Due to a lack of genetic tools, Clostridium cellulovorans DSM 743B has not been comprehensively explored as a putative strain platform for n-butanol production by consolidated bioprocessing (CBP). Based on the previous study of genetic tools, strain engineering of C. cellulovorans for the development of a CBP-enabling microbial chassis was demonstrated in this study. Metabolic engineering and evolutionary engineering were integrated to improve the n-butanol production of C. cellulovorans from the low-cost renewable agricultural waste of alkali-extracted, deshelled corn cobs (AECC). The n-butanol production from AECC was increased 138-fold, from less than 0.025 g/liter to 3.47 g/liter, which represents the highest titer of n-butanol produced using a single recombinant clostridium strain by CBP reported to date. This engineered strain serves as a promising chassis for n-butanol production from lignocellulose by CBP.
KEYWORDS: Clostridium, adaptive laboratory evolution, consolidated bioprocessing, metabolic engineering, n-butanol
ABSTRACT
Clostridium cellulovorans DSM 743B offers potential as a chassis strain for biomass refining by consolidated bioprocessing (CBP). However, its n-butanol production from lignocellulosic biomass has yet to be demonstrated. This study demonstrates the construction of a coenzyme A (CoA)-dependent acetone-butanol-ethanol (ABE) pathway in C. cellulovorans by introducing adhE1 and ctfA-ctfB-adc genes from Clostridium acetobutylicum ATCC 824, which enabled it to produce n-butanol using the abundant and low-cost agricultural waste of alkali-extracted, deshelled corn cobs (AECC) as the sole carbon source. Then, a novel adaptive laboratory evolution (ALE) approach was adapted to strengthen the n-butanol tolerance of C. cellulovorans to fully utilize its n-butanol output potential. To further improve n-butanol production, both metabolic engineering and evolutionary engineering were combined, using the evolved strain as a host for metabolic engineering. The n-butanol production from AECC of the engineered C. cellulovorans was increased 138-fold, from less than 0.025 g/liter to 3.47 g/liter. This method represents a milestone toward n-butanol production by CBP, using a single recombinant clostridium strain. The engineered strain offers a promising CBP-enabling microbial chassis for n-butanol fermentation from lignocellulose.
IMPORTANCE Due to a lack of genetic tools, Clostridium cellulovorans DSM 743B has not been comprehensively explored as a putative strain platform for n-butanol production by consolidated bioprocessing (CBP). Based on the previous study of genetic tools, strain engineering of C. cellulovorans for the development of a CBP-enabling microbial chassis was demonstrated in this study. Metabolic engineering and evolutionary engineering were integrated to improve the n-butanol production of C. cellulovorans from the low-cost renewable agricultural waste of alkali-extracted, deshelled corn cobs (AECC). The n-butanol production from AECC was increased 138-fold, from less than 0.025 g/liter to 3.47 g/liter, which represents the highest titer of n-butanol produced using a single recombinant clostridium strain by CBP reported to date. This engineered strain serves as a promising chassis for n-butanol production from lignocellulose by CBP.
INTRODUCTION
Due to the unsustainability of oil resources and global environmental deterioration caused by the excessive use of fossil fuels, considerable attention has recently been focused on lignocellulose biorefinery using a fermentation process for various chemicals and fuels (1). A promising fermentation product as a substitute for petroleum is butanol, which can be used as both an industrial commodity and a gasoline substitute (2). Therefore, attention has focused on the production of butanol from various lignocellulosic feedstocks by microbial fermentation. The traditional process involves the synthesis of cellulases, saccharification, and hexose/pentose co-utilization (3, 4). Alternatively, consolidated bioprocessing (CBP) can combine all these processes within one step, which would decrease the cost of capital investment for cellulase production and, thus, offer the potential of economical production of butanol at industrial scale (5). However, no natural CBP-enabling microorganisms or microbial consortia with the ability to produce butanol are currently available (6, 7).
The combination of both cellulolytic and butanol-producing phenotypes within a single microorganism is an appealing challenge. A number of explorations have attempted to engineer Escherichia coli or Saccharomyces cerevisiae strains that directly produce n-butanol/isobutanol from lignocellulose. Achieved advances have been recently reviewed (8–11). However, only a very low butanol titer could be obtained (8). An alternative strategy is to develop native cellulolytic or solventogenic clostridia as an initial microbial chassis (12). Several key milestones have already been accomplished in overexpressing cellulase-encoding genes or the in vivo assembly of chimeric minicellulosome in solventogenic clostridia (13–15); however, these recombinant strains are still not capable of efficient growth on lignocellulose. In contrast, introducing a butanol metabolic pathway into native cellulolytic clostridia represents a more meaningful progress toward the realization of CBP. The engineered Clostridium thermocellum has been reported to produce 5.4 g/liter of isobutanol from cellulose via a hybrid keto acid pathway (16). Similarly, other anaerobic cellulolytic bacteria, such as Clostridium cellulolyticum and Clostridium cellulovorans (12, 17, 18), have also been suggested to have the potential to produce butanol by CBP after metabolic engineering.
C. cellulovorans DSM 743B is an anaerobic, celluloytic mesophile that can directly produce butyric acid as the main metabolic product from lignocellulose (19, 20). The complete sequencing and annotation of the genome deepened the understanding of both the plant cell wall degradation system and the metabolic network in C. cellulovorans (21). Studies on nuclease sequence analysis, cellulosome component properties, exoproteome profiles, and crystal structures of key cellulases have also achieved significant progress (22–25). Other breakthroughs on C. cellulovorans include genetic system development and genetic tool verification, such as ClosTron and the CRISPR/Cas system, which provide a valuable foundation for metabolic engineering (6, 12, 26, 27). As a proof of concept, Yang et al. overexpressed an aldehyde/alcohol dehydrogenase (adhE2) in C. cellulovorans, which enabled the recombinant strain to produce 1.42 g/liter n-butanol within 252 h (28). This represented the first metabolic engineering exemplified in C. cellulovorans; however, several other approaches remain unexplored, such as complicated genetic modification, n-butanol tolerance evolution, and process control optimization, which may promote the strain development of C. cellulovorans. In a previous study, Wen et al. developed a twin clostridial consortium in which C. cellulovorans DSM 743B was comprehensively engineered to promote the butyrate supply for Clostridium beijerinckii NCIMB 8052 to achieve n-butanol production (6). However, the ability of C. cellulovorans to produce n-butanol was not exploited.
In this study, metabolic engineering and adaptive laboratory evolution were integrated to explore the n-butanol production of C. cellulovorans (pure culture) by CBP, as shown in Fig. 1. A coenzyme A (CoA)-dependent acetone-butanol-ethanol (ABE) pathway was introduced into C. cellulovorans to direct carbon flux from butyrate to n-butanol. Then, a novel evolutionary approach was developed to improve the n-butanol tolerance of C. cellulovorans, which may boost its n-butanol production potential. Finally, the ABE pathway was reconstructed in the evolved strains. The obtained strains produced far more butanol than unevolved recombinant strains.
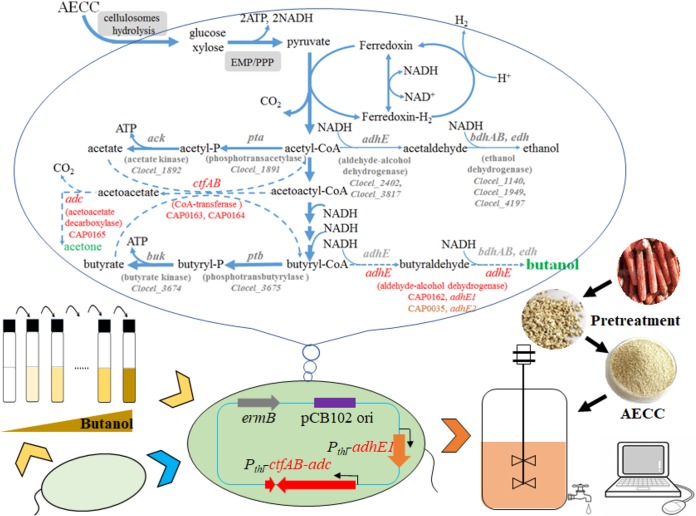
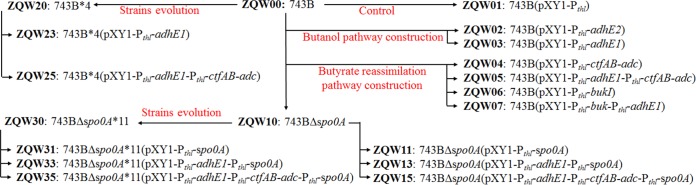
FIG 1.
Integrated metabolic and evolutionary engineering of Clostridium cellulovorans DSM 743B for n-butanol production from AECC by CBP. A CoA-dependent acetone-butanol-ethanol (ABE) pathway composed of adhE1 and ctfA-ctfB-adc (red font) from C. acetobutylicum ATCC 824 was constructed in C. cellulovorans. In parallel, C. cellulovorans was grown in medium with a serial enrichment of n-butanol to evolve and reinforce its butanol tolerance. To maximize butanol production, metabolic and evolutionary engineering were combined, using the evolved strain as a host for metabolic engineering.
RESULTS
Heterologous adhE1 introduction enables C. cellulovorans to produce n-butanol.
According to the prediction of KEGG, a complete CoA-dependent butanol synthesis pathway exists in C. cellulovorans. However, only less than 0.025 g/liter of n-butanol can be detected in broth (29). Low endogenous alcohol/aldehyde dehydrogenase activity was confirmed to be a bottleneck for n-butanol production (28). It has been reported that adhE1 overexpression (encoding an alcohol/aldehyde dehydrogenase in Clostridium acetobutylicum) can restore the n-butanol production to wild-type levels in the C. acetobutylicum M5 strain (a nonsporulating, non-solvent-producing mutant without the pSOL1 megaplasmid containing alcohol/aldehyde dehydrogenase and the acetone-formation genes) (30, 31). Accordingly, this study attempted to modify the n-butanol pathway in C. cellulovorans by introducing alcohol/aldehyde dehydrogenase genes.
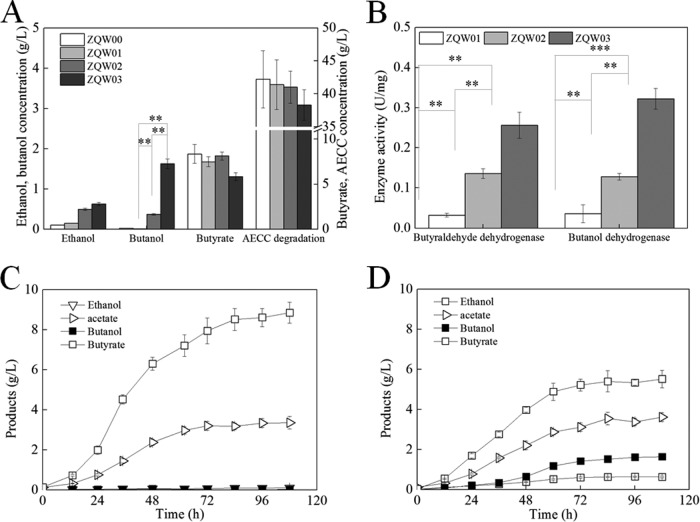
There are two homologous adhE genes: adhE1 (CA_p0162, located in the sol operon orfL-adhE1-ctfA-ctfB) and adhE2 (CA_p0035, a monocistronic operon) in the C. acetobutylicum ATCC 824 strain (32, 33). The effect of overexpressing either of these two genes on n-butanol production was evaluated (Fig. 2A). ZQW02 (743B/pXY1-Pthl-adhE2) produced 0.37 g/liter n-butanol with alkali-extracted, deshelled corn cobs (AECC) as the sole carbon source within 108 h. However, the titer of n-butanol produced by ZQW03 (743B/pXY1-Pthl-adhE1) reached 1.63 g/liter, which is almost 4-fold that achieved by ZQW02. The butyraldehyde dehydrogenase activity and the n-butanol dehydrogenase activity in ZQW03 were 88.2% and 152% higher than activities in ZQW02 and 7.97- and 13.6-fold higher than those in ZQW01 (743B/pXY1-Pthl) (as a control), which indicates why the n-butanol output by ZQW03 was higher than that by ZQW02 (Fig. 2B).
FIG 2.
Overexpression of adhE1 enabled C. cellulovorans to produce more n-butanol than overexpression of adhE2. (A) Effects of adhE1 and adhE2 introduction on butanol production. (B) Comparison of butyraldehyde dehydrogenase activities and butanol dehydrogenase activities between the ZQW02 and ZQW03 strains. (C and D) Fermentation profiles of ZQW01 and ZQW03, respectively, with AECC as the sole carbon source. The data in panels A and B are the means and standard deviations of three replicates (***, P ≤ 0.001; **, P ≤ 0.01; t test).
Then, the fermentation progress of ZQW03 was explored in a 3-liter bioreactor with ZQW01 as a control (Fig. 2C and D; see also Fig. S1 in the supplemental material). Less butyrate was accumulated in the broth by ZQW03 than by ZQW01. Moreover, n-butanol synthesis by ZQW03 coincides with butyrate accumulation, and butyrate hardly decreased in the fermentation anaphase, indicating that butyrate was almost not reassimilated by ZQW03 itself. Within 108 h, ZQW03 produced 1.64 g/liter n-butanol and 0.635 g/liter ethanol from 37.2 g/liter AECC with a residual of 5.52 g/liter butyrate and 8.78 g/liter total sugars (mainly pentose) in the broth. These results represent an acetone-uncoupled n-butanol production process by CBP, which indicates that C. cellulovorans has the potent to produce biofuel mixtures with a high n-butanol ratio, as previously suggested (34, 35).
Butyrate reassimilation by overexpressing ctfAB-adc to drive n-butanol production.
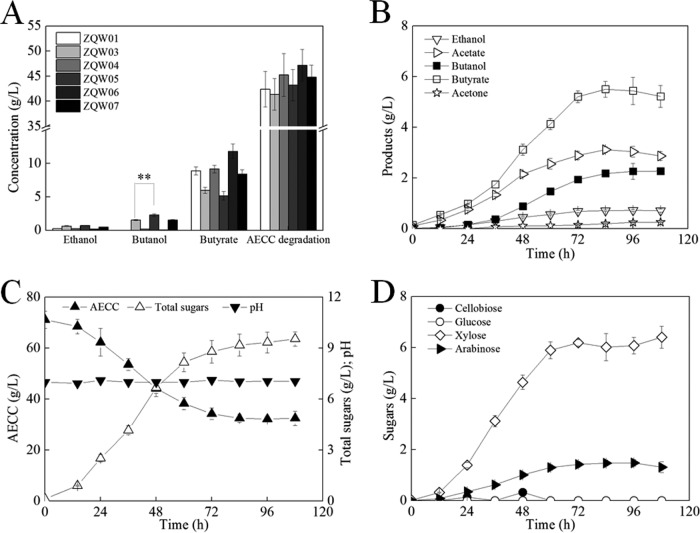
According to the working hypothesis, n-butanol titer and yield may be further promoted by reassimilating and transferring the butyrate accumulated by ZQW02 and ZQW03 to n-butanol. In C. acetobutylicum, it has been reported that the bifunctional BK-I encoded by bukI (CA_c3075) not only catalyzes butyrate formation but also is responsible for the uptake of butyrate without simultaneous production of acetone (36, 37). Therefore, bukI from C. acetobutylicum 824 was overexpressed coupled with adhE1 in C. cellulovorans. However, the n-butanol output of ZQW07 (743B/pXY1-Pthl-buk-Pthl-adhE1) did not noticeably change, while the residual butyrate increased by 32.6% (Fig. 3A). This indicates a much higher efficiency of butyrate synthesis than the reassimilation in C. cellulovorans. This further suggests that the knockout of endogenous buk in C. cellulovorans may help to direct the butyrate flux to n-butanol formation; however, a C. cellulovorans mutant deficient in buk (Clocel_3674, encoding butyrate kinase) could not be generated using ClosTron even with long-term screening.
FIG 3.
Overexpressing ctfAB-adc in ZQW03 dries n-butanol production by reassimilating butyrate. (A) Effects of ctfAB-adc and bukI introduction on n-butanol production and butyrate reassimilation. (B to D) Batch fermentation profile of ZQW05 with AECC as the sole carbon source. The data in panel A are the means and standard deviations of three replicates (**, P ≤ 0.01; t test).
Alternatively, a routine strategy for butyrate reassimilation in C. acetobutylicum and C. beijerinckii was used under solventogenesis. The strategy of overexpressing genes involved in the CoA transferase encoded by ctfA-ctfB (CA_p0163-0164 or Cbei_3833-3834) has been successfully verified in C. acetobutylicum, Clostridium tyrobutyricum, and C. beijerinckii (6, 38, 39). Both the adhE1 and ctfA-ctfB genes located in C. acetobutylicum’s sol operon were introduced, followed by its adjacent adc gene (CA_p0165, with native promoter) (33), since there are no known genes involved in the acetoacetate metabolism of C. cellulovorans. Interestingly, the heterologous ABE (acetone-butanol-ethanol) pathway worked very well in ZQW05 (743B/pXY1-Pthl-adhE1-Pthl-ctfAB-adc). Compared to ZQW03, the n-butanol output of ZQW05 was enhanced by 49.3%, while residual butyrate and acetate decreased by 13.2% and 17.3%, respectively (Fig. 3A). It is worth noting that the overexpression of ctfAB-adc (without adhE1) in wild-type C. cellulovorans showed no improvement of n-butanol production. This indirectly supports the hypothesis that low alcohol/aldehyde dehydrogenase activity maybe a bottleneck for the n-butanol synthesis in wild-type C. cellulovorans.
Furthermore, the fermentation progress of ZQW05 was investigated in a 3-liter bioreactor (Fig. 3B to D). Within 108 h, ZQW05 produced 2.27 g/liter n-butanol and 0.712 g/liter ethanol from 38.7 g/liter AECC. This was not a typical two-phase fermentation of acidogenesis followed by solventogenesis as in solventogenic clostridia (40). n-Butanol production coincides with butyrate accumulation during the first 72 h, after which acetone was also produced. Simultaneously, acetate and butyrate decreased by 8% and 5.1% from their peak titers, which were inferred to be reassimilated by the engineered C. cellulovorans strain harboring ctfA-ctfB genes (Fig. 3B). During the fermentation anaphase, AECC degradation, total sugar utilization, and n-butanol production almost ceased. This finding was in agreement with the results of Yang et al. when their engineered C. cellulovorans strain was cultured with glucose or cellobiose as the sole carbon source (28).
Adaptive laboratory evolution to improve the n-butanol tolerance of C. cellulovorans.
Butanol toxicity is a main barrier for solvent production in Clostridium (mainly butanol) with a high titer since it may cause microbial growth inhibition and cell death (41). Improvement in butanol tolerance sometimes leads to an increase in butanol production (42). The cellular responses and molecular mechanisms that can overcome butanol stress in Clostridium are complex and not yet fully understood (43). Laboratory-adaptive evolution has been shown to be an efficient strategy for the improvement of butanol tolerance (44, 45). In this study, two evolutionary approaches were adapted to boost the n-butanol tolerance of C. cellulovorans to improve n-butanol production.
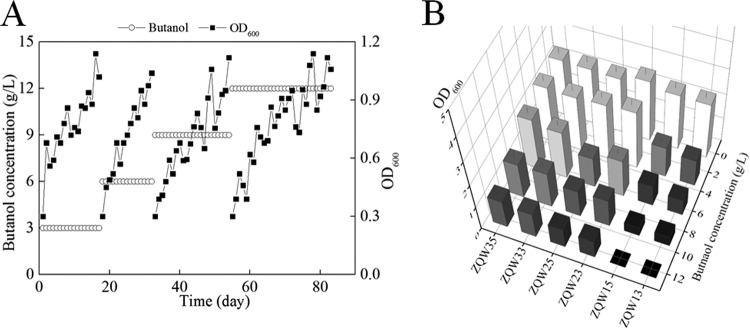
Wild-type ZQW00 (743B) and recombinant ZQW10 (743B Δspo0A) were chosen as parent strains for parallel adaptive evolution experiments. Both were cultured in medium supplemented with a serial enrichment of n-butanol (from 3 g/liter to 12 g/liter) to promote evolution. As expected, the culture concomitantly adapted to these intensified conditions. It took 17, 15, and 22 days for ZQW10 to evolve so that it could successfully resist 3 g/liter, 6 g/liter, and 9 g/liter of butanol, respectively (Fig. 4A); however, for ZQW00, it took 11, 11, and 25 days, respectively (Fig. S2). The offspring of ZQW10 needed about 29 days to achieve a stable biomass above 1.0 (optical density at 600 nm [OD600]), while ZQW00 could not further evolve in medium with added 12 g/liter of n-butanol (Fig. 4A and Fig. S2).
FIG 4.
Adaptive laboratory evolution improved n-butanol tolerance of C. cellulovorans. (A) The evolution process of ZQW10. ZQW10 was subcultured in medium supplemented with stepwise enrichment of butanol (3, 6, 9, and 12 g/liter) to evolve and strengthen its n-butanol tolerance. (B) Comparison of n-butanol tolerance levels of mutant strains derived from ZQW10, ZQW20, and ZQW30. Due to evolution, ZQW23, ZQW25, ZQW33, and ZQW35 can tolerate higher concentrations of n-butanol than ZQW13 and ZQW15. In addition, the n-butanol tolerance levels of ZQW33 and ZQW35 were superior to those of ZQW23 and ZQW25.
After four rounds of evolution over a total of 83 days, the resulting evolved ZQW10 and ZQW00 strains were harvested and spread on plates that were supplemented with 12 g/liter and 9 g/liter n-butanol (because the evolved ZQW00 cannot grow well in medium with 12 g/liter of n-butanol), respectively. The phenotype of several evolved colonies and selected strains ZQW30 (743B Δspo0A*11), derived from ZQW10, and ZQW20 (743B*4), derived from ZQW00, were tested for further assessment and engineering (Fig. S3). With an initial 12 g/liter of n-butanol in the medium, the 48-h biomass of ZQW30 achieved 45.2% of that cultured in blank Clocel medium (without n-butanol) (6, 49), while ZQW10 did not show noticeable growth. The n-butanol tolerance levels of mutants derived from ZQW00, ZQW10, ZQW20, and ZQW30 were evaluated (Fig. 4B and Fig. S4). In general, the strains derived from ZQW30 can resist more butanol than those derived from ZQW20. Since ZQW35 can resist more butanol than ZQW25, ZQW35 was selected for subsequent exploration (Fig. 4B).
spo0A inactivation seems to contribute to strain evolution but weakens AECC degradation by ZQW10 and ZQW30 (Fig. S5). This may be because the spo0A-deficient mutants were prone to cell lysis (46, 47). The spo0A gene was complemented using plasmid-based expression, driven by a thl promoter (48) in ZQW10 and ZQW30. Both ZQW11 (743B Δspo0A/pXY1-Pthl-spo0A) and ZQW31 (743B Δspo0A*11/pXY1-Pthl-spo0A) restored their AECC degradation abilities to a level similar to that of the wild-type (Fig. S5). Accordingly, spo0A complementation was implemented in all mutants derived from ZQW10 and ZQW30, and spo0A complementation did not strongly affect n-butanol tolerance (Fig. S4).
Combination of metabolic and evolutionary engineering to improve n-butanol production.
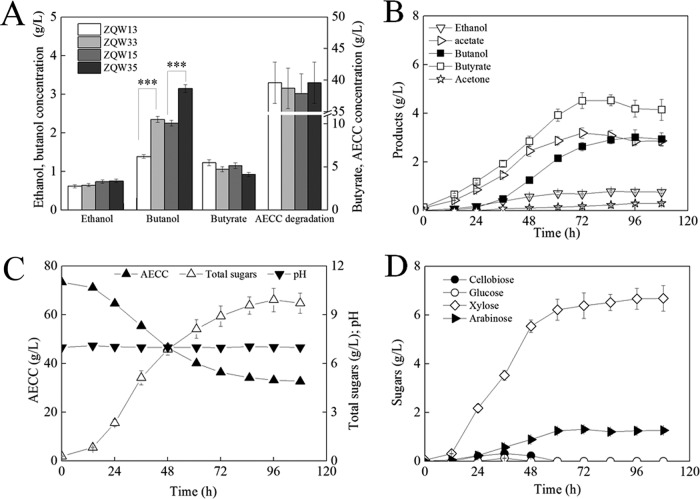
We decided to include more advantageous genetic modifications as described above in the evolved strains toward a further improvement of n-butanol production. The metabolic profiles were compared among mutants derived from different parent strains (Fig. 5 and 6A). The n-butanol output of ZQW33 (743B Δspo0A*11/pXY1-Pthl-adhE1-Pthl-spo0A) and ZQW35 (743B Δspo0A*11/pXY1-Pthl-adhE1-Pthl-ctfAB-adc-Pthl-spo0A) increased by 52.2% and 50.5% compared to the levels of ZQW13 (743B Δspo0A/pXY1-Pthl-adhE1-Pthl-spo0A) and ZQW15 (743B Δspo0A/pXY1-Pthl-adhE1-Pthl-ctfAB-adc-Pthl-spo0A), respectively. This indicates that the reinforced n-butanol tolerance improved n-butanol production.
FIG 5.
The dendrogram of genetically modified C. cellulovorans strains in this study.
FIG 6.
Improved butanol tolerance led an enhancement in n-butanol production. (A) Effects of laboratory-adaptive evolution on n-butanol production. Mutants ZQW33 and ZQW35 that were derived from evolved strain ZQW30 produced much more n-butanol than the mutants ZQW13 and ZQW15 that were derived from ZQW10. (B to D) Metabolic profile of batch fermentation of ZQW35 with AECC as the sole carbon source. The data in panel A are the means and standard deviations of three replicates (***, P ≤ 0.001; t test).
Batch fermentation in a 3-liter bioreactor was performed to investigate the metabolic profile of ZQW35. During the first 36 h, no obvious phenotype difference was found between strains before and after evolution. However, AECC degradation, butyrate reassimilation, and n-butanol production improved during the fermentation anaphase, suggesting relatively higher cell viability of ZQW35 than of ZQW05. During 96 h, ZQW35 produced 3.02 g/liter n-butanol and 0.777 g/liter ethanol from 40.2 g/liter AECC with 4.19 g/liter of residual butyrate (Fig. 6B to D). Compared to levels of ZQW05, the n-butanol production increased by 33%, and the residual butyrate decreased by 19.8%.
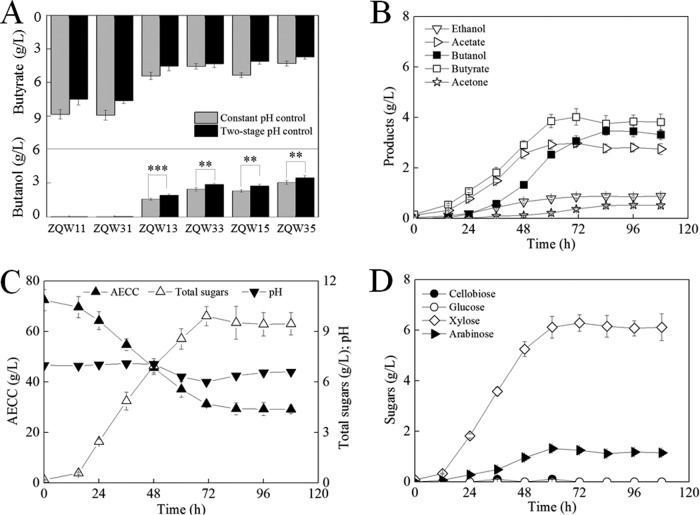
A two-stage pH control strategy was proposed to coordinate native cellular growth (pH of approximately 7.0) (28, 29) and cellulase production with the heterologous pathway of n-butanol synthesis and butyrate reassimilation (pH 4.5 to 6.0) in C. cellulovorans (33, 40, 49). This further improved n-butanol production. In such a strategy, pH was controlled at 7.0 during the first 48 h and then decreased to 6.0. In anaerobic shaken flasks, the n-butanol production of ZQW13, ZQW33, ZQW15, and ZQW35 increased by 22.4%, 17.3%, 19.1%, and 13.3%, respectively. Simultaneously, their butyrate secretion levels decreased by 16.3%, 5%, 23.4%, and 15.6%, respectively (Fig. 7A).
FIG 7.
Two-stage pH control strategy to improve n-butanol production further. (A) Effects of two-stage pH control strategy on n-butanol and butyrate production. (B to D) Metabolic profile of batch fermentation of ZQW35 using a two-stage pH control strategy with AECC as the sole carbon source. The data in panel A are the means and standard deviations of three replicates (***, P ≤ 0.001; **, P ≤ 0.01; t test).
A two-stage pH control strategy was implemented in a 3-liter bioreactor to maximize the butanol output of ZQW35 (Fig. 7B to D). Compared to the fermentation behavior shown in Fig. 6B, both the n-butanol accumulation and butyrate reassimilation clearly accelerated between 48 and 72 h but almost ceased after 84 h. The batch fermentation can be stopped 12 h earlier than that in the experiment shown in Fig. 6B. During 84 h, ZQW35 produced 3.47 g/liter n-butanol and 0.875 g/liter ethanol from 43.1 g/liter AECC, with 3.76 g/liter butyrate and 9.53 g/liter residual total sugars (mainly xylose and arabinose) in the broth (Fig. 7B to D). The n-butanol production increased by 14.9%, and the n-butanol productivity increased by 31.3% compared to that obtained under a control of constant neutral pH. This supports a beneficial two-stage pH control strategy. The n-butanol titer and productivity here were 2.44-fold and 7.32-fold higher than the levels obtained in a previous study (28).
DISCUSSION
In the present study, metabolic engineering and adaptive laboratory evolution were integrated to improve the n-butanol production of C. cellulovorans DSM 743B from AECC, a low-cost and renewable agricultural waste. This represents an application of a previous study of genetic tools of C. cellulovorans DSM 743B in CBP-enabling microbial chassis development (6).
To enable C. cellulovorans DSM 743B to produce n-butanol, the CoA-dependent ABE pathway, including adhE1 and ctfAB-adc genes from C. acetobutylicum ATCC 824, were introduced. The metabolically engineered strains showed increased butanol output compared to results in the study of Yang et al., who overexpressed only adhE2 in C. cellulovorans and eventually achieved 1.42 g/liter n-butanol production from cellulose (28). It is interesting that ZQW03 (743B/pXY1-Pthl-adhE1) exhibited higher n-butanol output, butyraldehyde dehydrogenase activity, and butanol dehydrogenase activity than ZQW02 (743B/pXY1-Pthl-adhE2) (Fig. 2B). The higher butanol dehydrogenase activity in ZQW03 may be attributed to C. cellulovorans itself. Since C. cellulovorans possesses several putative butanol dehydrogenase-encoding genes (Clocel_1140, Clocel_1949, Clocel_4197, Clocel_2402, and Clocel_3817), it is likely that one of them might be induced by the butyraldehyde generated by the heterogenous AdhE1. A similar phenomenon has been demonstrated in C. acetobutylicum under solventogenesis, with the exception that the butanol dehydrogenases expressed in C. acetobutylicum are NADPH dependent (32).
Previous studies confirmed Spo0A as a transcriptional regulator that is closely related to sporulation, solvent production, and butanol tolerance in Clostridium (50, 51). It plays a central role by mainly regulating sporulation-specific sigma factors, solvent genes, and heat shock protein genes (52). Overexpression of spo0A can enhance the solvent tolerance of Clostridium, thus increasing its solvent yield; however, this leads to the early onset of sporulation (50, 51).
We hypothesized that there may be further molecular mechanisms that lead to stress resistance in C. cellulovorans, such as several two-component systems or membrane transport proteins, that are not entirely dependent on spo0A (53, 54). When spo0A is knocked out, attenuated, or mutated, other mechanisms may be activated or reinforced for the strain to respond to butanol stress. Accordingly, two parallel evolutionary approaches that use the spo0A-knockout strain ZQW10 and wild-type ZQW00 as original strains were investigated and compared.
The evolved spo0A-deficient strain ZQW30 was demonstrated to tolerate 12 g/liter of butanol after four rounds, or a total of 83 days, of domestication. This implied putative molecular mechanisms independent of spo0A in C. cellulovorans that resist such high butanol concentrations. Comparative analysis of genome resequencing, transcriptional analysis, and functional genomics studies of ZQW30, ZQW20, and ZQW00, coupled with subsequent genetic and biochemical validation experiments, are currently being conducted to investigate the underlying molecular mechanism that is responsible for the higher n-butanol resistance in ZQW20 and ZQW30. The evolutionary approach based on the spo0A-knockout strain offers a new perspective for the exploration of diversities of stress response mechanisms in Clostridium.
In addition, spo0A inactivation helps to filter false positives benefiting from spo0A-related stress mechanisms, which leads to a more efficient and robust adaptive laboratory evolution. The inactivation of spo0A weakens the stress adaptability of Clostridium, which means that Clostridium is unable to survive (or resist butanol) by forming spores or by regulating spo0A expression levels (thus regulating target genes) (52). These circumstances drive the strain to either evolve or die. As shown in Fig. S3, far more survivors derived from ZQW10 than those derived from ZQW00 have real butanol tolerance phenotypes. Therefore, ZQW10 evolved more smoothly (or better) than ZQW00, and the n-butanol tolerance levels of the strains derived from ZQW30 (evolved ZQW10) were generally superior to those derived from ZQW20 (evolved ZQW00) (see Fig. 5; see also Fig. S4 in the supplemental material).
In this respect, the inactivation of genes involved in sporulation, such as spo0A in C. cellulovorans, provides a novel and reliable approach for adaptive laboratory evolution toward enhanced butanol tolerance of Clostridium, which may be extended to the study of lignocellulosic hydrolysate resistance.
The reinforced n-butanol tolerance indeed improved the n-butanol production from 2.27 g/liter of ZQW05 to 3.02 g/liter of ZQW35 (Fig. 3B and 6B). However, the toxicity of 3 g/liter n-butanol no longer significantly affected the fermentation phenotype of mutants derived from ZQW20 and ZQW30 (Fig. 4B). This provides an indication of why butanol outputs did not differ strongly between ZQW35 and ZQW25 (743B*4/pXY1-Pthl-adhE1-Pthl-ctfAB-adc) or between ZQW33 and ZQW23 (743B*4/pXY1-Pthl-adhE1) (Fig. 6A and Fig. S6). These results imply that n-butanol inhibition can limit the n-butanol output of the unevolved C. cellulovorans strain; however, this seems not to be the essential determinant for the achievement of high n-butanol titers.
The integration of metabolic and evolutionary engineering offered a stepwise promotion of n-butanol output (Table S1 and Fig. S7), which accelerated the overall development of a C. cellulovorans CBP-enabling microbial chassis for increased n-butanol production using lignocellulose or glucose as the sole carbon source. The n-butanol production of C. cellulovorans using AECC could be enhanced by 138-fold, from less than 0.025 g/liter to 3.47 g/liter (Fig. 8). Among CBPs for n-butanol production with a single recombinant clostridium strain using various lignocellulosic biomasses, the presented results represent significant progress in n-butanol titers and productivity (Table 1). In addition, the evolved strains may offer indications for new n-butanol tolerance mechanisms or target genes for reverse metabolic engineering. The engineered C. cellulovorans strain offers a promising microbial chassis for n-butanol production from lignocellulose by CBP.
FIG 8.
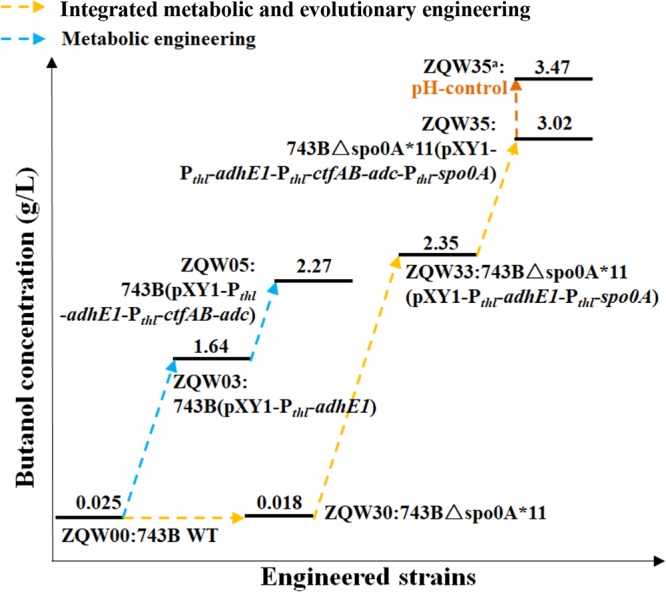
Integrated metabolic and evolutionary engineering enhanced n-butanol production of C. cellulovorans from AECC.
TABLE 1.
Comparison of n-butanol production with single recombinant clostridium strain by CBP
| Strain | Recombinant strategy | Substrate | Titer (g/liter)a | Productivity (g/liter/h) | Reference |
|---|---|---|---|---|---|
| C. acetobutylicum ATCC 824 | Overexpression optimization of chimeric cellulosomal operons | Crystalline cellulose | ND | ND | 15 |
| Grafting carrier modules at heterologous cellulases’ N termini | Crystalline cellulose | ND | ND | 60 | |
| Integrating synthetic cellulosomal operons into genome using allele-coupled exchange | Cellohexaose | ND | ND | 14 | |
| Anchoring the recombinant cellulosome to the cell surface using the native sortase system | Untreated wheat straw | ND | ND | 13 | |
| C. cellulolyticum ATCC 35319 | Introducing CoA-dependent pathway | Crystalline cellulose | 0.12 | 0.00025 | 17 |
| C. cellulovorans DSM 743B | Overexpressing adhE2 from C. acetobutylicum ATCC 824 | Crystalline cellulose | 1.42 | 0.0056 | 28 |
| Integrated metabolic and evolutionary engineering | Corn cobs (alkali extracted) | 3.47 | 0.0413 | This work |
ND, not determined.
However, the n-butanol titer and ratio in this study still offer room for improvement. As shown in Fig. 7B and D, a residual of 3.76 g/liter of butyrate and 6.16 g/liter of xylose, which should ideally have been converted to butanol, remained in the broth at the end of fermentation (84 h). To either avoid or decrease butyrate production, endogenous buk knockout was attempted in C. cellulovorans using ClosTron. However, no mutant of C. cellulovorans with inactivated buk was obtained. This may be due to an inherent limitation of group II intron technology, the inactivation efficiency of which is gene specific (or dependent) (55, 56). In other studies, the buk gene in C. acetobutylicum ATCC 824 has been successfully inactivated using in-frame deletion methods; consequently, buk inactivation directed carbon flux from butyrate to butanol (57, 58). A similar approach may work for C. cellulovorans. With regard to xylose, metabolic engineering is ideally suited for C. cellulovorans to alleviate carbon catabolite repression (CCR) and promote butanol conversion. The reason for this is that insufficient uptake and utilization of xylose lead to slow growth and excessively long fermentation. Strategies such as overexpression of xylose symporter (XylT) and inactivation of xylose operon transcriptional repressor (XylR) have proven successful in C. beijerinckii and C. tyrobutyricum (48, 59).
Similar to the achievements reported for other clostridia, ABE pathway engineering (32, 58), pentose metabolic engineering (48, 59), cellulase engineering (13), and understanding the resistance mechanism of inhibitors (41, 42, 53) will likely promote the further development of the C. cellulovorans platform more effectively.
MATERIALS AND METHODS
Strains, plasmids, primers, and strain cultivation.
All bacterial strains, plasmids, and primers used in this study are listed in Tables 2 and 3 and Fig. 5. Escherichia coli DH5α and ER2275 were aerobically cultivated at 37°C in liquid or solidified Luria-Bertani (LB) medium, which was composed of 5g/liter of yeast extract (AngelYeast Co., Ltd., China), 10 g/liter of tryptone (AngelYeast Co., Ltd., China), and 10 g/liter of NaCl with 1.5% agar (wt/vol) if necessary. C. cellulovorans DSM 743B was cultivated and evolved in Clocel medium (6, 49). The Clocel solidified medium contained only 1.0% (wt/vol) agar. Previously published protocols were employed for both the cultivation and genetic modification of C. cellulovorans DSM 743B (6). All stock cultures were maintained in 25% glycerol and frozen at −80°C.
TABLE 2.
Strains and plasmids used in this study
| Strain or plasmida | Description or genotypeb | Source or reference(s) |
|---|---|---|
| Strains | ||
| E. coli strains | ||
| DH5α | Commercial transformation host | Gibco-BRL, Life Technologies |
| ER2275 | Strain used to premethylate plasmids for electron-transformation in C. acetobutylicum ATCC 824 and C. cellulovorans DSM 743B | New England Biolabs |
| C. acetobutylicum strains | ||
| ATCC 824 | Wild type | ATCC |
| ZQW00 | DSM 743B; wild type | DSMZ |
| ZQW01 | 743B/pXY1- Pthl | 6 |
| ZQW02 | 743B/pXY1-Pthl-adhE2 | This study |
| ZQW03 | 743B/pXY1-Pthl-adhE1 | This study |
| ZQW04 | 743B/pXY1-Pthl-ctfAB-adc | This study |
| ZQW05 | 743B/pXY1-Pthl-adhE1-Pthl-ctfAB-adc | This study |
| ZQW06 | 743B/pXY1-Pthl-bukI | This study |
| ZQW07 | 743B/pXY1-Pthl-bukI-Pthl-adhE1 | This study |
| ZQW11 | 743B/pXY1-Pthl-adhE1-Pthl-spo0A | This study |
| ZQW10 | 743B spo0A::intron | 6 |
| ZQW11 | 743B spo0A::intron/pXY1-Pthl-spo0A | 6 |
| ZQW13 | 743B spo0A::intron/pXY1-Pthl-adhE1-Pthl-spo0A | This study |
| ZQW15 | 743B Δspo0A::intron/pXY1-Pthl-adhE1-Pthl-ctfAB-adc-Pthl-spo0A | This study |
| ZQW20 | 743B*4; evolved strain from wild-type ZQW00 | This study |
| ZQW23 | 743B*4/pXY1-Pthl-adhE1 | This study |
| ZQW25 | 743B*4/pXY1-Pthl-adhE1-Pthl-ctfAB-adc | This study |
| ZQW30 | 743B Δspo0A*11; evolved strain from wild-type ZQW10 | This study |
| ZQW31 | 743B Δspo0A*11/pXY1-Pthl-spo0A | This study |
| ZQW33 | 743B Δspo0A*11/pXY1-Pthl-adhE1-Pthl-spo0A | This study |
| ZQW35 | 743B Δspo0A*11/pXY1-Pthl-adhE1-Pthl-ctfAB-adc-Pthl-spo0A | This study |
| Plasmids | ||
| pANS1 | Φ3TI gene, p15A origin; Specr | 61 |
| pXY1-Pthl | Ampr, MLSr, pCB102 ori, ColE1 origin, Thl (CA_c2873) promoter region of C. acetobutylicum; E. coli-C. cellulovorans shuttle vector for expressing genes in C. cellulovorans | 62 |
| pXY1-Pthl-adhE1 | Derived from pXY1-Pthl, with the adhE1 (CA_p0162) gene from C. acetobutylicum overexpression | This study |
| pXY1-Pthl-adhE2 | Derived from pXY1-Pthl, with adhE2 (CA_p0035) gene from C. acetobutylicum overexpression | This study |
| pXY1-Pthl-ctfAB-adc | Derived from pXY1-Pthl, with ctfAB-adc cassette (CA_p0163-0164-0165) from C. acetobutylicum overexpression | This study |
| pXY1-Pthl-adhE1-Pthl-ctfAB-adc | Derived from pXY1-Pthl, with adhE1 gene and ctfAB-adc cassette from C. acetobutylicum overexpression | This study |
| pXY1-Pthl-bukI | Derived from pXY1-Pthl, with bukI (CA_C3075) gene from C. acetobutylicum overexpression | This study |
| pXY1-Pthl-bukI-Pthl-adhE1 | Derived from pXY1-Pthl, with bukI and adhE1 gene from C. acetobutylicum overexpression | This study |
| pWJ1-spo0A | Derived from pWJ1 for intron insertion in C. cellulovorans spo0A gene (Clocel_1943) at 586/587 nt | 6, 63 |
| pXY1-Pthl-spo0A | Derived from pXY1-Pthl, with C. cellulovorans spo0A gene expression (complementation) | This study |
| pXY1-Pthl-adhE1-Pthl-spo0A | Derived from pXY1-Pthl, with adhE1 gene from C. acetobutylicum overexpression and C. cellulovorans spo0A gene complementation | This study |
| pXY1-Pthl-adhE1-Pthl-ctfAB-adc-Pthl-spo0A | Derived from pXY1-Pthl, with adhE1 gene and ctfAB-adc cassette from C. acetobutylicum overexpression, and C. cellulovorans spo0A gene complementation | This study |
ATCC, American Type Culture Collection, USA; DSMZ, Deutsche Sammlung von Mikroorganismen und Zellkulturen, Germany.
Specr, spectinomycin resistance; Ampr, ampicillin resistance; MLSr, macrolide-lincosamide-streptogramin resistance; pCB102 ori, Gram-positive origin of replication from Clostridium butyricum; Thl, thiolase.
TABLE 3.
Primers used in this study
| Primer name | Sequence (5′–3′) | Description |
|---|---|---|
| EBS universal | CGAAATTAGAAACTTGCGTTCAGTAAAC | Targetron primera |
| spo0A-586|587a-IBS | CCGCTCGAGATAATTATCCTTACTTTTCTTACAGGTGCGCCCAGATAGGGTG | spo0A Targetron primera |
| spo0A-586|587a-EBS1d | AGATTGTACAAATGTGGTGATAACAGATAAGTCTTACAGCTTAACTTACCTTTCTTTGT | spo0A Targetron primera |
| spo0A-586|587a-EBS2 | TGAACGCAAGTTTCTAATTTCGGTTAAAAGTCGATAGAGGAAAGTGTCT | spo0A Targetron primera |
| spo0A_447-467 | CGTATGAATCTAAGCCAGTAG | Forward primer inside spo0A from base 447 to 467 |
| spo0A_743-764 | TTGGTTTACCTTTTGATGTATG | Revise primer inside spo0A from base 743 to 764 |
| spo0A F | TAGGAGGTTAGTTAGAGGATCCCCATGGACAATTCAAAAATAAGTG | Forward primer for gene Clocel_1943 clone from 743B chromosome and assembly in plasmid pXY1-Pthl-spo0A |
| spo0A R | CGACGGCCAGTGAATTCCCTTAACTTACTTTGTTTTTAAGTC | Reverse primer for gene Clocel_1943 clone from 743B chromosome and assembly in plasmid pXY1-Pthl-spo0A |
| adhE1 F | AAATTTAGGAGGTTAGTTAGAGGATCCCCATGAAAGTCACAACAGTAAAGGAATTAGAT | Forward primer for gene adhE1 clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-adhE1 |
| adhE1 R | GACGTTGTAAAACGACGGCCAGTGAATTCCCTTAAGGTTGTTTTTTAAAACAATTTATA | Reverse primer for gene adhE1 clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-adhE1 |
| adhE2 F | ATTTAGGAGGTTAGTTAGAGGATCCCCATGAAAGTTACAAATCAAAAAGAACTAAAACA | Forward primer for gene adhE2 clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-adhE2 |
| adhE2 R | GTTGTAAAACGACGGCCAGTGAATTCCCTTAAAATGATTTTATATAGATATCCTTAAGT | Reverse primer for gene adhE2 clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-adhE2 |
| bukI F | ATTTAGGAGGTTAGTTAGAGGATCCCCATGTATAGATTACTAATAATCAATCCTGGCTC | Forward primer for gene bukI clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-bukI |
| bukI R | CGTTGTAAAACGACGGCCAGTGAATTCCCTTATTTGTATTCCTTAGCTTTTTCTTCTCC | Reverse primer for gene bukI clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-bukI |
| bukI F | ATTTAGGAGGTTAGTTAGAGGATCCCCATGTATAGATTACTAATAATCAATCCTGGCTC | Forward primer for gene bukI clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-bukI-Pthl-adhE1 |
| Pthl-adhE1-bukI R | TTATTATTTTTATCAATATATTTTGTTAAAAATTATTTGTATTCCTTAGCTTTTTCTTC | Reverse primer for gene bukI clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-bukI-Pthl-adhE1 |
| bukI-Pthl-adhE1 F | GAAGAAAAAGCTAAGGAATACAAATAATTTTTAACAAAATATATTGATAAAAATAATAA | Forward primer for cassette Pthl-adhE1clone from pXY1-Pthl-adhE1 and assembly in plasmid pXY1-Pthl-bukI-Pthl-adhE1 |
| adhE1 R | GACGTTGTAAAACGACGGCCAGTGAATTCCCTTAAGGTTGTTTTTTAAAACAATTTATA | Reverse primer for cassette Pthl-adhE1clone from pXY1-Pthl-adhE1 and assembly in plasmid pXY1-Pthl-bukI-Pthl-adhE1 |
| ctfAB-adc F | ATTTAGGAGGTTAGTTAGAGGATCCCCATGAACTCTAAAATAATTAGATTTGAAAATTT | Forward primer for cassette ctfAB-adc clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-ctfAB-adc |
| ctfAB-adc R | GACGTTGTAAAACGACGGCCAGTGAATTCCCTAAGTTTATATAAATCTATTATGCAGAA | Reverse primer for cassette ctfAB-adc clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-ctfAB-adc |
| adhE1 F | AAATTTAGGAGGTTAGTTAGAGGATCCCCATGAAAGTCACAACAGTAAAGGAATTAGAT | Forward primer for gene adhE1 clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-adhE1-Pthl-ctfAB-adc |
| Pthl-ctfAB-dhE R | TATTATTTTTATCAATATATTTTGTTAAAAATTAAGGTTGTTTTTTAAAACAATTTATA | Reverse primer for gene adhE1 clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-adhE1-Pthl-ctfAB-adc |
| adhE-ctfAB-adc F | TATAAATTGTTTTAAAAAACAACCTTAATTTTTAACAAAATATATTGATAAAAATAATA | Forward primer for cassette Pthl-ctfAB-adc clone from pXY1-Pthl-ctfAB-adc and assembly in plasmid pXY1-Pthl-adhE1-Pthl-ctfAB-adc |
| ctfAB-adc R | GACGTTGTAAAACGACGGCCAGTGAATTCCCTAAGTTTATATAAATCTATTATGCAGAA | Reverse primer for cassette Pthl-ctfAB-adc clone from pXY1-Pthl-ctfAB-adc and assembly in plasmid pXY1-Pthl-adhE1-Pthl-ctfAB-adc |
| adhE1 F | AAATTTAGGAGGTTAGTTAGAGGATCCCCATGAAAGTCACAACAGTAAAGGAATTAGAT | Forward primer for gene adhE1 clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-adhE1-Pthl-spo0A |
| Pthl-spo0A-adhE1 R | TATTATTTTTATCAATATATTTTGTTAAAAATTAAGGTTGTTTTTTAAAACAATTTATA | Reverse primer for gene adhE1 clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-adhE1-Pthl-spo0A |
| adhE1-Pthl-spo0A F | TATAAATTGTTTTAAAAAACAACCTTAATTTTTAACAAAATATATTGATAAAAATAATA | Forward primer for cassette Pthl-spo0A clone from pXY1-spo0A and assembly in plasmid pXY1-Pthl-adhE1-Pthl-spo0A |
| spo0A R | CGACGGCCAGTGAATTCCCTTAACTTACTTTGTTTTTAAGTC | Reverse primer for cassette Pthl-spo0A clone from pXY1-spo0A and assembly in plasmid pXY1-Pthl-adhE1-Pthl-spo0A |
| adhE1 F | AAATTTAGGAGGTTAGTTAGAGGATCCCCATGAAAGTCACAACAGTAAAGGAATTAGAT | Forward primer for gene adhE1 clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-adhE1-Pthl-ctfAB-adc-Pthl-spo0A |
| Pthl-ctfAB-adhE1 R | TATTATTTTTATCAATATATTTTGTTAAAAATTAAGGTTGTTTTTTAAAACAATTTATA | Reverse primer for gene adhE1 clone from C. acetobutylicum genome and assembly in plasmid pXY1-Pthl-adhE1-Pthl-ctfAB-adc-Pthl-spo0A |
| adhE1-ctfAB-adc F | TATAAATTGTTTTAAAAAACAACCTTAATTTTTAACAAAATATATTGATAAAAATAATA | Forward primer for cassette Pthl-ctfAB-adc clone from pXY1-Pthl-ctfAB-adc and assembly in plasmid pXY1-Pthl-adhE1-Pthl-ctfAB-adc-Pthl-spo0A |
| Pthl-spo0A-ctfAB-adc R | TATTATTTTTATCAATATATTTTGTTAAAAATAAGTTTATATAAATCTATTATGCAGAA | Reverse primer for cassette Pthl-ctfAB-adc clone from pXY1-Pthl-ctfAB-adc and assembly in plasmid pXY1-Pthl-adhE1-Pthl-ctfAB-adc-Pthl-spo0A |
| ctfAB-adc-Pthl-spo0A F | TTCTGCATAATAGATTTATATAAACTTATTTTTAACAAAATATATTGATAAAAATAATA | Forward primer for gene Clocel_1943 clone from 743B chromosome and assembly in plasmid pXY1-Pthl-adhE1-Pthl-ctfAB-adc-Pthl-spo0A |
| spo0A R | CGACGGCCAGTGAATTCCCTTAACTTACTTTGTTTTTAAGTC | Reverse primer for gene Clocel_1943 clone from 743B chromosome and assembly in plasmid pXY1-Pthl-adhE1-Pthl-ctfAB-adc-Pthl-spo0A |
Designed by online tools (www.clostron.com).
DNA manipulation and vector construction.
All DNA sequencing and oligonucleotide primers syntheses were conducted by Shanghai RuiDi Biological Technology Co., Ltd. (Shanghai, China). Previously described standard procedures were used to accomplish all recombinant DNA manipulations, including genomic DNA preparation, DNA fragment amplification, digestion, ligation, transformation, colony PCR, plasmid extraction, and identification (6).
The construction of the ClosTron knockout plasmid pWJ1-spo0A derived from pWJ1 and all overexpression (or complementation) plasmids derived from pXY1-Pthl was conducted according to previously published standard procedures (6).
Generation and screening of genetically modified strains.
Electrocompetent cell preparation and electro-transformation of C. cellulovorans DSM 743B (here referred to as 743B) were conducted using previously described procedures (6). Positive transformants were screened and identified by colony PCR. Specially, for transformants that were generated from the electro-transformation of pWJ1-spo0A, PCR products of the desired mutants were approximately 0.9 kb longer than those of the wild type due to insertion of the group II intron (6). ClosTron knockout plasmids were cured by repeated subculture on plates without erythromycin until a single colony that was sensitive to erythromycin had been isolated (6). Figure 5 shows the derivative relationship of all genetically modified C. cellulovorans strains in this work.
Butanol tolerance evolutionary engineering and growth tolerance assay.
Evolutionary engineering was accomplished by four stages with a stepwise increment of n-butanol (3, 6, 9, and 12 g/liter) supplemented to the medium. ZQW10 (743B Δspo0A) and ZQW00 (743B) were grown in tubes with 10 ml of liquid medium in an anaerobic chamber (Thermo Forma, Inc., Waltham, MA, USA) at 37°C. Cultures were inoculated to an OD600 of 0.3, grown for 24 h, and then harvested by centrifugation (3,000 × g, 2 min) for fresh inoculation. Evolution in medium with higher n-butanol concentrations was not launched unless an OD600 of 1.0 was achieved within 24 h. Samples from the last batch were spread on solidified medium for clone selection.
A growth assay was used to evaluate the n-butanol tolerance of C. cellulovorans. The genetically modified or evolved C. cellulovorans strains were inoculated in medium with various concentrations of n-butanol (0, 3, 6, 9, and 12 g/liter) and grown for 48 h. During this time, their OD600 values were monitored and compared.
Batch fermentation of C. cellulovorans with constant pH and two-stage pH control.
Alkali-extracted corn cobs (AECC) were manufactured according to a previously described protocol, including alkali extraction, neutralization, thorough washing, and drying. The particles varied from a 30- to 40-mesh size (0.45 to 0.60 mm). Cellulose, hemicellulose, and lignin contents were 69.8%, 27.4%, and 1.47% (wt/wt), respectively (6). All batch fermentations with glucose or AECC as the sole carbon source for phenotype comparison (expressed as bar charts) were conducted in 500-ml anaerobic shaken flasks (customized from Guxin Biotech Co., Ltd., Shanghai, China) with a 400-ml working volume. All batch fermentations for the study of the metabolic progress (expressed as line charts) were conducted in a 3-liter BioFlo 110 bioreactor (New Brunswick Scientific Co., Inc., NJ, USA) with a 1.6-liter working volume. The pH was controlled via automatic addition of 5 N NaOH, while temperature and stirring speed were kept at 37°C and 150 rpm, respectively. The medium was supplemented with 15 mg/liter erythromycin for mutants that harbored pXY1 or pWJ1 series plasmids. Samples were collected in an anaerobic chamber at regular intervals, and enzyme activity and biomass, as well as substrate and product concentration, were analyzed. The inoculum size of C. cellulovorans DSM 743B was 10% (vol/vol) if not indicated otherwise (49).
Analysis.
A DU730 spectrophotometer (Beckman Coulter) was used to monitor cell growth on glucose at an optical density of 600 nm (OD600). The cell mass on AECC particles and the AECC concentration were calculated according to previously reported procedures (49). Volatile solvents and organic acids (ethanol, n-butanol, acetone acetate, and butyrate) were determined by gas chromatography (7890 A; Agilent, Wilmington, DE, USA), while lactate, monosaccharides, and disaccharides were measured by a high-performance liquid chromatography (HPLC) system (1200 series; Agilent, Wilmington, DE, USA) (6). The assay of butyraldehyde dehydrogenase and butanol dehydrogenase activities in ZQW01, ZQW02, and ZQW03 were accomplished according to the protocol of Yang et al. except that the crude cell extract was obtained using a French press (Constant Systems Limited, UK) (28). The carbon recovery of C2, C3, and C4 products (acetate, ethanol, lactate, butyrate, n-butanol, and acetone) was calculated as previously described (6).
Supplementary Material
ACKNOWLEDGMENTS
Zhiqiang Wen, Yu Jiang, and Sheng Yang performed the studies and drafted the manuscript; Rodrigo Ledesma-Amaro and Jianping Lin drafted and revised the manuscript.
This work is supported by grants from the National Natural Science Foundation of China (21706133, 21825804, and 20876141), the Fundamental Research Funds for the Central Universities (30918011310), Natural Science Foundation of Shanghai (Shanghai Natural Science Foundation, 18ZR1446500), Key Laboratory of Biomass Chemical Engineering of Ministry of Education, Zhejiang University (2018BCE003), and the Program for Zhejiang Leading Team of S&T Innovation (2011R50002).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02560-18.
REFERENCES
- 1.Ren C, Wen Z, Xu Y, Jiang W, Gu Y. 2016. Clostridia: a flexible microbial platform for the production of alcohols. Curr Opin Chem Biol 35:65–72. doi: 10.1016/j.cbpa.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Chen CT, Liao JC. 2016. Frontiers in microbial 1-butanol and isobutanol production. FEMS Microbiol Lett 363:fnw020. doi: 10.1093/femsle/fnw020. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert LA, Horlbeck MA, Adamson B, Villalta JE, Chen Y, Whitehead EH, Guimaraes C, Panning B, Ploegh HL, Bassik MC, Qi LS, Kampmann M, Weissman JS. 2014. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159:647–661. doi: 10.1016/j.cell.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang Y, Liu J, Jiang W, Yang Y, Yang S. 2015. Current status and prospects of industrial bio-production of n-butanol in China. Biotechnol Adv 33:1493–1501. doi: 10.1016/j.biotechadv.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Olson DG, McBride JE, Shaw AJ, Lynd LR. 2012. Recent progress in consolidated bioprocessing. Curr Opin Biotechnol 23:396–405. doi: 10.1016/j.copbio.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Wen Z, Minton NP, Zhang Y, Li Q, Liu J, Jiang Y, Yang S. 2017. Enhanced solvent production by metabolic engineering of a twin-clostridial consortium. Metab Eng 39:38–48. doi: 10.1016/j.ymben.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Brethauer S, Studer MH. 2014. Consolidated bioprocessing of lignocellulose by a microbial consortium. Energy Environ Sci 7:1446. doi: 10.1039/c3ee41753k. [DOI] [Google Scholar]
- 8.Salehi Jouzani G, Taherzadeh MJ. 2015. Advances in consolidated bioprocessing systems for bioethanol and butanol production from biomass: a comprehensive review. Biofuel Res J 5:152–195. doi: 10.18331/BRJ2015.2.1.4. [DOI] [Google Scholar]
- 9.Hasunuma T, Ishii J, Kondo A. 2015. Rational design and evolutional fine tuning of Saccharomyces cerevisiae for biomass breakdown. Curr Opin Chem Biol 29:1–9. doi: 10.1016/j.cbpa.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 10.den Haan R, van Rensburg E, Rose SH, Görgens JF, van Zyl WH. 2015. Progress and challenges in the engineering of non-cellulolytic microorganisms for consolidated bioprocessing. Curr Opin Biotechnol 33:32–38. doi: 10.1016/j.copbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Yamada R, Hasunuma T, Kondo A. 2013. Endowing non-cellulolytic microorganisms with cellulolytic activity aiming for consolidated bioprocessing. Biotechnol Adv 31:754–763. doi: 10.1016/j.biotechadv.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Yang X, Chen C-C, Yang S-T. 2014. Engineering clostridia for butanol production from biorenewable resources: from cells to process integration. Curr Opin Chem Biol 6:43–54. doi: 10.1016/j.coche.2014.09.003. [DOI] [Google Scholar]
- 13.Willson BJ, Kovacs K, Wilding-Steele T, Markus R, Winzer K, Minton NP. 2016. Production of a functional cell wall-anchored minicellulosome by recombinant Clostridium acetobutylicum ATCC 824. Biotechnol Biofuels 9:109. doi: 10.1186/s13068-016-0526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovács K, Willson BJ, Schwarz K, Heap JT, Jackson A, Bolam DN, Winzer K, Minton NP. 2013. Secretion and assembly of functional mini-cellulosomes from synthetic chromosomal operons in Clostridium acetobutylicum ATCC 824. Biotechnol Biofuels 6:117. doi: 10.1186/1754-6834-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mingardon F, Chanal A, Tardif C, Fierobe HP. 2011. The issue of secretion in heterologous expression of Clostridium cellulolyticum cellulase-encoding genes in Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 77:2831–2838. doi: 10.1128/AEM.03012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin PP, Mi L, Morioka AH, Yoshino KM, Konishi S, Xu SC, Papanek BA, Riley LA, Guss AM, Liao JC. 2015. Consolidated bioprocessing of cellulose to isobutanol using Clostridium thermocellum. Metab Eng 31:44–52. doi: 10.1016/j.ymben.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Gaida SM, Liedtke A, Jentges AH, Engels B, Jennewein S. 2016. Metabolic engineering of Clostridium cellulolyticum for the production of n-butanol from crystalline cellulose. Microb Cell Fact 15:6. doi: 10.1186/s12934-015-0406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higashide W, Li Y, Yang Y, Liao JC. 2011. Metabolic engineering of Clostridium cellulolyticum for production of isobutanol from cellulose. Appl Environ Microbiol 77:2727–2733. doi: 10.1128/AEM.02454-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoseyov O, Doi RH. 1990. Essential 170-kDa subunit for degradation of crystalline cellulose by Clostridium cellulovorans cellulase. Proc Natl Acad Sci U S A 87:2192–2195. doi: 10.1073/pnas.87.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shoseyov O, Takagi M, Goldstein MA, Doi RH. 1992. Primary sequence analysis of Clostridium cellulovorans cellulose binding protein A. Proc Natl Acad Sci U S A 89:3483–3487. doi: 10.1073/pnas.89.8.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamaru Y, Miyake H, Kuroda K, Nakanishi A, Kawade Y, Yamamoto K, Uemura M, Fujita Y, Doi RH, Ueda M. 2010. Genome sequence of the cellulosome-producing mesophilic organism Clostridium cellulovorans 743B. J Bacteriol 192:901–902. doi: 10.1128/JB.01450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu CG, Huang RR, Teng L, Jing XY, Hu JQ, Cui GZ, Wang YL, Cui Q, Xu J. 2015. Cellulosome stoichiometry in Clostridium cellulolyticum is regulated by selective RNA processing and stabilization. Nat Commun 6:6900. doi: 10.1038/ncomms7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon SD, Kim SJ, Park SH, Choi G-W, Han SO. 2015. Hydrolytic effects of scaffolding proteins CbpB and CbpC on crystalline cellulose mediated by the major cellulolytic complex from Clostridium cellulovorans. Bioresour Technol 191:505–511. doi: 10.1016/j.biortech.2015.02.071. [DOI] [PubMed] [Google Scholar]
- 24.Bianchetti CM, Brumm P, Smith RW, Dyer K, Hura GL, Rutkoski TJ, Phillips GN. 2013. Structure, dynamics, and specificity of endoglucanase D from Clostridium cellulovorans. J Mol Biol 425:4267–4285. doi: 10.1016/j.jmb.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morisaka H, Matsui K, Tatsukami Y, Kuroda K, Miyake H, Tamaru Y, Ueda M. 2012. Profile of native cellulosomal proteins of Clostridium cellulovorans adapted to various carbon sources. AMB Express 2:37. doi: 10.1186/2191-0855-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou YJ, Buijs NA, Zhu Z, Gomez DO, Boonsombuti A, Siewers V, Nielsen J. 2016. Harnessing yeast peroxisomes for biosynthesis of fatty-acid-derived biofuels and chemicals with relieved side-pathway competition. J Am Chem Soc 138:15368–15377. doi: 10.1021/jacs.6b07394. [DOI] [PubMed] [Google Scholar]
- 27.Yang X, Xu M, Yang ST. 2016. Restriction modification system analysis and development of in vivo methylation for the transformation of Clostridium cellulovorans. Appl Microbiol Biotechnol 100:2289–2299. doi: 10.1007/s00253-015-7141-9. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Xu M, Yang ST. 2015. Metabolic and process engineering of Clostridium cellulovorans for biofuel production from cellulose. Metab Eng 32:39–48. doi: 10.1016/j.ymben.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Sleat R, Mah RA, Robinson R. 1984. Isolation and characterization of an anaerobic, cellulolytic bacterium, Clostridium cellulovorans sp. nov. Appl Environ Microbiol 48:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JY, Jang Y-S, Lee J, Papoutsakis ET, Lee SY. 2009. Metabolic engineering of Clostridium acetobutylicum M5 for highly selective butanol production. Biotechnol J 4:1432–1440. doi: 10.1002/biot.200900142. [DOI] [PubMed] [Google Scholar]
- 31.Sillers R, Chow A, Tracy B, Papoutsakis ET. 2008. Metabolic engineering of the non-sporulating, non-solventogenic Clostridium acetobutylicum strain M5 to produce butanol without acetone demonstrate the robustness of the acid-formation pathways and the importance of the electron balance. Metab Eng 10:321–332. doi: 10.1016/j.ymben.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Yoo M, Croux C, Meynial-Salles I, Soucaille P. 2016. Elucidation of the roles of adhE1 and adhE2 in the primary metabolism of Clostridium acetobutylicum by combining in-frame gene deletion and a quantitative system-scale approach. Biotechnol Biofuels 9:92. doi: 10.1186/s13068-016-0507-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durre P. 2008. Fermentative butanol production: bulk chemical and biofuel. Ann N Y Acad Sci 1125:353–362. doi: 10.1196/annals.1419.009. [DOI] [PubMed] [Google Scholar]
- 34.Gong F, Bao G, Zhao C, Zhang Y, Li Y, Dong H. 2016. Fermentation and genomic analysis of acetone-uncoupled butanol production by Clostridium tetanomorphum. Appl Microbiol Biotechnol 100:1523–1529. doi: 10.1007/s00253-015-7121-0. [DOI] [PubMed] [Google Scholar]
- 35.Sabra W, Groeger C, Sharma PN, Zeng AP. 2014. Improved n-butanol production by a non-acetone producing Clostridium pasteurianum DSMZ 525 in mixed substrate fermentation. Appl Microbiol Biotechnol 98:4267–4276. doi: 10.1007/s00253-014-5588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jang YS, Im JA, Choi SY, Lee JI, Lee SY. 2014. Metabolic engineering of Clostridium acetobutylicum for butyric acid production with high butyric acid selectivity. Metab Eng 23:165–174. doi: 10.1016/j.ymben.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann D, Radomski N, Lütke-Eversloh T. 2012. New insights into the butyric acid metabolism of Clostridium acetobutylicum. Appl Microbiol Biotechnol 96:1325–1339. doi: 10.1007/s00253-012-4109-x. [DOI] [PubMed] [Google Scholar]
- 38.Yu L, Zhao J, Xu M, Dong J, Varghese S, Yu M, Tang IC, Yang S-T. 2015. Metabolic engineering of Clostridium tyrobutyricum for n-butanol production: effects of CoA transferase. Appl Microbiol Biotechnol 99:4917–4930. doi: 10.1007/s00253-015-6566-5. [DOI] [PubMed] [Google Scholar]
- 39.Jang YS, Lee JY, Lee JM, Lee SY. 2009. Metabolic engineering of Clostridium acetobutylicum M5 for butanol-ethanol production by complementation of adhE1 and ctfAB genes. New Biotechnol 25:S273–S274. doi: 10.1016/j.nbt.2009.06.616. [DOI] [Google Scholar]
- 40.Moon HG, Jang Y-S, Cho C, Lee J, Binkley R, Lee SY. 2016. One hundred years of clostridial butanol fermentation. FEMS Microbiol Lett 363:fnw001. doi: 10.1093/femsle/fnw001. [DOI] [PubMed] [Google Scholar]
- 41.Peabody GL, Kao KC. 2016. Recent progress in biobutanol tolerance in microbial systems with an emphasis on Clostridium. FEMS Microbiol Lett 363:fnw017. doi: 10.1093/femsle/fnw017. [DOI] [PubMed] [Google Scholar]
- 42.Nicolaou SA, Gaida SM, Papoutsakis ET. 2010. A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: From biofuels and chemicals, to biocatalysis and bioremediation. Metab Eng 12:307–331. doi: 10.1016/j.ymben.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Qureshi N, Hughes SR. 2017. Progress and perspectives on improving butanol tolerance. World J Microbiol Biotechnol 33:51. doi: 10.1007/s11274-017-2220-y. [DOI] [PubMed] [Google Scholar]
- 44.Dragosits M, Mattanovich D. 2013. Adaptive laboratory evolution—principles and applications for biotechnology. Microb Cell Fact 12:64. doi: 10.1186/1475-2859-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang S-T, Zhao J. May 2013. Adaptive engineering of Clostridium for increased butanol production. US Patent US8450093B1.
- 46.Li Y, Xu T, Tschaplinski TJ, Engle NL, Yang Y, Graham DE, He Z, Zhou J. 2014. Improvement of cellulose catabolism in Clostridium cellulolyticum by sporulation abolishment and carbon alleviation. Biotechnol Biofuels 7:25. doi: 10.1186/1754-6834-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kodama T, Endo K, Ara K, Ozaki K, Kakeshita H, Yamane K, Sekiguchi J. 2007. Effect of Bacillus subtilis spo0A mutation on cell wall lytic enzymes and extracellular proteases, and prevention of cell lysis. J Biosci Bioeng 103:13–21. doi: 10.1263/jbb.103.13. [DOI] [PubMed] [Google Scholar]
- 48.Xiao H, Li Z, Jiang Y, Yang Y, Jiang W, Gu Y, Yang S. 2012. Metabolic engineering of D-xylose pathway in Clostridium beijerinckii to optimize solvent production from xylose mother liquid. Metab Eng 14:569–578. doi: 10.1016/j.ymben.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Wen Z, Wu M, Lin Y, Yang L, Lin J, Cen P. 2014. Artificial symbiosis for acetone-butanol-ethanol (ABE) fermentation from alkali extracted deshelled corn cobs by co-culture of Clostridium beijerinckii and Clostridium cellulovorans. Microb Cell Fact 13:92. doi: 10.1186/s12934-014-0092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris LM, Welker NE, Papoutsakis ET. 2002. Northern, morphological, and fermentation analysis of spo0A inactivation and overexpression in Clostridium acetobutylicum ATCC 824. J Bacteriol 184:3586–3597. doi: 10.1128/JB.184.13.3586-3597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alsaker KV, Spitzer TR, Papoutsakis ET. 2004. Transcriptional analysis of spo0A overexpression in Clostridium acetobutylicum and its effect on the cell's response to butanol stress. J Bacteriol 186:1959–1971. doi: 10.1128/JB.186.7.1959-1971.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Al-Hinai MA, Jones SW, Papoutsakis ET. 2015. The Clostridium sporulation programs: diversity and preservation of endospore differentiation. Microbiol Mol Biol Rev 79:19–37. doi: 10.1128/MMBR.00025-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu M, Zhao J, Yu L, Tang IC, Xue C, Yang S-T. 2015. Engineering Clostridium acetobutylicum with a histidine kinase knockout for enhanced n-butanol tolerance and production. Appl Microbiol Biotechnol 99:1011–1022. doi: 10.1007/s00253-014-6249-7. [DOI] [PubMed] [Google Scholar]
- 54.Liu J, Lin Q, Chai X, Luo Y, Guo T. 2018. Enhanced phenolic compounds tolerance response of Clostridium beijerinckii NCIMB 8052 by inactivation of Cbei_3304. Microb Cell Fact 17:35. doi: 10.1186/s12934-018-0884-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP. 2007. The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods 70:452–464. doi: 10.1016/j.mimet.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 56.Enyeart PJ, Mohr G, Ellington AD, Lambowitz AM. 2014. Biotechnological applications of mobile group II introns and their reverse transcriptases: gene targeting, RNA-seq, and non-coding RNA analysis. Mob DNA 5:2. doi: 10.1186/1759-8753-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Green EM, Boynton ZL, Harris LM, Rudolph FB, Papoutsakis ET, Bennett GN. 1996. Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142:2079–2086. doi: 10.1099/13500872-142-8-2079. [DOI] [PubMed] [Google Scholar]
- 58.Yoo M, Croux C, Meynial-Salles I, Soucaille P. 2017. Metabolic flexibility of a butyrate pathway mutant of Clostridium acetobutylicum. Metab Eng 40:138–147. doi: 10.1016/j.ymben.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Fu H, Yang S-T, Wang M, Wang J, Tang IC. 2017. Butyric acid production from lignocellulosic biomass hydrolysates by engineered Clostridium tyrobutyricum overexpressing xylose catabolism genes for glucose and xylose co-utilization. Bioresour Technol 234:389–396. doi: 10.1016/j.biortech.2017.03.073. [DOI] [PubMed] [Google Scholar]
- 60.Chanal A, Mingardon F, Bauzan M, Tardif C, Fierobe HP. 2011. Scaffoldin modules serving as “cargo” domains to promote the secretion of heterologous cellulosomal cellulases by Clostridium acetobutylicum. Appl Environ Microbiol 77:6277–6280. doi: 10.1128/AEM.00758-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mermelstein L, Papoutsakis E. 1993. In vivo methylation in Escherichia coli by the Bacillus subtilis phage phi 3T I methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 59:1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang N, Shao L, Jiang Y, Gu Y, Li Q, Liu J, Jiang W, Yang S. 2015. I-SceI-mediated scarless gene modification via allelic exchange in Clostridium. J Microbiol Methods 108:49–60. doi: 10.1016/j.mimet.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Xiao H, Gu Y, Ning Y, Yang Y, Mitchell WJ, Jiang W, Yang S. 2011. Confirmation and elimination of xylose metabolism bottlenecks in glucose phosphoenolpyruvate-dependent phosphotransferase system-deficient Clostridium acetobutylicum for simultaneous utilization of glucose, xylose, and arabinose. Appl Environ Microbiol 77:7886–7895. doi: 10.1128/AEM.00644-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.