant strain demonstrated negligible nitrogenase activity and hydrogen production (29). Despite these successes in Cyanothece 7822, genetic manipulation in this strain remains difficult and unpredictable. In this study, we have established a straightforward and reproducible system of genetic manipulation in Cyanothece 51142.
In order to generate genetic mutants in Cyanothece 51142, we used a modified conjugation protocol that allowed cells to be grown in liquid BG11 medium and plated on BG11 plates, in contrast to previous work in which cells were grown in ASP2 liquid and solid media. A main difference between the two types of solid media lies in the type of agar used; standard bacterial agar is used in BG11 plates, whereas ASP2 plates typically incorporate Phytagel as the solidifying agent. In our experience, Cyanothece 51142 cells did not grow reproducibly at low titer and did not form colonies well on ASP2 plates made with Phytagel, and they could not grow at all on ASP2 plates made with many other solidifying agents. The relative ease with which cells could be plated on BG11 plates and form colonies and the colonies could be picked and transferred to new plates was important for the overall success of our strategy. Therefore, the ability to grow Cyanothece 51142 on BG11 medium provided a considerable advantage in terms of cell growth after conjugation and subsequent colony formation. For growth experiments and other assays, ASP2 medium, the original growth medium for Cyanothece 51142, was used (5).
The presence of the pRL623 plasmid (30) containing the AvaI, AvaII, and AvaIII methylase genes was found to be necessary for successful conjugation in Cyanothece 51142, and an examination of the restriction modification (RM) system in Cyanothece 51142 showed that these three components are present and likely play important roles in the RM system. In Cyanothece 51142, carbon is accumulated in large bodies found to be semiamylopectin, in contrast to the smaller, more conventional, glycogen granules found in some other Cyanothece strains, as well as in most other bacteria (24). The accumulation and subsequent utilization of these granules as part of the diurnal cycle have been well documented in Cyanothece 51142 (25, 31). We sought to understand the physiological characteristics of a strain in which the process of polysaccharide storage granule degradation was modified. We focused on the glgX gene because it existed in a single copy in Cyanothece 51142 and phylogenetic analysis showed that this gene is highly conserved in cyanobacteria (see Fig. S1 in the supplemental material).
The ΔglgX strain was generated by insertional inactivation of the glgX gene, and colony PCR showed that the resulting kanamycin-resistant strains were fully segregated after about 2 weeks (Fig. 1). The ΔglgX strain displayed several important phenotypic changes. The observed reduction in the phycocyanin peak in the ΔglgX strain (Fig. 2A) is in agreement with previous work (32) that demonstrated upregulation of genes involved in phycobilisome degradation (including nblA) when cells had greater carbon contents. Another important change was faster growth under nitrogen-sufficient conditions with 3% CO2, compared to the WT strain (Fig. 2B). Glycogen contents in the cells would be expected to be low under such growth conditions, and the faster growth suggests that the absence of GlgX in the modified strain enables CO2 to be used more efficiently. Interestingly, in the only other ΔglgX strain created to date in cyanobacteria, insertional inactivation of glgX in Synechococcus elongatus PCC 7942, a nondiazotrophic strain, resulted in similar growth rates for the mutant and WT strains (18). It may be relevant that Synechococcus 7942 forms conventional cyanobacterial glycogen granules, not semiamylopectin as in Cyanothece 51142.
In Cyanothece 51142, carbon is accumulated and stored throughout the light period, reaching a peak at the end of the period, when it is mobilized for use throughout the dark period. This general pattern is observed in both the WT and ΔglgX strains, although the peak is shifted in the mutant (Fig. 4). However, the ΔglgX strain consistently maintains a greater amount of glycogen throughout the diurnal cycle, a result that is consistent with the findings for a ΔglgX mutant in Escherichia coli (33).
The most striking feature of the ΔglgX strain was the large increase in nitrogen fixation levels (Fig. 5). Under aerobic conditions, the ΔglgX strain showed up to 3-fold increases in nitrogen fixation, compared to the WT strain; with argon sparging, up to 6-fold increased rates were observed. When glycerol was added as a carbon source, a greater increase in nitrogen-fixing ability was seen in the WT strain than in the ΔglgX strain. This finding might indicate that a larger pool of carbon is available in the mutant cells, which allows higher rates of nitrogen fixation. Larger carbon pools in Cyanothece 51142 cells have been shown to lead to higher rates of nitrogenase activity (32). It is likely that the structure of the polysaccharide storage product is changed in the absence of glgX, and this modified storage product may facilitate greater nitrogen fixation by an unidentified process. Interestingly, another gene (cce_3194) in Cyanothece 51142 is listed as a glycogen-debranching enzyme; it has no significant similarity to glgX (cce_3465). Although cce_3194 is widely found in cyanobacteria, to our knowledge the role of this gene has not been characterized in any strain, and the gene is a candidate for future analysis.
MATERIALS AND METHODS
Strain cultivation and genetic modification.
Cyanothece sp. strain ATCC 51142 was acquired from the American Type Culture Collection (ATCC) (Manassas, VA). The ATCC maintained and shipped Cyanothece 51142 on 616 medium, which has the same composition as BG11 medium (34). We found that the strain could grow on both BG11 medium and ASP2 medium (5). For conjugation experiments, Cyanothece 51142 cells were grown in BG11 medium with added nitrate, with shaking at ∼150 rpm, under 30 µmol photons m−2 s−1 of white light in ambient air at 30°C. For physiological experiments, Cyanothece 51142 cells were grown in ASP2 liquid medium with or without added nitrate as required.
The ΔglgX strain was generated by insertion of a kanamycin resistance cassette into the glgX gene. The suicide plasmid pRL271-glgX was constructed by cloning ∼2 kb of the glgX gene (cce_3465) into the SacI and XhoI sites in pRL271, and then a kanamycin resistance gene was cloned into the internal AgeI site in the glgX gene. Triparental conjugation was performed using pRL443 as the conjugal plasmid and pRL623 as the helper plasmid, with pRL271-glgX transformed into the HB101 strain already containing pRL623 (27). Overnight cultures of the E. coli strains were mixed with Cyanothece 51142 cultures grown in BG11 medium (200 µl at an optical density at 730 nm [OD730] of 1.5, as measured with a µQuant plate reader [BioTek Instruments, Winooski, VT]). Mixed cells were incubated together for 2 h under low light at 30°C and then were plated onto HATF08250 filters (Millipore-Sigma, St. Louis, MO) on BG11 plates containing 5% (vol/vol) LB without antibiotic. After 3 days, filters were transferred to BG11 plates containing 50 µg/ml kanamycin without LB; colonies appeared after ∼9 days. Colonies were picked and grown on BG11 plates containing 10 µg/ml kanamycin.
Measurement of cell growth.
For growth measurements, Cyanothece cultures were maintained in ASP2 medium, with shaking at ∼150 rpm, under 30 µmol photons m−2 s−1 of white light in ambient air at 30°C. Culture aliquots were then diluted to an OD730 of 0.05 in a Multi-Cultivator MC 1000-OD device (Photon Systems Instruments, Drasov, Czech Republic), and growth under continuous light-emitting diode (LED) light of 200 µmol photons m−2 s−1 was measured at 730 nm for 72 h or longer.
Transmission electron microscopy.
Cells were grown in liquid ASP2 medium without nitrate, under 12-h light/dark conditions with 30 µmol photons m−2 s−1, at 30°C for 5 days. Cells were harvested at the appropriate time points by centrifugation, resuspended in a small volume, and transferred to aluminum planchettes with 100-µm-deep wells. Cells were fixed by ultrarapid high pressure freezing using a Bal-Tec high pressure freezer (Bal-Tec, Manchester, NH), followed by freeze substitution in 2% osmium/acetone and embedding in Spurr’s resin. Freeze substitution was performed for 3 days at −80°C, followed by slow thawing to room temperature. Thin sections (∼80 nm) were cut and stained with uranyl acetate and lead citrate and were imaged using a LEO 912AB transmission electron microscope equipped with a ProScan digital camera. Length and area measurements were performed using iTEM software (Olympus Soft Imaging Solutions, Lakewood, CO).
Determination of glycogen contents.
Cells were grown in liquid ASP2 medium without nitrate, under 12-h light/dark conditions with 30 µmol photons m−2 s−1, at 30°C and collected for glycogen measurements at the appropriate time points, and the glycogen contents were measured using a hexokinase assay kit (Millipore-Sigma). Cells were pelleted and 30% (wt/vol) KOH was added to remove free glucose, followed by incubation at 95°C for 90 min. Glycogen was precipitated by the addition of absolute ethanol on ice for 2 h and was collected by centrifugation. Pellets were washed with absolute ethanol, dried at 60°C, and resuspended in 300 µl of 100 mM sodium acetate buffer (pH 4.75). A 25-µl volume of sample was retained for measurement of the glucose background level. The remaining glycogen sample was digested with amyloglucosidase for 25 min at 55°C. For the enzyme assay, 25 µl of sample was mixed with the assay reagent in a light-proof microtiter plate and incubated for 15 min at room temperature. NADPH levels were measured at 340 nm with a µQuant plate reader (BioTek Instruments).
Nitrogen fixation assay.
Nitrogenase activity was measured using the acetylene reduction assay, as detailed in reference 14. Cultures were grown in liquid ASP2 medium without nitrate, under 12-h light/dark conditions with ∼100 µmol photons m−2 s−1, at 30°C for 5 days. Ten-milliliter or 5-ml culture volumes were transferred to airtight 36-ml glass vials and incubated in a 5% acetylene atmosphere under light at 100 µmol photons m−2 s−1 at 30°C for 24 h. For anaerobic incubation, the glass vials were flushed with argon for 10 min. Gas samples were withdrawn from the vials, and ethylene production was measured using an Agilent 6890N gas chromatograph equipped with a Poropak N column and a flame ionization detector, with argon as the carrier gas.
Phylogenetic analysis.
GlgX protein sequences were obtained from the JGI/IMG microbial database and aligned using ClustalW within MEGA 7. The phylogenetic tree was generated using MEGA 7 (maximum likelihood method). The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches.
Supplementary Material
ACKNOWLEDGMENTS
We thank all members of the Pakrasi laboratory for helpful discussions. We thank Howard Berg at the Donald Danforth Plant Science Center’s Imaging and Microscopy Facility for TEM assistance.
This work was supported by funding from the Gordon and Betty Moore Foundation through grant GBMF5760 to H.B.P.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02887-18.
REFERENCES
- 1.Zehr JP, Ward BB. 2002. Nitrogen cycling in the ocean: new perspectives on processes and paradigms. Appl Environ Microbiol 68:1015–1024. doi: 10.1128/AEM.68.3.1015-1024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar K, Mella-Herrera RA, Golden JW. 2010. Cyanobacterial heterocysts. Cold Spring Harb Perspect Biol 2:a000315. doi: 10.1101/cshperspect.a000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandyopadhyay A, Elvitigala T, Welsh E, Stöckel J, Liberton M, Min H, Sherman LA, Pakrasi HB. 2011. Novel metabolic attributes of the genus Cyanothece, comprising a group of unicellular nitrogen-fixing cyanobacteria. mBio 2:e00214-11. doi: 10.1128/mBio.00214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Sherman DM, Bao S, Sherman LA. 2001. Pattern of cyanophycin accumulation in nitrogen-fixing and non-nitrogen-fixing cyanobacteria. Arch Microbiol 176:9–18. doi: 10.1007/s002030100281. [DOI] [PubMed] [Google Scholar]
- 5.Reddy KJ, Haskell JB, Sherman DM, Sherman LA. 1993. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. J Bacteriol 175:1284–1292. doi: 10.1128/jb.175.5.1284-1292.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newie J, Neumann P, Werner M, Mata RA, Ficner R, Feussner I. 2017. Lipoxygenase 2 from Cyanothece sp. controls dioxygen insertion by steric shielding and substrate fixation. Sci Rep 7:2069. doi: 10.1038/s41598-017-02153-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Plooy SJ, Anandraj A, White S, Perissinotto R, Du Preez DR. 2018. Robust photosystem I activity by Cyanothece sp. (cyanobacteria) and its role in prolonged bloom persistence in Lake St. Lucia, South Africa. Extremophiles 22:639–650. doi: 10.1007/s00792-018-1025-8. [DOI] [PubMed] [Google Scholar]
- 8.Aryal UK, Ding Z, Hedrick V, Sobreira TJP, Kihara D, Sherman LA. 2018. Analysis of protein complexes in the unicellular cyanobacterium Cyanothece ATCC 51142. J Proteome Res 17:3628–3643. doi: 10.1021/acs.jproteome.8b00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito M, Endo K, Kobayashi K, Watanabe M, Ikeuchi M, Murakami A, Murata N, Wada H. 2018. High myristic acid content in the cyanobacterium Cyanothece sp. PCC 8801 results from substrate specificity of lysophosphatidic acid acyltransferase. Biochim Biophys Acta Mol Cell Biol Lipids 1863:939–947. doi: 10.1016/j.bbalip.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Welsh EA, Liberton M, Stöckel J, Loh T, Elvitigala T, Wang C, Wollam A, Fulton RS, Clifton SW, Jacobs JM, Aurora R, Ghosh BK, Sherman LA, Smith RD, Wilson RK, Pakrasi HB. 2008. The genome of Cyanothece 51142, a unicellular diazotrophic cyanobacterium important in the marine nitrogen cycle. Proc Natl Acad Sci U S A 105:15094–15099. doi: 10.1073/pnas.0805418105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stöckel J, Welsh EA, Liberton M, Kunnvakkam R, Aurora R, Pakrasi HB. 2008. Global transcriptomic analysis of Cyanothece 51142 reveals robust diurnal oscillation of central metabolic processes. Proc Natl Acad Sci U S A 105:6156–6161. doi: 10.1073/pnas.0711068105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stöckel J, Jacobs JM, Elvitigala TR, Liberton M, Welsh EA, Polpitiya AD, Gritsenko MA, Nicora CD, Koppenaal DW, Smith RD, Pakrasi HB. 2011. Diurnal rhythms result in significant changes in the cellular protein complement in the cyanobacterium Cyanothece 51142. PLoS One 6:e16680. doi: 10.1371/journal.pone.0016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberton M, Austin JR, Berg RH, Pakrasi HB. 2011. Unique thylakoid membrane architecture of a unicellular N2-fixing cyanobacterium revealed by electron tomography. Plant Physiol 155:1656–1666. doi: 10.1104/pp.110.165332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandyopadhyay A, Stöckel J, Min H, Sherman LA, Pakrasi HB. 2010. High rates of photobiological H2 production by a cyanobacterium under aerobic conditions. Nat Commun 1:139. doi: 10.1038/ncomms1139. [DOI] [PubMed] [Google Scholar]
- 15.Bandyopadhyay A, Elvitigala T, Liberton M, Pakrasi HB. 2013. Variations in the rhythms of respiration and nitrogen fixation in members of the unicellular diazotrophic cyanobacterial genus Cyanothece. Plant Physiol 161:1334–1346. doi: 10.1104/pp.112.208231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnakumar S, Gaudana SB, Digmurti MG, Viswanathan GA, Chetty M, Wangikar PP. 2015. Influence of mixotrophic growth on rhythmic oscillations in expression of metabolic pathways in diazotrophic cyanobacterium Cyanothece sp. ATCC 51142. Bioresour Technol 188:145–152. doi: 10.1016/j.biortech.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Min H, Sherman LA. 2010. Genetic transformation and mutagenesis via single-stranded DNA in the unicellular, diazotrophic cyanobacteria of the genus Cyanothece. Appl Environ Microbiol 76:7641–7645. doi: 10.1128/AEM.01456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki E, Umeda K, Nihei S, Moriya K, Ohkawa H, Fujiwara S, Tsuzuki M, Nakamura Y. 2007. Role of the GlgX protein in glycogen metabolism of the cyanobacterium, Synechococcus elongatus PCC 7942. Biochim Biophys Acta 1770:763–773. doi: 10.1016/j.bbagen.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Cano M, Holland SC, Artier J, Burnap RL, Ghirardi M, Morgan JA, Yu J. 2018. Glycogen synthesis and metabolite overflow contribute to energy balancing in cyanobacteria. Cell Rep 23:667–672. doi: 10.1016/j.celrep.2018.03.083. [DOI] [PubMed] [Google Scholar]
- 20.Deschamps P, Colleoni C, Nakamura Y, Suzuki E, Putaux JL, Buleon A, Haebel S, Ritte G, Steup M, Falcon LI, Moreira D, Loffelhardt W, Raj JN, Plancke C, d'Hulst C, Dauvillee D, Ball S. 2008. Metabolic symbiosis and the birth of the plant kingdom. Mol Biol Evol 25:536–548. doi: 10.1093/molbev/msm280. [DOI] [PubMed] [Google Scholar]
- 21.Ball S, Colleoni C, Cenci U, Raj JN, Tirtiaux C. 2011. The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J Exp Bot 62:1775–1801. doi: 10.1093/jxb/erq411. [DOI] [PubMed] [Google Scholar]
- 22.Cenci U, Chabi M, Ducatez M, Tirtiaux C, Nirmal-Raj J, Utsumi Y, Kobayashi D, Sasaki S, Suzuki E, Nakamura Y, Putaux JL, Roussel X, Durand-Terrasson A, Bhattacharya D, Vercoutter-Edouart AS, Maes E, Arias MC, Palcic M, Sim L, Ball SG, Colleoni C. 2013. Convergent evolution of polysaccharide debranching defines a common mechanism for starch accumulation in cyanobacteria and plants. Plant Cell 25:3961–3975. doi: 10.1105/tpc.113.118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura Y, Takahashi J, Sakurai A, Inaba Y, Suzuki E, Nihei S, Fujiwara S, Tsuzuki M, Miyashita H, Ikemoto H, Kawachi M, Sekiguchi H, Kurano N. 2005. Some cyanobacteria synthesize semi-amylopectin type α-polyglucans instead of glycogen. Plant Cell Physiol 46:539–545. doi: 10.1093/pcp/pci045. [DOI] [PubMed] [Google Scholar]
- 24.Welkie DG, Lee BH, Sherman LA. 2015. Altering the structure of carbohydrate storage granules in the cyanobacterium Synechocystis sp. strain PCC 6803 through branching-enzyme truncations. J Bacteriol 198:701–710. doi: 10.1128/JB.00830-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman LA, Meunier P, Colon LMS. 1998. Diurnal rhythms in metabolism: a day in the life of a unicellular, diazotrophic cyanobacterium. Photosynth Res 58:25–42. doi: 10.1023/A:1006137605802. [DOI] [Google Scholar]
- 26.Welkie DG, Sherman DM, Chrisler WB, Orr G, Sherman LA. 2013. Analysis of carbohydrate storage granules in the diazotrophic cyanobacterium Cyanothece sp. PCC 7822. Photosynth Res 118:25–36. doi: 10.1007/s11120-013-9941-z. [DOI] [PubMed] [Google Scholar]
- 27.Elhai J, Wolk CP. 1988. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol 167:747–754. doi: 10.1016/0076-6879(88)67086-8. [DOI] [PubMed] [Google Scholar]
- 28.Arnon DI, McSwain BD, Tsujimoto Y, Wada K. 1974. Photochemical activity and components of membrane preparation from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta 357:231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Sherman DM, Sherman LA. 2014. The uptake hydrogenase in the unicellular diazotrophic cyanobacterium Cyanothece sp. strain PCC 7822 protects nitrogenase from oxygen toxicity. J Bacteriol 196:840–849. doi: 10.1128/JB.01248-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elhai J, Vepritskiy A, Muro-Pastor AM, Flores E, Wolk CP. 1997. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J Bacteriol 179:1998–2005. doi: 10.1128/jb.179.6.1998-2005.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneegurt MA, Sherman DM, Sherman LA. 1997. Composition of the carbohydrate granules of the cyanobacterium Cyanothece sp. strain ATCC 51142. Arch Microbiol 167:89–98. doi: 10.1007/s002030050420. [DOI] [PubMed] [Google Scholar]
- 32.Stöckel J, Elvitigala T, Liberton M, Pakrasi HB. 2013. Carbon availability affects diurnally controlled processes and cell morphology of Cyanothece 51142. PLoS One 8:e56887. doi: 10.1371/journal.pone.0056887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dauvillée D, Kinderf IS, Li Z, Kosar-Hashemi B, Samuel MS, Rampling L, Ball S, Morell MK. 2005. Role of the Escherichia coli glgX gene in glycogen metabolism. J Bacteriol 187:1465–1473. doi: 10.1128/JB.187.4.1465-1473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen MM. 1968. Simple conditions for growth of unicellular blue-green algae on plates. J Phycol 4:1–4. doi: 10.1111/j.1529-8817.1968.tb04667.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.